ABSTRACT
Although nivolumab is associated with a significant improvement in overall survival and progression-free survival, only 20 to 40% of patients experience long-term benefit. It is therefore of great interest to identify a predictive marker of clinical benefit for nivolumab. To address this issue, the frequencies of CD4+ T cell subsets (Treg, Th1, Th2, Th9, Th17 and Th22), CD8+ T cells, and serum cytokine levels (IFNγ, IL-4, IL-9, IL-10, TGF-β) were assessed in 46 patients with melanoma. Eighteen patients responded to nivolumab, and the other 28 patients did not. An early increase in Th9 cell counts during the treatment with nivolumab was associated with an improved clinical response. Before the first nivolumab infusion, the responders displayed elevated serum concentrations of TGF-β compared to non-responders. Th9 induction by IL-4 and TGF-β was enhanced by PD-1/PD-L1 blockade in vitro. The role of IL-9 in disease progression was further assessed using a murine melanoma model. In vivo IL-9 blockade promoted melanoma progression in mice using an autochthonous mouse melanoma model, and the cytotoxic ability of murine melanoma-specific CD8+ T cells was enhanced in the presence of IL-9 in vitro. These findings suggest that Th9 cells, which produce IL-9, play an important role in the successful treatment of melanoma patients with nivolumab. Th9 cells therefore represent a valid biomarker to be further developed in the setting of anti-PD-1 therapy.
KEYWORDS: Anti-PD-1 antibody, melanoma, nivolumab, Th9
Introduction
Effective immune checkpoint blockades have improved the overall survival of patients with metastatic melanoma. The monoclonal antibody nivolumab blocks programmed cell death 1 (PD-1), an inhibitory immune checkpoint receptor expressed on activated T cells.1 Nivolumab is associated with a significant improvement in overall survival and progression-free survival, and 20 to 40% of patients experience long-term benefit.1-3 Although the expression of programmed cell death 1 ligand 1 (PD-L1) in tumor cells has been associated with responsiveness to the blockade of this immune checkpoint, 4 the objective measurement of PD-L1 protein levels reveals heterogeneity within tumors and prominent interassay variability or discordance.5 The pharmacodynamic biomarkers of nivolumab, however, remain unknown to date. It is therefore of great interest to find a predictive marker of clinical benefit for nivolumab and a parameter that can be validated as a surrogate marker of response or survival benefit.
Recently, interleukin (IL)-9-producing CD4+ T helper cells (Th9) have been identified as a new subset of CD4+ T helper cells mediating both proinflammatory events and the induction of tolerance.6,7 Although Th1 cells, which produce interferon (IFN)-γ, have a clear role in cancer immune surveillance and in promoting antitumor responses, 8 reports on the role of Th9 cells in tumor development remain contradictory.9 For instance, IL-9 is known to promote the proliferation, migration, and adhesion of human lung cancer cells, 10 but IL-9 seems to have the opposite effect on melanoma proliferation and migration in the B16 melanoma murine model.11
Herein, we analyzed the immunological profile of peripheral blood in patients receiving nivolumab treatment in order to identify clinically useful biomarkers. We discovered that Th9 cells but not Th1 cells are increased in melanoma patients who were successfully treated with nivolumab. Using the autochthonous mouse melanoma model, we found that IL-9 treatment suppressed melanoma progression and increased granzyme B and perforin in CD8+ T cells. We therefore propose that elevated levels of Th9 cells may represent a valid biomarker in the setting of anti-PD-1 therapy. Our data support the idea that boosting IL-9 in itself might be a therapeutic avenue.
Results
Th9 cell frequency is increased in responders to nivolumab treatment
Forty-six melanoma patients who received nivolumab were prospectively included in this study (Table 1). The group contained 18 males and 28 females. The median age of the patients was 66 y (ranging from 34 to 89 y). Patients were divided into two groups, responders (SD, PR, and CR) and non-responders (PD) to nivolumab treatment. Eighteen patients responded to the treatment, and the other 28 patients did not. We found no difference in the total number of whole blood cells and lymphocytes, and in serum lactate dehydrogenase levels between responders and non-responders (Table S1).
Table 1.
Characteristics of 46 melanoma patients.
| All patients n = 46 | CR n = 1 | PR n = 3 | SD n = 14 | PD n = 28 | |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 28 | 1 | 1 | 12 | 14 |
| Male | 18 | 0 | 2 | 2 | 14 |
| Age | 67 (34–89) | 83 | 72 (68–79) | 69 (57–78) | 65 (34–89) |
| Stage | |||||
| I | 0 | 0 | 0 | 0 | 0 |
| II | 11 | 0 | 0 | 5 | 6 |
| III | 21 | 1 | 3 | 7 | 10 |
| IV | 14 | 0 | 0 | 2 | 12 |
Forty-six patients participated. The clinical response was defined as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD), based on response evaluation criteria in solid tumors (RECIST, v1.1).
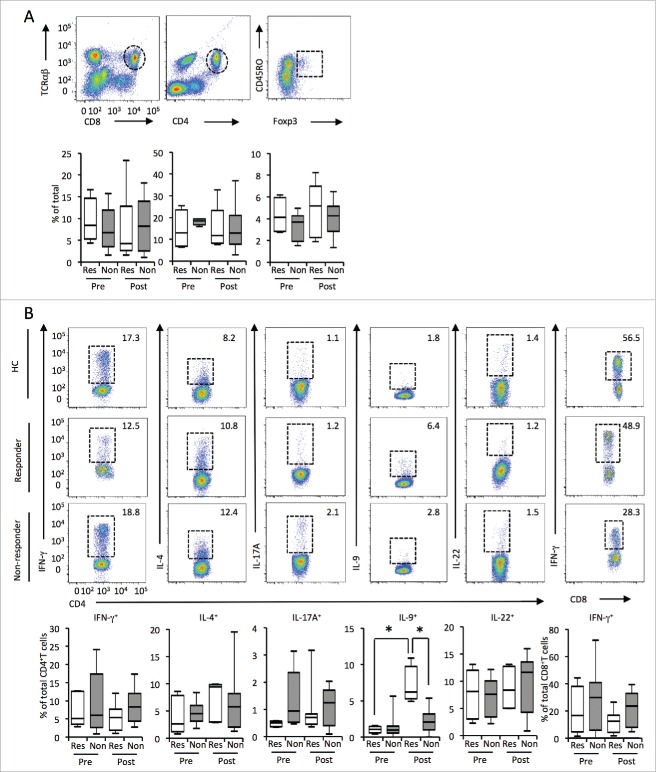
To investigate the candidates for biomarkers related to treatment response, we first compared the frequencies of CD8+ T cells, CD4+ T cells, and Tregs in the peripheral blood between responders and non-responders. We found no significant differences among these cell populations before and after treatment (Fig. 1A). We further compared CD4+ T cell subsets in responders and non-responders. In addition to Th1 and Th2 subsets, we investigated Th9, Th17, and Th22 subsets since recent studies showed that these Th subsets might also play some roles in tumor immunity.12-14 Although there was no significant difference in Th1, Th2, Th17, and Th22 cells, Th9 cells were significantly increased in the responder group (Fig. 1B). IFNγ-producing CD8+ T cell numbers before and after nivolumab treatment were comparable between responders and non-responders (Fig. 1B). These results suggest that Th9 cells may play some role in nivolumab-induced antitumor immunity.
