ABSTRACT
Leukemia relapse and chronic graft-versus-host disease (cGVHD) are still major obstacles of allogeneic hematopoietic stem cell transplantation (allo-HSCT). The numbers and activity of natural killer (NK) and T-regulatory cells can be increased post-transplantation by exposure to interleukin-2 (IL-2). We tested whether administering low-dose IL-2 would decrease leukemia relapse while reducing cGVHD after allotransplantation. This controlled, open-label randomized trial included 90 recipients of allotransplants. Subjects were randomized in a 1:1 ratio to either receive or not receive low-dose IL-2 during the early post-transplantation period. Patients in the IL-2 arm received a subcutaneous injection of low-dose IL-2 (1×106 U/d) on day 60 after allo-HSCT. IL-2 was administered daily for 14 d followed by a 14-d hiatus. The primary endpoint was the cumulative incidence of leukemia relapse (CIR). Three-year CIRs for the IL-2 arm and control arm were 23% (range 16–30%) and 11% (range 6–15%; p = 0.20), respectively. Minimal residual disease-positive (MRD+) tests were more common in the IL-2 arm compared to the control arm (36% [range 29–44%] vs. 15% [range 10–20%], p = 0.03). The cumulative incidence of moderate-to-severe chronic GVHD (cGVHD) was lower in the IL-2 arm compared to the control arm (33% [range 26–39%] vs. 57% [range 49–64%), p = 0.02). Therefore, the 3-y GVHD-free and GVHD progression-free survival (GPFS) rates were significantly higher in the IL-2 arm compared to the control arm (47% [range 39–55%] vs. 31% [range 25–38%], p = 0.048). Blood Tregs, NK cells, and NK-cell cytotoxicity were increased in subjects in the IL-2 arm between 3 mo and 6 mo post-transplantation. Administration of low-dose IL-2 during the immediate post-transplantation period was associated with a higher GPFS but did not decrease the CIR.
KEYWORDS: cGVHD, IL-2, MRD, NK, Treg
Introduction
Leukemia relapse is still a major obstacle for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Natural killer (NK) cells are of major importance in allo-HSCT because of their ability to recognize and destroy leukemic cells.1,2 NK cells could also prevent the occurrence of graft-versus-host disease (GVHD).3 Previous studies have demonstrated that CD4+Foxp3+ regulatory T cells (Treg) can suppress autoreactive lymphocytes and control innate and adaptive immune responses.4 Treg cell impairment is associated with the loss of tolerance, increased autoimmunity and cGVHD.5 Interleukin-2 (IL-2) is a pleiotropic cytokine that promotes cytolytic activity in CD8+ T cells and NK cells.6,7 Moreover, IL-2 is essential for the development and maintenance of Treg cells and for activation-induced cell death, thereby, mediating tolerance and limiting inappropriate immune responses.8 Thus, IL-2 treatment might decrease the likelihood of leukemia relapse after transplantation and has the potential to separate GVHD from graft-versus-leukemia (GVL) effect.
Based on the above information, we performed a randomized clinical trial (RCT) to determine whether administration of a low dose of IL-2 at early stages post-transplantation could decrease the incidence of leukemia relapse and reduce the incidence of cGVHD.
Results
Study population
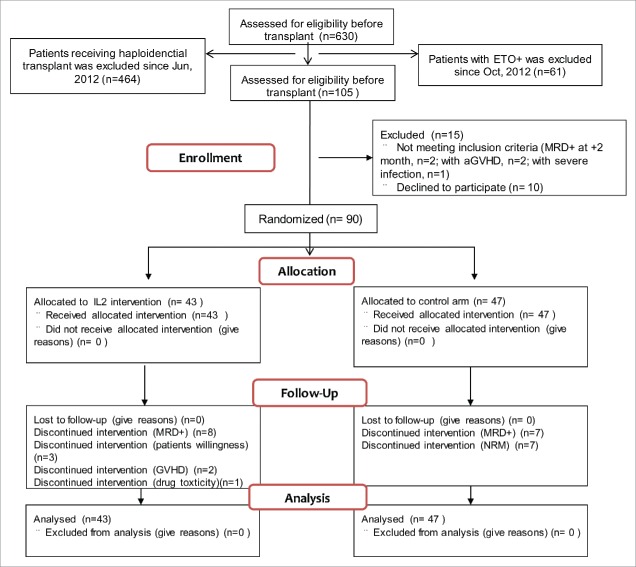
A total of 105 consecutive patients were screened (Fig. 1). Among these, 10 declined participation, another 5 were excluded because of severe acute GVHD (aGVHD) (n = 2), a positive MRD test (n = 2), or severe infection (n = 1). Of the enrolled subjects, 43 were randomized to receive IL-2 treatment and the remaining 47 were assigned to the control cohort.
Figure 1.
Flowchart of study design and patient enrollment.
The two groups had equivalent patient and donor characteristics (Table 1). Median follow-up was 1234 d (range, 587–1596 d). All of the subjects in the IL-2 cohort received ≥1 cycle of IL-2; 29 received ≥4 cycles. The detailed flowchart of patients enrolled in the IL-2 and control arms of this trial and their reasons for exiting the study has been described in Fig. S1.
Table 1.
Patient and donor characteristics.
