Biomarkers of inflammation, coagulation cascade activation, and fibrosis predict serious non-AIDS during antiretroviral therapy (ART). We found increased biomarker levels in acute human immunodeficiency virus infection. Several did not normalize despite early ART initiation.
Keywords: acute HIV infection, antiretroviral therapy, inflammation, monocyte activation, sIL-6R
Abstract
Background. Serious non-AIDS events cause substantial disease and death despite human immunodeficiency virus (HIV) suppression with antiretroviral therapy (ART). Biomarkers of inflammation, coagulation cascade activation, and fibrosis predict these end-organ events. We aimed to determine whether ART initiation during acute HIV infection would attenuate changes in these biomarker levels.
Methods. Plasma samples were obtained from participants starting ART during acute or chronic HIV infection and from HIV-uninfected participants from Bangkok, Thailand. Biomarkers of inflammation (C-reactive protein [CRP], interleukin 6, soluble interleukin 6 receptor [sIL-6R], soluble gp130, tumor necrosis factor [TNF]), enterocyte turnover (intestinal fatty acid binding protein [I-FABP]), lipopolysaccharide-induced monocyte activation (soluble CD14 [sCD14]), coagulation cascade activation [D-dimer], and fibrosis (hyaluronic acid [HA]) were measured at baseline and through 96 weeks of ART.
Results. CRP, TNF, sIL-6R, I-FABP, sCD14, D-dimer, and HA levels were elevated in acute HIV infection. Early ART was associated with increased I-FABP levels but normalization of TNF, sIL-6R, and D-dimer levels. CRP, sCD14, and HA levels decreased during ART but remained elevated compared with HIV-uninfected participants. Higher sCD14, CRP, and D-dimer levels were associated with higher peripheral blood mononuclear cell and gut integrated HIV DNA levels. Decreases in sCD14 and CRP levels were correlated with increases in CD4 T-cell counts.
Conclusions. ART initiated in early acute HIV infection was associated with normalization of the coagulation cascade and several systemic inflammatory biomarkers, but the acute-phase response, enterocyte turnover, monocyte activation, and fibrosis biomarkers remained elevated. Additional interventions to attenuate inflammation may be needed to optimize clinical outcomes in persons with HIV infection.
Serious non-AIDS events, including cardiovascular, liver, and renal disease and non-AIDS malignancies, have emerged as leading causes of disease and death in human immunodeficiency virus (HIV)–infected persons on combination antiretroviral therapy (ART) [1]. Levels of plasma viremia and CD4 T-cell counts are invaluable markers of risk and outcomes of opportunistic infections, but less so for noninfectious complications. In contrast, biomarkers of inflammation and coagulation are strong predictors of all-cause disease and death in HIV-infected persons, even with suppressive ART [2–5]. These pathologies highlight the critical role of chronic inflammation despite ART-mediated HIV suppression.
Chronic immune activation and inflammation in treated HIV infection is probably driven by residual HIV replication, coinfections such as cytomegalovirus, gut mucosal injury with translocation of microbial products, tissue fibrosis that destroys the lymphocyte niche, and possibly cytokines that drive lymphocyte proliferation in persistent lymphopenia [6]. Initiation of ART at high CD4 T-cell counts and/or within the first year of seroconversion results in more robust immune restoration and less chronic immune activation [1, 7], whereas lower nadir CD4 T-cell counts are associated with higher risk of serious non-AIDS events [8, 9]. However, whether ART initiation during acute HIV infection could eliminate increased inflammation in chronic HIV infection remains unknown.
We hypothesized that initiation of ART during the earliest stages of acute HIV infection would normalize levels of biomarkers associated with disease and death in chronic HIV infection. We evaluated biomarkers of inflammation and coagulation at baseline and over the subsequent 96 weeks in participants of an ongoing, prospective study of ART initiation in acute HIV infection in Bangkok, Thailand (RV254/SEARCH010) [10].
METHODS
Study Design and Participants
In RV254/SEARCH010 (NCT00796146), high-risk participants presenting to voluntary testing and counseling centers at the Thai Red Cross Anonymous Clinic were screened for acute HIV infection in real time by means of pooled nucleic acid testing (NAT) and sequential immunoassay (IA) [11]. Participants with nonreactive HIV immunoglobulin G (n = 78) were enrolled and categorized as fourth-generation IA (4thG) stage 1 (4thG1; NAT positive, 4thG IA negative, and third-generation [3rdG] IA negative), 4thG stage 2 (4thG2; NAT and 4thG IA positive and 3rdG IA negative), and 4thG stage 3 (4thG3; NAT, 4thG IA, and 3rdG IA positive; Western blot negative or indeterminate) [10, 12]. Samples were collected before ART (baseline) and over 96 weeks of ART. Thai HIV-uninfected controls were prevaccinated age- and sex-matched participants from RV114 (n = 99), an HIV vaccine trial [13], and RV304/SEARCH013 (n = 29), a tissue sampling study (NCT01397669) [14]. Chronically infected Thai participants were enrolled in SEARCH011, a study of neurocognitive impairment in persons with varying monocyte proviral burden (n = 27) [15], or the RV217 natural history cohort (n = 14) [16]. Individuals with unthawed samples were selected. Samples were collected from SEARCH011 before ART and after 48 weeks of ART and from RV217 before ART. All participants provided signed informed consent.