Figure 1.
The Th9 frequency in the peripheral blood was increased in responders after nivolumab treatment. (A) Flow cytometry (top) of the PBMCs from patients before (pre) and after (post) treatment with nivolumab. The frequency of CD4+ T cells, CD8+ T cells, and Tregs in the PBMCs of patients assessed by flow cytometry. (B) The frequency of Th1, Th1, Th17, Th9, Th22, and cytotoxic T lymphocyte in the PBMCs of patients as in (A), assessed by flow cytometry. Small horizontal lines indicate the mean (± SD). *p < 0.05 (two-tailed Student's t-test).
Since Th9 cells can be generated in the presence of TGF-β and IL-4, 15 we next evaluated serum profiles between responders and non-responders by ELISA. Indeed, serum TGF-β levels were significantly higher in responders than in non-responders before nivolumab treatment (Fig. 2A). Serum IL-4 levels were below the detection limit of ELISA. However, more IL-4-producing CD4+ T cells were detected following nivolumab treatment in responder and non-responder patients (Fig. 2B). We found no difference in IFNγ levels between responders and non-responders before treatment (responders; 2.9 ± 4.2 pg/mL, non-responders; 5.8 ± 16.7 pg/mL, mean ± SD) or after treatment (responders; 7.7 ± 11.3 pg/mL, non-responders; 2.7 ± 7.6 pg/mL, mean ± SD). In addition, serum IL-9, IL-10, and IL-17 levels were below the limit of detection of ELISA in both groups (IL-4 < pg/mL, IL-9 <1 pg/mL, IL-10 <2 pg/mL, IL-17 <15 pg/mL).
Figure 2.
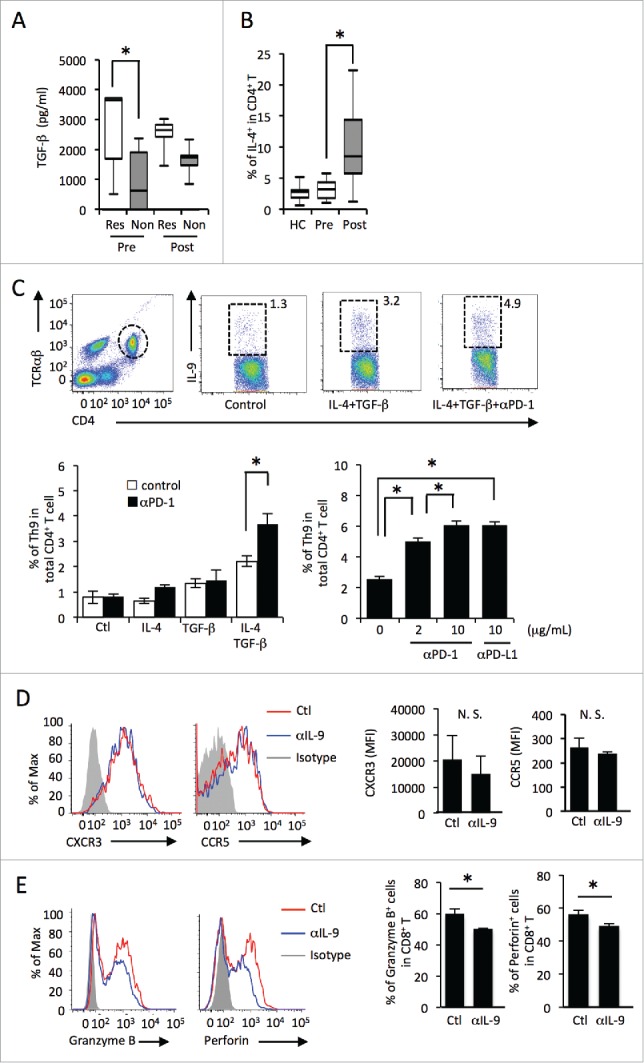
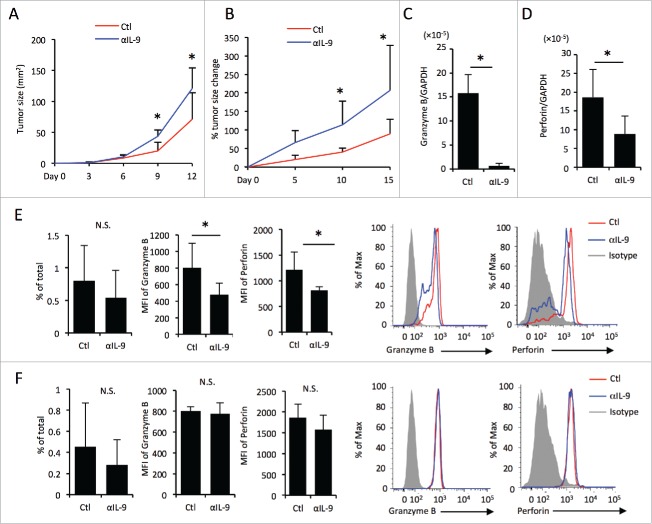
PD-1/PD-L1 blockade promotes Th9 differentiation by IL-4 and TGF-β. (A) Serum levels of TGF-β from melanoma patients (“Res” representing responders, “Non” non-responders) before (pre) and after (post) treated with nivolumab evaluated by ELISA. (B) The frequency of IL-4-producing Th2 cells in the peripheral blood of healthy controls (HC), melanoma patients before treatment (pre), and those after treatment (post). (C) Th9 differentiation assay by IL-4 and TGF-β. The frequency in CD4+ T cells was analyzed by flow cytometry. (D) The histogram shows the expression levels of CXCR3 and CCR5 on CD8+ T cells in the presence or absence of anti-IL-9 neutralizing antibody. The bar graph shows MFI levels of CXCR3 and CCR5 on CD8+ T cells. (E) The histogram shows the expression levels of granzyme B and perforin in CD8+T cells in the presence or absence of anti-IL-9 neutralizing antibody. The bar graph shows the frequency of granzyme B or perforin positive cells out of CD8+ T cells.
