Abstract
Phase III evidence in the shape of a series of randomized controlled trials and meta-analyses has shown that laparoscopic gastrectomy is safe and gives better short-term results with respect to the traditional open technique for early-stage gastric cancer. In fact, in the East laparoscopic gastrectomy has become routine for early-stage gastric cancer. In contrast, the treatment of advanced gastric cancer through a minimally invasive way is still a debated issue, mostly due to worries about its oncological efficacy and the difficulty of carrying out an extended lymphadenectomy and intestinal reconstruction after total gastrectomy laparoscopically. Over the last ten years the introduction of robotic surgery has implied overcoming some intrinsic drawbacks found to be present in the conventional laparoscopic procedure. Robot-assisted gastrectomy with D2 lymphadenectomy has been shown to be safe and feasible for the treatment of gastric cancer patients. But unfortunately, most available studies investigating the robotic gastrectomy for gastric cancer compared to laparoscopic and open technique are so far retrospective and there have not been phase III trials. In the present review we looked at scientific evidence available today regarding the new high-tech surgical robotic approach, and we attempted to bring to light the real advantages of robot-assisted gastrectomy compared to the traditional laparoscopic and open technique for the treatment of gastric cancer.
Keywords: Gastric cancer, Gastric resection, Minimally invasive surgery, Robot-assisted gastrectomy
Core tip: Laparoscopic gastrectomy has been shown to be a viable option for early gastric cancer, showing survival rates comparable to those of open procedure. However, there has been criticism concerning the routine use of laparoscopy in patients with advanced gastric cancer, principally because it adapts poorly to complex maneuvers like D2 lymphadenectomy. Robotic surgery has been shown to make certain laparoscopic procedures easier and safer. Reports have recently shown the ever increasing feasibility and safety of robotic assisted laparoscopic gastrectomy for gastric cancer, in some cases even proving superior to traditional laparoscopy.
INTRODUCTION
In 1991, Kitano et al[1] performed the first laparoscopically assisted gastrectomy for gastric cancer. Subsequently, under the impulse of level III studies providing the evidence of the safety of laparoscopic assisted distal gastrectomy (LADG) for distal early-stage gastric cancer, several authors reported comparative studies with better short-term results in favor of this technique with respect to traditional open[2]. As a consequence laparoscopic gastrectomy (LG) has progressively spread worldwide, especially in the East, for the treatment of early gastric cancer[3,4]. On the other hand, the treatment of patients with advanced gastric cancer has always been considered difficult laparoscopically, thus techniques such as laparoscopic assisted total gastrectomy (LATG) and laparoscopic extended lymphadenectomy did not meet the same enthusiasm. As a result, the spread of laparoscopic surgery as a means of performing total gastrectomy and managing advanced gastric cancer was limited. This was mainly due to the technical difficulties and complexity of the D2 lymphadenectomy and the intestinal reconstruction after total gastrectomy[5,6].
Robot-assisted techniques have brought about improvements to certain surgical procedures, particularly those which require precise dissection, making it possible to resolve some of the innate limitations of laparoscopy. So over the years, robot-assisted gastrectomy (RAG) has become increasingly considered as a valid, yet still debatable, alternative to executing gastrectomy for gastric cancer, in particular for total gastric resection and extended lymph node dissection in advanced tumours[7-9]. We analyzed high-quality clinical trials by systematically reviewing the literature published so far in Pubmed comprehending robotic case series, as well as those studies that have compared RAG with LG and/or open gastrectomy for gastric cancer. Our intent is to verify if at present there is actual evidence of an advantage to robotic compared to laparoscopic and traditional open gastrectomy for gastric cancer.
Rational basis of robotic surgery as improvement of laparoscopy
Areas of surgery necessitating precise movements have employed Robotic technology. In 1994 the da Vinci® Surgical System (Intuitive Surgical, Sunnyvale, California, United States) gained the approval of the United States Food and Drug Administration (FDA). The da Vinci® Surgical Robotic has undergone constant improvement over recent years, and now includes additional features including near-infrared technology, and facilitated set-up. The latest generation, which was released in 2014 and is known as the da Vinci Xi™ system, is less bulky and its arms are more ergonomic (Figure 1).
Figure 1.
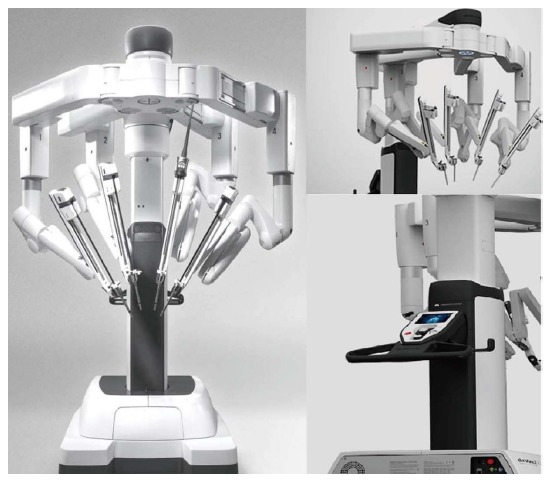
New-generation da Vinci Xi™; the system is more versatile and better manoeuvrable, the robotic arms are thinner and arranged in a more ergonomic way, enabling multiquadrant procedures without repositioning the system.
Robotic surgery eliminates some of the disadvantages of conventional laparoscopy. The principal drawbacks of conventional laparoscopy from a technical standpoint are: The instability of the two-dimensional (2D) camera; instruments with limited movement which augment the physiologic tremor of the surgeon’s hand, therefore limiting manipulative actions and increasing ergonomic discomfort.
The robotic surgery system has the upper hand over laparoscopy when fine dissection is needed, eliminating the traces of physiologic human tremor, increasing dexterity through its typical internal articulated endoscopic wrist (EndoWrist™ System), and providing stereoscopic vision with 3D high-resolution images[10]. This allows surgeons to perform minimally invasive surgery with greater ease and safety, and more ergonomically. As a consequence it probably makes it possible for more surgeons to complete complex procedure in a minimally invasive fashion.
Moreover, even if laparoscopic surgery may have an effect on the robotic gastrectomy learning process, robotic surgery appears to globally need less time to master compared to a laparoscopic procedure traditionally requiring a steep learning curve[11-14].
Main drawbacks of conventional laparoscopy in gastric cancer surgery
Delicate maneuvers which necessitate excellent visualization and total precision such as intra-corporeal anastomosis and dissection of extra-perigastric lymph nodes along the major arterial structures are the principal pitfalls of conventional laparoscopic gastrectomy for gastric cancer.
The far from perfect and often shallow angulation of the traditional unergonomic laparoscopic technique render the D2 lymphadenectomy especially hard and demanding even for minimally-invasive surgeons who have been solidly trained. Areas which are quite hard to reach during laparoscopic lymphadenectomy include lymph node stations 4, 6, 9, 11p and 12a[15]. It may be linked to the risk of important blood loss which can occur particularly during the lymph node dissection around the infra pyloric area and the inferior mesenteric vein, including stations 6 and 14, and the supra pancreatic area including stations 7, 8, and 9[16]. Miura et al[15] indicated a far inferior amount of harvested lymph nodes obtained by laparoscopy in comparison to open surgery along the major gastric curvature (Nos. 4 and 6) and second tier nodes along the celiac and splenic arteries (Nos. 9 and 11). Similarly, Bouras et al[17] showed a greatly inferior amount of lymph nodes harvested along the common hepatic artery in a series of laparoscopic distal gastrectomy procedures compared to open distal gastrectomy (ODG).
