Abstract
Rationale
The mosquito-borne Zika virus (ZIKV) is now recognized as a blood-borne pathogen, raising an important question about how the virus gets into human bloodstream. The imminent threat of the ZIKV epidemic to the global blood supply also demands novel therapeutics to stop virus transmission though transfusion.
Objective
We intend to characterize ZIKV tropism for human endothelial cells (ECs) and provide potential targets for intervention.
Methods and Results
We conducted immunostaining, plaque assay, and quantitative RT-PCR of ZIKV RNA to evaluate the possible infection of ECs by ZIKV. Both the African and the South American ZIKV strains readily infect human umbilical vein endothelial cells (HUVECs) and human ECs derived from aortic and coronary artery as well as the saphenous vein. Infected ECs released infectious progeny virus. Compared to the African strains, South American ZIKV isolates replicate faster in ECs and are partially cytopathic, suggesting enhanced virulence of these isolates. Flow cytometric analyses showed that the susceptibility of ECs positively correlated with the cell-surface levels of AXL receptor tyrosine kinase. Gain- and loss-of-function studies further revealed that AXL is required for ZIKV entry at a post-binding step. Lastly, small molecule inhibitors of the AXL kinase significantly reduced ZIKA infection of ECs.
Conclusions
We identified EC as a key cell type for ZIKV infection. These data support the view of hematogenous dissemination of ZIKV and implicate AXL as a new target for antiviral therapy.
Keywords: Zika virus, endothelial cells, productive infection, AXL receptor tyrosine kinase, blood, virus entry
Subject Terms: Basic Science Research, Endothelium/Vascular Type, Vascular Disease
INTRODUCTION
Zika virus (ZIKV) is an emerging arbovirus of the Flaviviridae family 1. First isolated from a febrile rhesus macaque in 1947 in Uganda, ZIKV has not been recognized as a major viral pathogen until ZIKV infection in pregnant women in the Americas was confirmed as the cause of microcephaly and other birth defects seen in neonates 2, 3. Indeed, studies in mouse models confirmed ZIKV can be transmitted from the pregnant mother to the fetus 4–6. Additionally, ZIKV may be transmitted during sex 7–9 or a blood transfusion 10. To date, ZIKV has been detected in the human central nervous system 11, 12, blood, saliva 13, semen 14 and urine 15, suggesting the virus has developed mechanisms to reach multiple tissues. The Centers for Disease Control (CDC) has now placed ZIKV on the list of blood-borne pathogens. The detection of ZIKV in blood further raises serious concerns about the risk of transfusion-related transmission and, in particular, sparking fear about the potential for severe outcomes in at-risk recipient populations.
Conceptually, ZIKV may be carried into bloodstream by infected macrophages or dendritic cells after local mosquito bites 16. Here we report that ZIKV directly infects human endothelial cells (ECs) and disseminates infectious progeny virus. Specifically, both the African and the South American ZIKV strains readily infect primary human ECs isolated from umbilical veins, the aorta, the coronary arteries, and the saphenous veins, leading to the release of infectious virus. The candidate receptor of entry appears to be the AXL receptor tyrosine kinase because specific small molecular inhibitors of AXL blocked ZIKV infection. Our results support a model that ZIKV spreads through infected endothelial cells and bypasses the barriers that would otherwise restrict viral infection.
METHODS
A detailed Methods section is provided in the Online Supplement.
RESULTS
The interior surfaces of vascular and lymphatic vessels are lined with endothelium, forming an interface between circulating blood or lymph in the lumen and the rest of the vessel wall. Endothelial cells (ECs) are also major components of the blood-brain barrier (BBB) and part of the placental blood barrier (PBB), preventing circulating virus from entering the brain and the fetal tissues, respectively. To investigate whether ZIKV directly infects endothelial cells, we cultured the immortalized human BBB endothelial cell line hCMEC/D3, which is a well-characterized BBB cell system 17. The African MR766 and IbH 30656 (IbH) ZIKV strains (ZIKVAF), as well as the South American ZIKV isolates (ZIKVSA) PRVABC59 (PRV) and FLR, but not Dengue virus (DENV-2 strain Thailand 16681), readily infected hCMEC/D3 cells and produced infectious virus with titers comparable to that of the C6/36 cell line (Online Figure I).