PD-1/PD-L1 blockade promotes Th9 differentiation
Our data showed that Th9 cells and serum TGF-β levels are increased in responders to nivolumab treatment. We therefore hypothesized that nivolumab enhanced tumor immunity by promoting Th9 differentiation. To this end, we evaluated the effect of anti-PD-1 antibody on Th9 induction in vitro. Human PBMCs were stimulated with recombinant IL-4 and recombinant TGF-β in the presence or absence of anti-PD-1 blocking antibody. After 48 h of stimulation, the frequency of Th9 cells was evaluated by flow cytometry. IL-4 and TGF-β-induced Th9 differentiation was enhanced by anti-PD-1 antibody in a dose-dependent manner (Fig. 2C). In addition, anti-PD-L1 antibody also enhanced Th9 differentiation (Fig. 2C). These results suggest that PD-1 signaling blockade by nivolumab may promote IL-4 and TGF-β-dependent Th9 differentiation.
Anti-IL-9 neutralizing antibody downregulates granzyme B and perforin expression in CD8+ T cells in vitro
Since our data suggest that Th9 cells may promote antitumor immunity, we next investigated the effects of IL-9 on immune cells. A previous report demonstrated that Th9 cells promote the recruitment of dendritic cells (DCs) to the tumor tissues.11 We hypothesized that Th9 cells also promoted the recruitment of CD8+ T cells to melanoma tissues. We evaluated the effect of IL-9 on the expression levels of chemokine receptors, which are responsible for CD8+ T cells. Because CXCR3 and CCR5 are reportedly important chemokine receptors for the recruitment of T cells to melanoma,16,17 we investigated the expression levels of these receptors on CD8+ T cells cultured with or without anti IL-9 neutralizing antibody. However, we found that CXCR3 and CCR5 expression on CD8+ T cells was not affected by anti-IL-9 neutralizing antibody (Fig. 2D).
Granzyme B and perforin are cytolytic molecules produced by cytotoxic CD8+ T cells, and they have activity against a variety of tumors.18 We therefore evaluated next the effect of IL-9 on granzyme B and perforin expression in CD8+ T cells in vitro. Human PBMCs were cultured with or without anti-IL-9 neutralizing antibody, and their expression levels were evaluated analyzed via flow cytometry. The expression levels of granzyme B and perforin in CD8+ T cells were reduced in the presence of anti-IL-9 neutralizing antibody (Fig. 2E). These results suggest that IL-9 promotes the expression of granzyme B and perforin in CD8+ T cells.
In vivo IL-9 blockade augments tumor progression in melanoma-bearing mice
To investigate the effect of IL-9 on anti-melanoma immunity in vivo, we next evaluated melanoma progression through the administration of anti-IL-9 neutralizing antibody to melanoma-bearing mice. We found that anti-IL-9 neutralizing antibody administration promoted tumor progression in a melanoma cell line, the B16 melanoma cell line injection model (Fig. 3A).
Figure 3.
In vivo IL-9 blockade promotes melanoma progression in mice. Tumor growth of the B16 melanoma injection model (A) and Braf/Pten mutation model (B). (A) The tumor size of B16 melanoma injection model was evaluated by length and width (mm2). (B) The change (%) in tumor size of Braf/Pten mutation model based on the size at day 0. (C, D) mRNA expression levels of granzyme B (C) and perforin (D) in the melanoma tissues of Braf/Pten mutation mice with or without injection of anti-IL-9 neutralizing antibody were evaluated by RT-PCR. (E, F) The flow cytometric analysis of CD8+T cells (E) and NK cells (F) infiltrating into the melanoma lesion (Braf/Pten mutation model). The histograms show the MFI levels of granzyme B and perforin in CD8+ T cells (E) and NK cells (F). The bar graphs indicate the frequency in the total cell number of CD8+ T cells (E) and NK cells (F), and the MFI levels of granzyme B and perforin in CD8+ T cells (E) and NK cells (F). The histograms show representative data.
The subcutaneous inoculation of B16 cells mirrors human disease development poorly because tumor cells are an artificially inoculated and so it has low immunogenicity.19 We next used the Baf/Pten autochthonous mouse melanoma model, in which melanoma develops de novo within the murine skin.20 Consistent with the B16 injection model, the administration of anti-IL-9 neutralizing antibody also promoted tumor progression in the Braf/Pten model (Fig. 3B, Table S2).
To exclude the possibility that IL-9 directly inhibits melanoma progression but does not modulate tumor immunity, we next performed a tumor proliferation assay. B16 melanoma cells were cultured with or without recombinant murine IL-9. We found that there was no significant difference in the proliferation of B16 cells between the two groups (Fig. S1). These results support the notion that IL-9 suppresses melanoma progression via immune modulation.
