Abstract
AIM
To determine the effect of tempol in normal rats fed high salt on arterial pressure and the balance between antagonist components of the renal renin-angiotensin system.
METHODS
Sprague-Dawley rats were fed with 8% NaCl high-salt (HS) or 0.4% NaCl (normal-salt, NS) diet for 3 wk, with or without tempol (T) (1 mmol/L, administered in drinking water). Mean arterial pressure (MAP), glomerular filtration rate (GFR), and urinary sodium excretion (UVNa) were measured. We evaluated angiotensin II (Ang II), angiotensin 1-7 (Ang 1-7), angiotensin converting enzyme 2 (ACE2), mas receptor (MasR), angiotensin type 1 receptor (AT1R) and angiotensin type 2 receptor (AT2R) in renal tissues by immunohistochemistry.
RESULTS
The intake of high sodium produced a slight but significant increase in MAP and differentially regulated components of the renal renin-angiotensin system (RAS). This included an increase in Ang II and AT1R, and decrease in ACE-2 staining intensity using immunohistochemistry. Antioxidant supplementation with tempol increased natriuresis and GFR, prevented changes in blood pressure and reversed the imbalance of renal RAS components. This includes a decrease in Ang II and AT1R, as increase in AT2, ACE2, Ang (1-7) and MasR staining intensity using immunohistochemistry. In addition, the natriuretic effects of tempol were observed in NS-T group, which showed an increased staining intensity of AT2, ACE2, Ang (1-7) and MasR.
CONCLUSION
These findings suggest that a high salt diet leads to changes in the homeostasis and balance between opposing components of the renal RAS in hypertension to favour an increase in Ang II. Chronic antioxidant supplementation can modulate the balance between the natriuretic and antinatriuretic components of the renal RAS.
Keywords: Kidney, Angiotensin II, Tempol, Angiotensin 1-7, High sodium diet
Core tip: This study explored the effect of tempol on arterial pressure and the balance between antagonist components of the renal renin-angiotensin system in rats fed high salt for 3 wk. A high salt diet altered the balance between opposing components of renal renin-angiotensin system (RAS), favouring the angiotensin II arm. Tempol supplementation improves the balance between the natriuretic and antinatriuretic components of the renal RAS.
INTRODUCTION
Several studies have shown that the current dietary intake of salt in Western societies is an important factor for the genesis of hypertension and may even cause blood pressure-independent target organ damage, including the kidney[1,2]. It is well known that a high salt intake increases the oxidative stress in the kidney of normal and salt-sensitive rats[3-5]. In this regard, we have reported that the administration of tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl), a permeate superoxide dismutase mimetic[6] commonly used to scavenge superoxide anion, prevented oxidative stress and produced a natriuretic and diuretic effect in Sprague Dawley rats fed a high salt diet[7].
The renin-angiotensin system (RAS) essentially controls the sodium homeostasis and blood pressure[8]. Furthermore, a local RAS that is expressed in the kidneys and operate independently of the systemic RAS, may also contribute to long-term blood pressure control and cause renal injury when it is associated with a high salt diet[7,9-11]. Angiotensin II (Ang II), the main effector of RAS, acts through two receptor subtypes named angiotensin II type-1 receptor (AT1R) and type-2 receptor (AT2R). It is well known that the triad angiotensin I converting enzyme (ACE)/Ang II/AT1R is closely involved in signaling pathways that mediate vasoconstriction, antinatriuresis, release of cytokines and accumulation of inflammatory cells in the kidney, all factors which together tend to increase blood pressure[12,13]. On the other hand, activation of AT2R is associated with vasodilatation, apoptosis, anti-proliferation and natriuresis, which contribute to lower blood pressure[14]. Therefore, AT2R is considered a physiological antagonist of AT1R. The discovery of the Ang II breakdown enzyme, angiotensin I converting enzyme 2 (ACE2) in addition to ACE, enhanced the complexity and understanding of generation and degradation of Ang II in hypertension[15,16]. ACE2 leads to generation of Ang-(1-7) that stimulates Mas receptor (MasR), antagonizing AT1R-mediated effects, and favouring vasodilatation, natriuresis, anti-fibrogenic and anti-proliferative actions[17,18]. Hyper-activation of the RAS, mainly via enhanced AT1R function, contributes to excessive sodium reabsorption in the kidney, like in salt-sensitive hypertension[19]. There is evidence that AT1R activation decreases ACE2 activity and Ang-(1-7) production[16]. These findings suggest the presence of an imbalance between opposing components of the renal RAS, namely the hypertensive axis, ACE-Ang II-AT1R vs the anti-hypertensive axis, AT2 and ACE2-Ang (1-7)-MasR in the development of hypertension.
Although it is well known that the intake of sodium is a regulatory factor to control the activity of renal axis ACE-Ang II-AT1R components, the regulation of renal axis AT2 and ACE2-Ang (1-7)-MasR in response to high sodium intake is not fully understood to date. Hereby, it remains unclear whether the increased oxidative stress observed in salt-sensitive hypertension is associated to an imbalance of renal RAS components or not.
We hypothesized that a high sodium intake, through oxidative stress development, may induce an imbalance between the hypertensive and anti-hypertensive components of the renal RAS, contributing to the pathogenesis of hypertension. Considering this assertion, we explored the effect of tempol (used as an inhibitor of oxidative stress) in normal rats fed a high salt intake on the balance between the antagonist components of the renal RAS.