| Characteristics | IL-2 arm | Control group | p value |
|---|---|---|---|
| No. of patients | 43 | 47 | |
| Median age (range), years | 38 (17–52) | 36 (15–56) | 0.971 |
| Patient sex, male, no. (%) | 25 (58.1%) | 27 (57.4%) | 0.833 |
| Donor sex, male, no. (%) | 22 (53.7%) | 16 (35.6%) | 0.091 |
| Donor–recipient, sex-matched grafts, no. (%) | 22 (53.7%) | 18 (40%) | 0.205 |
| Donor–recipient sex combination, no. (%) | 0.25 | ||
| Male–male | 13 (31.7%) | 7 (15.6%) | |
| Male–female | 9 (22%) | 9 (20%) | |
| Female–male | 10 (24.4%) | 19 (40%) | |
| Female–female | 9 (22%) | 11 (24.4%) | |
| Diagnosis, no. | 0.139 | ||
| AML | 30 (69.8%) | 39 (83%) | |
| ALL | 13(30.2%) | 8(17%) | |
| Disease status, standard risk, no. (%) | 43 (100%) | 47 (100%) | |
| Disease risk index, no. (%) | 0.338 | ||
| Low | 4 (9.3%) | 2 (4.3%) | |
| Intermediate | 39 (90.7%) | 45 (95.7%) | |
| High | 0 | 0 | |
| HCT-CI | 0.063 | ||
| 0 | 36 (83.7%) | 37 (78.7%) | |
| 1 | 2 (4.7%) | 9 (19.1%) | |
| 2 | 2 (4.7%) | 0 (0%) | |
| ≥3 | 3 (7.0%) | 1 (2.1%) | |
| Conditioning regimen, no. | 0.505 | ||
| Bu/Cy | 41 (95.3%) | 46 (97.9%) | |
| Bu/Cy+ATG | 2 (4.7%) | 1 (2.1%) | |
| Types of transplants | 0.632 | ||
| HLA-matched sibling-related | 39 (90.7%) | 45 (95.7%) | |
| Related haploidentical | 2 (4.7%) | 1 (2.1%) | |
| Unrelated matched | 2 (4.7%) | 1 (2.1%) | |
| Graft type, no. (%) | 1 | ||
| GBM+PBSCs | 40 (93%) | 43 (91.5%) | |
| PBSCs | 3 (7%) | 4 (8.5%) | |
| HLA-A, HLA-B, HLA-DR mismatched grafts, no. | 0.604 | ||
| 0 | 41 (95.3%) | 46 (97.9%) | |
| 1 | 0 | 0 | |
| 2 | 2 (4.7%) | 1 (2.1%) | |
| 3 | 0 | 0 | |
| ABO matched grafts, no. (%) | 0.184 | ||
| Matched | 31 (72.1%) | 25 (53.2%) | |
| Major mismatch | 5 (11.6%) | 12 (25.5%) | |
| Minor mismatch | 5 (11.6%) | 9 (19.1) | |
| Bidirectional mismatch | 2 (4.7%) | 1 (2.1%) | |
| Cell composition in allografts, median (range) | |||
| Infused CD34+ cells, 106/kg | 2.23 (0.18–8.35) | 2.40 (0.52–9.09) | 0.495 |
| Infused lymphocytes, 108/kg | 2.47 (0.42–5.79) | 2.71 (0.33–9.02) | 0.347 |
| Infused CD3+ cells, 108/kg | 1.74 (0.30–3.67) | 1.83 (0.20–5.54) | 0.547 |
| Infused CD4+ cells, 108/kg | 0.94 (0.15–2.0) | 0.98 (0.14–2.11) | 0.744 |
| Infused CD8+ cells, 108/kg | 0.64 (0.09–1.68) | 0.59(0.04–2.58) | 0.597 |
| Infused CD4−CD8− cells, 108/kg | 0.099 (0.035–0.40) | 0.12 (0.014–0.81) | 0.355 |
| Infused CD14+ cells, 108/kg | 1.20 (0.14–2.34) | 1.43 (0.22–4.22) | 0.206 |
| DLI within 100 days after transplantation, no. (%) | 0 | 1 (2.1%) | 1 |
| DLI later than 100 days after transplantation, no. (%) | 8 (18.6%) | 4 (8.5%) | 0.218 |
| ANC engraftment | 15 (10–22) | 15 (11–22) | 0.674 |
| PLT engraftment | 12 (7–50) | 12 (7–152) | 0.254 |
| HBV infection | |||
| Recipient (n, %) | 0.112 | ||
| HBsAg−/HBeAb−/HBcAb− | 11 | 11 | |
| HBsAg+/HBeAb+/HBcAb+ | 2 | 8 | |
| HBsAb+/HBeAb+/HBcAb+ | 30 | 28 | |
| Donor (n, %) | 0.673 | ||
| HBsAg−/HBeAb−/HBcAb− | 12 | 13 | |
| HBsAg+/HBeAg+/HBcAb+ | 0 | 2 | |
| HBsAg+/HBeAb+/HBcAb+ | 2 | 2 | |
| HBsAb+/HBeAb+/HBcAb+ | 29 | 30 | |
| Follow-up days | 1301 (674–1,596) | 1217 (587–1,591) | 0.30 |
Abbreviations: ABO, ABO blood type; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; DLI, donor lymphocyte infusions; GBM, G-CSF mobilized bone marrow; PBSCs, G-CSF mobilized peripheral blood stem cells.
In total, 11 patients (4 control arm and 7 IL-2 arm) required DLI because of a positive MRD test, which occurred at a median of 193 d (range, 102–298 d) after transplantation, 1 patient required DLI because of relapse (day 420, IL-2 arm) and 1 patient accepted DLI because of GF (day 56, control arm).
IL-2 therapy
The IL-2 therapy was well tolerated. The planned total dose delivered ranged from 64 × 106 U to 84 × 106 U. The planned total dose has been delivered to 67% patients. The most common adverse event was fever. Details are shown in Table 2.
Table 2.
Toxicities of IL-2.
| Drug-related toxicities | Number of cases (n, %) | Number of patients unable to tolerate (n) |
|---|---|---|
| Fever | 11 (25.6%) | |
| Flu-like symptoms (e.g., malaise and fatigue) | 5 (11.6%) | |
| Nausea and/or vomiting | 5 (11.6%) | 1* |
| Diarrhea | 1 (2.3%) | |
| Allergic reaction | 2 (4.6%) | |
| Pain at the infection site | 6 (14.0%) | |
| Dyspnea | 1 (2.3%) | 1* |
One patient had significant symptoms of the upper digestive tract and dyspnea simultaneously.
Leukemia relapse
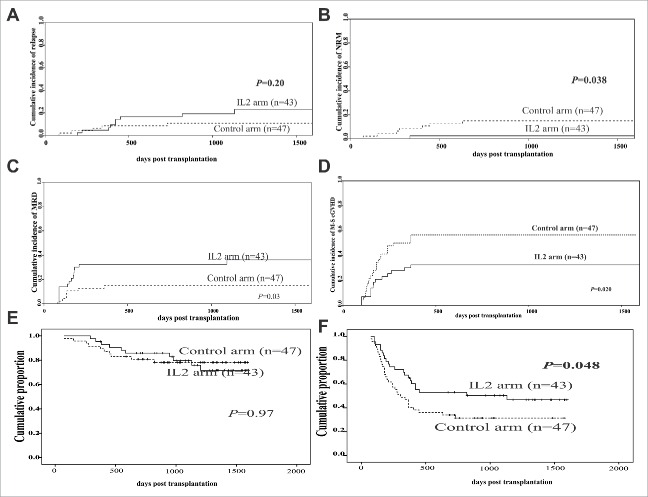
Nine subjects in the IL-2 cohort relapsed vs. five in the control cohort. The cumulative incidence of relapse (CIR) in the IL-2 cohort was 23% (range 16–30%) vs. 11% (range 6–15%; p = 0.20; Fig. 2A and Table 3) in the control arm. Of nine subjects with a prior positive MRD test in the IL-2 arm, six relapsed, as did three of five subjects with a prior positive MRD test in the control arm.
Figure 2.
The clinical outcomes between the IL-2 and control arms. (A) Relapse, (B) non-relapse mortality (NRM), (C) minimal residual disease (MRD), (D) moderate-to-severe chronic GVHD, (E) overall survival (OS) and (F) GVHD-free and relapse-free survival (GPFS). Patient cohorts: IL-2 group (n = 43) and control group (n = 47).