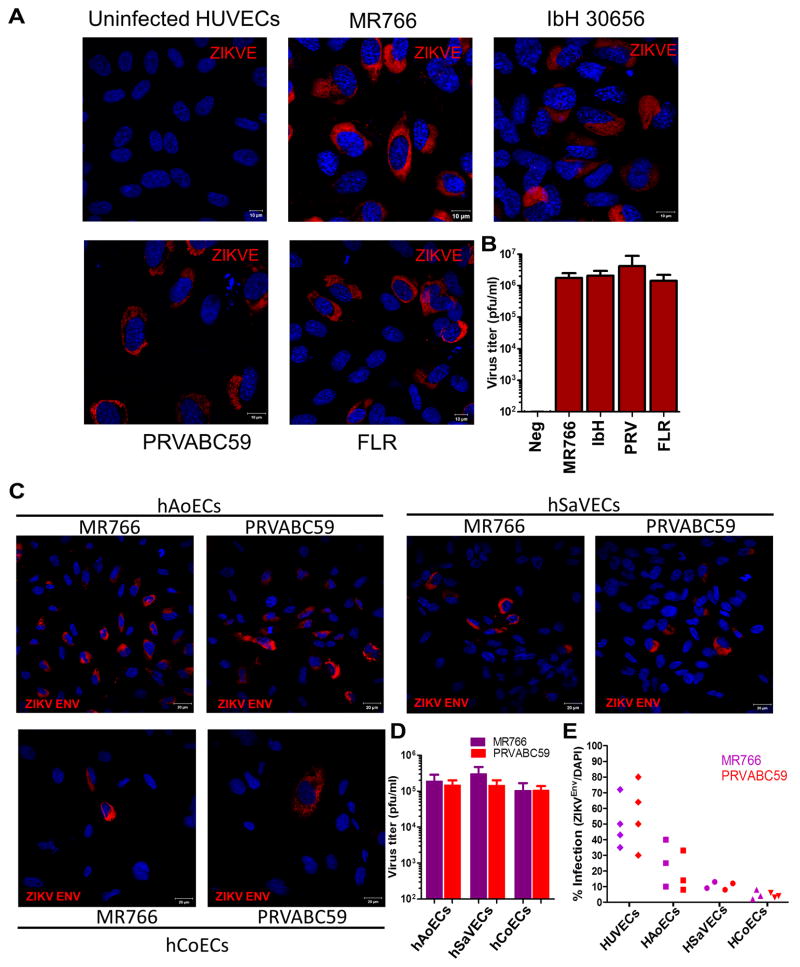
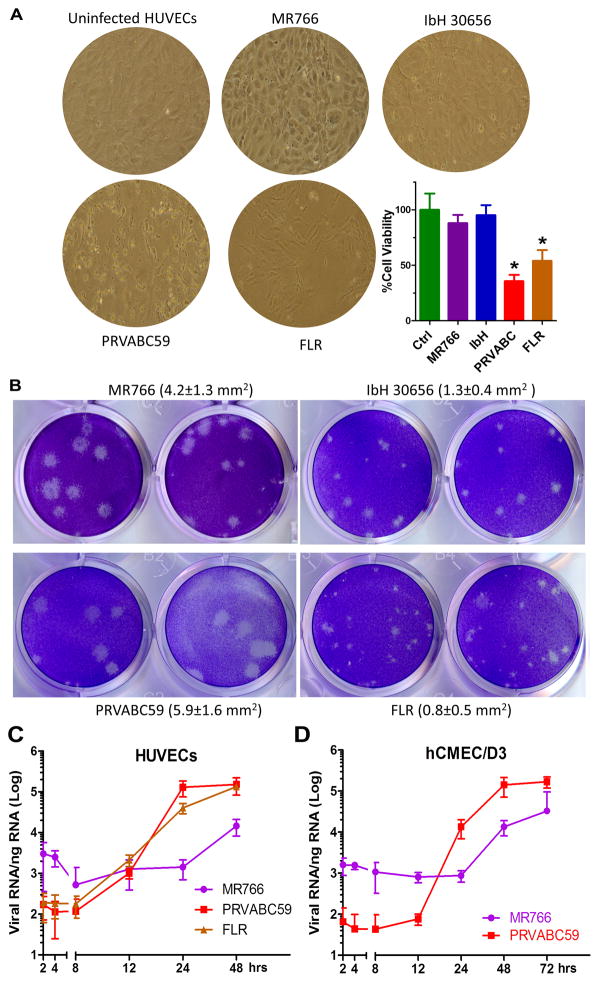
To determine the extent of ZIKV EC infection, we used cultures of human ECs at low passages (passage numbers <7) isolated from umbilical veins (HUVECs), aorta (HAoECs), coronary artery (HCoECs), and saphenous vein (HSaVECs) (Online Figure II). For comparison, we also obtained human lymphatic endothelial cells (Online Figure III). Low-passage ECs were inoculated with the MR766 and IbH 30656 (IbH) ZIKV strains, and the PRV and FLR. Both ZIKVAF and ZIKVSA strains, but not DENV2, infected all vascular ECs from multiple donors, with HUVECs being significantly most susceptible (Figure 1A–E). Notably, ZIKV entry into vascular ECs also led to productive infection and the release of infectious progeny virus (Figure 1B, D). While the ZIKVAF strains MR766 and IbH caused minimal morphological change, infection of HUVEC cells with the two ZIKVSA isolates induced significant cell death, suggesting enhanced virulence of these isolates (Figure 2A). The PRV isolate also formed noticeably larger plaques on plaque assays (Figure 2B). Indeed, when the kinetics of viral RNA replication was measured, the ZIKVSA isolates displayed faster growth rates (Figure 2C, D). While we cannot completely rule out that the African isolates have been cultured for decades in the lab and hence adapted to be less cytopathic, our observation is consistent with a recent report that pups born from a Brazilian ZIKV (ZIKVBR) isolate-infected SJL pregnant mice displayed abnormalities resembling the microcephaly seen in humans4. These findings highlight the differences between the original ZIKVAF strain, which causes no or very mild symptoms, and the circulating ZIKVSA strains that appear to be more pathogenic. The fact that HUVECs are most susceptible and the partial cytopathic effect of ZIKVSA isolates on these cells also suggest the potential involvement of ECs during vertical transmission of ZIKV.