MATERIALS AND METHODS
Animal model
Male Sprague Dawley rats, 5-6 wk-old (180-200 g body weight), were used in the experiments. The animals were housed in steel cages in a controlled room temperature at 23 °C ± 2 °C, exposed to a daily 12-h light-dark cycle (light on 07:00 a.m. to 07:00 p.m), fed for three weeks with the diets described below, and given tap water ad libitum. Experiments were conducted in accordance with the institutional University of Buenos Aires guidelines for the care and use of research animals. The experiments were performed in animals randomly divided in four groups (n = 6 each group): (1) NS (control): Animals fed with a normosodic diet (0.4% NaCl); (2) HS: Fed with a hypersodic diet (8% NaCl); (3) NS-T: Fed with a normosodic diet (0.4% NaCl), plus 1 mmol/L tempol (Sigma-Aldrich Inc, St. Louis, Missouri, United States), administered in the drinking water; (4) HS-T: Fed with a hypersodic diet (8% NaCl), plus 1 mmol/L tempol administered in the drinking water. After a 3-wk diet, the rats were intraperitoneally anaesthetized with urethane (1.2 g/kg). A PE-90 tubing (3 cm long) was inserted into the trachea to maintain an open airway. The left femoral vein was catheterized with a Silastic cannula (0.12 mm i.d.) for continuous infusion. The right carotid artery was also catheterized with a T4 tube for blood sampling and for continuous mean arterial pressure recording (MAP) by means of a Statham GOULD P23ID transducer coupled to a Grass Polygraph 79D during all the procedures. The bladder was cannulated for urine collection using a PE-75 cannula. A femoral vein infusion with isotonic saline solution, 0.15 mol/L NaCl (ISS) was performed at a 0.04 mL/min rate (Syringe Infusion Pump, SageTM, Orion) for 60 min to allow reaching a steady diuresis and permitting urine collection in all groups. Then, ISS infusion continued for another 60 min at the same rate as an experimental period. Blood samples were collected at 30 min and urine samples were collected along the 60 min of ISS infusion for sodium, potassium and creatinine measurement.
Urine and blood measurements
Urinary and plasmatic sodium and creatinine were measured by standard methods using an autoanalyzer. Creatinine clearance was assessed in order to evaluate glomerular filtration rate (GFR). GFR and sodium fractional excretion (FENa) were calculated according to a standard formula. Urinary sodium excretion is expressed as μmol/min per kilogram, GFR as mL/min and FENa as percentage.
Kidney processing for histological examination
At the end of the infusion period, the left kidney was perfused with ISS through the abdominal aorta until the blood was washed out and the parenchyma showed a pale appearance. The kidney was rapidly excised, decapsuled, longitudinally cut and harvested for immunohistochemical studies. Tissues were fixed in phosphate-buffered 10% formaldehyde (pH 7.2) and included in paraffin. For immunohistochemistry, renal sections were deparaffined and rehydrated, and endogenous peroxidase activity was blocked by treatment with 0.5 % H2O2 in methanol for 20 min. Local Ang II, ACE 2, AT1R, AT2, Ang (1-7) and MasR were detected using the following specific antibodies: human anti-Ang II (Peninsula, CA; United States, dilution of 1:500), rabbit anti-Ang (1-7) (Santa Cruz Biotechnology, Inc, United States; dilution: 1:200), rabbit anti-ACE2 (Santa Cruz Biotechnology, Inc, United States; dilution: 1:200), rabbit anti-AT1 (Santa Cruz Biotechnology, Inc, United States; dilution: 1:200), rabbit anti-AT2 (Santa Cruz Biotechnology, Inc; dilution: 1:200) and rabbit anti-MasR (Abcam, Cambridge, MA, United States, dilution: 1:100). Immunostaining was carried out by means of a commercial modified avidin-biotin-peroxidase complex technique (Vectastain ABC kit, Universal Elite, Vector Laboratories, CA, United States) and counterstained with hematoxylin. Histological sections were observed in a Nikon E400 light microscope (Nikon Instrument Group, Melville, New York, United States). The antibodies used in this study worked well in nephron segments, especially in tubules[20-25]. Immunoreactivities for Ang II, ACE 2, AT1R, AT2R, Ang (1-7) and MasR in renal tissue are expressed as integrated optical density (IOD) ± SEM using a model for automated computer image analysis to quantify immunohistochemical stains in hematoxylin counterstained histological sections[26].
Statistical analysis
Results from urine, blood measurements and MAP are expressed as mean ± SEM. Gaussian distribution was evaluated by the Kolmogorov and Smirnov method and comparisons between groups were carried out using ANOVA followed by the Newman-Keuls test. P values < 0.05 were considered significant.
RESULTS
MAP, GFR and excretory function
In Table 1, it can be observed that HS diet increased MAP, UVNa and FENa(%), but did not alter GFR, respect to NS fed animals. Interestingly, even though the administration of Tempol to NS group did not modify MAP levels and FENa(%), it increased GFR and UVNa respect to NS group. Moreover, tempol administration to HS group, restored MAP to control NS levels, increased GFR to similar levels of NS-T group and increased further UVNa and FENa values.
Table 1.
High-salt diet increased mean arterial pressure, urinary sodium excretion and sodium fractional excretion (%), but did not alter glomerular filtration rate, respect to normal salt fed animals
| NS | NS-T | HS | HS-T | |
| MAP (mmHg) | 92 ± 3 | 94 ± 3 | 108 ± 3a | 95 ± 2c |
| GFR (mL/min) | 1.50 ± 0.1 | 3.2 ± 0.5c | 1.52 ± 0.2 | 3.1 ± 0.6c |
| UVNa (μmol/min per kilogram) | 0.25 ± 0.1 | 2.03 ± 0.90c | 3.56 ± 0.70a | 13.2 ± 1.2ac |
| FENa (%) | 0.06 ± 0.02 | 0.10 ± 0.06 | 0.50 ± 0.08a | 1.20 ± 0.15ac |
All values are mean ± SEM (n = 5-6 per group),
P < 0.05 vs respective NS group,
P < 0.05 vs respective group without tempol. NS: Normal salt diet group; HS: High salt diet group; NS-T: Normal salt diet plus tempol group; HS-T: High salt diet plus tempol group; MAP: Mean arterial pressure; GFR: Glomerular filtration rate; UVNa: Urinary sodium excretion; FENa: Fractional sodium excretion.