IL-9 blockade leads to the downregulation of granzyme B and perforin in CD8+ T cells but not in NK cells in mice
To further investigate the mechanism of IL-9, we used the Braf/Pten melanoma model and analyzed the immune cells infiltrating into the tumor in mice treated with or without anti-IL-9 neutralizing antibody. First, the expression of granzyme B and perforin in the whole melanoma tissues was investigated by means of real-time polymerase chain reaction (RT-PCR). We found that granzyme B and perforin expression were reduced in mice treated with anti-IL-9 neutralizing antibody (Figs. 3C and D), suggesting that IL-9 promotes the expression of granzyme B and perforin in the melanoma tissues. Since both CD8+ T cells and NK cells produce granzyme B and perforin, we next analyzed the effect of IL-9 on granzyme B and perforin expression in CD8+ T cells and NK cells by flow cytometry. We already demonstrated that the expression levels of chemokine receptor responsible for tissue infiltration were not changed by anti-IL-9 treatment with human samples (Fig. 2D), suggesting that lymphocytes infiltration into the skin is not regulated by IL-9. Consistent with the above findings, there was no significant difference in the frequency of CD8+ T cells (Fig. 3E, left) or NK cells (Fig. 3F, left) infiltrating into murine melanoma tissues treated with or without anti-IL-9 antibody. Next, we evaluated the expression levels of granzyme B and perforin in CD8+ T cells and NK cells in melanoma tissues. The mean fluorescence intensity (MFI) levels of granzyme B and perforin in CD8+ T cells were significantly lower after IL-9 blockade (Fig. 3E, right), whereas MFI levels of granzyme B and perforin in NK cells were unaltered (Fig. 3F, right). These results suggest that IL-9 causes an increase in granzyme B and perforin in tumor-infiltrating CD8+ T cells.
IL-9 enhances cytotoxicity of tumor-specific mouse CD8+ T cells
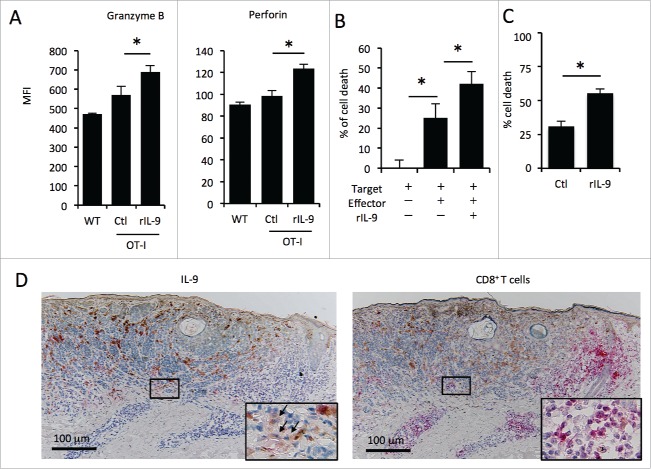
We evaluated the effect of IL-9 on the cytotoxic ability of tumor specific CD8+ T cells in vitro. First, we co-cultured B16 murine melanoma cells stably transduced with OVA (termed MO4 cells) and whole lymph node cells from OT-I mice (which have OVA-specific CD8+ T cells), in the presence or absence of recombinant IL-9. The MFI levels of granzyme B and perforin in CD8+ T cells increased significantly after recombinant IL-9 exposure (Fig. 4A). In addition, the cytotoxic assay showed that immune cells from inguinal lymph nodes of MO4 tumor-bearing OT-I mice killed melanoma cells more effectively when cultured with recombinant IL-9 (Fig. 4B). Furthermore, we prepared purified CD8+ T cells from inguinal lymph nodes of MO4 tumor-bearing OT-I mice, and confirmed that the tumor-specific cytotoxic ability of CD8+ T cells increased by recombinant IL-9 (Fig. 4C), while IL-9 did not directly affect the proliferation of B16 (Fig. S1). We next evaluated that the effect of IL-9 on the ability of proliferation and antigenicity of human melanoma cell lines using two human melanoma cell lines, A375 and SK-MEL-28. We found that IL-9 did not affect the proliferation of these cell lines (Fig. S2). The expression levels of HLA-ABC and HLA-DR were quantified by means of flow cytometry to evaluate the antigenicity. The expression of PD-L1 and IL-9 receptor (IL-9R) was also assessed to evaluate the effect of IL-9 on these cell lines. The results showed that IL-9 did not affect these expression levels (Fig. S2).
Figure 4.
The tumor-specific cytotoxicity of CD8+ T cells is enhanced in the presence of IL-9 in vitro. (A, B) The effect of IL-9 on tumor-specific cytotoxicity was evaluated by means of cytotoxic assay in the presence or absence of rIL-9, using MO4 cells as target cells and lymph node cells from tumor-bearing OT-I mice or C57B6/N mice (WT) as effector cells. (A) The MFI levels of granzyme B and peroforin in CD8+ T cells after the assay analyzed by flow cytometry. (B) The bar graph shows the % of target cell death. (C) The effect of IL-9 on the cytotoxic ability of tumor-specific CD8+ T cells. (D) Immunohistochemistry analysis of IL-9 (left) and CD8+ (right) in the human melanoma tissues. The figure shows the representative data (patient #8).
IL-9 is highly expressed in human melanoma lesions
Finally, we assessed 10 melanoma samples by immunohistochemistry to evaluate the localization of IL-9+ cells and CD8+ T within the tumor before nivolumab treatment. We analyzed sequential sections for these two stains using 10 samples selected from both responders and non-responders. All samples showed that high IL-9 expression and CD8+ T cell infiltration were observed in the peritumoral lesion (Fig. 4D, Fig. S3). These findings suggest that IL-9 may be related to some extent to antitumor immunity by CD8+ T cells in the lesional area of human melanoma.
Discussion
In this study, we demonstrated that Th9 cells in peripheral blood were significantly increased in the responders to nivolumab treatment. In addition, the serum level of TGF-β, which contributes to the development of Th9, was significantly higher in responders compared to non-responders before nivolumab treatment. Moreover, anti-PD-1 antibody enhanced Th9 differentiation in vitro. Using melanoma-bearing mice, we showed that anti-IL-9 antibody decreased granzyme B and perforin in CD8+ T cells. Furthermore, in vitro experiments revealed that IL-9 enhanced the cytotoxic ability of tumor-specific CD8+ T cells in mice. Finally, we demonstrated that IL-9-positive cells existed near CD8+ T cells in human melanoma tissues. These results suggest that Th9 cells may play an essential role in anti-melanoma immunity and that anti-PD-1 antibody may elicit an antitumor effect through upregulating Th9 differentiation in melanoma patients successfully treated with nivolumab.