Table 3.
Incidence of adverse events and transplantation outcomes for patients who underwent allogeneic stem cell transplantation.
| Parameter | IL-2 arm | Control group | p value |
|---|---|---|---|
| Overall aGVHD at day 100 post-transplantation | 16.3% (10.9–21.7%) | 27.7% (21.4–33.9%) | 0.25 |
| Grade 2–4 aGVHD at day 100 post-transplantation | 11.6% (7.0–16.3%) | 21.3% (15.6–27.0%) | 0.24 |
| CMV reactivation at day 180 post-transplantation | 30.2% (23.6–36.9%) | 42.6% (35.8–49.4%) | 0.23 |
| EBV reactivation at day 180 post-transplantation | 0 | 2.1% (0.1–4.2%) | 0.339 |
| IFI at day 100 post-transplantation | 4.7% (1.6–7.7%) | 2.2% (0.1–1.6%) | 0.928 |
| 3-y cumulative incidence of overall chronic GVHD | 53.5% (46.1–60.9%) | 65.2% (58.4–72.0%) | 0.33 |
| 3-y cumulative incidence of moderate-to-severe chronic GVHD | 32.6% (25.7–39.5%) | 56.5% (49.5–63.6%) | 0.02 |
| 3-y cumulative incidence of MRD | 36.2% (28.8–43.6%) | 14.9% (9.9–19.9%) | 0.03 |
| 3-y cumulative incidence of relapse | 23.1% (16.4–29.9%) | 10.8% (6.4–15.2%) | 0.20 |
| 3-y cumulative incidence of NRM | 2.3% (0.1–4.5%) | 14.89% (9.9–19.9%) | 0.038 |
| 3-y cumulative incidence of LFS | 74.6% (67.8–81.4%) | 74.3% (68.2–80.4%) | 0.72 |
| 3-y cumulative incidence of GPFS | 46.8% (39.2–54.4%) | 31.4% (24.8–38.0%) | 0.048 |
| 3-y cumulative incidence of OS | 71.7% (64.1–79.3%) | 78.2% (72.4–84.0%) | 0.696 |
Note: All data are given as incidence (95% CI) unless otherwise noted and were analyzed with Gray's method and a log-rank test.
Abbreviations: CMV, cytomegalovirus; EBV, Epstein–Barr virus; GVHD, graft-versus-host disease; GPFS, GVHD progression free survival; IFI, invasive fungal infection; LFS, leukemia-free survival; MRD, minimal residual disease; NRM, non-relapse mortality; OS, overall survival.
Second endpoints
NRM
One subject in the IL-2 cohort died of non-relapse mortality (NRM) compared with seven subjects in the control cohort (p = 0.038). Five subjects died of severe cGVHD (IL-2 cohort n = 1; control cohort n = 4). Three other subjects died of CMV-related hepatitis, HBV-related hepatitis, and lung infection. The median intervals to NRM were 336 d in the IL-2 cohort and 321 d in the control cohort (range, 73–819 d). The NRM rates were lower in the IL-2 cohort than in the control arm (2% (range 0–5%) vs. 15% (range 10–21%); p = 0.038; Fig. 2B and Table 3).
Positive MRD tests
Twenty subjects became MRD+, including fifteen in the IL-2 cohort and seven in the control cohort. The median intervals from randomization to a positive MRD test was 198 d (range, 90–1093 d) in the IL-2 cohort and 166 d (range, 83–360 d) in the control cohort (p = 0.745). The cumulative incidence of a positive MRD test was higher in IL-2 cohort compared with the control cohort (38% [range 29–44%] vs. 15% [range 10–20%]; p = 0.03; Fig. 2C). Multivariate analysis showed that IL-2 treatment during the early post-transplantation period significantly increased the incidence of positive MRD tests compared with the control arm (hazard ratio [HR] = 3.3; 95% CI, 1.2–9.1; p = 0.022; Table 3). The interventions for recurrent leukemia and a positive MRD test are shown in Fig. S2.
cGVHD status
A total of 23 subjects in the IL-2 cohort developed cGVHD compared with 30 subjects in the control cohort. Median intervals to developing cGVHD were 186 d in the IL-2 cohort and 197 d in the control cohort. The cumulative incidences of all stages of cGVHD were 53% [range 46–61%] vs. 65% [range 58–72%] (p = 0.33), but the cumulative incidence of moderate-to-severe cGVHD was lower in the IL-2 cohort (33% [range 26–39%] vs. 56% [range 49–64%]; p = 0.02; Fig. 2D). Multivariate analysis showed that IL-2 treatment during the early post-transplantation period significantly decreased the incidence of moderate-to-severe cGVHD compared with the control arm (HR = 0.5; 95% CI, 0.3–0.9; p = 0.033; Table 4). The sites of involvement are shown in Table S1.
Table 4.