Intrarenal Ang II expression
Figure 1 shows Ang II immunoexpression in renal tissues. Immunohistochemical analysis of renal sections revealed positive staining for Ang II in all tubular sections of the nephron examined. HS diet increased Ang II expression with respect to NS group. Tempol administration, which did not reduce Ang II staining significantly in NS-T, decreased Ang II staining in HS-T animals, but remaining higher in NS-T group.
Figure 1.
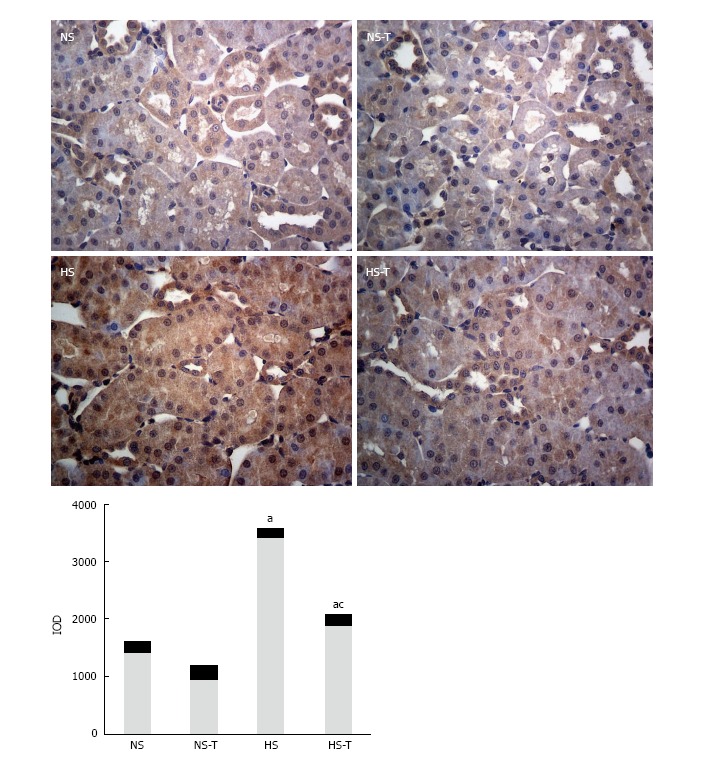
The graphs show the quantitative evaluation of Angiotensin II immunostaining in renal tissue. Data are expressed as mean ± SEM; aP < 0.05 vs the respective NS group, cP < 0.05 vs the respective group without tempol. The photomicrographs represent Ang II immunostaining in NS, HS, NS-T and HS-T groups. Original magnification: 400 ×. IOD: Integrated optic density; NS: Normal salt diet group; HS: High salt diet group; NS-T: Normal salt diet plus tempol group; HS-T: High salt diet plus tempol group.
Intrarenal ACE2 expression
Figure 2 shows ACE2 immunoexpression in renal tissues. The study focused on tubular expression, where ACE2 staining was more evident, whereas in glomeruli it was very low. ACE2 appeared to be expressed in the endothelium of larger vessels which was closer to the renal hilum. In renal tubules where the differences between groups are more significant, ACE2 staining was expressed, in order of density, in proximal convolute tubules, distal convolute tubules located in the juxtamedullary area, and in collecting tubules.
Figure 2.
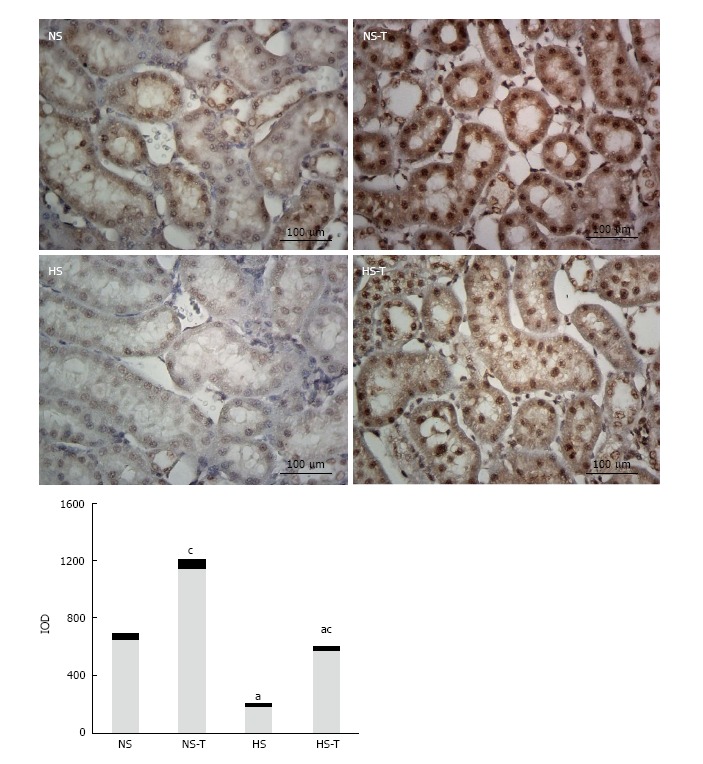
The graphs show the quantitative evaluation of angiotensin converting enzyme 2 immunostaining in renal tissue. Data are expressed as mean ± SEM; aP < 0.05 vs the respective NS group, cP < 0.05 vs the respective group without tempol. The photomicrographs represent ACE2 immunostaining in NS, HS, NS-T and HS-T groups. Original magnification: 400 ×. IOD: Integrated optic density; NS: Normal salt diet group; HS: High salt diet group; NS-T: Normal salt diet plus tempol group; HS-T: High salt diet plus tempol group; ACE2: Angiotensin converting enzyme 2.
HS diet decreased ACE2 immunoexpression compared to NS fed animals. Tempol administration nearly duplicated ACE2 staining in both groups, NS-T and HS-T respect to NS and HS groups, respectively, showing in HS-T group similar levels than those in NS control group.
Intrarenal Ang (1-7) expression
Figure 3 shows Ang 1-7 immunoexpression in renal tissues. Positive staining for Ang 1-7 was observed in all tubular sections examined. HS diet did not modify Ang (1-7) expression with respect to NS group. Tempol increased Ang 1-7 staining in NS-T animals and further increased in HS-T group.