Main technical advantages of robotics over traditional laparoscopy in gastric cancer
The majority of resectable gastric cancer patients are advised to undergo gastrectomy with D2 lymph node dissection surgical procedure[18]. Thus, in gastric cancer treatment, in order to fit oncological criteria, minimally invasive procedures must entail proper lymphadenectomy, as in its traditional open counterpart.
It is widely accepted that D2 lympadenectomy is one of the most difficult steps of the laparoscopic gastrectomy procedure for gastric cancer. The certain advantage produced by the robotic system could be decisive in gastric cancer surgery, mainly ensuring an extremely precise and safe lymphadenectomy with reduced risk of vessel injury[19], thus making this phase a principal indicator for the robot-assisted technique. The advantages of robotic surgery, such as tremor filtration and articulated function of wristed instruments, would be particularly suitable for enabling more complete dissection in demanding areas such as the dorsal part of the pancreas and behind splenic vessels at the hilum, which are not easily identified and are difficult to reach with current laparoscopic instruments and camera system[20]. It is extremely hard to reach the back of the suprapancreatic lymphatic area laparoscopically, and the downward compression of the pancreas which is particular prominent through the laparoscopic instruments may lead to pancreatic damage and pancreatitis. In these sites especially, the EndoWrist® robotic property and a far more stable vision allow the surgeon to complete this surgical step more easily and safely in comparison to the laparoscopic counterpart.
Robotic surgery also has the advantage of making intra-corporeal anastomosis easier, and therefore overcomes one of the greatest limitations of traditional laparoscopy from a technical standpoint in carrying out digestive restoration. This is particularly true after total gastrectomy, otherwise made possible by extracorporeal anastomosis with a small mini-laparotomy. Placing a hand-sewn purse-string suture on the esophagus is made easier by using robotic assistance, and esophageal anastomosis can subsequently be carried out by using a circular stapler, as in open surgery[7,19]. Another option would be to carry out a full robotic hand-sewn esophagojejunal anastomosis[21], possible because the robotic system gives surgeons the chance to suture more easily and with greater precision compared to laparoscopy, particularly in deep and narrow areas. Thus, increased know-how and confidence with the robotic system will enable the surgeon to perform high-precision and safer intra-corporeal sutures for patients undergoing digestive anastomosis.
LITERATURE EVIDENCE
Studies of feasibility and safety
The earliest reports of robot-assisted gastrectomy (RAG) were published in 2003 by Hashizume et al[22] and Giulianotti et al[23]. Recent reports have shown the safety and viability of robotic gastrectomy for treating gastric cancer[24,25]. Table 1 summarizes some of the robotic case series published to date[7-9,26-34]. Most of the experience so far derives from non-randomized retrospective studies, while only one available clinical trial to date has been prospectively conducted[34]. The studies mainly hail from the East. In the western countries, reports on RAG are fewer and usually limited to smaller series. In 2007, in the United States Anderson et al[7] reported the results of the first western series including 7 gastric cancer patients who were submitted to robot-assisted subtotal gastrectomy, demonstrating that robotic gastrectomy was viable, even if no direct comparison with laparoscopy was made[7].
Table 1.
Robot-assisted laparoscopic gastrectomy series for treatment of gastric cancer
| Ref. | Country | Patients (n) | Stage disease |
Resection type |
Operative time1 (min ± SD) | Blood loss1 (mL ± SD) | Open conversion (%) | Harvested nodes1 (n ± SD) | Morbidity (%) | Mortality (%) | Hospital stay1 (d ± SD) | |
| Total | Subtotal | |||||||||||
| Anderson et al[7] | United States | 7 | 0-I-II | - | 7 | 420 ± NR | 300 ± NR | 0 | 24 ± NR | 11.1 | 0 | 4 ± NR |
| Patriti et al[8] | Italy | 13 | I-II-III | 4 | 9 | 286 ± 32.6 | 103 ± 87.5 | 0 | 28.1 ± 8.3 | 7.7 | 0 | 11.2 ± 4.3 |
| Song et al[9] | South Korea | 100 | I-II-III | 33 | 67 | 231.3 ± 43.2 | 128.2 ± 217.5 | 0 | 36.7 ± NR | 13 | 1 | 7.8 ± 17.1 |
| Pugliese et al[26] | Italy | 18 | All stages | - | 18 | 344 ± 62 | 90 ± 48 | 12 | 25 ± 4.5 | 6 | 6 | 10 ± 3 |
| Lee et al[27] | South Korea | 12 | I | - | 12 | 253.7 ± 53.0 | 135.8 ± 133.9 | 0 | 46.0 ± 25.5 | 8.3 | 0 | 6.6 ± 1.6 |
| D’Annibale et al[28] | Italy | 24 | I-II-III | 11 | 13 | 267.5 ± NR | 30 ± NR | 0 | 28 ± NR | 8.3 | 0 | 6 ± NR |
| Jiang et al[29] | China | 120 | I-II-III | 35 | 85 | 245 ± 50 | 70 ± 45 | 0.9 | 22.5 ± 10.7 | 5 | 0 | 6.3 ± 2.6 |
| Isogaki et al[30] | Japan | 61 | Not reported | 14 | 47 | 520 ± 177 TG 388 ± 85 SDG | 150 ± 234 TG 61.8 ± 46.5 SDG | 0 | 43 ± 14 TG 42 ± 18 SDG | 4.9 | 1.6 | 13.3 ± NR |
| Liu et al[31] | China | 104 | I-II-III | 54 | 50 | 272.52 ± 53.91 | 80.78 ± 32.37 | 2 | 23.1 ± 5.3 | 11.5 | 0 | 6.2 ± 2.5 |
| Park et al[32] | South Korea | 200 | All stages | 46 | 154 | 248.8 ± 55.6 | 146.1 ± 130.3 | 7 | 37.9 ± NR | 10 | 0.5 | 8.0 ± 3.7 |
| Coratti et al[33] | Itlay | 98 | All stages | 38 | 60 | 296.1 ± NR | 105.4 ± NR | 7.1 | 30.6 ± NR | 12.1 | 4.1 | 8.7 ± NR |
| Tokunaga et al[34] | Japan | 120 | I | 12 | 108 | 348.5 ± NR | 19 ± NR | 2.5 | 44 ± NR | 14.2 | 0 | 9 ± NR |
Mean value. SD: Standard deviation; NR: Not reported; TG: Total gastrectomy; SDG: Subtotal distal gastrectomy.
Several authors worldwide reported their experience on RAG for cancer and the largest single institution series investigating clinical and oncological outcomes so far include (Table 1): Song et al[9] in 2009, Jiang et al[29] in 2012, Liu et al[31] and Park et al[32] in 2013, Tokunaga et al[34] in 2015, which included respectively 100, 120, 104, 200 and 120 patients. These studies confirmed the safety and feasibility of RAG for cancer, essentially reporting a suitable amount of lymph nodes retrieved, but they did not furnish long-term survival data. Globally, among these various studies RAG appears to be safe in terms of the incidence and severity of postoperative complications. The morbidity rate ranges between 4.9% to 13%, with a mortality rate of 0%-6%, comparable to those of conventional gastric cancer surgery. Among reported potential advantages of the robotic procedure, Tokunaga et al[34] noted a very low incidence of intra-abdominal infectious complications (3.3%) in a large cohort of gastric cancer patients (n = 120) submitted to total or subtotal gastrectomy.