Biomarker Measurements
Intestinal fatty acid binding protein (I-FABP), soluble CD14 (sCD14) (R&D Biosystems), and hyaluronic acid (HA) (Corgenix) were measured with enzyme-lined immunosorbent assay; C-reactive protein (CRP) with electrochemiluminescence assay (Meso Scale Discovery), and D-dimer with Enzyme Linked Fluorescent Assay (bioMerieux). Tumor necrosis factor (TNF), soluble interleukin 6 (IL-6) receptor (sIL-6R), soluble gp130 (sgp130), and high-sensitivity IL-6 were measured using the Luminex platform (Millipore). All biomarkers were measured in samples from participants with acute HIV infection, HIV-uninfected participants, and untreated participants with untreated chronic HIV infection. sCD14, I-FABP, D-dimer, HA, and CRP were measured in SEARCH011 samples, and IL-6, TNF, sIL-6R, and sgp130 in RV217 samples. sCD14, HA, CRP, and D-dimer were measured in available unthawed samples from treated participants with chronic infection. All biomarkers were performed in duplicate on cryopreserved acid citrate dextrose plasma according to the manufacturers' instructions and for research purposes only. All assays were performed after the first thaw except I-FABP (second thaw).
Total and Integrated HIV DNA Measurements
Sigmoid biopsies were performed in the RV254 cohort. The frequencies of cells harboring total and integrated HIV DNA in peripheral blood mononuclear cells (PBMCs) and sigmoid colon gut biopsies were measured as described elsewhere [17, 18].
Statistical Analysis
Data were reported as median (interquartile range) values. Comparisons between 4thG groups were performed using the Mann–Whitney U test for continuous variables and ϰ2 or Fisher exact test for categorical variables. To account for differences between acute and chronic infection, we used a linear regression model adjusted by age and sex. Comparisons within groups across time points were performed using the Wilcoxon signed rank test. Biomarker levels were compared between participants who received 48 weeks of ART starting during acute or chronic HIV infection, and between participants who received 96 weeks of ART and HIV-uninfected participants. The Spearman rank test was used to determine correlations. Statistical tests were 2 sided and were differences were considered statistically significant at P < .05. Analyses were performed using StataCorp 2013 (StataCorp) and Prism (version 6.0; GraphPad) software.
RESULTS
Participant Characteristics
Acutely infected participants had a median age of 28 years (interquartile range, 24–33 years) and were 92.3% male, compared with 35 (30–42) years and 37.0% male for chronically infected and 27 (22–37) years and 77.1% male for HIV-uninfected (Table 1). The median CD4 T-cell count was 384 cells/mm3 and the median HIV RNA level 5.6 log10 copies/mL for acutely infected participants, compared with 230 cells/mm3 and 4.7 log10 copies/mL for chronically infected participants. For acutely infected participants, 14 were in 4thG1, 22 in 4thG2, and 42 in 4thG3. Age, sex, CD4 T-cell counts, and HIV RNA levels did not differ significantly across stages. The median time since self-reported HIV exposure was 12 days in 4thG1, 17 in 4thG2, and 18 in 4thG3. Sixty-one participants (78.2%) had ≥3 symptoms of acute retroviral syndrome. The median time to ART initiation was 2 days. Seventy-six participants received tenofovir, emtricitabine, and efavirenz. Forty-four also received maraviroc and raltegravir (mega-HAART [highly active ART]) [10]. Two participants who opted not to take ART were excluded from the longitudinal analysis. One participant who reported nonadherence remained viremic and was excluded from the longitudinal analysis at week 48. Participants with blips were included in the analysis, including 5 participants at week 24 and 1 at week 48 (all with HIV RNA levels <2.3 log10 copies/mL).
Table 1.
Baseline Characteristics
| Baseline Characteristic | HIV Uninfected (n = 128) | Acute HIV Infectiona
|
Chronic HIV-Infectionb (n = 41) | |||
|---|---|---|---|---|---|---|
| All Stages (n = 78) | 4thG1 (n = 14) | 4thG2 (n = 22) | 4thG3 (n = 42) | |||
| Age, median (IQR), y | 27 (22–37) | 28 (23–32) | 28 (23–37) | 28 (25–34)c | 26 (23–30)c | 29 (23–37) |
| Male sex, No. (%) | 84 (77.1) | 72 (92.3) | 12 (85.7)c | 21 (95.5)c | 39 (92.9)c | 24 (58.5) |
| Time from HIV exposure (per participant), median (IQR), d | … | 16 (12–21) | 12 (9–14.5) | 17 (14–23) | 18 (13–22) | NA |
| Antiretroviral syndrome, No. (%)d | … | 61 (78.2) | 5 (35.7) | 17 (77.3)e | 39 (92.9)e | |
| CD4 T-cell count, median (IQR), cells/µL | … | 384 (294–523) | 494 (347–561)c | 323 (265–443)c | 386 (298–496)c | 230 (106–331) |
| Plasma HIV RNA, median (IQR), log10 copies/mL | … | 5.6 (5.3–6.3) | 5.6 (4.9–6.4)c | 5.6 (5.1–6.6)c | 5.6 (5.2–6.2)c | 4.7 (4.5–5.1) |
Abbreviations: 4thG1, fourth-generation (4thG) stage 1; 4thG2, 4thG stage 2; 4thG3, 4thG stage 3; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not available.