Figure 3.
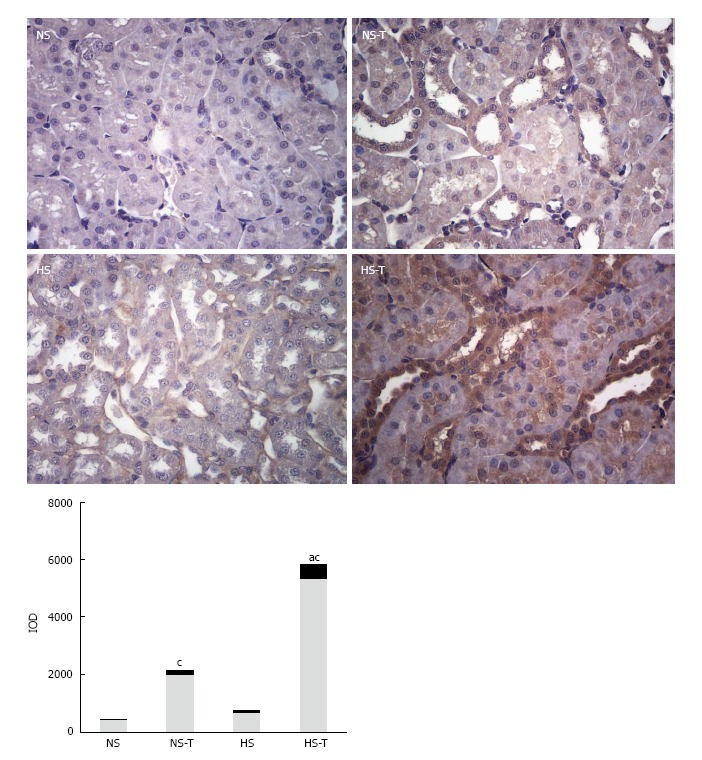
The graphs show the quantitative evaluation of Angiotensin 1-7 immunostaining in renal tissue. Data are expressed as mean ± SEM; aP < 0.05 vs the respective NS group, cP < 0.05 vs the respective group without tempol. The photomicrographs represent Ang 1-7 immunostaining in NS, HS, NS-T and HS-T groups. Original magnification: 400 ×. IOD: Integrated optic density; NS: Normal salt diet group; HS: High salt diet group; NS-T: Normal salt diet plus tempol group; HS-T: High salt diet plus tempol group.
Intrarenal MasR expression
Figure 4 shows MasR immunoexpression in renal tissues. HS diet decreased MasR staining respect to NS control group, while tempol nearly doubled in MasR staining in NS-T and H-T groups compared to NS and HS groups, respectively. MasR expression remained lower in HS-T group respect to NS-T group.
Figure 4.
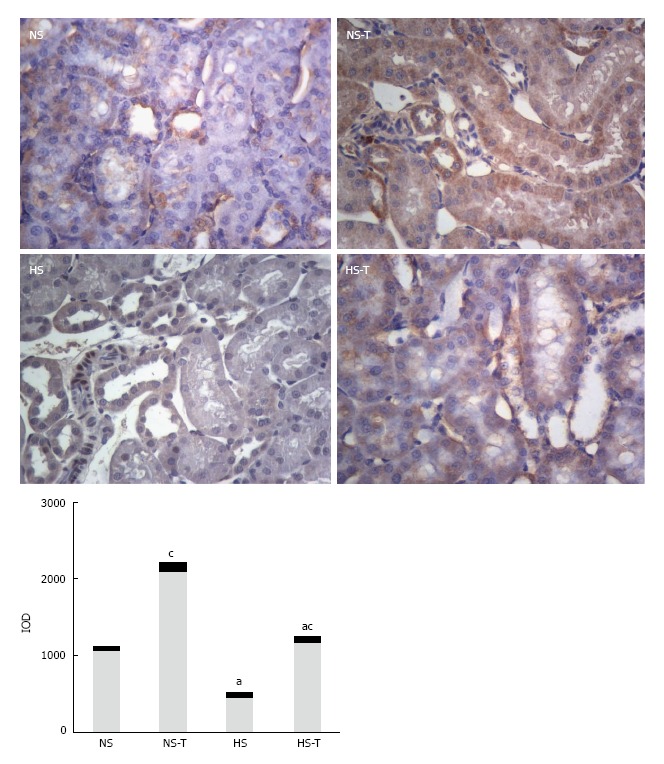
The graphs show the quantitative evaluation of Mas receptor immunostaining in renal tissue. Data are expressed as mean ± SEM; aP < 0.05 vs the respective NS group, cP < 0.05 vs the respective group without tempol. The photomicrographs represent MasR immunostaining in NS, HS, NS-T and HS-T groups. Original magnification: 400 ×. IOD: Integrated optic density; NS: Normal salt diet group; HS: High salt diet group; NS-T: Normal salt diet plus tempol group; HS-T: High salt diet plus tempol group; MasR: Mas receptor.
Intrarenal AT1R expression
Figure 5 shows AT1R immunoexpression in renal tissues. HS diet increased AT1R expression compared to NS group. Tempol administration prevented changes in AT1R staining in HS-T group with respect to HS group. No differences were observed in AT1R staining between NS-T and NS groups.
Figure 5.