Comparative studies
Despite the existence of numerous reports regarding the safety and feasibility of RAG, only few robotic comparative analysis investigated RAG vs laparoscopic and/or open gastrectomy (Table 2)[11,12,24,25,35-50]. Most studies comparing robotic gastrectomy with open and laparoscopic surgery are retrospective case-control studies, almost all of these with sample sizes of fewer than 100 cases. Only one multi-centre comparative study was prospectively conducted: Kim et al[50], compared a total of 434 gastric cancer patients submitted to robotic and laparoscopic gastrectomy (223 vs 211 respectively), and showed similar overall complications rate with no operative mortality in either group, at the expense of significantly higher operative time and higher costs of the robotic group.
Table 2.
Comparative case-control studies of robot-assisted gastrectomy vs laparoscopic assisted gastrectomy and/or open gastrectomy
| Ref. | Subject | Stage disease |
Patients (n) |
Operation time (min)1 | Blood loss (mL)1 | Harvested nodes (n)1 | Morbidity (%) | Mortality (%) | Hospital stay (d)1 | ||
| RAG | LAG | OG | |||||||||
| Song et al[35] | RAG vs iLAG2 vs rLAG2 | I-II | 202 | 202 202 | - | 230 vs 289.5 vs 134.1 (RAG < iLAG > rLAG)3 | 94.8 RAG vs 39.5 rLAG (NS) | 35.3 vs 31.5 vs 42.7 (NS) | 5 vs 5 vs 10 (NS) | 0 vs 0 vs 0 | 5.7 vs 7.7 vs 6.2 (RAG < iLAG)3 (RAG~rLAG, NS) |
| Kim et al[36] | RAG vs LAG vs OG | I-II-III | 16 | 11 | 12 | 259.2 vs 203.9 vs 126.7 (RAG > LAG > OG)3 | 30.3 vs 44.7 vs 78.8 (RAG < LAG < OG)3 | 41.1 vs 37.4 vs 43.3 (NS) | 0 vs 10 vs 20 (NS) | 0 vs 0 vs 0 | 5.1 vs 6.5 vs 6.7 (RAG < LAG < OG)3 |
| Eom et al[37] | RAG vs LAG | I-II-III | 30 | 62 | - | 229.1 vs 189.4 (RAG > LAG)3 | 152.8 vs 88.3 (NS) | 30.2 vs 33.4 (NS) | 13.3 vs 6.6 (NS) | 0 vs 0 | 7.9 vs 7.8 (NS) |
| Woo et al[25] | RAG vs LAG | I-II-III | 236 | 591 | - | 219.5 vs 170.7 (RAG > LAG)3 | 91.6 vs 147.9 (RAG < LAG)3 | 39.0 vs 37.4 (NS) | 11 vs 13.7 (NS) | 0.4 vs 0.3 (NS) | 7.7 vs 7.0 (RAG > LAG)3 |
| Caruso et al[38] | RAG vs OG | All stages | 29 | - | 120 | 290 vs 222 (RAG > OG)3 | 197.6 vs 386.1 (RAG < OG)3 | 28.0 vs 31.7 (RAG~OG) | 10.34 vs 10.04 (NS) | 0 vs 3.3 (NS) | 9.6 vs 13.4 (RAG < OG)3 |
| Huang et al[39] | RAG vs LAG vs OG | I-II-III | 39 | 64 | 586 | 430 vs 350 vs 320 (RAG > LAG > OG)3 | 50 vs 100 vs 400 (RAG < LAG < OG)3 | 32 vs 26 vs 34 (RAG = OG > LAG)3 | 15.4 vs 15.6 vs 14.7 (NS) | 1.4 vs 1.6 vs 2.6 (NS) | 7 vs 11 vs 12 (RAG < LAG < OG)3 |
| Uyama et al[40] | RAG vs LAG | All stages | 25 | 225 | - | 361 vs 345 (NS) | 51.8 vs 81.0 (RAG < LAG)3 | 44.3 vs 43.2 (NS) | 11.2 vs 16.9 (NS) | 0 vs 0 | 12.1 vs 17.3 (RAG < LAG)3 |
| Kang et al[12] | RAG vs LAG | I-II-III | 100 | 282 | - | 202.05 vs 173.45 (RAG > LAG)3 | 93.25 vs 173.45 (RAG < LAG)3 | NR | 14.0 vs 10.3 (NS) | 0 vs 0 | 9.81 vs 8.11 (RAG > LAG)3 |
| Kim et al[41] | RAG vs LAG vs OG | 0-I-II-III | 436 | 861 | 4542 | 226 vs 176 vs 158 (RAG > LAG > OG)3 | 85 vs 112 vs 192 (RAG = LAG < OG)3 | 40.2 vs 37.6 vs 40.5 (RAG = OG > LAG)3 | 10.1 vs 10.4 vs 10.7 (NS) | 0.5 vs 0.3 vs 0.5 (NS) | 7.5 vs 7.8 vs 10.2 (RAG = LAG < OG)3 |
| Yoon et al[42] | RAG vs LAG | I-II-III | 36 | 65 | - | 305.8 vs 210.2 (RAG > LAG)3 | NR | 42.8 vs 39.4 (NS) | 16.7 vs 15.4 (NS) | 0 vs 0 | 8.8 vs 10.3 (NS) |
| Hyun et al[43] | RAG vs LAG | I-II-III | 38 | 83 | - | 234.4 vs 220.0 (NS) | 131.3 vs 130.48 (NS) | 32.8 vs 32.8 (NS) | 13.14 vs 16.84 (NS) | 0 vs 0 | 10.5 vs 11.9 (NS) |
| Kim et al[11] | RAG vs LAG | I-II-III | 172 | 481 | - | 206.4 vs 167.1 (RAG > LAG)3 | 59.8 vs 134.9 (RAG < OG)3 | 37.3 vs 36.8 (NS) | 5.2 vs 4.2 (NS) | 0 vs 0.6 (NS) | 7.1 vs 6.7 (NS) |
| Kim et al[44] | RAG vs LAG | I-II-III | 87 | 288 | - | 248.4 vs 230.0 (RAG > LAG)3 | NR | 37.1 vs 34.1 (RAG > LAG)3 | 5.7 vs 9.0 (RAG < LAG)3 | 1.1 vs 0.3 (NS) | 6.7 vs 7.4 (RAG < LAG)3 |
| Son et al[45] | RAG vs LAG | I-II-III | 51 | 58 | - | 264.1 vs 210.3 (RAG > LAG)3 | 163.4 vs 210.7 (NS) | 47.2 vs 42.8 (NS) | 16 vs 22 (NS) | 1.9 vs 0 (NS) | 8.6 vs 7.9 (NS) |
| Park et al[46] | RAG vs LAG | I-II-III | 30 | 120 | - | 218 vs 140 (RAG > LAG)3 | 75 vs 60 (NS) | 34 vs 35 (NS) | 17 vs 7.5 (NS) | 0 vs 0 | 7.0 vs 7.0 (NS) |
| Junfeng et al[24] | RAG vs LAG | I-II-III | 120 | 394 | - | 234.8 vs 221.3 (RAG > LAG)3 | 118.3 vs 137.6 (RAG < LAG)3 | 34.6 vs 32.7 (RAG > LAG)3 | 5.8 vs 4.3 (NS) | NR | 7.8 vs 7.9 (NS) |
| Seo et al[47] | RAG vs LAG | I-II-III | 40 | 40 | - | 243 vs 224 (NS) | 76 vs 227 (RAG < LAG)3 | 40.4 vs 35.4 (NS) | NR | NR | 6.75 vs 7.37 (RAG < LAG)3 |
| Shen et al[48] | RAG vs LAG | I-II-III | 93 | 330 | - | 257.1 vs 226.2 (RAG > LAG)3 | 176.6 vs 212.5 (RAG < LAG)3 | 33.0 vs 31.3 (RAG > LAG)3 | 9.8 vs 10.0 (NS) | NR | 9.4 vs 10.6 (NS) |
| Suda et al[49] | RAG vs LAG | All stages | 88 | 438 | - | 381 vs 361 (RAG > LAG)3 | 46 vs 34 (RAG > LAG)3 | 40 vs 38 (NS) | 2.3 vs 11.4 (RAG < LAG)3 | 1.1 vs 0.2 (NS) | 14 vs 15 (RAG < LAG)3 |
| Kim et al[50] | RAG vs LAG | I-II-III | 223 | 211 | - | 226 vs 180 (RAG > LAG)3 | 50 vs 60 (NS) | 33 vs 32 (NS) | 13.5 vs 14.2 (NS) | 0 vs 0 | 7.8 vs 7.9 (NS) |
Mean value;
The authors compared 20 gastric cancer patients who underwent robotic gastrectomy with 20 initial patients who underwent laparoscopic subtotal gastrectomy (iLAG) and 20 recent laparoscopic subtotal gastrectomy performed during the same period as the 20 robotic gastrectomy (rLAG);
Difference statistically significant, P < 0.05;
Major complications rate base on Clavien-Dindo classification ≥ 3, such as anastomotic and duodenal leakage. RAG: Robot-assisted laparoscopic gastrectomy; LAG: Laparoscopic assisted gastrectomy; OG: Open gastrectomy; NR: Not reported; NS: Not statistically significant difference.