Univariate and multivariate analysis of prophylactic use of low-dose interleukin-2 on allogeneic stem cell transplantation outcomes.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Covariate | HR | 95% CI | p | HR | 95% CI | p |
| Acute GVHD | ||||||
| HLA mismatch | 4.5 | 2.3–8.8 | <0.0001 | 5.1 | 2.6–10.2 | <0.0001 |
| CMV infection | 2.7 | 0.9–7.5 | 0.063 | |||
| Infused lymphocyte/kg recipient weight | 1.0 | 1.0–1.1 | 0.02 | |||
| Infused CD3+ cells/kg recipient weight | 1.01 | 1.0–1.01 | 0.021 | |||
| Infused CD4+ cells/kg recipient weight | 1.01 | 1 to 1.02 | 0.047 | |||
| Infused CD8+ cells/kg recipient weight | 1.01 | 1.0–1.02 | 0.02 | 1.01 | 1.0–1.02 | 0.008 |
| Infused CD14+ cells/kg recipient weight | 1.01 | 1.0–1.01 | 0.008 | |||
| Overall chronic GVHD | ||||||
| Donor sex (female vs. male) | 2.1 | 1.2–3.7 | 0.011 | 2.1 | 1.2–3.7 | 0.012 |
| MRD-positive | 1.5 | 1.0–2.2 | 0.06 | 1.9 | 1.0–3.5 | 0.045 |
| Moderate-to-severe chronic GVHD | ||||||
| Donor sex (female vs. male) | 2.5 | 1.3–4.9 | 0.009 | 2.4 | 1.2–4.8 | 0.011 |
| Donor–recipient sex mismatch | 1.3 | 1.0–1.7 | 0.088 | |||
| CMV infection | 0.5 | 0.2–1.0 | 0.063 | |||
| MRD-positive | 1.5 | 0.9–2.4 | 0.089 | |||
| Presence or absence of prophylactic IL-2 | 0.5 | 0.3–0.9 | 0.027 | 0.5 | 0.3–0.9 | 0.033 |
| MRD | ||||||
| Donor–recipient sex mismatch | 0.4 | 0.1–1.0 | 0.051 | |||
| Presence or absence of prophylactic IL-2 | 0.4 | 0.2–1.0 | 0.049 | 3.3 | 1.2–9.1 | 0.022 |
| Infused CD34+ cells/kg recipient weight | 1.2 | 1.0–1.5 | 0.065 | |||
| Relapse | ||||||
| Donor–recipient sex mismatch | 0.2 | 0–0.9 | 0.034 | |||
| MRD-positive | 2.5 | 1.2–5.2 | 0.019 | 8.1 | 2.4–27.9 | 0.001 |
| Moderate-to-severe cGVHD | 0.2 | 0–0.8 | 0.027 | 0.2 | 0–0.8 | 0.028 |
| Non-relapse mortality | ||||||
| Presence or absence of prophylactic IL-2 | 0.2 | 0–1.2 | 0.076 | 0.2 | 0–1.2 | 0.076 |
| GPFS | ||||||
| Donor sex (female vs. male) | 2.1 | 1.2–3.7 | 0.014 | |||
| DLI | 1.8 | 0.9–3.5 | 0.085 | |||
| Presence or absence of prophylactic IL-2 | 0.6 | 0.3–1.0 | 0.052 | |||
| MRD-positive | 1.7 | 1.2–2.5 | 0.002 | 1.8 | 1.2–2.6 | 0.002 |
| Infused lymphocyte/kg recipient weight | 0.9 | 0.9–1.0 | 0.064 | |||
| LFS | ||||||
| CMV infection | 2.0 | 0.9–4.7 | 0.099 | 2.2 | 0.9–5.0 | 0.073 |
| MRD-positive | 2.4 | 1.0–5.7 | 0.04 | 2.6 | 1.1–6.1 | 0.029 |
Note: All variables were first included in the univariate analysis; only variables with p < 0.1 were included in the Cox proportional hazards regression model.
Abbreviations: DLI, donor lymphocyte infusion; GVHD graft-versus-host disease; GPFS, GVHD progression free survival; HR, hazard ratio; LFS, leukemia-free survival; MRD, minimal residual disease; NRM, non-relapse mortality.
Survival
The 3-y leukemia-free survival (LFS) was 75% [range 68–81%] in the IL-2 cohort vs. 74% [range 68–80%] in the control cohort (p = 0.72, Table 2), and the 3-y overall survival (OS) rates were 72% [range 64–79%] vs. 78% [range 72–84%] (p = 0.70; Fig. 2E and Table 2). However, the 3-y GPFS rates were significantly higher in the IL-2 cohort compared to the control cohort (47% [range 39–55%] vs. 31% [range 25–38%], p = 0.048; Fig. 2F and Table 2). Multivariate analysis showed that a positive MRD test post-transplantation significantly decreased the incidence of LFS (hazard ratio [HR], 2.6; 95% CI, 1.1–6.1; p = 0.029) and GPFS (hazard ratio [HR], 1.8; 95% CI, 1.2–2.5; p = 0.002; Table 3).
Impact of low-dose IL-2 on treg and NK cells
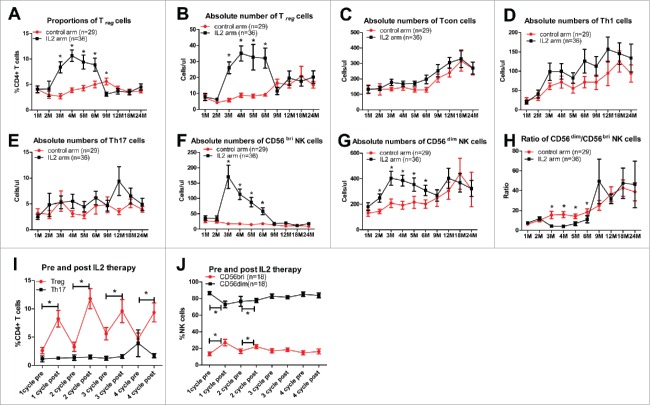
The reconstitution of CD4+CD25highCD127−/lowFoxP3+Treg cells was significantly higher in the IL-2 arm than in the control arm post-transplantation (Fig. 3A and B). No significant differences were found in the reconstitution of CD4+CD25+CD127+ conventional (Tcon) cells, Th1 cells, or Th17 cells in the IL-2 and control arms post-transplantation (Fig. 3C–E). Both of the absolute numbers of CD56bri and CD56dim NK cells were significantly higher in the IL-2 arm than in the control arm from 3 m to 6 m post-transplantation (Fig. 3F and G). Meanwhile, the ratio of CD56dim and CD56bri was significantly decreased in the IL-2 group (Fig. 3H). Both the proportions and absolute numbers of Treg and NK cells were expanded one week after IL-2 therapy, and peaked by two weeks, then rapidly decreased after halting IL-2 treatment (Fig. 3I and J).
Figure 3.
CD4+CD25highCD127−/lowFoxP3+ Treg cell and NK cell reconstitution is expanded by a low dose of IL-2. Reconstitution of peripheral blood Treg cells and NK cells at different time points post-transplantation between the IL-2 and control groups: (A) proportion of Treg cells within CD4+ T cells; (B) absolute numbers of Treg cells (cells/µL); (C) absolute numbers of CD4+CD25+CD127high conventional T cells (Tcon) (cells/µL); (D) absolute numbers of CD3+CD8−IFNγ+ T cells (Th1) (cells/µL); (E) absolute numbers of CD3+CD8−IL17A+ T cells (Th17) (cells/µL); (F) absolute numbers of CD56bright NK cells (cells/µL); (G) absolute numbers of CD56dim NK cells (cells/µL); and (H) ratio of CD56dim/CD56bri. (I) The proportion of Treg cells and Th17 cells within the CD4+ T cell population and (J) the proportion of CD56bri NK and CD56dim NK cells within the total NK cell population before and after IL-2 treatment.
No significant differences in the functional capacity of suppressed Treg cells in vitro were found in IL-2-expanded Treg cells and Treg cells from a healthy donor (Fig. S3A). Cytotoxicity and IFNγ secretion from NK cells against K562 cells were significantly higher in the IL-2 group than those of the control arm (Fig. S3B and C). The IL-2 expanded CD56bri NK cells and CD56dim NK cells, showing promoted cytotoxicity against resting or activated conventional T cells (Fig. S3D–G).
Patients with moderate-to-severe cGVHD had lower absolute numbers of Treg cells from 1 m to 3 m post-transplantation and a lower ratio of CD56dim/CD56bri cells from 2 m to 6 m post-transplantation in the control group (Fig. S4A and B).