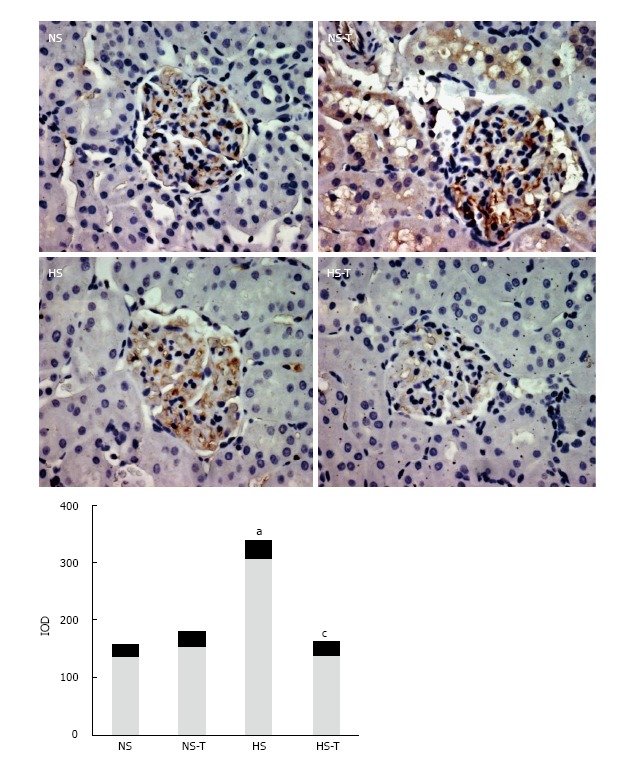
The graphs show the quantitative evaluation of angiotensin type 1 receptor immunostaining in renal tissue. Data are expressed as mean ± SEM; aP < 0.05 vs the respective NS group, cP < 0.05 vs the respective group without tempol. The photomicrographs represent AT1R immunostaining in NS, HS, NS-T and HS-T groups. Original magnification: 400 ×. IOD: Integrated optic density; NS: Normal salt diet group; HS: High salt diet group; NS-T: Normal salt diet plus tempol group; HS-T: High salt diet plus tempol group; AT1R: Angiotensin type 1 receptor.
Intrarenal AT2R expression
Figure 6 shows AT2R immunoexpression in renal tissues. High salt diet did not change AT2R expression compared to NS group. Tempol administration increased AT2R staining in NS-T and H-T groups respect to NS and HS groups, respectively. No differences were observed in AT2R staining between NS-T and HS-T groups.
Figure 6.
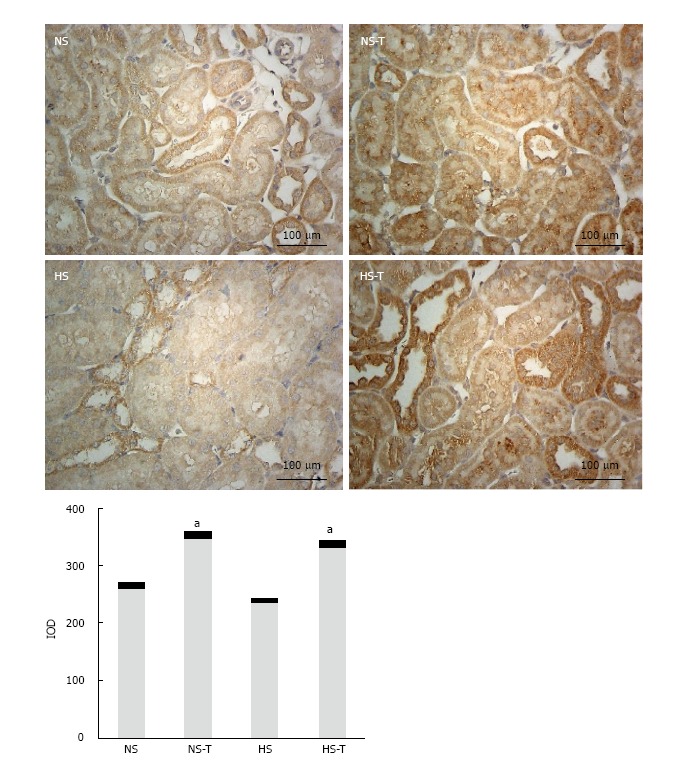
The graphs show the quantitative evaluation of angiotensin type 2 receptor immunostaining in renal tissue. Data are expressed as mean ± SEM; aP < 0.05 vs the respective group without tempol. The photomicrographs represent AT2R immunostaining in NS, HS, NS-T and HS-T groups. Original magnification: 400 ×. IOD: Integrated optic density; NS: Normal salt diet group; HS: High salt diet group; NS-T: Normal salt diet plus tempol group; HS-T: High salt diet plus tempol group; AT2R: Angiotensin type 2 receptor.
DISCUSSION
Our current study shows that a high sodium intake, besides increasing blood pressure, may also deregulate the components of the renal RAS. In HS diet-fed animals, we observed increased Ang II and AT1R, and decreased ACE-2 immunoexpression, suggesting an altered production and/or degradation of Ang II. Antioxidant supplementation with tempol in rats which were fed a high salt diet, not only prevented the increase in blood pressure, but also reversed the imbalance of renal RAS components. Indeed, tempol decreased Ang II and AT1R overexpression and increased AT2, ACE2, Ang 1-7 and MasR expression. This result was associated with enhanced diuresis and natriuresis, which may justify the decrease in blood pressure observed in these animals. In addition, the natriuretic effect of tempol was also observed in NS-T group, which simultaneously showed increased AT2, ACE2, Ang (1-7) and MasR immunoexpression.
Many reports indicate that oxidative stress is an important contributing factor in hypertension[27-31]. Hypertensive patients and animal models of hypertension have shown increased reactive oxygen species production and decreased antioxidant capacity[32,33]. Tempol, has been shown to reduce oxidative stress and attenuate hypertension in obese Zucker rats, Dahl salt-sensitive and spontaneously hypertensive rats (SHR)[34-38]. In addition, we have previously reported that tempol can prevent the increase in renal oxidative stress and arterial pressure in Sprague Dawley rats fed a high salt diet[7].
It is well known that the proximal tubule synthesizes and secretes Ang II to the lumen in the kidney[39,40]. Our current study, using a model of oxidative stress in rats fed a high salt diet, showing that Ang II expression increased and ACE2 expression decreased in renal tissues. Considering that ACE2 is the enzyme that metabolizes Ang II to Ang 1-7, one might assume that the higher expression of Ang II is due to reduced expression of ACE2. With this in mind, Deshotels et al[41] have described that Ang II is able to up-regulate ACE and down-regulate ACE2 expressions in human kidney tubular cells, associated with activation of extracellular regulated (ERK)1/2 and p38 mitogen-activated protein (MAP) kinases, a component that was blocked by the angiotensin II AT1R antagonist losartan, but not by the AT2 receptor blocker PD123319.