However, initial outcome demonstrated comparable or superior short-term results of RAG than the results achieved by open and laparoscopic procedures, at the price of generally longer operation time, as well as higher cost. The prolonged operation time is attributable also to the additional time docking the robotic system, however that time decreases gradually as the expertise of the team increases, and robotic devices are upgraded[9]. Multiple series have reported various ranges in morbidity (5%-17%) after RAG (Table 2). Essentially, outcomes shown in these studies are satisfactory and similar to those of traditional surgical procedures (Table 2). Aforementioned outcomes demonstrate the clinical feasibility in using robotic radical gastrectomy for gastric adenocarcinoma in comparison with the conventional open and traditional minimally invasive laparoscopic approach, in some cases with potential clinical advantages also. For example, Kim et al[44] and Suda et al[49] showed a statistically significant improvement of the postoperative morbidity rate in gastric cancer patients submitted to RAG compared to LAG. In particular, Suda et al[49] noted that local (particularly pancreatic fistula, robotic 0% vs conventional laparoscopy 4.3%, P = 0.029) rather than systemic complication rates were attenuated using the surgical robot. Also Seo et al[47] reported an advantages of RAG in comparison to LAG in terms of a reduction of the incidence of postoperative pancreatitis or pancreatic fistula, which has been attributed to what is assumed to be a more gentle and steady pancreatic compression through the robotic system compared to laparoscopy during the suprapancreatic lymph nodes dissection.
For the first time Kim et al[36] reported the results achieved with robotic surgery with respect to laparoscopic and open gastrectomy for the treatment of early gastric cancer. They compared 16 patients who underwent robotic procedure with 11 and 12 laparoscopic and open gastrectomy respectively, revealing longer operative times of the robotic group, but less bleeding and reduced length of hospital stay. With regards to number of harvested lymph nodes and post-operative outcomes amongst the groups no difference was demonstrated.
The biggest (not meta-analyzed) comparative study so far was carried out by Kim et al[41]. They retrospectively looked at data on surgical complications of 5839 gastric cancer patients (4542 open, 861 laparoscopic and 436 robotic gastrectomies), and found no significant differences between the three groups with regards to post-operative complication and morbidity.
In another large single institute comparative study[25] the authors made a comparison between 236 patients who had undergone robotic curative resection of gastric cancer and 591 laparoscopic surgery patients (Table 2). The authors revealed a statistical significance difference, the mean duration of surgery was 49 min longer in the robotic group, whereas blood loss was 56.3 mL less. Morbidity, mortality and number of lymph nodes retrieved per level were comparable.
In yet another large comparative study (39 patients with gastric cancer undergoing robotic, 586 open and 64 laparoscopic gastrectomies)[39], RAG was linked to diminished bleeding and reduced hospital stay, but with longer operative time than was necessary for both open and laparoscopic gastrectomy. The amount of harvested lymph nodes was also similar between the open and robotic groups, but less in the laparoscopic group (Table 2). The authors especially underlined that robotic instruments made it a great deal more simple to carry out the lymph node dissection, rather than the conventional laparoscopic approach, more so in the infra-pyloric and supra-pancreatic stations.
Junfeng et al[24] retrospectively compared 120 vs 394 gastric cancer patients who had undergone RAG and laparoscopic assisted gastrectomy (LAG) respectively, revealing similar results. However, it is interesting to note that the authors showed, in addition to once more less intra operative bleeding and longer RAG operative time compared to the laparoscopic counterpart, that the numbers of harvested lymph nodes were notably superior in the RAG group at tier 2. In the same way, Kim et al[44] commented that, with regard to their experience achieved on 87 gastric cancer patients who had undergone robot-assisted distal gastrectomy (RADG) compared to 288 submitted to LADG, RADG seemed to be advantageous over LADG in performing the dissection of the second level lymph nodes, in particular those located in the suprapancreatic space and those around the splenic artery. Also Son’s et al[45] showed that robotic gastric surgery gave a much larger amount of harvested lymph nodes around splenic vessels in comparison to lymph nodes retrieved during laparoscopic procedure. This current medical research evidence, albeit initial, seems to consolidate the advantage of robotic surgery over LAG in its ability to perform a more complete D2 lymphadenectomy, probably making it possible to overcome one of the greatest surgical drawbacks of the laparoscopy in the treatment of gastric cancer.
An advantage of RAG compared to LAG has been reported in terms of a reduction of the incidence of postoperative pancreatitis or pancreatic fistula. This has been attributed to what is assumed to be a more gentle and constant pancreatic compression obtained using the robotic system compared to laparoscopy during the suprapancreatic lymph nodes dissection, i.e., at station 9 and 11[47].
Review and meta-analysis studies
To date, several review articles[10,19,51-55] have been published which provide a critical appraisal of the effectiveness of RAG for gastric cancer, but they are not systematic research and do not actually supply any statistical comparative analysis. Thus, the usefulness of these articles is essentially of scientific expounding and debating, they do not add any new knowledge to that so far evidenced by clinical studies.
On the other hand, 9 meta-analysis[20,56-63] conducted using a systematic method have been published to date in literature trying to focus on RAG utility in treating gastric cancer (Table 3). One meta-analysis included certain reports which compared RAG to OG[57]; 5 meta-analyses utilized high quality studies which compared RAG and LG[56,59-61,63]; and the remaining 3 meta-analyses contained a systematic review and meta-analysis of studies investigating short-term results of RAG vs LG and OG[20,58,62]. Exclusively prospective and retrospective studies were included in these meta-analysis, while no randomized controlled trials (RCTs) were found. The aforementioned meta-analysis showed that the RAG short-term clinical results were basically to be compared to LG and OG results. In terms of bleeding in particular, RAG was superior to both LG and OG, in spite of longer operation time. In addition RAG and LG groups did not show differences with regards to the number of harvested lymph nodes and conversion to open rates; RAG comported slightly inferior hospital stay or similar to that for LAG, but much less than OG; complications occurring after the operation were similar for all three operating methods.