This is the first report showing that Th9 cells in peripheral blood can be a pharmacodynamic biomarker for nivolumab efficacy in melanoma patients. The literature suggests the diverse effects of anti-PD-1 antibody treatment on antitumor immunity. Anti-PD-1 antibody caused an increase in the frequency of CD8+ T cells in the melanoma lesion of patients who responded to the treatment.21,22 In addition, anti-PD-1 antibody mediates antitumor effects through augmented T cell proliferation, increased IFNγ, and IFNγ inducible chemokine production at the tumor site.23 We also demonstrated that nivolumab increases Th9 cells both in vivo and in vitro. It has been reported that the PD-1 blockade in itself enhanced T cell migration into the tumor lesion,23 and our study proposes the possibility that nivolumab may promote antitumor immunity in the melanoma lesion via CD8+ T cell activation by Th9 cells. This is supported by our observation that IL-9+ cells and CD8+ T cells co-localized in melanoma lesion. We are currently working to evaluate whether IL-9+ cells are increased in the melanoma lesion in responders using available samples, such as in-transit metastases.
Recent studies have shown that genetic markers might be useful as biomarkers for the effectiveness of immune checkpoint blockades in the treatment of melanoma.24-26 However, no serum cytokines have been reported as useful and effective biomarkers. We demonstrated that serum TGF-β levels were significantly higher in responders compared to non-responders before nivolumab treatment. Our study therefore suggests that TGF-β may also be a good biomarker for nivolumab treatment. Several human cancer cells express high levels of TGF-β, which influences the microenvironment and promotes tumor growth, invasiveness, and metastases.27 Increased expression and secretion of TGF-β in melanoma cell lines have been reported.28,29 Interestingly, the expression levels of TGF-β in melanoma are different in among melanoma cell lines and patient disease stages.29,30 Although the mechanisms that caused high serum TGF-β levels in responders remain unclear in our study, one hypothesis is that TGF-β expression may be related to mutations in the melanoma cells. In human hepatocellular carcinoma, Kras mutation deregulates the TGF-β signaling pathway.31 Above all, the differences in TGF-β expression in melanoma may have a genetic basis. Further studies are needed to investigate the relationship between TGF-β expression and mutation burden.
The role of Th9 cells has remained controversial in tumor immunity. It has been reported that IL-9 acts directly to drive tumor growth and contributes to the establishment of an immunosuppressive environment.9 For example, IL-9 promotes the proliferation of human lymphoid tumors, such as Hodgkin's lymphoma, diffuse large B-cell lymphoma, and NK T-cell lymphoma.32-34 Furthermore, IL-9 is known to inhibit adaptive immunity and promote tumor progression using colon carcinoma and breast cancer in mice.9 Other studies have suggested the beneficial roles of IL-9 in preventing tumor progression through a multivariate effector response.11,12 In this study, we observed an antitumor effect of IL-9 against melanoma in two different murine melanoma models, the B16 injection model and the Braf/Pten model. This is the first report demonstrating the beneficial roles of IL-9 for tumor immunity using the autochthonous mouse melanoma Braf/Pten model. Consistent with our study, some recent reports refer to the importance of IL-9 and Th9 cells in suppressing melanoma progression. For example, one report showed that exogenous rIL-9 inhibited melanoma growth in Rag1−/− mice but not in mast-cell-deficient mice, suggesting that mast cells are essential for IL-9-mediated antitumor immunity.12 Another report demonstrated that Th9 cells elicited strong cytotoxic T cell responses by promoting the recruitment of DCs to the tumor tissues.11 As for the origin of Th9 cells, one study showed that IL-1β induced Th9 cells, which in turn exerted potent anticancer functions in an interferon regulatory factor 1 (IRF1)- and IL-21-dependent manner.35 Our study proposes a new mechanism whereby IL-9 directly enhances tumor-specific cytotoxic activity of CD8+ T cells by increasing their levels of granzyme B and perforin.
In addition, we observed that PD-1/PD-L1 blockade promotes Th9 differentiation in the present study. Several studies report that PD-1 modulates the metabolic program of T cells.36 For example, PD-1 ligation is known to prevent T cell development by altering metabolic reprogramming of cells.37 Since glycolytic activation is required for the differentiation of Th9 cells,38 the PD-1/PD-L1 blockade might promote Th9 differentiation by modulating the cell metabolism. Further investigation is required on this subject.
In conclusion, we present data to show that the frequency of Th9 cells can serve as a pharmacodynamic biomarker for anti-PD-1 therapy. Of note, we found an increase in Th9 cells after the third infusion of nivolumab and provided evidence that Th9 cells promote anti-melanoma immunity. Therefore, we propose that Th9 cells may represent a biomarker to be further developed in the setting of anti-PD-1 therapy.
Materials and methods
Patients, treatment, and clinical evaluation
This observational immunomonitoring study included 46 metastatic melanoma patients receiving nivolumab (Ono Pharmaceutical) at Kyoto University Hospital and other collaborating hospitals in Japan. This study was approved by the ethic committee of the Kyoto University Graduate School of Medicine (R0251). Patients were included if they (i) had a confirmed diagnosis of stage IV melanoma according to the 2009 American Joint Committee on Cancer (AJCC) melanoma staging and classification, (ii) were alive 12 weeks after the first nivolumab perfusion, and (iii) were receiving at least four courses of nivolumab over 90 min at a dose of 2 mg/kg of body weight every 3 weeks.39 Other inclusion criteria were: at least 20 y of age and no specific melanoma therapy during the previous 28 d. All histological types of melanoma, including mucosal and uveal melanoma, were eligible for inclusion. Exclusion criteria were the presence of an autoimmune disease, HIV, hepatitis B or C, pregnancy, or concomitant systemic therapy or any history of prior immunotherapy for melanoma. Treatment efficacy was assessed using contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography-CT (PET-CT) after the third nivolumab infusion and clinical response was defined based on response evaluation criteria in solid tumors (Response Evaluation Criteria in Solid Tumors, Version 1.1 (RECIST, v1.1)). A clinical response was defined as complete response (CR), partial response (PR), or stable disease (SD).