Figure 1. Productive infection of primary human endothelial cells by ZIKV.
(A) HUVECs from ATCC (passage 2) were infected by indicated ZIKV strains/isolates at MOI of 1 for 48 hrs followed by immunostaining of ZIKV Env protein. (B) Infectious titers of supernatants collected at 48 hrs post-infection from cells in a (n=3). (C) ECs were infected by ZIKV MR766 or PRVABC59 at MOI of 1 and then imaged for the presence of viral Env protein at 48hrs post-infection. (D) Infectious titers of supernatants collected at 48 hrs post-infection from cells in b (n=3). (E) Summary of infection efficiencies of ECs from different donors by MR766 or PRVABC59. Each solid shape represents one donor. Data are shown as the percent of ZIKV Env-positive cells relative to the total number of nuclei (as assessed by DAPI).
Figure 2. Enhanced virulence of South American ZIKV isolates.
(A) Representative phase-contrast images of infected HUVEC cells (ATCC, passage 2) from Fig. 1a at 48 hrs post-infection. The percentage of cell viability was quantified using the CellTiter-Glo Luminescent Cell Viability Assay (Promega). N = 3, *P<0.05. (B) Representative photographs of plaques using supernatants from above cells. Plaque sizes were indicated in the brackets. (C and D) HUVECs or hCMEC/D3 cells were infected by ZIKV MR766, PRVABC59 or FLR at MOI of 0.1 for 1hr. After extensive wash in PBS, cellular RNA was extracted at indicated time points post-infection for quantitative RT-PCR to determine the kinetics of virus RNA replication (n=4, each experiment contains 3 technical replicates; error bars, s.d.).