Table 3.
Meta-analysis comparing robot-assisted gastrectomy with laparoscopic assisted gastrectomy and/or open gastrectomy in the treatment gastric cancer
| Ref. | Subject |
Patients (n) |
Operation time (min)1 | Blood loss (mL)1 | Harvested nodes (n)1 | Morbidity (%) | Mortality (%) | Hospital stay (d)1 | ||
| RAG | LAG | OG | ||||||||
| Xiong et al[56] | RAG vs LAG | 268 | 650 | - | 68.772 (RAG > LAG)3 | -41.882 (RAG < LAG)3 | -0.712 (NS) | 0.744 (NS) | 1.804 (NS) | -0.542 (NS) |
| Liao et al[57] | RAG vs OG | 520 | - | 5260 | 65.732 (RAG > LAG)3 | -126.082 (RAG < LAG)3 | -0.782 (NS) | 0.984 (NS) | 0.984 (NS) | -2.872 (RAG < LAG)3 |
| Hyun et al[58] | RAG vs LAG RAG vs OG | 634 558 | 1236 - | - 5301 | 61.992 (RAG > LAG)3 65.732 (RAG > OG)3 | -6.082 (NS) -154.182 (RAG < OG)3 | -0.252 (NS) -1.132 (NS) | 1.124 (NS) 1.374 (NS) | NR NR | -0.602 (NS) -2.182 (RAG < OG)3 |
| Marano et al[20] | RAG vs OG RAG vs LAG | 404 404 | - 845 | 718 - | 95.832 (RAG > OG)3 63.702 (RAG > LAG)3 | -225.582 (NS) -35.532 (RAG < LAG)3 | -2.682 (NS) 0.502 (NS) | 0.934 (NS) 0.874 (NS) | NR NR | -2.922 (RAG < OG)3 -0.602 (NS) |
| Xiong et al[59] | RAG vs LAG | 736 | 1759 | - | 48.642 (RAG > LAG)3 | -33.562 (RAG < LAG)3 | 1.282 (NS) | 1.134 (NS) | 1.664 (NS) | -1.162 (NS) |
| Liao et al[60] | RAG vs LAG | 762 | 1473 | - | 50.02 (RAG > LAG)3 | -46.972 (RAG < LAG)3 | 1.612 (NS) | 0.884 (NS) | 0.454 (NS) | -0.52 (NS) |
| Shen et al[61] | RAG vs LAG | 506 | 1369 | - | 48.462 (RAG > LAG)3 | -38.432 (RAG < LAG)3 | 1.062 (NS) | 0.954 (NS) | NR | -1.02 (NS) |
| Zong et al[62] | RAG vs OG RAG vs LAG | 481 997 | - 2207 | 4674 - | 68.472 (RAG > OG)3 57.152 (RAG > LAG)3 | -106.632 (RAG < OG)3 -28.592 (NS) | -0.782 (NS) -0.632 (NS) | 0.924 (NS) 1.064 (NS) | 0.724 (NS) 1.054 (NS) | -2.492 (RAG < OG)3 -0.162 (NS) |
| Chuan et al[63] | RAG vs LAG | 551 | 1245 | - | 42.92 (RAG > LAG)3 | -16.072 (RAG < LAG)3 | 2.452 (NS) | 1.054 (NS) | NR | -1.982 (RAG < LAG)3 |
Mean value;
Weighted mean difference;
Difference statistically significant, P < 0.05;
Odds ratio. RAG: Robot-assisted laparoscopic gastrectomy; LAG: Laparoscopic assisted gastrectomy; OG: Open gastrectomy; NR: Not reported; NS: Not statistically significant difference.
Robotic surgery lasts longer mainly because of the additional set-up and docking-time necessary for the robotic system. However, it must be said that operating time noticeably diminished as surgical experience in robotic gastrectomy increased[9,32,46]. Moreover, there are major limits to how these meta-analysis are interpreted. All data came from non-randomized controlled trials, and the included studies are essentially limited in number and with small sample sizes. Moreover, significant heterogeneity exists among the included studies deriving from several factors, such as different surgeon skill levels, different types of gastrectomy, different extent of lymph node dissection, different tumour stage, different rate of adjuvant treatment, and different protocols of post-operative management and discharge of patients. Thus, the overall level of clinical evidence of this pooled data was low and, since there have been no randomized comparative studies, even if a meta-analysis is performed, it seems difficult to reach a clear conclusion.
Long term outcome
At the present time, long-term benefits of RAG for the treatment of gastric cancer are under reported in literature. Pugliese et al[26] are among the few who have reported long term results in their minimally invasive surgical experience in gastric cancer patients. Among a cohort-case study of 70 patients who underwent minimally invasive subtotal gastrectomy with D2 lymphadenectomy, the authors included also 18 patients submitted to the robotic procedure. The authors did not provide data specifically referred to the robotic group only, however, always on the basis of analogous short-term results between groups undergoing laparoscopic and robotic procedures, the reported 5-year survival was 81% for the whole cohort. Coratti et al[33] were the first to report long-term survival data specifically referring to gastric cancer patients submitted to robot-assisted gastrectomies. They analyzed survival results in a group of 98 patients with either early and advanced gastric cancer submitted to RAG. In a mean follow-up of 46.9 mo, they registered a cumulative 5-year survival rate of 73.3%. Son et al[45] carried out the longest follow-up study till now available. They evaluated the survival rates in a cohort-study group of 51 gastric cancer patients submitted to robotic total gastrectomy with D2 lymph nodes dissection and compared it to 58 patients who underwent analogous surgery but through the laparoscopic approach. In a median long-term follow-up of 70 mo, the authors did not find significant differences in overall survival and disease-free survival between the two groups. Specifically, the authors reported a 5-year overall survival rate of 89.5% for the robotic group, which was not statistically significant different with respect to the rate revealed in the laparoscopic group (91.1%).
The aforementioned results are comforting, but it must be said that the case studies were limited, and selection bias is a real worry as it was a non-randomized study design. Follow-up periods longer than 5 years are needed to show oncological results, and so further RCTs are required in order to validate definitive conclusions.
DISCUSSION
The relative new technological advance in surgery through the introduction of minimally invasive technique can be accepted as an alternative to open surgery, which usually confers better short-term post-operative results, only if the oncologic parameters are as sufficiently respected as for the traditional open approach. Obviously, at the same time the long-term survival rates should not be adversely affected either.
With specific reference to gastric cancer one of the most important oncological criterion is the quality of lymphadenectomy, thus in order for laparoscopic or robot-assisted laparoscopic gastric surgery to be considered adequate, at least the same extent of lymph node dissection as in traditional surgery should be achieved, and moreover favorable postoperative results should also be evident.