Collection of human samples and analysis of serum
Peripheral blood was taken one to 7 d before the first nivolumab infusion (pre) and within one to 3 weeks after the third infusion (post). In most cases, nivolumab was administered every 3 weeks in Japan in conformity to the national health insurance and thus the PBMCs after treatment were obtained from 10 to 12 weeks after the first infusion. Peripheral blood mononuclear cells (PBMCs) were obtained from venous blood anticoagulated with EDTA by density gradient centrifugation using lymphocyte separation solution (Nacalai Tesque). Isolated cells were cryopreserved in Bambanker (Nippon Genetics) at −80°C or washed with FACS buffer (PBS containing BSA and sodium azide) for flow cytometry as previously described.40,41 Serum levels of IFNγ, IL-4, IL-9, IL-10, IL-17, and transforming growth factor (TGF)-β were measured by enzyme-linked immunosorbent assay (ELISA) kits for quantitative detection of each cytokine (eBioscience, San Diego). All measurements were made in duplicate and mean values were obtained.
Analysis of peripheral blood samples
The following fluorescent-labeled monoclonal antibodies were used for surface or intracellular staining: TCRαβ-PerCP/Cy5.5, CD8+-Pacific Blue, CD45RO-PE, CD193 (CCR3)-APC/Cy7, IFNγ-FITC, IL-17A-Brilliant-Violet421, perforin-APC (from BioLegend), TCRαβ-PE/Cy7, CD4+-eFluor450, CD184 (CXCR4)-APC, IL-4-PE, IL-9-PerCP, IL-22-APC, Foxp3-FITC (from eBioscience), and granzyme B-FITC (from BD Biosciences). For intracellular staining of cytokines, PBMCs were stimulated for 3 h with BD Leukocyte Activation Cocktail (Ionomycin, Brefeldin A, and phorbol myristic acetate (PMA)) in the presence of BD GolgiStop. To detect intracellular protein, cells were permeabilized, fixed, and stained according to the manufacturer's instructions using the Cytofix/Cytoperm kit (BD Biosciences) for cytoplasmic targets or the Foxp3 Staining Buffer Set (eBioscience, San Diego, CA) for the nuclear target FoxP3. Tregs were defined as FOXP3+CD45RO+CD4+TCRαβ+ T cells.
Acquisition was performed by eight-color flow cytometry using FACS Fortessa with FACS Diva software (both from BD Biosciences). The compensation control was performed with BD CompBeads (BD Biosciences). FlowJo software (Tree Star) was used for analysis. Data were expressed as dot plots.
Mouse melanoma model
C57B6/N mice (8 weeks old) were obtained from Shimizu Laboratory Supplies, Kyoto, Japan. B6.Cg-Braftm1Mmcm Ptentm1Hwu Tg (Tyr-cre/ERT2) 13Bos/BosJ (Braf/Pten) mice 20 (8–12 weeks old) were obtained from Jackson Laboratories, Bar Harbor, Maine. B16F10 (B16) murine melanoma cell lines 42 were commercially purchased from ATCC. The B16 murine melanoma cell line stably transduced with OVA (termed MO4 43) was obtained from the Dana Farber Cancer Institute (Boston, MA). The cell lines were maintained in complete DMEM supplemented with 10% FCS/10 mM Hepes/100 units/mL penicillin/100 mg/mL streptomycin/2 mM L-glutamine (Invitrogen) at 5% CO2.
B16 melanoma cells were injected into C57/B6N mice subcutaneously at each lateral region by 10 × 5 cells in 100 μL of PBS per place. The tumor volume was measured every 3 d. Braf/Pten mice were treated topically with 10 μL of 1.9 mg/mL (5 mM) 4-Hydroxytamoxifen (4-HT, 70% Z-isomer, Sigma) in acetone at 6 to 8 weeks of age on both ears, the abdomen, and back. The tumor size (length × width, in mm2) was first measured at week 2 of tamoxifen treatment and every subsequent 5 d. Change in tumor size was expressed as percentage change compared to the first measurement. Anti-IL-9 neutralizing antibody (9C1, Bio X Cell, NH, USA) administration was started one day before the application of tamoxifen. It was injected intraperitoneally at 200 μg per mouse every 3 d for 2 weeks. Braf/Pten mice receiving control IgG or α-IL-9 every 2 d were analyzed on day 15 followed by a previous study using IL-9-neutralized melanoma mice.11
Th9 differentiation assay
PBMCs obtained as mentioned above from 10 healthy donors were cultured in round-bottom 96-well plates and stimulated with anti-CD3 antibody (0.5 μg/mL, BioLegend) or anti-CD3/CD28 beads (4 μL/well, Thermo Fisher Scientific). Th9 cell induction with or without anti-PD-1 antibody (2 μg/mL, 10 μg/mL, or 50 μg/mL, J105, eBioscience) or anti-PD-L1 antibody (10 μg/mL, B7H1, BioLegend) was assessed by flow cytometry after 72 h of culture with recombinant IL-4 (10 ng/mL, BioLegend), recombinant TGF-β (10 ng/mL, BioLegend), both, or neither as control.
Human IL-9 blocking assay and mouse cytotoxicity assays with recombinant IL-9
PBMCs from healthy donors were cultured with anti-CD3 antibody (0.5 μg/mL, BioLegend) for 48 h with or without anti-IL-9 neutralizing antibody (10 ng/mL, MH9D1, BioLegend). The expression levels of CXCR3, CCR5, Granzyme B, and Perforin of CD8+ T cells were evaluated by flow cytometry. Mouse T cell cytotoxicity with or without recombinant IL-9 was assayed using a Cytotox 96 nonradioactive kit (Promega) following the instructions provided.