Down-regulation of ACE2 by Ang II represents a novel positive feed-forward system as noted in the brain[42,43]. Our findings suggest that down-regulation of ACE2 in high salt fed animals may favour decreased Ang II degradation.
Moreover, the increase of ACE2 expression in tempol treated rats in HS-T group, could explain the decline of Ang II and the enhancement of Ang (1-7) expression observed in this group.
Interestingly, however, the increasing effects of tempol on ACE2 and Ang (1-7) expression were also observed in the rats fed with NS. Thus, we can suggest that these effects of tempol are independent of dietary sodium load. Moreover, these results suggest, that intrarenal mechanisms that regulate the differential expression of Ang II and ACE2 exacerbated by a high-sodium diet, would not be a dependent mechanism of oxidative stress. In this sense it has been shown that in physiological conditions, acute renal sympathetic nerve activity modulates sodium reabsorption by increasing intrarenal Ang II generation and activation of the luminal membrane AT1R[44]. It has been recently shown that activation of renal nervous system results in higher levels of intrarenal angiotensinogen and Ang II independent of changes in renal function and systemic RAS[45].
In this study results show that HS diet decreased MasR expression while tempol treatment increased Ang 1-7 and AT2R expression, and restored MasR levels. As tempol also increased MasR expression in rats fed with NS diet, this action of tempol seems to be independent from sodium overload in the diet. These changes may aid the natriuretic effect and normalization of blood pressure in HS-T animals.
Altogether, the results suggest that the signalling pathways of the renal RAS could be physiologically regulated by the redox state.
It is known that Ang (1-7) reduces vasoconstriction, water retention, salt intake, cell proliferation and reactive oxygen stress, displaying also a renoprotective effect[46]. Thus, our results suggest that in the renal RAS, the ACE2-Ang (1-7)-Mas axis counteracts the ACE-Ang II-AT1 axis.
It has been reported that Ang II may directly stimulate nuclear AT1 receptors to induce transcriptional responses, which may be associated with stimulation of tubular epithelial sodium transport and inflammatory response[47,48]. When these effects are inhibited by tempol, they may increase distal sodium overload, which in turn may increase the tubule-glomerular feedback, thus decreasing the GFR. However, tempol also reduces sodium signalling in the macula dense[49], as well as afferent arteriole responses[50], contributing to moderate the tubule-glomerular feedback response and to increase GFR, as we observed in the current study.
Finally, our results are in line with reports of studies performed in obesity-related hypertension, where it was suggest that a high sodium intake that provokes hypertension is also able to regulate differentially and shift the balance between opposing components of the renal RAS[51]. Additionally, antioxidant treatment with tempol reversed the imbalance of renal RAS components and led to diuresis and natriuresis, lowering obesity-related hypertension. Furthermore, a high salt diet in SHR produced glomerular hypertrophy and decreased ACE2 and nephrin expressions[52].
In conclusion, our results show that a high sodium diet may alter the physiological balance between opposing components of the renal RAS, favouring increased Ang II and down-regulation of ACE2. Chronic antioxidant supplementation that attenuates oxidative stress can improve the imbalance between the natriuretic and antinatriuretic components of the renal RAS and decrease hypertensive blood pressure levels.
COMMENTS
Background
The renin-angiotensin system (RAS) is a key regulator of renal function and hydrosaline balance controlling arterial pressure. The aim of this study was to determine the effect of tempol on antagonist components of RAS in renal tissue in rats a high salt intake.
Research frontiers
To date, no study has been undertaken to assess the effectiveness of tempol in rats with imbalance between opposing components of the renal RAS on hypertension and moderate renal impairment. Further studies are required to elucidate whether oxidative stress directly contributes to altered blood pressure by high salt consume.
Innovations and breakthroughs
This study found that chronic antioxidant supplementation that attenuates oxidative stress can improve the imbalance between the natriuretic and antinatriuretic components of the renal RAS and decrease hypertensive blood pressure levels, suggesting that tempol may regulate renal function through renal RAS regulation.
Applications
Although this study provides promising data regarding oxidative stress as a possible target for renal inflammation and hypertension, it should be noted that there are no selective superoxide inhibitors approved for human use. Drugs that possess antioxidant properties, such as apocynin, may be potential therapies, but require further investigation in experimental models of high salt diet.
Terminology
The altered function of the RAS could be a contributing factor to the renal alterations induced by salt excess. To further explore this issue, the authors evaluated the consequences of chronic tempol administration on the in vivo levels of Ang II, Ang-(1-7), angiotensin I converting enzyme 2, and Mas receptor in kidney of rats fed a high salt diet. In tissues such as kidney, heart, and vasculature, Ang II, through the AT1 receptor, promotes vasoconstriction, reactive oxygen species production, and extracellular matrix remodeling, and can activate multiple intracellular signaling pathways leading to inflammatory response and tissue injury. In many cases, the AT2 receptor has been shown to counterbalance the actions exerted through the AT1 receptor. Advances in the field led to the recognition of other active components of the RAS metabolism, such as Ang- (1-7), ACE2, a homolog of classic ACE that forms Ang-(1-7) directly from Ang II and indirectly from Ang I, and the Ang-(1-7)-specific G-protein-coupled receptor Mas. The ACE2/Ang-(1-7)/Mas receptor axis opposes the vascular and proliferative effects of Ang II and exerts complex renal actions in chronic renal diseases and hypertension.
Peer-review
The paper is interesting and well written.
Footnotes
Supported by A grant from the Universidad de Buenos Aires (UBACYT 20020130200105BA).