Over the last two decades LG with lymph node dissection has developed as minimally invasive surgery for gastric cancer and it has been principally applied to early gastric cancer. Certain RCTs and meta-analysis showed that laparoscopic gastrectomy did not have inferior oncologic results compared to open surgery for early-stage gastric cancer, with instead improved results in the short term[3,64,65]. In fact, laparoscopic extended D1 lymphadenectomy may be seen as sufficient for almost all early gastric cancer in which lymph node metastases rarely occur, and is today the recommended approach in the East. On the other hand, only few high quality reports investigating the oncological adequacy of laparoscopic minimally invasive techniques for advanced gastric cancer are available to date. Recently, some meta-analyses related to this have been published, but there have been contrasting outcomes, particularly regarding complications after total gastrectomy and the actual adequacy of D2 lymphadenectomy in patients affected by advanced-stage of gastric cancer[4,64,66-69]. Even though a complete LG and extended lymph nodes dissection has been demonstrated by several experts to be feasible laparoscopically[5,6,26,70], due to some intrinsic limiting drawbacks of the laparoscopic technique, important oncologic preoccupations have been raised. When in the meta-analysis studies data not restricted to LADG solely for early gastric cancer was considered, but instead included advanced-stage tumour too, it was not possible to guarantee the same amount of lymph node dissection as in conventional surgical procedures[71,72]. Thus, the laparoscopic techniques cannot be considered a standard validated procedure for all gastric cancer sufferers.
Certain inherent drawbacks of conventional laparoscopy may be eliminated by robotics by increasing the use of minimally invasive procedures, especially when more extended lymph nodes dissection and complicated reconstruction are required. In light of this, the introduction of robotic technologies could lead to the improvement of health care and final results. Particularly during typical difficult maneuvers in laparocopy, such as the dissection of the lymphatic tissue around major abdominal vessels (gastric, gastroepiploic, common hepatic, and celiac artery lymph nodes), robotics offers some indisputable advantages, which make it possible to perform the dissection more safely and easily. Consequently, robotic techniques should be viewed more as a technical advancement and auxiliary tool of the traditional minimally invasive laparoscopic approach, rather than an independent device system.
Most surgeons who are experts in robotics reported in their experience amounts of retrieved lymph nodes during RAG similar to those obtainable by the classic open counterpart procedure and sometimes more than those achieved by laparoscopy[8,20,38,56-63]. However, it must be said that the explanation of available comparable data among RAG, LG and OG has notable limitations. The principal issue that could affect the interpretation of these data is essentially the lack of a comprehensive comparative RCT. However, we have also to consider that the number of published high quality observational and retrospective studies is limited, and globally the sample sizes in each singular trial is poor. Ultimately, but not less importantly from the point of view of oncological adequacy, the duration of follow up is almost always limited.
CONCLUSION
RAG appears to be a safe and feasible alternative to conventional open or laparoscopic gastrectomy for the treatment of early stage gastric carcinoma, having demonstrated satisfactory perioperative outcomes and oncological adequacy. The number of collected lymph nodes when comparing RAG to open and laparoscopic gastrectomy are essentially similar when considering early-stage gastric cancer only, while an advantageous lower blood loss estimation was revealed in comparison with the other two approaches.
Basically the robotic system simplifies certain hard conventional laparoscopy techniques and renders them safer, in addition simultaneously possessing a learning curve and reproducibility that appear to be briefer than conventional laparoscopy[11-14]. These results, albeit initial, are promising, but the superiority of robotic gastric surgery over the traditional laparoscopic approach has not yet been solidly proved and its validation is still a long way off for all gastric cancer patients. The main controversial issue regards the possibility of demonstrating that the supposed superiority of RAG with respect to laparoscopy in carrying out a more adequate extended lymphadenectomy could lead to potential oncological benefit, probably true in gastric cancer of a more advanced stage.
Unfortunately, due to inadequate long-term follow-up results and a limited number of studies to date available, larger and randomized prospective trials are required to draw definitive conclusion.
Footnotes
Conflict-of-interest statement: All authors disclose any potential or actual personal, political or financial conflict of interest in the material, information or techniques described in the paper.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: June 27, 2016
First decision: August 11, 2016
Article in press: October 27, 2016
P- Reviewer: Aoyagi K, Arigami T, Jonaitis L, Terashima M, Zhu YL S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
References
- 1.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 2.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yakoub D, Athanasiou T, Tekkis P, Hanna GB. Laparoscopic assisted distal gastrectomy for early gastric cancer: is it an alternative to the open approach? Surg Oncol. 2009;18:322–333. doi: 10.1016/j.suronc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Deng Y, Zhang Y, Guo TK. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: A meta-analysis based on seven randomized controlled trials. Surg Oncol. 2015;24:71–77. doi: 10.1016/j.suronc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230–234. doi: 10.1007/s101200050069. [DOI] [PubMed] [Google Scholar]
- 7.Anderson C, Ellenhorn J, Hellan M, Pigazzi A. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc. 2007;21:1662–1666. doi: 10.1007/s00464-007-9266-0. [DOI] [PubMed] [Google Scholar]
- 8.Patriti A, Ceccarelli G, Bellochi R, Bartoli A, Spaziani A, Di Zitti L, Casciola L. Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc. 2008;22:2753–2760. doi: 10.1007/s00464-008-0129-0. [DOI] [PubMed] [Google Scholar]
- 9.Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg. 2009;249:927–932. doi: 10.1097/01.sla.0000351688.64999.73. [DOI] [PubMed] [Google Scholar]
- 10.Alimoglu O, Atak I, Eren T. Robot-assisted laparoscopic (RAL) surgery for gastric cancer. Int J Med Robot. 2014;10:257–262. doi: 10.1002/rcs.1566. [DOI] [PubMed] [Google Scholar]