Discussion
The purpose of this study was to prove that prophylactic use of low-dose IL-2 early after transplantation would reduce the incidence of leukemia relapse and cGVHD through amplification of NK and Treg cells. However, this study was halted early because of futility. In fact, we observed a higher rate of relapse in subjects receiving IL-2. To our knowledge, this is the first report of this observed result. This increase was not significant, but our study was not sufficiently powered to detect an increase. cGVHD has been inversely correlated with relapse risk in acute leukemia in many studies9,10 and in this study. The etiology of the increased relapse risk is unclear but may relate to the significantly decreased incidence of cGVHD we observed. The cumulative incidence of MRD+ in the control arm was similar to that of our previous report (14.6% vs. 12.9%). Moreover, the conditioning regimen, transplant source, disease status, and other patient characteristics were all comparable between the IL-2 and control arms. Obviously, IL-2 administration resulted in the increase in MRD+. Although there was enhanced NK cytotoxicity against K562 cells post-transplantation, in vivo IL-2 administration did not show any effect on leukemia prevention. Thus, it is worth discussing whether NK cells display a predominant role in an allogeneic anti-leukemia effect. Recently, Hallett et al. conducted long-term tumor outgrowth experiments and showed that prior depletion of Tregs before IL-2 administration led to improved antitumor effects compared with administration of either treatment alone.11 Bachanova et al. conducted an adoptive NK cell infusion clinical trial, which demonstrated that depletion of host regulatory T cells with IL-2 diphtheria toxin fusion protein (IL-2DT) improves the efficacy of haploidentical (HID) NK-cell therapy for patients with refractory AML.12 Therefore, higher levels of Treg cells in the tumor environment might inhibit the GVL effects of NK cells in vivo as well as other cytotoxic T cells post-transplantation.13 These data indicated that even though IL-2 could prevent cGVHD, relapse is still an important problem that should be addressed.
Consistent with previously published data,14,15 we found that NK and Treg cells had profoundly expanded in patients receiving IL-2 treatment. In addition, the data showed that the incidence of cGVHD was decreased after IL-2 treatment. Similar to previous publications, the number of Treg cells was negatively correlated with cGVHD occurrence in the control arm,5 which indicated that IL-2 might prevent cGVHD by expanding Tregs and therefore displaying immunoregulatory function. To our knowledge, this is the first randomized study showing that prophylactic use of low-dose IL-2 might reduce the incidence of cGVHD. Although more MRD+ patients received interventional treatment (including DLI) that would increase cGVHD, the IL-2 arm still showed a lower incidence of cGVHD. This indicated the strength of our conclusion. Koreth et al. reported that low-dose IL-2 could effectively improve the symptoms of cGVHD.14,16 Therefore, treatment with IL-2 plays not only a therapeutic role but also a prophylactic role on cGVHD. Previous studies explored the roles of Treg cells in cGVHD that were mainly obtained from the patient's sample after the diagnosis of cGVHD.5,17,18 In this study, we prospectively accrued and serially evaluated a cohort of patients after allo-HSCT to evaluate the “natural history” of cGVHD after unmanipulated transplantation either with or without IL-2 treatment, which would provide direct evidence to further clarify the protective roles of Treg cells as well as the protective effect of IL-2 treatment on cGVHD.
With the decrease in moderate-to-severe cGVHD, the cumulative incidence of NRM was also reduced; therefore, the GPFS rate was significantly increased in the IL-2 arm. Previous studies showed that most NK cells are likely more immunoregulatory than cytotoxic (CD56bright CD16 cells) within 30 d after transplantation.19-21 In our study, CD56bright NK cells were significantly expanded after in IL-2 treatment. In vitro cytotoxicity showed that the expanded NK cells, regardless of whether they were CD56dim or CD56bri NK cells, could kill self-resting or self-activated conventional T cells. This suggested that the early expansion of CD56bri NK cells could exert a protective effect on the pathogenesis of cGVHD in patients with moderate-to-severe cGVHD. Bielekova et al. showed that CD56bri NK cells could inhibit T-cell survival by a contact-dependent mechanism.22 Kheav et al. demonstrated that a high NK cell count at month 3 was associated with a lower incidence of cGVHD.23 Functional analysis further demonstrated that expanded CD56bri NK cells retained IFNγ production, which is known to promote antimicrobial immunity through the maturation of dendritic cells and the induction of Th1 responses.24,25 Our previous study also found that rapid reconstitution of CD56bri NK cells was associated with a lower TRM post-transplantation.21 Therefore, the IL-2-induced expansion of CD56bri NK cells could not only decrease the incidence of moderate-to-severe cGVHD but also enhance the anti-infection capacity of patients, which could explain its contribution to the lower incidence of NRM in the IL-2 arm. Although a higher frequency of MRD+ tests and a trend of increased relapse in the IL-2 cohort were observed, the OS and LFS were comparable between the two groups, and GPFS was significantly increased in the IL-2 arm.
Finally, we must note that there are several limitations in this RCT study. First, we only evaluated the different effects either with or without IL-2 treatment on clinical outcomes instead of using a placebo as a control. Second, based on our previous study, MRD+ had preceded relapse post-transplantation. Therefore, most MRD+ patients received an intervention to prevent relapse, which would influence the effect of IL-2 treatment on relapse and cGVHD.
In summary, low-dose IL-2 administered post-transplantation did not reduce the CIR but was associated with a higher frequency of a positive MRD test, a lower frequency of cGVHD, and a lower frequency of NRM as well as a higher GPFS. Thus, prophylactic administration of IL-2 still requires caution.
Methods
Eligibility criteria
We conducted an open-label, prospective, randomized trial in patients with acute leukemia who underwent unmanipulated allo-HSCT at the Peking University Institute of Hematology between January 2012 and December 2014. Patients aged 15–65 y with acute leukemia in complete remission (CR) who received myeloablative allo-HSCT were eligible for inclusion in this trial. The patients also met the following criteria: (1) diagnosis with non-Ph+ALL or T-ALL; (2) negative for minimal residual disease (MRD) at day 60 post-transplantation; and (3) no active GVHD or severe infection. The IL-2 trial is registered at the US National Institutes of Health (NIH) (ClinicalTrials.gov, #NCT01517347). Before May 2012, patients that underwent haploidentical, unrelated, and HLA-matched sibling transplantation (MSD) were all enrolled in this study. After May 2012, we revised the inclusion criteria of protocol for patients that underwent HLA-MSD to maintain the uniformity of the clinical trial.