a For the 4thG acute HIV infection stages: 4thG1 was defined as nucleic acid testing (NAT) positive and 4thG and third-generation (3rdG) immunoassay (IA) negative; 4thG2, as NAT and 4thG IA positive and 3rdG IA negative; and 4thG3, as NAT, 4thG IA, and 3rdG IA positive and Western blot negative or indeterminate.
b Before antiretroviral therapy.
c P < .05 (comparison with chronically HIV-infected population; before antiretroviral therapy or after 48 weeks).
d Antiretroviral syndrome was defined by ≥3 of the following: fever, adenopathy, headache, fatigue, myalgia, arthralgia, pharyngitis, oral ulcer, genital ulcer, weight loss, anorexia, nausea and vomiting, diarrhea, odynophagia, rash, oral candidiasis, vaginal candidiasis, and neurologic symptoms.
e P < .05 (comparison with 4thG1; the difference between 4thG2 and 4thG3 was not significant).
Inflammation
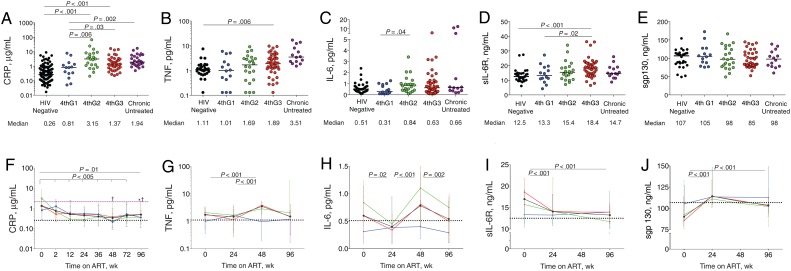
Plasma levels of CRP, an acute-phase reactant associated with cardiovascular events and death [3, 4, 19], were significantly higher in HIV-infected participants from 4thG2 and 4thG3 than in HIV-uninfected participants (P < .001 for both) (Table 2; Figure 1 A; Supplementary Table 1). CRP levels were significantly lower in 4thG1 than in 4thG2 (P = .006) or 4thG3 (P = .03). Baseline plasma TNF levels were significantly higher in 4thG3 than in HIV-uninfected participants (P = .006; Figure 1 B).
Table 2.
Biomarker Levels in Participants With Acute HIV Infection
| Biomarker | Participants With Acute HIV Infection by 4thG Stage and Week of ART |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Stages |
4thG1 |
4thG2 |
4thG3 |
|||||||||||||||||||||
| ARTa | 0 (n = 75) | 2 | 12 | 24 | 48 | 96 | 0 (n = 14) | 2 | 12 | 24 | 48 | 96 | 0 (n = 20) | 2 | 12 | 24 | 48 | 96 | 0 (n = 41) | 2 | 12 | 24 | 48 | 96 |
| CRP, µg/mL | 1.34 | 0.77 | 0.51 | 0.45 | 0.34 | 0.49 | 0.81 | 1.25 | 0.51 | 0.55 | 0.52 | 0.51 | 3.15 | 0.69 | 0.29 | 0.28 | 0.25 | 0.35 | 1.34 | 0.52 | 0.59 | 0.48 | 0.38 | 0.49 |
| TNF, pg/mL | 1.72 | NA | NA | 1.46 | 3.52 | 1.50 | 1.01 | NA | NA | 1.65 | 0.96 | 1.21 | 2.10 | NA | NA | 1.69 | 2.89 | 2.05 | 1.84 | NA | NA | 1.24 | 3.95 | 1.50 |
| IL-6, pg/mL | 0.60 | NA | NA | 0.40 | 0.78 | 0.54 | 0.31 | NA | NA | 0.39 | 0.4 | 0.29 | 0.84 | NA | NA | 0.45 | 1.14 | 0.75 | 0.60 | NA | NA | 0.34 | 0.77 | 0.50 |
| sIL-6R, ng/mL | 17.0 | NA | NA | 14.1 | NA | 13.3 | 13.3 | NA | NA | 13.2 | NA | 13.9 | 15.7 | NA | NA | 14.1 | NA | 11.7 | 18.6 | NA | NA | 14.1 | NA | 13.6 |
| sgp130, ng/mL | 90 | NA | NA | 114 | NA | 104 | 105 | NA | NA | 114 | NA | 113 | 92 | NA | NA | 111 | NA | 102 | 85 | NA | NA | 114 | NA | 104 |
| I-FABP, pg/mL | 1004 | 2661 | 2702 | 2009 | 2869 | 2528 | 812 | 2698 | 2441 | 1426 | 2921 | 2979 | 1041 | 2607 | 3492 | 2792 | 3173 | 3715 | 1044 | 2668 | 2702 | 1734 | 2642 | 2254 |
| sCD14, µg/mL | 1.51 | 1.32 | 1.18 | 1.16 | 1.04 | 1.15 | 1.20 | 1.57 | 1.20 | 1.20 | 0.98 | 1.24 | 1.57 | 1.32 | 1.06 | 1.06 | 1.06 | 1.17 | 1.57 | 1.30 | 1.19 | 1.22 | 1.01 | 1.11 |
| D-dimer, µg/mL | 0.28 | 0.23 | 0.15 | 0.15 | 0.17 | 0.18 | 0.17 | 0.32 | 0.15 | 0.17 | 0.19 | 0.25 | 0.30 | 0.34 | 0.17 | 0.19 | 0.16 | 0.13 | 0.33 | 0.22 | 0.13 | 0.15 | 0.16 | 0.18 |
| HA, ng/mL | 18.4 | 10.2 | 12.5 | 18.5 | 15.7 | 12.2 | 12.5 | 14.7 | 11.3 | 15.9 | 18.1 | 11.8 | 15.3 | 9.0 | 15.9 | 21.4 | 17.7 | 18.5 | 23.7 | 9.0 | 12.0 | 17.6 | 15.6 | 12.1 |
Abbreviations: 4thG, fourth-generation; 4thG1, 4thG stage 1; 4thG2, 4thG stage 2; 4thG3, 4thG stage 3; ART, antiretroviral therapy; CRP, C-reactive protein; D-dimer, coagulation cascade activation; HA, hyaluronic acid; HIV, human immunodeficiency virus; I-FABP, intestinal fatty acid binding protein; IL-6, interleukin 6; NA, not available; sCD14, soluble CD14; sgp130, soluble gp130; sIL-6R, soluble IL-6 receptor; TNF, tumor necrosis factor.