OT-I mice, MHC class I-restricted TCR transgenic line specific for ovalbumin (OVA),44 were inoculated with 1 × 106 MO4 tumor cells into the skin of both lateral regions. On day 10 after tumor inoculation, draining lymph nodes (inguinal) were excised, mashed, and washed with RPMI in order to obtain single-cell suspensions. Purified CD8+ T cells were obtained from the lymph node cells using CD8a+ T cell isolation kit (Miltenyi Biotec KK, Tokyo, Japan). Whole lymph node cells or purified CD8+ T cells were plated as the effector cells in 96-well plates at the effector/target ratio of 10/1 using 5 × 103 MO4 target cells per well in RPMI lacking phenol red for 4 h at 37°C. Lactate dehydrogenase release was subsequently assessed by incubation of the supernatants with the provided substrate for 30 min and the absorbance was read at 490 nm using a Thermomax plate reader (Molecular Devices). Percentage cytotoxicity was calculated as follows: (experimental effectorspontaneous − target spontaneous/targetmaximum − target spontaneous) × 100. All cytotoxicity assays were reproducible in at least three separate assays.
Immunohistochemistry
Ten paraffinized human primary melanoma samples from 10 melanoma patients were cut into 5-μm-thick sections. Their clinical information is shown in Table S3. Antigens were retrieved by boiling in citrate buffer, pH 6.0, using a microwave. Non-specific binding of immunoglobulin G was blocked by normal goat serum (Vector Laboratories, Burlingame, CA). The sections were incubated with rabbit anti-IL-9 antibody (polyclonal, Abcam, Tokyo, Japan) overnight at 4°C. Then, they were incubated with biotinylated goat-anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA). Secondary antibodies were visualized using the Vectastain ABC-AP kit (Vector Laboratories, Burlingame, CA).
Statistical analysis
Unless otherwise indicated, data are presented as the means ± standard deviation (SD) and are a representative of three independent experiments. p-values were calculated with the two-tailed Student's t-test or one-way analysis of variance (ANOVA) followed by the Dunnett multiple comparison test. p-values less than 0.05 were considered to be statistically significant and are denoted by asterisks (*) in the figures.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Ms. Hiromi Doi and Ms. Kaori Tomari for technical assistance.
Funding
This work was supported in part by the Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research (15H05790), Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research on Innovative Areas (15H1155), Japan Society for the Promotion of Science, Grant-in-Aid for challenging Exploratory Research (15K15417), Japan Science and Technology Agency, Precursory Research for Embryonic Science and Technology (PRESTO) (16021031300), and Japan Agency for Medical Research and Development (AMED) (16ek0410011h0003, 16he0902003h0002).
References
- 1.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E et al.. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320-30; PMID:25399552; http://dx.doi.org/ 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD et al.. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32:1020-30; PMID:24590637; http://dx.doi.org/ 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116:1757-66; PMID:20143437; http://dx.doi.org/ 10.1002/cncr.24899 [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelekanou V, Rehman J, Velcheti V, Herbst R, LoRusso P, Rimm DL. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol 2016; 2:46-54; PMID:26562159; http://dx.doi.org/ 10.1001/jamaoncol.2015.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC et al.. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol 2008; 9:1347-55; PMID:18997793; http://dx.doi.org/ 10.1038/ni.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol 2008; 9:1341-6; PMID:18931678; http://dx.doi.org/ 10.1038/ni.1659 [DOI] [PubMed] [Google Scholar]
- 8.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006; 6:836-48; PMID:17063185; http://dx.doi.org/ 10.1038/nri1961 [DOI] [PubMed] [Google Scholar]
- 9.Hoelzinger DB, Dominguez AL, Cohen PA, Gendler SJ. Inhibition of adaptive immunity by IL9 can be disrupted to achieve rapid T-cell sensitization and rejection of progressive tumor challenges. Cancer Res 2014; 74:6845-55; PMID:25297635; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-0836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushita K, Arima N, Ohtsubo H, Fujiwara H, Hidaka S, Fukumori J, Tanaka H. Frequent expression of interleukin-9 mRNA and infrequent involvement of interleukin-9 in proliferation of primary adult T-cell leukemia cells and HTLV-I infected T-cell lines. Leuk Res 1997; 21:211-6; PMID:9111165; http://dx.doi.org/ 10.1016/S0145-2126(96)00109-9 [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Hong S, Li H, Park J, Hong B, Wang L, Zheng Y, Liu Z, Xu J, He J, et al.. Th9 cells promote antitumor immune responses in vivo. J Clin Invest 2012; 122:4160-71; PMID:23064366; http://dx.doi.org/ 10.1172/JCI65459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK et al.. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med 2012; 18:1248-53; PMID:22772464; http://dx.doi.org/ 10.1038/nm.2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuang DM, Xiao X, Zhao Q, Chen MM, Li XF, Liu RX, Wei Y, Ouyang FZ, Chen DP, Wu Y et al.. B7-H1-expressing antigen-presenting cells mediate polarization of protumorigenic Th22 subsets. J Clin Invest 2014; 124:4657-67; PMID:25244097; http://dx.doi.org/ 10.1172/JCI74381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009; 31:787-98; PMID:19879162; http://dx.doi.org/ 10.1016/j.immuni.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol 2009; 183:7169-77; PMID:19890056; http://dx.doi.org/ 10.4049/jimmunol.0901906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedognetti D, Spivey TL, Zhao Y, Uccellini L, Tomei S, Dudley ME, Ascierto ML, De Giorgi V, Liu Q, Delogu LG et al.. CXCR3/CCR5 pathways in metastatic melanoma patients treated with adoptive therapy and interleukin-2. Br J Cancer 2013; 109:2412-23; PMID:24129241; http://dx.doi.org/ 10.1038/bjc.2013.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Martin A, Mira E, Manes S. CCR5 in cancer immunotherapy: More than an “attractive” receptor for T cells. Oncoimmunology 2012; 1:106-8; PMID:22720226; http://dx.doi.org/ 10.4161/onci.1.1.17995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 2015; 15:388-400; PMID:25998963; http://dx.doi.org/ 10.1038/nri3839 [DOI] [PubMed] [Google Scholar]