- 11.Kim HI, Park MS, Song KJ, Woo Y, Hyung WJ. Rapid and safe learning of robotic gastrectomy for gastric cancer: multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur J Surg Oncol. 2014;40:1346–1354. doi: 10.1016/j.ejso.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Kang BH, Xuan Y, Hur H, Ahn CW, Cho YK, Han SU. Comparison of Surgical Outcomes between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: The Learning Curve of Robotic Surgery. J Gastric Cancer. 2012;12:156–163. doi: 10.5230/jgc.2012.12.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508–7511. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heemskerk J, van Gemert WG, de Vries J, Greve J, Bouvy ND. Learning curves of robot-assisted laparoscopic surgery compared with conventional laparoscopic surgery: an experimental study evaluating skill acquisition of robot-assisted laparoscopic tasks compared with conventional laparoscopic tasks in inexperienced users. Surg Laparosc Endosc Percutan Tech. 2007;17:171–174. doi: 10.1097/SLE.0b013e31805b8346. [DOI] [PubMed] [Google Scholar]
- 15.Miura S, Kodera Y, Fujiwara M, Ito S, Mochizuki Y, Yamamura Y, Hibi K, Ito K, Akiyama S, Nakao A. Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection: a critical reappraisal from the viewpoint of lymph node retrieval. J Am Coll Surg. 2004;198:933–938. doi: 10.1016/j.jamcollsurg.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Kim MC, Choi HJ, Jung GJ, Kim HH. Techniques and complications of laparoscopy-assisted distal gastrectomy (LADG) for gastric cancer. Eur J Surg Oncol. 2007;33:700–705. doi: 10.1016/j.ejso.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Bouras G, Lee SW, Nomura E, Tokuhara T, Tsunemi S, Tanigawa N. Comparative analysis of station-specific lymph node yield in laparoscopic and open distal gastrectomy for early gastric cancer. Surg Laparosc Endosc Percutan Tech. 2011;21:424–428. doi: 10.1097/SLE.0b013e3182367dee. [DOI] [PubMed] [Google Scholar]
- 18.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 19.Coratti A, Annecchiarico M, Di Marino M, Gentile E, Coratti F, Giulianotti PC. Robot-assisted gastrectomy for gastric cancer: current status and technical considerations. World J Surg. 2013;37:2771–2781. doi: 10.1007/s00268-013-2100-z. [DOI] [PubMed] [Google Scholar]
- 20.Marano A, Choi YY, Hyung WJ, Kim YM, Kim J, Noh SH. Robotic versus Laparoscopic versus Open Gastrectomy: A Meta-Analysis. J Gastric Cancer. 2013;13:136–148. doi: 10.5230/jgc.2013.13.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hur H, Kim JY, Cho YK, Han SU. Technical feasibility of robot-sewn anastomosis in robotic surgery for gastric cancer. J Laparoendosc Adv Surg Tech A. 2010;20:693–697. doi: 10.1089/lap.2010.0246. [DOI] [PubMed] [Google Scholar]
- 22.Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am. 2003;83:1429–1444. doi: 10.1016/S0039-6109(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 23.Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–784. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 24.Junfeng Z, Yan S, Bo T, Yingxue H, Dongzhu Z, Yongliang Z, Feng Q, Peiwu Y. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg Endosc. 2014;28:1779–1787. doi: 10.1007/s00464-013-3385-6. [DOI] [PubMed] [Google Scholar]
- 25.Woo Y, Hyung WJ, Pak KH, Inaba K, Obama K, Choi SH, Noh SH. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg. 2011;146:1086–92. doi: 10.1001/archsurg.2011.114. [DOI] [PubMed] [Google Scholar]
- 26.Pugliese R, Maggioni D, Sansonna F, Costanzi A, Ferrari GC, Di Lernia S, Magistro C, De Martini P, Pugliese F. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc. 2010;24:2594–2602. doi: 10.1007/s00464-010-1014-1. [DOI] [PubMed] [Google Scholar]
- 27.Lee HH, Hur H, Jung H, Jeon HM, Park CH, Song KY. Robot-assisted distal gastrectomy for gastric cancer: initial experience. Am J Surg. 2011;201:841–845. doi: 10.1016/j.amjsurg.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 28.D’Annibale A, Pende V, Pernazza G, Monsellato I, Mazzocchi P, Lucandri G, Morpurgo E, Contardo T, Sovernigo G. Full robotic gastrectomy with extended (D2) lymphadenectomy for gastric cancer: surgical technique and preliminary results. J Surg Res. 2011;166:e113–e120. doi: 10.1016/j.jss.2010.11.881. [DOI] [PubMed] [Google Scholar]
- 29.Jiang ZW, Zhao K, Wang G, Bao Y, Xie LF, Liu FT, Pan HF, Zhang XL, Ruan H, Li N, et al. [Application of surgical robotic system in patients with gastric cancer: a report of 120 cases] Zhonghua Weichang Waike Zazhi. 2012;15:801–803. [PubMed] [Google Scholar]
- 30.Isogaki J, Haruta S, Man-I M, Suda K, Kawamura Y, Yoshimura F, Kawabata T, Inaba K, Ishikawa K, Ishida Y, et al. Robot-assisted surgery for gastric cancer: experience at our institute. Pathobiology. 2011;78:328–333. doi: 10.1159/000330172. [DOI] [PubMed] [Google Scholar]
- 31.Liu XX, Jiang ZW, Chen P, Zhao Y, Pan HF, Li JS. Full robot-assisted gastrectomy with intracorporeal robot-sewn anastomosis produces satisfying outcomes. World J Gastroenterol. 2013;19:6427–6437. doi: 10.3748/wjg.v19.i38.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JY, Kim YW, Ryu KW, Eom BW, Yoon HM, Reim D. Emerging Role of Robot-assisted Gastrectomy: Analysis of Consecutive 200 Cases. J Gastric Cancer. 2013;13:255–262. doi: 10.5230/jgc.2013.13.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coratti A, Fernandes E, Lombardi A, Di Marino M, Annecchiarico M, Felicioni L, Giulianotti PC. Robot-assisted surgery for gastric carcinoma: Five years follow-up and beyond: A single western center experience and long-term oncological outcomes. Eur J Surg Oncol. 2015;41:1106–1113. doi: 10.1016/j.ejso.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Tokunaga M, Makuuchi R, Miki Y, Tanizawa Y, Bando E, Kawamura T, Terashima M. Late phase II study of robot-assisted gastrectomy with nodal dissection for clinical stage I gastric cancer. Surg Endosc. 2016;30:3362–3367. doi: 10.1007/s00464-015-4613-z. [DOI] [PubMed] [Google Scholar]
- 35.Song J, Kang WH, Oh SJ, Hyung WJ, Choi SH, Noh SH. Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg Endosc. 2009;23:1204–1211. doi: 10.1007/s00464-009-0351-4. [DOI] [PubMed] [Google Scholar]
- 36.Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc. 2010;24:610–615. doi: 10.1007/s00464-009-0618-9. [DOI] [PubMed] [Google Scholar]
- 37.Eom BW, Yoon HM, Ryu KW, Lee JH, Cho SJ, Lee JY, Kim CG, Choi IJ, Lee JS, Kook MC, et al. Comparison of surgical performance and short-term clinical outcomes between laparoscopic and robotic surgery in distal gastric cancer. Eur J Surg Oncol. 2012;38:57–63. doi: 10.1016/j.ejso.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Caruso S, Patriti A, Marrelli D, Ceccarelli G, Ceribelli C, Roviello F, Casciola L. Open vs robot-assisted laparoscopic gastric resection with D2 lymph node dissection for adenocarcinoma: a case-control study. Int J Med Robot. 2011;7:452–458. doi: 10.1002/rcs.416. [DOI] [PubMed] [Google Scholar]
- 39.Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Hsieh MC, Li AF, Chiou SH, Wu CW. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1303–1310. doi: 10.1007/s11605-012-1874-x. [DOI] [PubMed] [Google Scholar]
- 40.Uyama I, Kanaya S, Ishida Y, Inaba K, Suda K, Satoh S. Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg. 2012;36:331–337. doi: 10.1007/s00268-011-1352-8. [DOI] [PubMed] [Google Scholar]
- 41.Kim KM, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg. 2012;99:1681–1687. doi: 10.1002/bjs.8924. [DOI] [PubMed] [Google Scholar]
- 42.Yoon HM, Kim YW, Lee JH, Ryu KW, Eom BW, Park JY, Choi IJ, Kim CG, Lee JY, Cho SJ, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc. 2012;26:1377–1381. doi: 10.1007/s00464-011-2043-0. [DOI] [PubMed] [Google Scholar]