a All biomarker.
Figure 1.
Inflammatory biomarker levels in human immunodeficiency virus (HIV)–uninfected participants from Thailand (black dots); in participants with acute HIV infection diagnosed in fourth-generation stage 1 (4thG1; blue), stage 2 (4thG2; green), or stage 3 (4thG3; red); and in participants with chronic untreated HIV infection (purple). A–E, Plasma biomarker levels at study entry before starting antiretroviral therapy (ART), including C-reactive protein (CRP) (A), tumor necrosis factor (TNF) (B), interleukin 6 (IL-6) (C), soluble IL-6 receptor (sIL-6R) (D), and soluble gp130 (sgp130) (E). Horizontal bars represent median values. F–J, Changes in the same plasma biomarker levels over 96 weeks of ART initiated during acute HIV infection. Purple dashed line represents median biomarker level in participants during treated chronic HIV infection; black dotted lines, median biomarker levels in HIV-uninfected participants from Thailand. † P < .05 for comparison with treated chronically HIV-infected participants; *P < .05 for comparison with HIV-uninfected participants. Median values are shown with interquartile range bars.
Plasma IL-6 levels, associated with increased morbidity and mortality risk [3, 4, 19], were significantly higher in 4thG2 compared with 4thG1 (P = .04) but not compared with HIV-uninfected participants (Figure 1 C). Levels of sIL-6R, which mediates IL-6 signaling in cells without IL-6R [20], were significantly increased in 4thG3 compared with HIV-uninfected participants (P < .001; Figure 1 D) and 4thG1 (P = .02) but not compared with 4thG2. Levels of sgp130, which inactivates the IL-6/sIL-6R complex, did not differ significantly among HIV-infected groups or compared with HIV-uninfected participants (Figure 1 E).
Next, we evaluated the effects of ART on these biomarkers. The median CRP level for all acutely HIV-infected participants decreased after 2 weeks of ART (P < .001), driven by changes in 4thG2 and 4thG3 (Figure 1 F). CRP levels remained significantly lower than in treated chronically HIV-infected participants at week 48 (P < .001), even after adjustment for age and sex (Supplementary Table 2), but they were elevated compared with those in HIV-uninfected participants at week 96 (P = .04). TNF levels increased after 48 weeks of ART (P < .001; Figure 1 G) but decreased by week 96 to levels similar to those in HIV-uninfected participants.
Median IL-6 levels varied over time receiving ART (Figure 1 H). After 96 weeks of ART, IL-6 levels were similar in participants treated during acute HIV infection and HIV-uninfected participants. Levels of sIL-6R decreased significantly after 24 and 96 weeks of ART (P < .001 for both; Figure 1 I), with decreases primarily in the 4thG2 and 4thG3 groups. sIL-6R levels at 96 weeks were similar to those in HIV-uninfected participants, regardless of stage at diagnosis. The sgp130 levels increased significantly by week 24 (P < .001; Figure 1 J) in all 4thG groups and remained higher compared with baseline at 96 weeks (P < .001), particularly in the 4thG3 group (P = .001). After 96 weeks of ART, all groups had sgp130 levels comparable to those in HIV-uninfected participants. Participants with lower sgp130 levels at baseline were more likely to have persistently increased IL-6 levels (odds ratio, 4.0 [95% CI, 1.3–12.1] for sgp130 below vs above the median; P = .01). Together, these data show that ART started during very early acute HIV infection normalized TNF and mediators of IL-6 signaling but not CRP levels.