Institutional review board statement: The study was reviewed and approved by the Universidad de Buenos Aires (UBACYT 20020130200105BA) and the Institutional Committee for Care and Use of Laboratory Animals of the School of Pharmacy and Biochemistry of University of Buenos Aires.
Institutional animal care and use committee statement: All animal procedures and experimental protocols were approved by the Institutional Committee for Care and Use of Laboratory Animals of the School of Pharmacy and Biochemistry of University of Buenos Aires (Protocol Number: 2100-15; file 0035638/15). Animals were used following international guiding principles and local regulations regarding the care and use of laboratory animals for biomedical research as well as the “International Ethical Guiding Principles for Biomedical Research on Animals” established by the CIOMS (Council for International Organizations of Medical Sciences).
Conflict-of-interest statement: The authors have no conflicts of interest to disclose.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: July 12, 2016
First decision: August 11, 2016
Article in press: November 2, 2016
P- Reviewer: Fujigaki Y, Yong D S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
References
- 1.Koliaki C, Katsilambros N. Dietary sodium, potassium, and alcohol: key players in the pathophysiology, prevention, and treatment of human hypertension. Nutr Rev. 2013;71:402–411. doi: 10.1111/nure.12036. [DOI] [PubMed] [Google Scholar]
- 2.Titze J, Ritz E. Salt and its effect on blood pressure and target organ damage: new pieces in an old puzzle. J Nephrol. 2009;22:177–189. [PubMed] [Google Scholar]
- 3.Majid DS, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2007;34:946–952. doi: 10.1111/j.1440-1681.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- 4.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension. 2006;48:467–472. doi: 10.1161/01.HYP.0000236646.83354.51. [DOI] [PubMed] [Google Scholar]
- 5.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD. NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1858–R1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosón MI, Della Penna SL, Cao G, Gorzalczany S, Pandolfo M, Cerrudo C, Fernández BE, Toblli JE. High-sodium diet promotes a profibrogenic reaction in normal rat kidneys: effects of Tempol administration. J Nephrol. 2011;24:119–127. doi: 10.5301/jn.2010.5824. [DOI] [PubMed] [Google Scholar]
- 8.Bader M, Ganten D. Update on tissue renin-angiotensin systems. J Mol Med (Berl) 2008;86:615–621. doi: 10.1007/s00109-008-0336-0. [DOI] [PubMed] [Google Scholar]
- 9.Franco M, Martínez F, Rodríguez-Iturbe B, Johnson RJ, Santamaría J, Montoya A, Nepomuceno T, Bautista R, Tapia E, Herrera-Acosta J. Angiotensin II, interstitial inflammation, and the pathogenesis of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2006;291:F1281–F1287. doi: 10.1152/ajprenal.00221.2006. [DOI] [PubMed] [Google Scholar]
- 10.Ferrão FM, Lara LS, Lowe J. Renin-angiotensin system in the kidney: What is new? World J Nephrol. 2014;3:64–76. doi: 10.5527/wjn.v3.i3.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey RM. The intrarenal renin-angiotensin system in hypertension. Adv Chronic Kidney Dis. 2015;22:204–210. doi: 10.1053/j.ackd.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl(-) cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–F669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 13.Li XC, Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-beta1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol. 2008;294:C1034–C1045. doi: 10.1152/ajpcell.00432.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumners C, de Kloet AD, Krause EG, Unger T, Steckelings UM. Angiotensin type 2 receptors: blood pressure regulation and end organ damage. Curr Opin Pharmacol. 2015;21:115–121. doi: 10.1016/j.coph.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu CX, Hu Q, Wang Y, Zhang W, Ma ZY, Feng JB, Wang R, Wang XP, Dong B, Gao F, et al. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol Med. 2011;17:59–69. doi: 10.2119/molmed.2010.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol. 2008;172:1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XC, Zhuo JL. Mechanisms of AT1a receptor-mediated uptake of angiotensin II by proximal tubule cells: a novel role of the multiligand endocytic receptor megalin. Am J Physiol Renal Physiol. 2014;307:F222–F233. doi: 10.1152/ajprenal.00693.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varagic J, Ahmad S, Nagata S, Ferrario CM. ACE2: angiotensin II/angiotensin-(1-7) balance in cardiac and renal injury. Curr Hypertens Rep. 2014;16:420. doi: 10.1007/s11906-014-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou MS, Adam AG, Jaimes EA, Raij L. In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin II. Hypertension. 2003;42:945–951. doi: 10.1161/01.HYP.0000094220.06020.C8. [DOI] [PubMed] [Google Scholar]
- 20.Giani JF, Miquet JG, Muñoz MC, Burghi V, Toblli JE, Masternak MM, Kopchick JJ, Bartke A, Turyn D, Dominici FP. Upregulation of the angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas receptor axis in the heart and the kidney of growth hormone receptor knock-out mice. Growth Horm IGF Res. 2012;22:224–233. doi: 10.1016/j.ghir.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaziri ND, Bai Y, Ni Z, Quiroz Y, Pandian R, Rodriguez-Iturbe B. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J Pharmacol Exp Ther. 2007;323:85–93. doi: 10.1124/jpet.107.123638. [DOI] [PubMed] [Google Scholar]
- 22.Li XC, Hopfer U, Zhuo JL. Novel signaling mechanisms of intracellular angiotensin II-induced NHE3 expression and activation in mouse proximal tubule cells. Am J Physiol Renal Physiol. 2012;303:F1617–F1628. doi: 10.1152/ajprenal.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yim HE, Ha KS, Bae IS, Yoo KH, Hong YS, Lee JW. Postnatal early overnutrition dysregulates the intrarenal renin-angiotensin system and extracellular matrix-linked molecules in juvenile male rats. J Nutr Biochem. 2012;23:937–945. doi: 10.1016/j.jnutbio.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin-(1-7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol. 2010;299:F983–F990. doi: 10.1152/ajprenal.00371.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosón MI, Cavallero S, Della Penna S, Cao G, Gorzalczany S, Pandolfo M, Kuprewicz A, Canessa O, Toblli JE, Fernández BE. Acute sodium overload produces renal tubulointerstitial inflammation in normal rats. Kidney Int. 2006;70:1439–1446. doi: 10.1038/sj.ki.5001831. [DOI] [PubMed] [Google Scholar]