Because vascular ECs form physical barriers with tight junctions between cells, we conducted confocal microscopy and Western blotting to evaluate the effects of ZIKV infection on endothelium integrity. Transendothelial electrical resistance was measured for the assessment of endothelial barrier function. Infection by ZIKV did not directly disrupt the tight junctions or the barrier function of HUVEC or hCMEC/D3 cells (Online Figure IV). We therefore reason that the loss of endothelium integrity probably only occurs when significant cell death is caused by ZIKV infection.
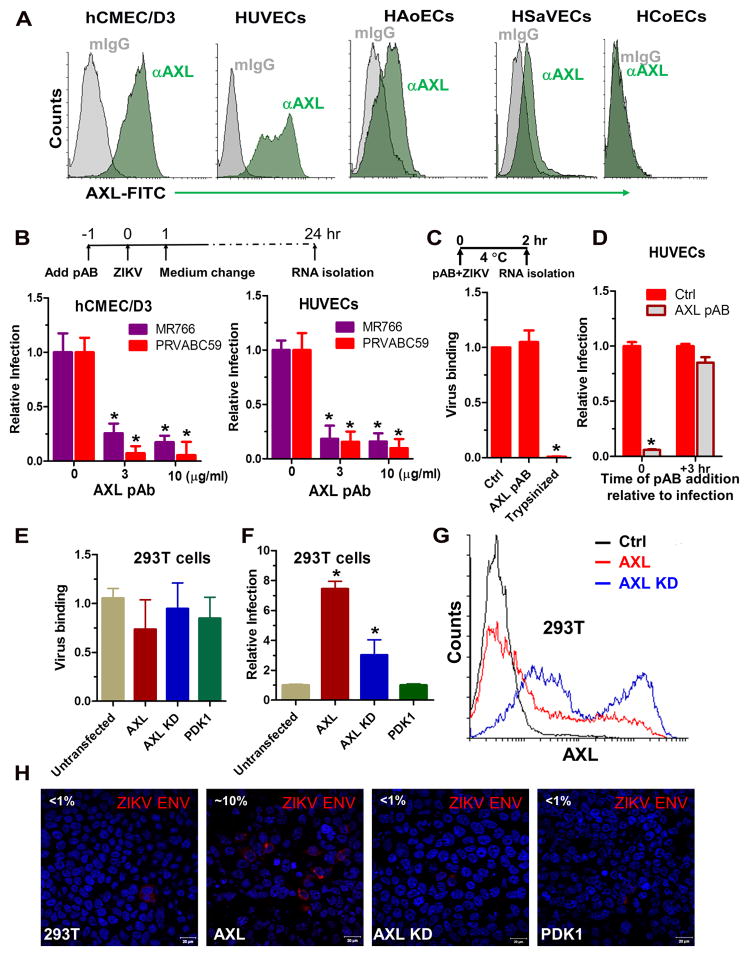
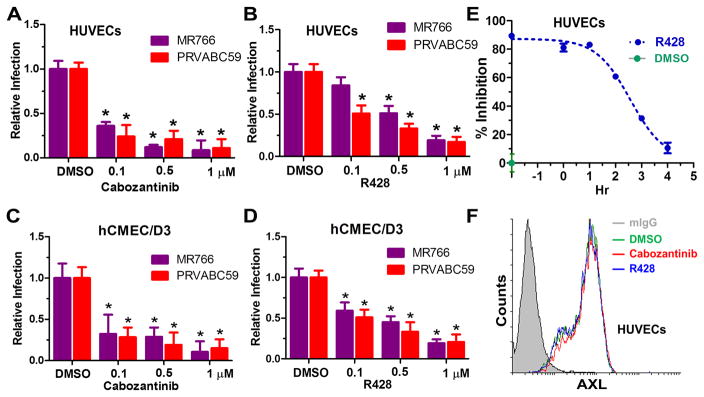
ZIKV is known to utilize multiple cell surface receptors, including DC-SIGN, AXL, Tyro3, and TIM-1, to gain entry with a major role for the receptor tyrosine kinase (RTK) AXL16. A recent single-cell expression analysis revealed that the candidate entry receptor AXL is highly expressed in neural stem cells and endothelial cells in developing human cortex 18. To probe into the potential involvement of AXL, we first performed flow cytometric analysis and determined the AXL expression levels on low-passage cultures of human endothelial cells. Notably, while most endothelial cells express AXL, HUVECs appear to express AXL to the highest level, followed by HAoECs and HSaVECs (Figure 3A). By contrast, HCoECs expressed very little AXL. Although we cannot completely exclude potential donor variability, the cell-surface expression levels of AXL positively correlate with cellular susceptibilities to ZIKV (Figure 1E and Figure 3A). AXL is a receptor tyrosine kinase that transduces signals from the extracellular matrix into the cytoplasm by binding to the vitamin K-dependent protein growth arrest-specific 6 (Gas6) gene 19. To explore the role of AXL in the observed ZIKV infection, we conducted three sets of experiments. First, a polyclonal antibody that recognizes the extracellular portion