Intestinal Permeability and Microbial Translocation
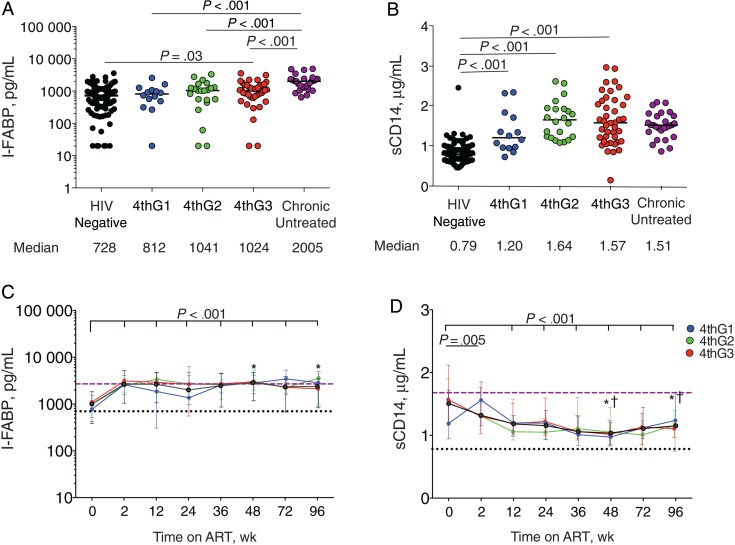
Intestinal damage and microbial translocation start in acute infection [21]. Plasma levels of I-FABP, a marker of enterocyte turnover associated with death [19], were significantly higher at baseline than in HIV-uninfected participants only in the 4thG3 group (P = .03) (Figure 2 A). Plasma sCD14 levels, reflecting monocyte activation and predictive of death [2], were significantly higher at baseline in all HIV-infected groups compared with HIV-uninfected participants (P < .001 for all; Figure 2 B) and did not differ among 4thG stages.
Figure 2.
Biomarkers of enterocyte turnover (intestinal fatty acid binding protein [I-FABP]) (A, C) and lipopolysaccharide-induced monocyte activation (soluble CD14 [sCD14]) (B, D). A, B, Plasma biomarker levels at diagnosis before initiation of antiretroviral therapy (ART). 4thG1, fourth-generation (4thG) stage 1; 4thG2, 4thG stage 2; 4thG3, 4thG stage 3. C, D, Changes in plasma biomarker levels over 96 weeks of ART initiated during acute human immunodeficiency virus (HIV) infection. Purple dashed lines represent median biomarker levels in participants starting ART during chronic HIV infection; black dotted lines, median biomarker levels in HIV-uninfected participants from Thailand. † P < .05 for comparison with treated chronically HIV-infected participants; *P < .05 for comparison with HIV-uninfected participants. Median values are shown with interquartile range bars.
We evaluated biomarker changes after starting ART during early acute HIV infection. I-FABP levels increased during the first 2 weeks of ART (P < .001) before plateauing at levels comparable to those in treated chronically HIV-infected participants at 48 weeks, even after adjustment for age and sex (Supplementary Table 2), and significantly higher than those in HIV-uninfected participants after 96 weeks of ART (P < .001) (Figure 2 C). In contrast, sCD14 levels decreased after 2 weeks of ART (P = .005), primarily driven by changes in 4thG2 and 4thG3, and remained significantly lower through week 96 compared with baseline (P < .001 for all; Figure 2 D). sCD14 levels were significantly lower in participants treated during acute HIV infection after 48 weeks of ART than in participants treated during chronic HIV infection (P < .001), even after adjustment for age and sex (Supplementary Table 2). Nonetheless, sCD14 levels remained significantly higher than in HIV-uninfected participants after 96 weeks of ART (P < .001). In summary, increased enterocyte turnover persisted and monocyte activation decreased but did not normalize in participants starting ART during acute HIV infection.
Coagulation and Fibrosis
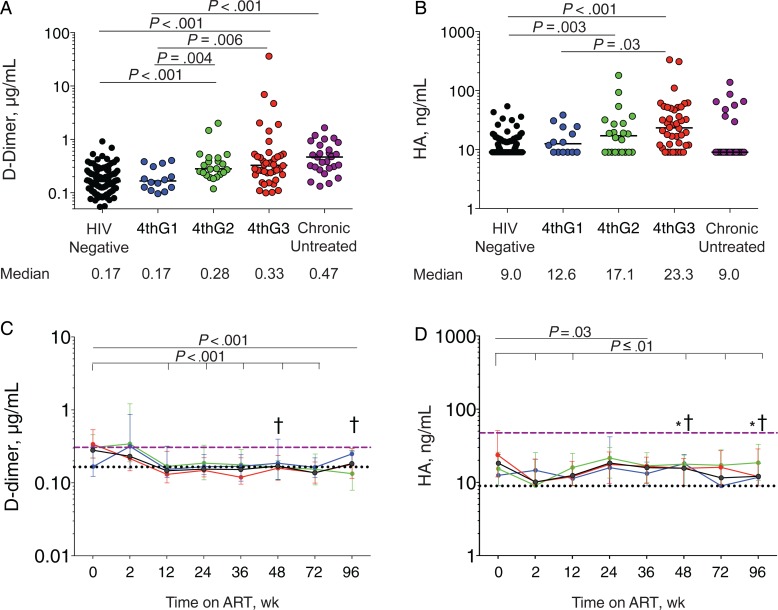
Increased levels of D-dimer, reflecting coagulation cascade activation, and HA, reflecting fibrosis, are also associated with disease progression and death [3–5]. D-dimer levels were significantly increased in the 4thG2 and 4thG3 groups compared with HIV-uninfected participants (P < .001 for both; Figure 3 A) and the 4thG1 group (P = .004 and P = .006, respectively). D-dimer levels in the 4thG1 group were comparable to those in HIV-uninfected participants. Plasma HA levels were increased in 4thG2 and 4thG3 groups compared with HIV-uninfected participants (P = .003 and P < .001, respectively; Figure 3 B), and in 4thG3 compared with 4thG1 (P = .03). Next, we assessed the impact of ART initiation during acute infection on these biomarkers. After 12 weeks, plasma D-dimer levels decreased significantly compared with baseline (P < .001) and then stabilized through 96 weeks of ART (Figure 3 C). Circulating D-dimer levels in participants treated during acute HIV infection were significantly lower than in treated chronically HIV-infected participants after 48 weeks of ART (P < .001), even after adjustment for age and sex (Supplementary Table 2). Compared with those in HIV-uninfected participants, D-dimer levels were not significantly different after 96 weeks of ART. In contrast, after 2 weeks of ART, HA levels decreased significantly (P = .003; Figure 3 D). HA levels were lower than in treated chronically HIV-infected participants after 48 weeks of ART (P < .001), even after adjustment for age and sex (Supplementary Table 2) but remained higher than in HIV-uninfected participants after 96 weeks (P < .001). Thus, whereas coagulation biomarkers may normalize when ART is started during acute HIV infection, a profibrotic state persists.