- 19.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med 2004; 200:771-82; PMID:15381730; http://dx.doi.org/ 10.1084/jem.20041130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 2009; 41:544-52; PMID:19282848; http://dx.doi.org/ 10.1038/ng.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568-71; PMID:25428505; http://dx.doi.org/ 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E, Seja E, Kivork C, Siebert J, Kaplan-Lefko P et al.. PD-1 Blockade Expands Intratumoral Memory T Cells. Cancer Immunol Res 2016; 4:194-203; PMID:26787823; http://dx.doi.org/ 10.1158/2326-6066.CIR-15-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizée G, Radvanyi L et al.. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res 2012; 72:5209-18; PMID:22915761; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS et al.. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371:2189-99; PMID:25409260; http://dx.doi.org/ 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Church SE, Galon J. Tumor microenvironment and immunotherapy: the whole picture is better than a glimpse. Immunity 2015; 43:631-3; PMID:26488814; http://dx.doi.org/ 10.1016/j.immuni.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 26.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Geukes Foppen MH, Goldinger SM et al.. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015; 350:207-11; PMID:26359337; http://dx.doi.org/ 10.1126/science.aad0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leivonen SK, Kahari VM. Transforming growth factor-β signaling in cancer invasion and metastasis. Int J Cancer 2007; 121:2119-24; PMID:17849476; http://dx.doi.org/ 10.1002/ijc.23113 [DOI] [PubMed] [Google Scholar]
- 28.Albino AP, Davis BM, Nanus DM. Induction of growth factor RNA expression in human malignant melanoma: markers of transformation. Cancer Res 1991; 51:4815-20; PMID:1716514 [PubMed] [Google Scholar]
- 29.Javelaud D, Alexaki VI, Mauviel A. Transforming growth factor-beta in cutaneous melanoma. Pigment Cell Melanoma Res 2008; 21:123-32; PMID:18426405; http://dx.doi.org/ 10.1111/j.1755-148X.2008.00450.x [DOI] [PubMed] [Google Scholar]
- 30.Van Belle P, Rodeck U, Nuamah I, Halpern AC, Elder DE. Melanoma-associated expression of transforming growth factor-beta isoforms. Am J Pathol 1996; 148:1887-94; PMID:8669474 [PMC free article] [PubMed] [Google Scholar]
- 31.Ye H, Zhang C, Wang BJ, Tan XH, Zhang WP, Teng Y, Yang X. Synergistic function of Kras mutation and HBx in initiation and progression of hepatocellular carcinoma in mice. Oncogene 2014; 33:5133-8; PMID:24213574; http://dx.doi.org/ 10.1038/onc.2013.468 [DOI] [PubMed] [Google Scholar]
- 32.Merz H, Houssiau FA, Orscheschek K, Renauld JC, Fliedner A, Herin M, Noel H, Kadin M, Mueller-Hermelink HK, Van Snick J et al.. Interleukin-9 expression in human malignant lymphomas: unique association with Hodgkin's disease and large cell anaplastic lymphoma. Blood 1991; 78:1311-7; PMID:1908723 [PubMed] [Google Scholar]
- 33.Lv X, Feng L, Fang X, Jiang Y, Wang X. Overexpression of IL-9 receptor in diffuse large B-cell lymphoma. Int J Clin Exp Pathol 2013; 6:911-6; PMID:23638223 [PMC free article] [PubMed] [Google Scholar]
- 34.Nagato T, Kobayashi H, Kishibe K, Takahara M, Ogino T, Ishii H, Oikawa K, Aoki N, Sato K, Kimura S et al.. Expression of interleukin-9 in nasal natural killer/T-cell lymphoma cell lines and patients. Clin Cancer Res 2005; 11:8250-7; PMID:16322282; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-1426 [DOI] [PubMed] [Google Scholar]
- 35.Vegran F, Berger H, Boidot R, Mignot G, Bruchard M, Dosset M, Chalmin F, Rébé C, Dérangère V, Ryffel B et al.. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat Immunol 2014; 15:758-66; PMID:24973819; http://dx.doi.org/ 10.1038/ni.2925 [DOI] [PubMed] [Google Scholar]
- 36.Ho PC, Liu PS. Metabolic communication in tumors: a new layer of immunoregulation for immune evasion. J Immunother Cancer 2016; 4:4; PMID:26885366; http://dx.doi.org/ 10.1186/s40425-016-0109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P et al.. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 2015; 6:6692; PMID:25809635; http://dx.doi.org/ 10.1038/ncomms7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Bi Y, Chen X, Li C, Li Y, Zhang Z, Wang J, Lu Y, Yu Q, Su H et al.. Histone deacetylase SIRT1 negatively regulates the differentiation of interleukin-9-producing CD4(+) T cells. Immunity 2016; 44:1337-49; PMID:27317260; http://dx.doi.org/ 10.1016/j.immuni.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 39.Dummer R, Schadendorf D, Ascierto PA, Larkin J, Lebbe C, Hauschild A. Integrating first-line treatment options into clinical practice: what's new in advanced melanoma? Melanoma Res 2015; 25:461-9; PMID:26426764; http://dx.doi.org/ 10.1097/CMR.0000000000000200 [DOI] [PubMed] [Google Scholar]
- 40.Huang D, Tani M, Wang J, Han Y, He TT, Weaver J, Charo IF, Tuohy VK, Rollins BJ, Ransohoff RM. Pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 overexpression in mice. J Neurosci 2002; 22:10633-42; PMID:12486156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elhofy A, Wang J, Tani M, Fife BT, Kennedy KJ, Bennett J, Huang D, Ransohoff RM, Karpus WJ. Transgenic expression of CCL2 in the central nervous system prevents experimental autoimmune encephalomyelitis. J Leukoc Biol 2005; 77:229-37; PMID:15539456; http://dx.doi.org/ 10.1189/jlb.0804465 [DOI] [PubMed] [Google Scholar]
- 42.Kreider JW, Schmoyer ME. Spontaneous maturation and differentiation of B16 melanoma cells in culture. J Natl Cancer Inst 1975; 55:641-7; PMID:1159842 [DOI] [PubMed] [Google Scholar]
- 43.Falo LD Jr, Kovacsovics-Bankowski M, Thompson K, Rock KL. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat Med 1995; 1:649-53; PMID:7585145; http://dx.doi.org/ 10.1038/nm0795-649 [DOI] [PubMed] [Google Scholar]
- 44.Clarke SR, Barnden M, Kurts C, Carbone FR, Miller JF, Heath WR. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol Cell Biol 2000; 78:110-7; PMID:10762410; http://dx.doi.org/ 10.1046/j.1440-1711.2000.00889.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.