of human AXL blocked the entry of virus into HUVECs and hCMEC/D3 cells (Figure 3B). Notably, addition of the antibody did not reduce the attachment of ZIKV to cells (Figure 3C) and was unable to block ZIKV infection if added 3 hours after the infection had been initiated (Figure 3D). This data suggests that AXL is a ZIKV entry factor required at a late stage during entry. Second, ectopic expression of AXL in 293T cells promoted ZIKV infection without enhancing virus binding (Figure 3E, F, H). By contrast, the kinase-dead AXL, which carries a K567R mutation that destroys an ATP-binding site and inhibits Axl phosphorylation and signaling 20–22, is significantly impaired its ability to confer permissiveness to 293T cells (Figure 3F, H). These observations reinforced the idea that AXL promotes ZIKV entry at a post-binding step and also imply that AXL-mediated signaling is needed for ZIKV entry. Lastly, two known inhibitors of AXL phosphorylation, Cabozantinib 23 and R428 24, significantly impaired ZIKA infection of hCMEC/D3 and the HUVECs in a dose-dependent manner (Figure 4A–D). By contrast, RTK inhibitors Sunitinib malate and Sorafenib had none or marginal inhibition at one micromolar (Online Figure V). These results indicate that AXL receptor tyrosine kinase activity is potentially important to its function in ZIKV entry. The kinetics of R428-mediated inhibition, generated from a time-of-addition experiment, showed that the compound remained inhibitory even when added at 1 hour after the virus was added but drastically lost its effect if added at 2 hours after infection had been initiated (Figure 4E). Therefore, R428 interferes with a post-binding process during ZIKV entry. Of note, treatment of HUVECs with AXL inhibitors neither altered AXL cell surface expression (Figure 4F) nor reduced cell viability (Online Figure VC).
Figure 3. AXL is a required factor for ZIKV infection of human endothelial cells at a post-binding step.