Figure 3.
Biomarkers of coagulation cascade activation (D-dimer) (A, C) and a profibrotic state (hyaluronic acid [HA]) (B, D). A, B, Plasma biomarker levels at diagnosis before starting antiretroviral therapy (ART). 4thG1, fourth-generation (4thG) stage 1; 4thG2, 4thG stage 2; 4thG3, 4thG stage 3. C, D, Changes in plasma biomarker levels over 96 weeks of ART initiated during acute human immunodeficiency virus (HIV) infection. Purple dashed lines represent median biomarker levels in participants starting ART during chronic HIV infection; black dotted lines, median biomarker levels in HIV-uninfected participants from Thailand. † P < .05 for comparison with treated chronically HIV-infected participants; *P < .05 for comparison with HIV-uninfected participants. Median values are shown with interquartile range bars.
Relationships Among CD4 T-Cell Counts, HIV Burden, and Biomarkers
To assess whether inflammation was associated with HIV disease state, we performed correlation analyses of these biomarkers with CD4 T-cell count and HIV burden in the acute HIV cohort. We pooled all stages to encompass the breadth of disease (Supplementary Table 3). As reported elsewhere, total and integrated PBMC HIV DNA levels are higher at baseline in the later stages and decrease to 1 and 0.1 log10 copies/106 PBMCs, respectively, after 48 weeks of ART; the sigmoid colon has a similar pattern [18]. Among acutely infected participants at baseline, higher plasma HIV RNA levels were correlated with higher sIL-6R, I-FABP, sCD14, HA, and D-dimer levels. A similar pattern was observed for total and integrated PBMC HIV DNA levels (Supplementary Figure 1 A–C). Higher integrated HIV DNA levels in the gut were correlated with higher baseline sCD14, CRP, and D-dimer levels. Low baseline peripheral CD4 T-cell counts were correlated with high CRP levels, but low sigmoid colon CD4 T-cell counts were associated with high plasma HIV RNA, sCD14, and HA levels (Supplementary Figure 1 D–F).
To determine whether improvement in inflammation was associated with improvement in HIV disease state in the acute infection cohort, we correlated changes in inflammatory biomarkers with changes in CD4 T-cell count and HIV burden. After 24 weeks of ART, the median CD4 T-cell count increased to 600 cells/mm3. Increases in CD4 T-cell counts were associated with decreases in sCD14 and CRP levels (Supplementary Figure 2 A and 2 B). Similarly, only fold decrease in D-dimer levels correlated with fold-decrease in plasma HIV RNA levels (r = 0.29; P = .01), total HIV DNA in PBMCs (r = 0.33; P = .005), and intestinal total HIV DNA levels (Supplementary Figure 2 C). Together, these data suggest an association of the coagulation cascade with HIV burden and of gut damage and persistent inflammation with slow CD4 T-cell recovery.
DISCUSSION
Although inflammation persists with ART started during early and chronic HIV infection, the effect of initiating ART during early acute HIV infection on chronic inflammation is unknown. Using age- and sex-matched controls from Bangkok, Thailand, we found that (1) acute HIV infection is associated with increased levels of biomarkers of intestinal damage, inflammation, coagulation, and fibrosis and with suppressive ART initiated during acute HIV infection; (2) the procoagulant state normalizes after early ART; (3) enterocyte damage persists during suppressive ART; (4) monocyte activation, systemic inflammation, and fibrosis remain increased but lower than in participants starting ART in chronic HIV infection; and (5) increases in CD4 T-cell populations correlate with decreases in sCD14 and CRP levels. These results show that some elements of very early immune damage by HIV are durable and potentially irreversible, CD4 T-cell reconstitution may be determined by reduction in inflammation, and additional immunomodulatory therapies during early infection may be necessary to avert these long-term consequences.