- 26.Pham NA, Morrison A, Schwock J, Aviel-Ronen S, Iakovlev V, Tsao MS, Ho J, Hedley DW. Quantitative image analysis of immunohistochemical stains using a CMYK color model. Diagn Pathol. 2007;2:8. doi: 10.1186/1746-1596-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol. 2012;590:5519–5528. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe M, O’Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW. Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol Renal Physiol. 2006;291:F350–F357. doi: 10.1152/ajprenal.00407.2005. [DOI] [PubMed] [Google Scholar]
- 29.Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O’Connor PM, Cowley AW. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab. 2012;15:201–208. doi: 10.1016/j.cmet.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banday AA, Lokhandwala MF. Oxidative stress-induced renal angiotensin AT1 receptor upregulation causes increased stimulation of sodium transporters and hypertension. Am J Physiol Renal Physiol. 2008;295:F698–F706. doi: 10.1152/ajprenal.90308.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayorh MA, Mann G, Walton M, Eatman D. Effects of enalapril, tempol, and eplerenone on salt-induced hypertension in dahl salt-sensitive rats. Clin Exp Hypertens. 2006;28:121–132. doi: 10.1080/10641960500468276. [DOI] [PubMed] [Google Scholar]
- 32.Bayorh MA, Ganafa AA, Socci RR, Silvestrov N, Abukhalaf IK. The role of oxidative stress in salt-induced hypertension. Am J Hypertens. 2004;17:31–36. doi: 10.1016/j.amjhyper.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Adler S, Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol. 2004;287:F907–F913. doi: 10.1152/ajprenal.00060.2004. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther. 2010;126:119–145. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai EY, Luo Z, Onozato ML, Rudolph EH, Solis G, Jose PA, Wellstein A, Aslam S, Quinn MT, Griendling K, et al. Effects of the antioxidant drug tempol on renal oxygenation in mice with reduced renal mass. Am J Physiol Renal Physiol. 2012;303:F64–F74. doi: 10.1152/ajprenal.00005.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobori H, Nishiyama A. Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem Biophys Res Commun. 2004;315:746–750. doi: 10.1016/j.bbrc.2004.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Patel NS, Caputi AP, Thiemermann C. Tempol reduces the activation of nuclear factor-kappaB in acute inflammation. Free Radic Res. 2004;38:813–819. doi: 10.1080/10715760410001710829. [DOI] [PubMed] [Google Scholar]
- 38.Feng MG, Dukacz SA, Kline RL. Selective effect of tempol on renal medullary hemodynamics in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1420–R1425. doi: 10.1152/ajpregu.2001.281.5.R1420. [DOI] [PubMed] [Google Scholar]
- 39.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 40.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64:1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol. 2006;290:C420–C426. doi: 10.1152/ajpcell.00409.2004. [DOI] [PubMed] [Google Scholar]
- 43.Gowrisankar YV, Clark MA. Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J Neurochem. 2016;138:74–85. doi: 10.1111/jnc.13641. [DOI] [PubMed] [Google Scholar]
- 44.Pontes RB, Girardi AC, Nishi EE, Campos RR, Bergamaschi CT. Crosstalk between the renal sympathetic nerve and intrarenal angiotensin II modulates proximal tubular sodium reabsorption. Exp Physiol. 2015;100:502–506. doi: 10.1113/EP085075. [DOI] [PubMed] [Google Scholar]
- 45.Johns EJ. The neural regulation of the kidney in hypertension and renal failure. Exp Physiol. 2014;99:289–294. doi: 10.1113/expphysiol.2013.072686. [DOI] [PubMed] [Google Scholar]
- 46.Lv LL, Liu BC. Role of non-classical renin-angiotensin system axis in renal fibrosis. Front Physiol. 2015;6:117. doi: 10.3389/fphys.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sevá Pessôa B, van der Lubbe N, Verdonk K, Roks AJ, Hoorn EJ, Danser AH. Key developments in renin-angiotensin-aldosterone system inhibition. Nat Rev Nephrol. 2013;9:26–36. doi: 10.1038/nrneph.2012.249. [DOI] [PubMed] [Google Scholar]
- 48.Thomson SC, Deng A, Wead L, Richter K, Blantz RC, Vallon V. An unexpected role for angiotensin II in the link between dietary salt and proximal reabsorption. J Clin Invest. 2006;116:1110–1116. doi: 10.1172/JCI26092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren Y, D’Ambrosio MA, Wang H, Peterson EL, Garvin JL, Carretero OA. Mechanisms of angiotensin II-enhanced connecting tubule glomerular feedback. Am J Physiol Renal Physiol. 2012;303:F259–F265. doi: 10.1152/ajprenal.00689.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Troncoso Brindeiro CM, Lane PH, Carmines PK. Tempol prevents altered K(+) channel regulation of afferent arteriolar tone in diabetic rat kidney. Hypertension. 2012;59:657–664. doi: 10.1161/HYPERTENSIONAHA.111.184218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo H, Wang X, Chen C, Wang J, Zou X, Li C, Xu Z, Yang X, Shi W, Zeng C. Oxidative stress causes imbalance of renal renin angiotensin system (RAS) components and hypertension in obese Zucker rats. J Am Heart Assoc. 2015;4:e001559. doi: 10.1161/JAHA.114.001559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berger RC, Vassallo PF, Crajoinas Rde O, Oliveira ML, Martins FL, Nogueira BV, Motta-Santos D, Araújo IB, Forechi L, Girardi AC, et al. Renal Effects and Underlying Molecular Mechanisms of Long-Term Salt Content Diets in Spontaneously Hypertensive Rats. PLoS One. 2015;10:e0141288. doi: 10.1371/journal.pone.0141288. [DOI] [PMC free article] [PubMed] [Google Scholar]