(A) AXL expression on various ECs used in Figure 1. (B) A polyclonal anti-human AXL antibody (AXL pAB) blocked ZIKV infection of hCMEC/D3 and HUVECs. Cells were preincubated with antibodies at indicated concentrations for 1 hour followed by virus infection (MOI 1) of 1 hour, after which a medium change occurred. Twenty-four hours post-infection cellular RNA was extracted for real-time RT-PCR quantification of entered vRNA. The obtained results were normalized against levels of GAPDH and the ZIKV RNA levels from cells without antibody treatment were set to 1. N = 3, *P<0.05. (C) AXL pAB (10 μg/ml) or control IgG was added together with ZIKV PRV isolate (MOI 1) to HUVECs cells at 4 °C for 2 hours, after which cells were washed or trypsinized (positive control) prior to real-time RT-PCR quantification of bound vRNA. N = 2, *P<0.05. (D) AXL pAB (10 μg/ml) was either added together with PRV at the time of inoculation (0 h) or 3 hours postinoculation after virus had been removed (+3 hr). Twenty-four hours postinfection, cells were lysed for ZIKV RNA quantitation. N = 2, *P<0.05. (E) Untransfected 293T cells or cells transfected with AXL, AXL kinase dead (KD, K567R) or control kinase PDK1 were incubated with ZIKV PRV (MOI 1) at 4 °C for two hrs. After extensive wash, bound viral RNA was quantified by RT-qPCR (n = 2). (F) Transfected 293T cells were inoculated with PRV (MOI 1) and then incubated for an additional 24 hours prior to quantification of viral RNA. N = 2, *P<0.05. (G) Expression of AXL on transfected 293T cells were quantified by flow cytometry using the anti-AXL antibody. (H) Representative confocal images of ZIKV infected 293T cells from (F). Percentages of infection were indicated at the upperleft corners.
Figure 4. AXL inhibitors block ZIKV infection.
HUVECs (A,B) or hCMEC/D3 cells (C,D) were pre-treated with Cabozantinib, R428, were pre-treated with Cabozantinib or R428 for 1 hour followed by ZIKV infection (MOI 1) of 1 hour, after which a medium change occurred. Twenty-four hours post-infection cellular RNA was extracted for real-time RT-PCR quantification of ZIKV RNA. The obtained results were normalized against levels of GAPDH and the ZIKV RNA levels from DMSO-treated cells were set to 1 (n=2; error bars, s.d.; *P<0.05.). (E) ZIKV PRV was added to HUVECs cells at 4 °C and incubated for 2 hours. Unbound virus was washed off with cold media, and the cells were shifted to 37°C (set as a 0-hour time point) to initiate synchronous infection. At the indicated time points, 1 μM R428 or DMSO was added into the media and incubated for 2 hours prior to removal (exception is t= −2 hours where R428 was added back after removal of the virus and incubated for additional 2 hours prior to removal). Infected cells were incubated at 37 °C for an additional 24 hours prior to RT-qPCR assay of ZIKV RNA. Inhibition was calculated as 100- % infection relative to infections containing no inhibitors. Fitted lines represent sigmoidal time-dependent curves (mean of n = 2; error bars, s.d). (F) Cell-surface levels of AXL on compound-treated HUVECs were quantified by flow cytometry using the anti-AXL antibody.
DISCUSSION
With the imminent threat of the ZIKV epidemic to pregnant women and to the global blood supply, this timely study provides mechanistic understanding of ZIKV tropism and pathogenicity. The significance of our findings is threefold: (1) human ECs are likely one of the principal cell types of ZKIV infection. In vivo, the release of infectious ZIKV by ECs would conceivably facilitate hematogenous dissemination of the virus and bypass the barriers that would otherwise restrict viral infection, and thus reach tissues where viruses typically cannot reach. Such a route allows the virus to rapidly enter or leave the bloodstream and potentially contributes to the intrauterine and transfusion-mediated ZIKV transmission (Online Figure VI). Although several human placental cell types, including cytotrophoblasts, epithelial cells, fibroblasts, Hofbauer macrophages were reportedly permissive to ZIKV 25, 26, Miner et. al. recently found that ZIKV-infected Ifnar−/− pregnant mice show signs of vascular damage in the placenta and fewer fetal blood vessels 5, evidencing ZIKV infection of fetal ECs in vivo. (2) The ZIKVSA isolates replicate faster in ECs than ZIKVAF strains. While the ZIKVAF strains MR766 and IbH have been around for many years and might have adapted more through continuous passages in cultures, faster replication kinetics could contribute to the enhanced virulence of ZIKVSA isolates. We speculate that the partial cytopathic effect of the ZIKVSA isolates may trigger vascular changes in vivo, from severe placental vascular damage and a reduction in fetal blood vessels early in pregnancy to hemorrhagic retinopathy and torpedo maculopathy 27. In this regard, it would be highly interesting to assess the prevalence of vascular complications among ZIKV infected individuals. (3) ZIKV tropism for ECs positively correlates with cell-surface levels of AXL. Despite previous studies have implicated a role of AXL in ZIKV entry, our results clearly demonstrated that AXL functions at a post-binding step, where its catalytic activity is required. Several AXL inhibitors, including Cabozantinib and R428 that are currently in clinical trials for anti-cancer activities, may serve as unique antiviral therapeutics that suppress ZIKV infection of ECs.