- 43.Hyun MH, Lee CH, Kwon YJ, Cho SI, Jang YJ, Kim DH, Kim JH, Park SH, Mok YJ, Park SS. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol. 2013;20:1258–1265. doi: 10.1245/s10434-012-2679-6. [DOI] [PubMed] [Google Scholar]
- 44.Kim YW, Reim D, Park JY, Eom BW, Kook MC, Ryu KW, Yoon HM. Role of robot-assisted distal gastrectomy compared to laparoscopy-assisted distal gastrectomy in suprapancreatic nodal dissection for gastric cancer. Surg Endosc. 2016;30:1547–1552. doi: 10.1007/s00464-015-4372-x. [DOI] [PubMed] [Google Scholar]
- 45.Son T, Lee JH, Kim YM, Kim HI, Noh SH, Hyung WJ. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc. 2014;28:2606–2615. doi: 10.1007/s00464-014-3511-0. [DOI] [PubMed] [Google Scholar]
- 46.Park JY, Jo MJ, Nam BH, Kim Y, Eom BW, Yoon HM, Ryu KW, Kim YW, Lee JH. Surgical stress after robot-assisted distal gastrectomy and its economic implications. Br J Surg. 2012;99:1554–1561. doi: 10.1002/bjs.8887. [DOI] [PubMed] [Google Scholar]
- 47.Seo HS, Shim JH, Jeon HM, Park CH, Song KY. Postoperative pancreatic fistula after robot distal gastrectomy. J Surg Res. 2015;194:361–366. doi: 10.1016/j.jss.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 48.Shen W, Xi H, Wei B, Cui J, Bian S, Zhang K, Wang N, Huang X, Chen L. Robotic versus laparoscopic gastrectomy for gastric cancer: comparison of short-term surgical outcomes. Surg Endosc. 2016;30:574–580. doi: 10.1007/s00464-015-4241-7. [DOI] [PubMed] [Google Scholar]
- 49.Suda K, Man-I M, Ishida Y, Kawamura Y, Satoh S, Uyama I. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc. 2015;29:673–685. doi: 10.1007/s00464-014-3718-0. [DOI] [PubMed] [Google Scholar]
- 50.Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, Park JM, An JY, Kim MC, Park S, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg. 2016;263:103–109. doi: 10.1097/SLA.0000000000001249. [DOI] [PubMed] [Google Scholar]
- 51.Son T, Hyung WJ. Robotic gastrectomy for gastric cancer. J Surg Oncol. 2015;112:271–278. doi: 10.1002/jso.23926. [DOI] [PubMed] [Google Scholar]
- 52.Terashima M, Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Miki Y, Makuuchi R, Honda S, Tatsubayashi T, Takagi W, et al. Robotic surgery for gastric cancer. Gastric Cancer. 2015;18:449–457. doi: 10.1007/s10120-015-0501-4. [DOI] [PubMed] [Google Scholar]
- 53.Baek SJ, Lee DW, Park SS, Kim SH. Current status of robot-assisted gastric surgery. World J Gastrointest Oncol. 2011;3:137–143. doi: 10.4251/wjgo.v3.i10.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marano A, Hyung WJ. Robotic gastrectomy: the current state of the art. J Gastric Cancer. 2012;12:63–72. doi: 10.5230/jgc.2012.12.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Obama K, Sakai Y. Current status of robotic gastrectomy for gastric cancer. Surg Today. 2016;46:528–534. doi: 10.1007/s00595-015-1190-7. [DOI] [PubMed] [Google Scholar]
- 56.Xiong B, Ma L, Zhang C. Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol. 2012;21:274–280. doi: 10.1016/j.suronc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Liao G, Chen J, Ren C, Li R, Du S, Xie G, Deng H, Yang K, Yuan Y. Robotic versus open gastrectomy for gastric cancer: a meta-analysis. PLoS One. 2013;8:e81946. doi: 10.1371/journal.pone.0081946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hyun MH, Lee CH, Kim HJ, Tong Y, Park SS. Systematic review and meta-analysis of robotic surgery compared with conventional laparoscopic and open resections for gastric carcinoma. Br J Surg. 2013;100:1566–1578. doi: 10.1002/bjs.9242. [DOI] [PubMed] [Google Scholar]
- 59.Xiong J, Nunes QM, Tan C, Ke N, Chen Y, Hu W, Liu X, Mai G. Comparison of short-term clinical outcomes between robotic and laparoscopic gastrectomy for gastric cancer: a meta-analysis of 2495 patients. J Laparoendosc Adv Surg Tech A. 2013;23:965–976. doi: 10.1089/lap.2013.0279. [DOI] [PubMed] [Google Scholar]
- 60.Liao GX, Xie GZ, Li R, Zhao ZH, Sun QQ, Du SS, Ren C, Li GX, Deng HJ, Yuan YW. Meta-analysis of outcomes compared between robotic and laparoscopic gastrectomy for gastric cancer. Asian Pac J Cancer Prev. 2013;14:4871–4875. doi: 10.7314/apjcp.2013.14.8.4871. [DOI] [PubMed] [Google Scholar]
- 61.Shen WS, Xi HQ, Chen L, Wei B. A meta-analysis of robotic versus laparoscopic gastrectomy for gastric cancer. Surg Endosc. 2014;28:2795–2802. doi: 10.1007/s00464-014-3547-1. [DOI] [PubMed] [Google Scholar]
- 62.Zong L, Seto Y, Aikou S, Takahashi T. Efficacy evaluation of subtotal and total gastrectomies in robotic surgery for gastric cancer compared with that in open and laparoscopic resections: a meta-analysis. PLoS One. 2014;9:e103312. doi: 10.1371/journal.pone.0103312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chuan L, Yan S, Pei-Wu Y. Meta-analysis of the short-term outcomes of robotic-assisted compared to laparoscopic gastrectomy. Minim Invasive Ther Allied Technol. 2015;24:127–134. doi: 10.3109/13645706.2014.985685. [DOI] [PubMed] [Google Scholar]
- 64.Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446–456. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 65.Ohtani H, Tamamori Y, Noguchi K, Azuma T, Fujimoto S, Oba H, Aoki T, Minami M, Hirakawa K. A meta-analysis of randomized controlled trials that compared laparoscopy-assisted and open distal gastrectomy for early gastric cancer. J Gastrointest Surg. 2010;14:958–964. doi: 10.1007/s11605-010-1195-x. [DOI] [PubMed] [Google Scholar]
- 66.Chen XZ, Hu JK, Yang K, Wang L, Lu QC. Short-term evaluation of laparoscopy-assisted distal gastrectomy for predictive early gastric cancer: a meta-analysis of randomized controlled trials. Surg Laparosc Endosc Percutan Tech. 2009;19:277–284. doi: 10.1097/SLE.0b013e3181b080d3. [DOI] [PubMed] [Google Scholar]
- 67.Liang Y, Li G, Chen P, Yu J, Zhang C. Laparoscopic versus open gastrectomy for early distal gastric cancer: a meta-analysis. ANZ J Surg. 2011;81:673–680. doi: 10.1111/j.1445-2197.2010.05599.x. [DOI] [PubMed] [Google Scholar]
- 68.Ding J, Liao GQ, Liu HL, Liu S, Tang J. Meta-analysis of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer. J Surg Oncol. 2012;105:297–303. doi: 10.1002/jso.22098. [DOI] [PubMed] [Google Scholar]
- 69.Martínez-Ramos D, Miralles-Tena JM, Cuesta MA, Escrig-Sos J, Van der Peet D, Hoashi JS, Salvador-Sanchís JL. Laparoscopy versus open surgery for advanced and resectable gastric cancer: a meta-analysis. Rev Esp Enferm Dig. 2011;103:133–141. doi: 10.4321/s1130-01082011000300005. [DOI] [PubMed] [Google Scholar]
- 70.Tanimura S, Higashino M, Fukunaga Y, Takemura M, Tanaka Y, Fujiwara Y, Osugi H. Laparoscopic gastrectomy for gastric cancer: experience with more than 600 cases. Surg Endosc. 2008;22:1161–1164. doi: 10.1007/s00464-008-9786-2. [DOI] [PubMed] [Google Scholar]
- 71.Hosono S, Arimoto Y, Ohtani H, Kanamiya Y. Meta-analysis of short-term outcomes after laparoscopy-assisted distal gastrectomy. World J Gastroenterol. 2006;12:7676–7683. doi: 10.3748/wjg.v12.i47.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Memon MA, Khan S, Yunus RM, Barr R, Memon B. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc. 2008;22:1781–1789. doi: 10.1007/s00464-008-9925-9. [DOI] [PubMed] [Google Scholar]


