Abstract
Certain abnormalities in behavioral performance and neural signaling have been attributed to a deficit of visual attention in amblyopia, a neurodevelopmental disorder characterized by a diverse array of visual deficits following abnormal binocular childhood experience. Critically, most have inferred attention's role in their task without explicitly manipulating and measuring its effects against a baseline condition. Here, we directly investigate whether human amblyopic adults benefit from covert spatial attention—the selective processing of visual information in the absence of eye movements—to the same degree as neurotypical observers. We manipulated both involuntary (Experiment 1) and voluntary (Experiment 2) attention during an orientation discrimination task for which the effects of covert spatial attention have been well established in neurotypical and special populations. In both experiments, attention significantly improved accuracy and decreased reaction times to a similar extent (a) between the eyes of the amblyopic adults and (b) between the amblyopes and their age- and gender-matched controls. Moreover, deployment of voluntary attention away from the target location significantly impaired task performance (Experiment 2). The magnitudes of the involuntary and voluntary attention benefits did not correlate with amblyopic depth or severity. Both groups of observers showed canonical performance fields (better performance along the horizontal than vertical meridian and at the lower than upper vertical meridian) and similar effects of attention across locations. Despite their characteristic low-level vision impairments, covert spatial attention remains functionally intact in human amblyopic adults.
Keywords: visual attention, endogenous and exogenous attention, amblyopia, strabismus, anisometropia, orientation discrimination
Introduction
Amblyopia (from the Greek amblos, blunt, and opia, vision), a neurodevelopmental disorder of spatial vision (Levi & Carkeet, 1993; Thompson, Chung, Kiorpes, Ledgeway, & McGraw, 2015), continues to be the leading cause of monocular vision loss in adults aged 20–70 years with an estimated prevalence ranging from 2% to 5% in the Western adult population (von Noorden, 1990). Individuals with amblyopia exhibit especially deficient perception in one eye, the “amblyopic” eye, compared to the other one, the “fellow” eye. Rather than a single clinical malady with a specific organic cause, it can arise from any condition that prevents one or both eyes from focusing clearly or from having a normal binocular interaction during a critical period, which manifests in the stunted development of early visual cortex (Daw, 1998). The amblyopic eye has impaired contrast sensitivity relative to neurotypical observers (Baker, Simard, Saint-Amour, & Hess, 2015; Kiorpes, 2006; Levi, 2006; McKee, Levi, & Movshon, 2003), and even the fellow eye of amblyopes has degraded contrast sensitivity compared to the eyes of neurotypical observers (Chatzistefanou et al., 2005; Koskela, 1986; Leguire, Rogers, & Bremer, 1990). Amblyopia is often categorized into four subtypes, depending on etiology: (a) strabismic, or “lazy eye,” wherein the brain suppresses visual input from a deviated eye to prevent diplopia; (b) anisometropic, caused by a large interocular difference in refractive error; (c) deprivation, which emerges when there is a literal interruption of visual input, often due to congenital cataracts, ptosis, or corneal haziness; and (d) mixed, in which the amblyope suffers from a combination of one or more of these conditions (Levi, 2006; Levi & Carkeet, 1993; von Noorden, 1990; von Noorden & Crawford, 1978).
The clinical characterization of amblyopia is typically a unilateral reduction in optotype (Snellen) acuity despite optimal optical correction (de Zarate & Tejedor, 2007; von Noorden, 1990). This and other low-level visual deficits, such as decreased contrast sensitivity and positional and grating acuity, are related to differences in the spatial properties and binocularity of neurons in the primary striate cortex of amblyopes, which may reliably differ according to their depth (a measure of the interocular difference), severity, and subtype (for reviews, see Kiorpes, 2006; Kiorpes & McKee, 1999; Levi, 2006; McKee et al., 2003). A myriad of human and animal studies have reported that amblyopes also show higher level visual impairments (for reviews, see Asper, Crewther, & Crewther, 2000a, 2000b; Kiorpes, 2006; Kiorpes & McKee, 1999; Levi, 2006; Levi, Knill, & Bavelier, 2015). These deficits include enhanced crowding as well as problems with stimulus localization, contour integration, texture and second-order pattern perception, shape discrimination, motion sensitivity, stereopsis, eye movements, and oculomotor coordination. Although typically characterized as a foveal visual disorder, amblyopia-related deficits and their neural markers are present across the visual field, including the perifovea (Bankó, Körtvélyes, Németh, & Vidnyánszky, 2014; Ho et al., 2006; Hou, Kim, Lai, & Verghese, 2016; Katz, Levi, & Bedell, 1984).