Supplementary Material
Novelty and Significance.
What Is Known?
Besides infecting the developing fetal brain, Zika virus has also been recognized as a blood-borne pathogen.
Endothelial cells are major components of the blood-brain barrier and part of the placental blood barrier, preventing circulating virus from entering the brain and the fetal tissues, respectively.
What New Information Does This Article Contribute?
Low passage human endothelial cells can be readily infected by Zika virus of the African and South American lineage and release infectious progeny virus.
South American Zika virus isolates replicate faster in human endothelial cells and are partially cytopathic.
The receptor tyrosine kinase AXL is required for Zika virus entry of endothelial cells at a post-binding step.
The endothelium is the key cellular barrier between the blood and interstitial space. We find that Zika virus use of the receptor tyrosine kinase AXL allows for entry into endothelium, in particular human umbilical vein endothelium. This work demonstrates that endothelial cells are key targets for ZIKA virus, and could be a novel pharmacological target. Critically, this work (1) strongly implies that screening of the stored blood supply should be a priority because of the direct contact between blood and endothelium, and (2) could explain the presence of the virus in embryos, in utero, and in stored blood.
Acknowledgments
We thank Dr. Matthew Kappes for critical reading of this manuscript. Zika Virus, MR 766, NR-50065 and IbH 30656, NR-50066 were obtained through BEI Resources, the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institutes of Health (NIH) as part of the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) program. The following reagents were obtained through BEI Resources, NIAID, NIH: Zika virus, PRVABC59, NR-50240 and FLR, NR-50183.
SOURCES OF FUNDING
This study was sponsored by NIH Grant R01DK088787 (to T.W.), R01HL088554 (B.E.I.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Nonstandard Abbreviations and Acronyms
- ZIKV
Zika virus
- ZIKVAF
African Zika virus stains
- ZIKVSA
south American Zika virus strains
- ECs
endothelial cells
- HUVECs
human umbilical vein endothelial cells
- HAoEC
human aortic endothelial cells
- HCoECs
human coronary artery derived endothelial cells
- HSaVECs
human saphenous vein derived endothelial cells
- AXL
Tyrosine-protein kinase receptor UFO
Footnotes
AUTHOR CONTRIBUTIONS
T. W. designed the overall experiments; L.D. prepared the primary human endothelial cells; S.L. and T.W. performed the experiments and analyzed the data; S. L., B.I., and T.W. wrote the manuscript. All authors read and approved the final manuscript. All authors have provided the corresponding author with written permission to be named in the manuscript.
DISCLOSURES
None.
COMPETING INTERESTS
The authors declare they have no competing interests.