Study design
All patients were randomly assigned to either the IL-2 arm or control arm post-transplantation. Patients in the IL-2 arm received a subcutaneous injection of low-dose IL-2 (1 × 106 U/d) on day 60 after allo-HSCT. IL-2 was administered daily for 14 d followed by a 14-d hiatus. This treatment cycle was repeated 4–6 times until one of the following conditions was met: (1) leukemia relapse; (2) MRD+; (3) toxic effects (a grade 3 or higher based on the National Cancer Institute's Common Terminology Criteria for Adverse Events [CTCAE]; (4) grade 3–4 aGVHD; and (5) participant demonstrated an inability or a low compliance with their medication regimen and/or documentation requirements. For patients in the control arm, the transplantation procedure was identical to that of the IL-2 arm except for administration of the IL-2 treatment. This study was approved by the Institutional Review Board of Peking University, and written informed consent was obtained from all of the patients before study entry in accordance with the Declaration of Helsinki. Finally, 43 patients and 47 patients were enrolled into the IL-2 arm and the control arm, respectively, after transplantation.
Randomization and masking
After obtaining written consent, the donor–recipient pairs were randomly assigned in a 1:1 ratio into the two arms (IL-2 arm and control arm) by the sub-investigator (treating physician of our transplant center) according to a computer-generated randomization system. Independent individuals assessing outcomes and analyzing data were masked to treatment allocation to eliminate the subjective bias regarding the aspects of assessment and care that could potentially affect the outcomes.
Transplants
The conditioning therapy for the HID or unrelated donor transplantation was as follows: cytarabine (4 g/m2 per day) i.v. on days −10 to −9; busulfan (3.2 mg/kg per day) i.v. on days −8 to −6; cyclophosphamide (1.8 g/m2 per day) i.v. on days −5 to −4; methyl chloride hexamethylene urea nitrate (Me-CCNU) (250 mg/m2 per day) orally once on day −3; and ATG (2.5 mg/kg per day; Sang Stat, Lyon, France) i.v. on days −5 to −2. Patients who underwent HLA-MSD received hydroxycarbamide (80 mg/kg) orally on day −10 and a lower dose of cytarabine (2 g/m2 per day) on day −9 in addition to the regimen described above with the exception of ATG administration. Allogeneic grafts were harvested and infused according to our previous protocol.26,27 Eighty-three patients received a combination of G-CSF-mobilized bone marrow (GBM) and peripheral blood stem cells (PBSCs), and seven patients received PBSC only because of either unrelated transplantation or the planned donor declined to donate bone marrow.
GVHD prophylaxis and treatment
All of the transplant recipients received cyclosporine A (CsA), mycophenolate mofetil (MMF), and short-term methotrexate (MTX) for GVHD prophylaxis. CsA was administered i.v. on day −9 at a dosage of 2.5 mg/kg that was adjusted to a blood concentration of 150–250 ng/mL. CsA treatment was reduced gradually and discontinued approximately 4–6 mo after HSCT. MTX (15 mg/m2) was administered i.v. on day +1, and 10 mg/m2 was administered on days +3, +5, and +11 in patients receiving either HID or unrelated transplantation but on days +3 and +6 in patients receiving MSD transplantation. MMF was administered orally (0.5 g every 12 h) from days −9 to +30 in HID patients but was discontinued upon engraftment in MSD patients. aGVHD was treated with steroids (methylprednisolone, 1 mg/kg per day) as the first-line therapy. Anti-CD25 monoclonal antibodies (basiliximab, Novartis Pharma AG, Basel, Switzerland) were administered as the second-line therapy.28-30 cGVHD was treated with CsA, MMF, or steroids.
Cytomegalovirus and epstein–barr virus monitoring and prevention
Levels of cytomegalovirus (CMV) and Epstein–Barr virus (EBV) were monitored, and infections were treated as described previously.31
MRD and relapse monitoring and intervention
After completion of the study treatment, BM samples were analyzed at 1, 2, 3, 4.5, 6, 9, and 12 mo after transplantation and at 6-mo intervals thereafter to monitor for MRD as previously reported.26,32,33 At Peking University, modified donor lymphocyte infusion (DLI) was administered before hematologic relapse as an interventional therapy (preemptive DLI) 3 mo post-HSCT following a trial of immunosuppressant withdrawal. The detailed criteria for preemptive DLI administration included the following: (1) an MRD+ score in patients within 1 y after transplantation, which is defined as having either two consecutive positive results using either flow cytometry or Wilms' tumor gene 1 or positive for both flow cytometry and Wilms' tumor gene 1 in a single sample; (2) no uncontrolled GVHD or life-threatening infections; and (3) donor availability and willingness. The modified DLI regimen was as previously described.33
When a hematological relapse was diagnosed after HSCT, post-transplantation immune suppression was immediately discontinued. If patients did not develop GVHD within two weeks, agreed to receive targeted therapeutic modified DLI, and had donors who also agreed to repeat the PB stem cell collection, the patients received chemotherapy followed by modified DLI; otherwise, the patients received chemotherapy alone.34
Donor lymphocyte infusion
Indications for DLI included hematologic leukemia relapse (i.e., patients received chemotherapy followed by DLI);34 molecular tests providing evidence of either persistent leukemia or a recurrence in patients without GVHD33; and graft failure (GF).35
Definitions and evaluation
Engraftment, infection, NRM, relapse, LFS, OS, and GPFS were defined as previously described.31,36,37 aGVHD was defined and graded from 0 to 4 based on the Seattle criteria.38 cGVHD was defined and graded according to the NIH criteria.39 Patient experience of relapse was defined on the basis of histological criteria. MRD was defined as previously described.32,33
End points
The primary endpoint was the cumulative incidence of hematological relapse. The secondary endpoints were MRD+, aGVHD, cGVHD, NRM, OS, DFS, and GPFS.
Sample size calculation
Power calculations dictated that 360 subjects were necessary to detect a 10% difference between groups in CIR based on our previous results (from 20% to 10%) with a one-sided α of 0.05 and a power of 0.80. The interim analysis after 1/4, 1/2, and 3/4 of the 360 cases was specified, and the conditional power for success was defined as 0.80. After the 90th patient enrolled in this study, we performed the first interim analysis. Because the conditional power was 0.083, the Data and Safety Monitoring Board recommended halting the study due to futility after the first interim analysis.
Blood sample preparation and immune reconstitution monitoring
The final 64 subjects (IL-2 n = 35; control n = 29) were prospectively monitored for reconstitution of Treg, NK, Tcon, Th1, and Th17 cells. Blood samples were obtained at 1, 2, 3, 4, 5, 6, 9, 12, and 24 mo after HSCT. In the IL-2 arm, samples were also obtained before and after every IL-2 treatment cycle to explore the effects of the IL-2 treatment more directly. All of the samples were treated with stains for cell surface markers and intracellular cytokines to detect the recovery of CD3+ T cells, CD56+ NK cells, CD4+CD25highCD127low/− Treg cells, CD4+CD25−/lowCD127+ conventional T cells, CD4+CD25+Foxp3+ Treg cells, Th17 cells, and Th1 cells. Functional analysis of the ex vivo suppression of either Treg cells or NK cells against self-conventional T cells as well as NK cells against the K562 cell response is described in the supplementary data.