There is not a parsimonious model of amblyopia to explain the diversity of its symptomology (for reviews, see Levi, 2013; Wong, 2012). Several hypotheses that have linked sensory losses to presumptive neurophysiological abnormalities partially account for visual losses. For example, the idea of “undersampling” by the amblyopic brain relies on a reduction of V1 neurons driven by the amblyopic eye at particular spatial scales (Levi, Klein, & Sharma, 1999); “positional jitter” could manifest as uncalibrated topographical and wiring scatter (Hess & Field, 1994); signal attenuation in amblyopic V1 neurons may underlie reduced contrast sensitivity (Baker, Meese, & Hess, 2008). Furthermore, amblyopic eyes show increased additive internal noise and deficient perceptual templates psychophysically (Baker et al., 2008; C. Huang, Tao, Zhou, & Lu, 2007; R. W. Li, Klein, & Levi, 2008; R. W. Li & Levi, 2004; Xu, Lu, Qiu, & Zhou, 2006) as well as abnormal binocular interactions (Hess & Thompson, 2015; C.-B. Huang, Zhou, Lu, Feng, & Zhou, 2009; C.-B. Huang, Zhou, Lu, & Zhou, 2011), wherein input from the amblyopic eye is not weighted as highly as that from the fellow eye, leading to a reduction in binocularly driven neurons in V1.
Interestingly, quantitative analyses show that the extent of neural abnormalities in V1 cannot explain the full range of visual deficits in amblyopia (Bi et al., 2011; Kiorpes, Kiper, O'Keefe, Cavanaugh, & Movshon, 1998; Kiorpes & McKee, 1999; Kiorpes & Movshon, 2004; Shooner et al., 2015). This may be due, at least in part, to a reduced strength of amblyopic eye input to higher level areas (Anderson, Holliday, & Harding, 1999; Anderson & Swettenham, 2006). Thus, whereas V1 anomalies may be at the root of amblyopic impairments, they are likely to be amplified by the progressive degradation of feed-forward neural signals in the dorsal and ventral pathways (Barnes, Hess, Dumoulin, Achtman, & Pike, 2001; Choi et al., 2001; Conner, Odom, Schwartz, & Mendola, 2007; El-Shamayleh, Kiorpes, Kohn, & Movshon, 2010; Goodyear, Nicolle, Humphrey, & Menon, 2000; Ho & Giaschi, 2009; Imamura et al., 1997; Kiorpes, 2006; Kiorpes et al., 1998; Kiorpes & Movshon, 1996; Levi, 2006; X. Li, Dumoulin, Mansouri, & Hess, 2007; Muckli et al., 2006; Secen, Culham, Ho, & Giaschi, 2011; Shooner et al., 2015; Sincich, Jocson, & Horton, 2012). Indeed, several studies have shown reduced levels of activation for amblyopes than neurotypical observers as far downstream as parietal and ventral temporal cortex (Ho & Giaschi, 2009; Hyvarinen, Hyvarinen, & Linnankoski, 1981; Lerner et al., 2006; Secen et al., 2011; review by Anderson & Swettenham, 2006).