The biomarkers reflect an inflammatory state at diagnosis, consistent with previous data showing a cytokine storm during acute HIV infection [22]. HIV itself probably contributes to this inflammation, because higher baseline plasma HIV RNA levels and total and integrated PBMC HIV DNA were correlated with higher sCD14, D-dimer, sIL-6R, and I-FABP levels. The normalization of D-dimer levels with early ART in association with decreased HIV burden suggests a reduced risk of prothrombotic events, such as deep-vein thromboses and pulmonary emboli. Conversely, the persistently elevated levels of biomarkers of monocyte activation (sCD14), systemic inflammation (CRP, IL-6, sIL-6R, TNF), and fibrosis (HA) during ART indicate that drivers of inflammation other than HIV itself persist even with early ART. The early differences in biomarker levels across 4thG stages did not persist in chronic infection, suggesting that starting ART even at the earliest stages of infection may not normalize inflammation. Conversely, a few days' delay in ART initiation may not adversely impact inflammation during chronic HIV infection.
Intestinal damage, increased regulatory T-cell infiltration, and lymph node collagen deposition occur within 1–2 weeks of infection [23], therefore preceding ART initiation. Recently, RV254 participants with acute HIV infection were shown to have increased neutrophil infiltration, immune activation (Ki67-positive cells), and inflammation (cells expressing TNF, Mx1, and indoleamine 2,3-dioxygenase 1) in the sigmoid colon. Despite decreasing intestinal inflammation, ART initiated during acute infection did not decrease intestinal neutrophil infiltration, a marker of gut damage [24]. Similarly, in this population, mucosal T-helper 17 cell depletion was demonstrated in Fiebig stage III but not stage I. Early ART restored T-helper 17 presence but not polyfunctionality [14], suggesting persistent immune and mucosal dysregulation, consistent with persistently elevated I-FABP and sCD14 levels. Similarly, early suppressive ART cannot halt the acute-phase reaction. The absence of increases in IL-6 levels may reflect a lack of sensitivity of the multiplex assay or the cross-sectional comparison. Nonetheless, increased IL-6 transsignaling by sIL-6R, persistently increased here, is associated with cardiac events in non-HIV populations and animal models [25]. The increased profibrotic state based on HA levels, despite early ART, may perpetuate fibrosis in lymphatic tissue, the liver, and other tissues [26]. However, the independent effect of ART on these biomarkers is unclear [27, 28].
Notably, levels of biomarkers predictive of cardiovascular events, such as sCD14, sIL-6R, and CRP, remain elevated compared with those in HIV-uninfected Thai participants despite ART initiation in acute infection, raising the question of whether these patients remain at increased risk for non-AIDS events. These biomarker levels, however, are lower than those observed in many Western cohorts and lower than those associated with end-organ disease, although variations in assays used and patient populations (eg, age, coinfections) may contribute to some differences [2, 4, 19, 29–32]. Thus, the clinical implications of this persistent low-level elevation remain unclear.
Our study has several limitations. Our chronically HIV-infected cohort was small and not matched for age or sex to the acutely HIV-infected cohort, potentially resulting in higher inflammatory marker levels. The acutely infected participants were 15–20 years younger than participants in many studies associating biomarkers with clinical outcomes and thus may have less inflammation [2, 3, 5, 19]. No major end-organ events have occurred to determine the clinical significance of our findings. Nonetheless, this study provides important insight into the evolution of inflammation in acute HIV infection and the potential impact of early ART.
In conclusion, initiation of ART during acute HIV infection is insufficient to resolve the chronic inflammation associated with increased all-cause morbidity and mortality risk in treated HIV infection. Initiating ART during acute infection, however, attenuates inflammation more than initiating ART in chronic HIV infection. These data are consistent with findings of the Strategic Timing in AntiRetroviral Therapy (START) study, which confirmed a decreased event rate in persons starting ART with CD4 T-cell counts >500/µL [33]. Whether persistent residual inflammation will translate into increased end-organ disease remains to be determined. If so, further interventions to attenuate inflammation, even among patients treated during acute HIV infection, may be necessary to optimize clinical outcomes.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgements. The study team is grateful to the individuals who volunteered to participate in this study and the staff at the Thai Red Cross AIDS Research Centre and the Department of Retrovirology, US Army Medical Component, Armed Forces Research Institute of Medical Sciences. We thank Silvia Ratto-Kim for providing samples from the RV114 study, and we also thank the RV254/SEARCH 010, RV304/SEARCH 013 and SEARCH 011 Protocols team members that include from SEARCH/TRCARC/HIV-NAT: Praphan Phanuphak, Nipat Teeratakulpisarn, Supanit Pattanachaiwit, Thep Chalermchai, Mark de Souza, Eugene Kroon, Carlo Sacdalan, Phillip Chan, Donn Colby, Duanghathai Sutthichom, Somprartthana Rattanamanee, Peeriya Prueksakaew, Sasiwimol Ubolyam, Pacharin Eamyoung, Naphassanant Laopraynak, Suwanna Puttamaswin, Nitiya Chomchey, Putthachard Karnsomlap, Tarandeep Singh, Chattiya Nitpolprasert; from Pharmongkut Klao Hospital: Pasiri Sithinamsuwan; from AFRIMS: Sorachai Nitayaphan, Somchai Sriplienchan, Rapee Trichavaroj, Alexandra Schuetz, Nicos Karasavvas, Sandhya Vasan, Yuwadee Phuang-Ngern, Surt Jongrakthaitae, Werrawan Chuenaron, Rapee Trichavaroj, Nantana Tantibul, Hathairat Savadsuk, Tanya Wansom, Siriwat Akapirat, Bessara Nuntapinit; from MHRP: Trevor Crowell, Madelaine Ouellette, Oratai Butterworth; from University of Montreal: Remi Fromentin.