Statistical analyses
Three groups were compared with the χ2 statistic for categorical variables and the Mann–Whitney test for continuous variables. Cumulative incidence curves were used in a competing risk setting, with relapse treated as a competing event, to calculate NRM probabilities, and with death from any cause as a competing risk for GVHD, engraftment, EBV or CMV reactivation, and relapse. Time to GVHD was defined as the time from transplantation to the onset of GVHD of any grade. The probabilities of LFS, GPFS, and OS were estimated using the Kaplan–Meier method. All of the variables in Table 1 were included in the univariate analysis. Only variables with p < 0.1 were then included in a Cox proportional hazards regression model with time-dependent variables. Unless otherwise specified, p values were based on two-sided hypothesis tests, and α was set at 0.05. Most analyses were performed with SPSS (version 16.0; Chicago, IL). The final follow-up was conducted on April 30, 2016.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Professors Robert Peter Gale (Imperial College London) kindly reviewed the manuscript. The authors thank all of the core facilities at the Peking University Institute of Hematology for sample collection.
Funding
This work was partly supported by grants from the National Natural Science Foundation of China (Grant Nos. 81670166, 81530046, 81270644 and 81230013), the Beijing Talents fund (No. 2015000021223ZK26), the Major State Basic Research Development Program of China (973 Program No. 2013CB733700), the Milstein Medical Asian American Partnership (MMAAP) Foundation Research Project Award in Haematology and the Collaborative Innovation Center of Haematology, Peking University, China, and the Beijing Municipal Science and Technology Program (Grant Nos. Z151100001615020 and Z141100000214011) and the Clinical Characteristic Study of Capital Project (Z121107001012085).
Author contributions
H-XJ, Z-XY, and Z-XS designed the study and the protocol and contributed to the critical review and revision of the manuscript. H-XJ was principal investigator of this trial. Z-XY and Z-XS contributed to the study protocol and led the laboratory analyses. Z-XY and Z-XS contributed to data acquisition, drafting of the manuscript, study supervision and revision of the manuscript. C-YH, X-LP, Z-XH, HW, CH, Y-CH, W-FR, W-JZ, S-YQ, L-KY, and C-YJ led and participated in the site work, including patient recruitment, data collection and data interpretation. W-YT contributed to the laboratory analyses and data interpretation. All of the authors reviewed and approved the final version of the manuscript. Z-XY and Z-XS contributed equally to this work.
References
- 1.Vago L, Forno B, Sormani MP, Crocchiolo R, Zino E, Di Terlizzi S, Stanghellini MTL, Mazzi B, Perna SK, Bondanza A et al.. Temporal, quantitative, and functional characteristics of single-KIR-positive alloreactive natural killer cell recovery account for impaired graft-versus-leukemia activity after haploidentical hematopoietic stem cell transplantation. Blood 2008; 112(8):3488-99; PMID:18645039; http://dx.doi.org/ 10.1182/blood-2007-07-103325 [DOI] [PubMed] [Google Scholar]
- 2.Zhao XY, Chang YJ, Zhao XS, Xu LP, Zhang XH, Liu KY, Li D, Huang XJ. Recipient expression of ligands for donor inhibitory KIRs enhances NK-cell function to control leukemic relapse after haploidentical transplantation. Eur J Immunol 2015; 45(8):2396-408; PMID:25952732; http://dx.doi.org/ 10.1002/eji.201445057 [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F et al.. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295(5562):2097-100; PMID:11896281; http://dx.doi.org/ 10.1126/science.1068440 [DOI] [PubMed] [Google Scholar]
- 4.Liston A, Gray DHD. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol 2014; 14(3):154-65; PMID:24481337; http://dx.doi.org/ 10.1038/nri3605 [DOI] [PubMed] [Google Scholar]
- 5.Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, Cutler C, Ho VT, Alyea EP, Antin JH et al.. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest 2010; 120(5):1479-93; PMID:20389017; http://dx.doi.org/24907378 10.1172/JCI41072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immun 2014; 192(12):5451-8; PMID:24907378; http://dx.doi.org/ 10.4049/jimmunol.1490019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao W, Lin JX, Leonard Warren J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013; 38(1):13-25; PMID:23352221; http://dx.doi.org/ 10.1016/j.immuni.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012; 12(3):180-90; PMID:22343569; http://dx.doi.org/ 10.1038/nri3156 [DOI] [PubMed] [Google Scholar]
- 9.Mohty M, Kuentz M, Michallet M, Bourhis JH, Milpied N, Sutton L, Jouet JP, Attal M, Bordigoni P, Cahn JY et al.. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation: long-term results of a randomized study. Blood 2002; 100(9):3128-34; PMID:12384409; http://dx.doi.org/ 10.1182/blood.V100.9.3128 [DOI] [PubMed] [Google Scholar]
- 10.Ringden O, Pavletic SZ, Anasetti C, Barrett AJ, Wang T, Wang D, Antin JH, Di Bartolomeo P, Bolwell BJ, Bredeson C et al.. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood 2009; 113(13):3110-8; PMID:19059878; http://dx.doi.org/ 10.1182/blood-2008-07-163212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallett WH, Ames E, Alvarez M, Barao I, Taylor PA, Blazar BR, Murphy WJ. Combination therapy using IL-2 and anti-CD25 results in augmented natural killer cell-mediated antitumor responses. Biol Blood Marrow Transplantation 2008; 14(10):1088-99; PMID:18804038; http://dx.doi.org/ 10.1016/j.bbmt.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K et al.. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 2014; 123(25):3855-63; PMID:24719405; http://dx.doi.org/22129252 10.1182/blood-2013-10-532531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verneris MR. Natural killer cells and regulatory T cells: how to manipulate a graft for optimal GVL. ASH Educ Program Book 2013; 2013(1):335-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, Armand P, Cutler C, Ho VT, Treister NS et al.. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Eng J Med 2011; 365(22):2055-66; PMID:22129252; http://dx.doi.org/ 10.1056/NEJMoa1108188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu MF, Liu H, Melenhorst J, Barrett AJ, Ito S, Foster A et al.. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res 2014; 20(8):2215-25; PMID:24573552; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koreth J, Kim HT, Jones KT, Lange PB, Reynolds CG, Chammas MJ, Dusenbury K, Whangbo J, Nikiforow S, Alyea EP 3rd et al.. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft vs. host disease. Blood 2016; 128(1):130-7; PMID:27073224; http://dx.doi.org/24577531 10.1182/blood-2016-02-702852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imanguli MM, Cowen EW, Rose J, Dhamala S, Swaim W, Lafond S, Yagi B, Gress RE, Pavletic SZ, Hakim FT. Comparative analysis of FoxP3+ regulatory T Cells in the target tissues and blood in chronic graft versus host disease. Leukemia 2014; 28(10):2016-2027; PMID:24577531; http://dx.doi.org/ 10.1038/leu.2014.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, Bellucci R, Alyea EP, Antin JH, Soiffer RJ et al.. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 2005; 106(8):2903-11; PMID:15972448; http://dx.doi.org/ 10.1182/blood-2005-03-1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 2009; 126(4):458-65; PMID:19278419; http://dx.doi.org/ 10.1111/j.1365-2567.2008.03027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang XJ, Chang YJ, Zhao XY. Maintaining hyporesponsiveness and polarization potential of T cells after in vitro mixture of G-CSF mobilized peripheral blood grafts and G-CSF primed bone marrow grafts in different proportions. Transpl Immunol 2007; 17(3):193-7; PMID:17331846; http://dx.doi.org/18275899 10.1016/j.trim.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 21.Chang YJ, Zhao XY, Huang XJ. Effects of the NK cell recovery on outcomes of unmanipulated haploidentical blood and marrow transplantation for patients with hematologic malignancies. Biol Blood Marrow Transplantation 2008; 14(3):323-34; PMID:18275899; http://dx.doi.org/ 10.1016/j.bbmt.2007.12.497 [DOI] [PubMed] [Google Scholar]
- 22.Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, McFarland H, Henkart PA, Martin R. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA 2006; 103(15):5941-6; PMID:16585503; http://dx.doi.org/25085354 10.1073/pnas.0601335103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kheav VD, Busson M, Scieux C, Peffault de Latour R, Maki G, Haas P, Mazeron M-C, Carmagnat M, Masson E, Xhaard A et al.. Favorable impact of natural killer cell reconstitution on chronic graft-versus-host disease and cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation. Haematologica 2014; 99(12):1860-1867; PMID:25085354; http://dx.doi.org/ 10.3324/haematol.2014.108407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 2003; 101(8):3052-7; PMID:12480696; http://dx.doi.org/ 10.1182/blood-2002-09-2876 [DOI] [PubMed] [Google Scholar]
- 25.Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, Kelsall B, Trinchieri G, Sher A. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 2012; 36(6):1047-59; PMID:22749354; http://dx.doi.org/ 10.1016/j.immuni.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao XS, Jin S, Zhu HH, Xu LP, Liu DH, Chen H, Liu KY, Huang XJ. Wilms' tumor gene 1 expression: an independent acute leukemia prognostic indicator following allogeneic hematopoietic SCT. Bone Marrow Transplant 2012; 47(4):499-507; PMID:21643023; http://dx.doi.org/ 10.1038/bmt.2011.121 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, Fan ZP, Wu DP, Huang XJ et al.. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood 2015; 125(25):3956-62; PMID:25940714; http://dx.doi.org/ 10.1182/blood-2015-02-627786 [DOI] [PubMed] [Google Scholar]
- 28.Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, Wang FR, Han W, Sun YQ, Yan CH et al.. Controlled, randomized, open-label trial of risk-stratified corticosteroid prevention of acute graft-versus-host disease after haploidentical transplantation. J Clin Oncol 2016; 34(16):1855-63; PMID:27091717; http://dx.doi.org/ 10.1200/JCO.2015.63.8817 [DOI] [PubMed] [Google Scholar]
- 29.Lai YR, Chen YH, Hu DM, Jiang M, Liu QF, Liu L, Hou J, Schwarzenberger P, Li QC, Zhang ZM et al.. Multicenter phase II study of a combination of cyclosporine a, methotrexate and mycophenolate mofetil for GVHD prophylaxis: results of the Chinese Bone Marrow Transplant Cooperative Group (CBMTCG). J Hematol Oncol 2014; 7:59; PMID:25139202; http://dx.doi.org/ 10.1186/s13045-014-0059-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao L, Zhang C, Gao L, Liu Y, Su Y, Wang S, Li B, Yang T, Yuan Z, Zhang X. Favorable outcome of haploidentical hematopoietic stem cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia: a multicenter study in Southwest China. J Hematol Oncol 2015; 8:90; PMID:26208715; http://dx.doi.org/ 10.1186/s13045-015-0186-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, Chen YH, Wang FR, Sun YQ, Tang FF et al.. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant 2014; 49(3):426-33; PMID:24292519; http://dx.doi.org/ 10.1038/bmt.2013.191 [DOI] [PubMed] [Google Scholar]
- 32.Zhao XS, Yan CH, Liu DH, Xu LP, Liu YR, Liu KY, Qin YZ, Wang Y, Huang XJ. Combined use of WT1 and flow cytometry monitoring can promote sensitivity of predicting relapse after allogeneic HSCT without affecting specificity. Annals Hematol 2013; 92(8):1111-9; PMID:23680867; http://dx.doi.org/ 10.1007/s00277-013-1733-1 [DOI] [PubMed] [Google Scholar]
- 33.Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, Han W, Wang Y, Qin YZ, Huang XJ. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012; 119(14):3256-62; PMID:22337715; http://dx.doi.org/ 10.1182/blood-2011-09-380386 [DOI] [PubMed] [Google Scholar]
- 34.Yan CH, Wang JZ, Liu DH, Xu LP, Chen H, Liu KY, Huang XJ. Chemotherapy followed by modified donor lymphocyte infusion as a treatment for relapsed acute leukemia after haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion: superior outcomes compared with chemotherapy alone and an analysis of prognostic factors. Eur J Haematol 2013; 91(4):304-14; PMID:23837640; http://dx.doi.org/ 10.1111/ejh.12168 [DOI] [PubMed] [Google Scholar]
- 35.Kong Y, Chang YJ, Wang YZ, Chen YH, Han W, Wang Y, Sun YQ, Yan CH, Wang FR, Liu YR et al.. Association of an impaired bone marrow microenvironment with secondary poor graft function after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplantation 2013; 19(10):1465-73; PMID:23879970; http://dx.doi.org/ 10.1016/j.bbmt.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 36.Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, Chen H, Wang FR, Mo XD, Zhang YY et al.. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol 2015; 8:84; PMID:26156584; http://dx.doi.org/ 10.1186/s13045-015-0182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, Blazar BR, MacMillan ML, Weisdorf DJ. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 2015; 125(8):1333-8; PMID:25593335; http://dx.doi.org/ 10.1182/blood-2014-10-609032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18(4):295-304; PMID:4153799; http://dx.doi.org/ 10.1097/00007890-197410000-00001 [DOI] [PubMed] [Google Scholar]
- 39.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D et al.. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplantation 2005; 11(12):945-56; PMID:16338616; http://dx.doi.org/ 10.1016/j.bbmt.2005.09.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.