References
- 1.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T. Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 4.Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandao WN, Rossato C, Andrade DG, de Faria DP, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PM, Peron JP, Muotri AR, Beltrao-Braga PC. The brazilian zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu KY, Zuo GL, Li XF, Ye Q, Deng YQ, Huang XY, Cao WC, Qin CF, Luo ZG. Vertical transmission of zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 2016;26:645–654. doi: 10.1038/cr.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, Leparc-Goffart I. Late sexual transmission of zika virus related to persistence in the semen. Lancet. 2016 doi: 10.1016/S0140-6736(16)30775-9. [DOI] [PubMed] [Google Scholar]
- 8.D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Leparc-Goffart I. Evidence of sexual transmission of zika virus. N Engl J Med. 2016;374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- 9.Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, Fiorentini C, Trotta M, Rizzo C, Mantella A, Rezza G, Bartoloni A. An autochthonous case of zika due to possible sexual transmission, florence, italy, 2014. Euro Surveill. 2016:21. doi: 10.2807/1560-7917.ES.2016.21.8.30148. [DOI] [PubMed] [Google Scholar]
- 10.Musso D, Stramer SL, Busch MP. Zika virus: A new challenge for blood transfusion. Lancet. 2016;387:1993–1994. doi: 10.1016/S0140-6736(16)30428-7. [DOI] [PubMed] [Google Scholar]
- 11.Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, Christian KM, Didier RA, Jin P, Song H, Ming GL. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiwanitkit V. Placenta, zika virus infection and fetal brain abnormality. Am J Reprod Immunol. 2016 doi: 10.1111/aji.12521. [DOI] [PubMed] [Google Scholar]
- 13.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of zika virus in saliva. J Clin Virol. 2015;68:53–55. doi: 10.1016/j.jcv.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson B, Hearn P, Afrough B, Lumley S, Carter D, Aarons EJ, Simpson AJ, Brooks TJ, Hewson R. Detection of zika virus in semen. Emerg Infect Dis. 2016;22:940. doi: 10.3201/eid2205.160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang FC, Li XF, Deng YQ, Tong YG, Qin CF. Excretion of infectious zika virus in urine. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)30070-6. [DOI] [PubMed] [Google Scholar]
- 16.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Despres P, Amara A, Yssel H, Misse D. Biology of zika virus infection in human skin cells. J Virol. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. Faseb J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 18.Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression analysis highlights axl as a candidate zika virus entry receptor in neural stem cells. Cell Stem Cell. 2016;18:591–596. doi: 10.1016/j.stem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham DK, DeRyckere D, Davies KD, Earp HS. The tam family: Phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer. 2014;14:769–785. doi: 10.1038/nrc3847. [DOI] [PubMed] [Google Scholar]
- 20.Braunger J, Schleithoff L, Schulz AS, Kessler H, Lammers R, Ullrich A, Bartram CR, Janssen JW. Intracellular signaling of the ufo/axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site. Oncogene. 1997;14:2619–2631. doi: 10.1038/sj.onc.1201123. [DOI] [PubMed] [Google Scholar]
- 21.Shimojima M, Ikeda Y, Kawaoka Y. The mechanism of axl-mediated ebola virus infection. J Infect Dis. 2007;196(Suppl 2):S259–263. doi: 10.1086/520594. [DOI] [PubMed] [Google Scholar]
- 22.Meyer AS, Zweemer AJ, Lauffenburger DA. The axl receptor is a sensor of ligand spatial heterogeneity. Cell Syst. 2015;1:25–36. doi: 10.1016/j.cels.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L, Liu XD, Sun M, Zhang X, German P, Bai S, Ding Z, Tannir N, Wood CG, Matin SF, Karam JA, Tamboli P, Sircar K, Rao P, Rankin EB, Laird DA, Hoang AG, Walker CL, Giaccia AJ, Jonasch E. Targeting met and axl overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35:2687–2697. doi: 10.1038/onc.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, Duan M, Torneros A, Yu J, Heckrodt TJ, Zhang J, Ding P, Apatira A, Chua J, Brandt R, Pine P, Goff D, Singh R, Payan DG, Hitoshi Y. R428, a selective small molecule inhibitor of axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70:1544–1554. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- 25.Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe. 2016 doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, Suthar MS. Zika virus infects human placental macrophages. Cell Host Microbe. 2016 doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda HA, 2nd, Costa MC, Frazao MA, Simao N, Franchischini S, Moshfeghi DM. Expanded spectrum of congenital ocular findings in microcephaly with presumed zika infection. Ophthalmology. 2016;123:1788–1794. doi: 10.1016/j.ophtha.2016.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.