Disclaimer. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above, the US Department of the Army, the US Department of Defense, or the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government or the Thai Red Cross AIDS Research Centre.
Financial support. This work was supported by the Intramural Research Program of the National Institutes of Health , National Institute of Allergy and Infectious Diseases; by a cooperative agreement (W81XWH-11-2-0174) between The Henry M. Jackson Foundation for the Advancement of Military Medicine and the US Department of Defense; and by the National Institutes of Health (grants R01MH095613 and R01NS061696 to SEARCH 010 and SEARCH 011, respectively). The Government Pharmaceutical Organization (GPO), Thailand, Gilead, Merck and ViiV Healthcare provided support for antiretroviral medications.
Potential conflicts of interest. N. C. is a board member of Theravectys. V. V. has consulted for Merck and ViiV Healthcare. J. A. has received honoraria from ViiV Healthcare, Merck, Gilead Sciences, and Roche Pharmaceuticals for her participation in advisory meetings. N. S. U. has consulted for Gilead Sciences, Entera Health, and Tobira Therapeutics and has been compensated for her participation in advisory meetings. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sandler NG, Wand H, Roque A et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuller LH, Tracy R, Belloso W et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boulware DR, Hullsiek KH, Puronen CE et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tenorio AR, Zheng Y, Bosch RJ et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu DC, Sereti I, Ananworanich J. Serious Non-AIDS events: Immunopathogenesis and interventional strategies. AIDS Res Ther 2013; 10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sandler NG, Sereti I. Can early therapy reduce inflammation? Curr Opin HIV AIDS 2014; 9:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Lelyveld SF, Gras L, Kesselring A et al. Long-term complications in patients with poor immunological recovery despite virological successful HAART in Dutch ATHENA cohort. AIDS 2012; 26:465–74. [DOI] [PubMed] [Google Scholar]
- 9. Rodger AJ, Lodwick R, Schechter M et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS 2013; 27:973–9. [DOI] [PubMed] [Google Scholar]
- 10. Ananworanich J, Schuetz A, Vandergeeten C et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7:e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ananworanich J, Phanuphak N, de Souza M et al. Incidence and characterization of acute HIV-1 infection in a high-risk Thai population. J Acquir Immune Defic Syndr 2008; 49:151–5. [DOI] [PubMed] [Google Scholar]
- 12. Ananworanich J, Fletcher JL, Pinyakorn S et al. A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology 2013; 10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pitisuttithum P, Nitayaphan S, Thongcharoen P et al. Safety and immunogenicity of combinations of recombinant subtype E and B human immunodeficiency virus type 1 envelope glycoprotein 120 vaccines in healthy Thai adults. J Infect Dis 2003; 188:219–27. [DOI] [PubMed] [Google Scholar]
- 14. Schuetz A, Deleage C, Sereti I et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog 2014; 10:e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valcour VG, Ananworanich J, Agsalda M et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One 2013; 8:e70164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robb ML, Eller LA, Kibuuka H et al. Prospective study of acute HIV-1 infection in adults in East Africa and Thailand. N Engl J Med 2016; 374:2120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ananworanich J, Chomont N, Fletcher JL et al. Markers of HIV reservoir size and immune activation after treatment in acute HIV infection with and without raltegravir and maraviroc intensification. J Virus Erad 2015; 1:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ananworanich J, Sacdalan CP, Pinyakorn S et al. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J Virus Erad 2016; 2:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunt PW, Sinclair E, Rodriguez B et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine 2014; 70:11–20. [DOI] [PubMed] [Google Scholar]
- 21. Estes JD, Harris LD, Klatt NR et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog 2010; 6:e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stacey AR, Norris PJ, Qin L et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 2009; 83:3719–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Estes JD. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol Rev 2013; 254:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deleage C, Schuetz A, Alvord WG et al. Impact of early cART in the gut during acute HIV infection. JCI Insight 2016; 1:e87065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moreno Velasquez I, Golabkesh Z, Kallberg H, Leander K, de Faire U, Gigante B. Circulating levels of interleukin 6 soluble receptor and its natural antagonist, sgp130, and the risk of myocardial infarction. Atherosclerosis 2015; 240:477–81. [DOI] [PubMed] [Google Scholar]
- 26. Musselwhite LW, Sheikh V, Norton TD et al. Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS 2011; 25:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hileman CO, Kinley B, Scharen-Guivel V et al. Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor-based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis 2015; 212:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hattab S, Guihot A, Guiguet M et al. Comparative impact of antiretroviral drugs on markers of inflammation and immune activation during the first two years of effective therapy for HIV-1 infection: an observational study. BMC Infect Dis 2014; 14:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Armah KA, McGinnis K, Baker J et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis 2012; 55:126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. French MA, Cozzi-Lepri A, Arduino RC et al. Plasma levels of cytokines and chemokines and the risk of mortality in HIV-infected individuals: a case-control analysis nested in a large clinical trial. AIDS 2015; 29:847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamlyn E, Fidler S, Stohr W et al. Interleukin-6 and D-dimer levels at seroconversion as predictors of HIV-1 disease progression. AIDS 2014; 28:869–74. [DOI] [PubMed] [Google Scholar]
- 32. Miller CJ, Baker JV, Bormann AM et al. Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS One 2014; 9:e95061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lundgren JD, Babiker AG, Gordin F et al. ; INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.