Previous studies of attention in amblyopia
In addition to the neuroimaging evidence, amblyopes have demonstrated psychophysical deficits in a few higher level tasks—e.g. the attentional blink (Popple & Levi, 2008), numerosity estimation (Sharma, Levi, & Klein, 2000), and multiple object tracking (Ho et al., 2006; Tripathy & Levi, 2008).1 These deficits may be a consequence of the anomalous visual input amblyopes receive during development, which leads to abnormal visual processing. However, the authors attributed their results to a deficit of visual attention in amblyopia, inferring its contribution to their tasks without directly manipulating it. Indeed, only two studies have used attentional cues to investigate voluntary attention in human strabismic amblyopes: Sharma et al. (2000) assessed its behavioral effects on numerosity estimation, and Hou et al. (2016) explored the underlying neural correlates in a contrast detection task.
Sharma et al. (2000) cued observers to the quadrant of the visual field for which they were asked to estimate the number of vertically oriented Gabors among an array of horizontal distractors with 80% validity. They found that amblyopes significantly underestimated the number of targets when using their amblyopic eye compared to the nonamblyopic eye and controls across all cueing conditions, which they argue to be evidence for a “high-level deficit.” Note that even though their amblyopes do exhibit a robust and reliable endogenous attention effect (valid vs. invalid cueing) of the same magnitude as that shown by visually intact observers, this study is often cited as one of the primary studies providing evidence for a visual attention deficit in amblyopia (Farzin & Norcia, 2011; Ho et al., 2006; Hou et al., 2016; Levi, 2013; Levi & Tripathy, 2006; McKee, Levi, Schor, & Movshon, 2016; Secen et al., 2011; Tripathy & Levi, 2008).
In the first study to directly investigate the underlying neural correlates of voluntary visual attention in strabismic amblyopia, Hou et al. (2016) used fMRI-informed EEG source imaging to measure the amplitudes of steady-state visual evoked potentials (SSVEPs) as observers were cued (100% validity) to voluntarily attend to one hemifield at a time during a contrast change detection task. Overall, SSVEP amplitudes corresponding to visual inputs from both eyes of the amblyopes were significantly reduced relative to visually intact controls. Further, attentional modulation of SSVEP amplitudes corresponding to visual input from both eyes was reduced in areas hV4 and hMT; however, reduced attentional modulation was found in V1 only for SSVEP amplitudes corresponding to visual input from the amblyopic eye. The authors concluded that these differences in neural signaling reflect a deficit of attentional modulation in the visual cortex.
Current study
This is the first investigation of both exogenous (involuntary, stimulus-driven) and endogenous (voluntary, goal-driven) visual attention with amblyopic observers. Here, we explicitly investigate the effects of covert spatial attention—the selective processing of visuospatial information without eye movements (Carrasco, 2011, 2014; Posner, 1980)—with human amblyopic adults. In two separate psychophysical experiments, we used peripheral and central attentional cueing to directly manipulate both exogenous (Experiment 1) and endogenous (Experiment 2) attention. Although their perceptual consequences are often the same, they can differ according to the specific task demands and test stimuli (for reviews, see Carrasco & Barbot, 2015; Carrasco & Yeshurun, 2009); both increase contrast sensitivity (Cameron, Tai, & Carrasco, 2002; Carrasco, Penpeci-Talgar, & Eckstein, 2000; Dosher & Lu, 2000a, 2000b; Herrmann, Montaser-Kouhsari, Carrasco, & Heeger, 2010; Ling & Carrasco, 2006a; Liu, Pestilli, & Carrasco, 2005; Lu & Dosher, 1998, 2000; Pestilli & Carrasco, 2005; Pestilli, Ling, & Carrasco, 2009), enhance spatial resolution (Carrasco, Loula, & Ho, 2006; Carrasco, Williams, & Yeshurun, 2002; Golla, Ignashchenkova, Haarmeier, & Thier, 2004; Montagna, Pestilli, & Carrasco, 2009), accelerate the rate of visual information processing (Carrasco, Giordano, & McElree, 2006; Carrasco & McElree, 2001; Giordano, McElree, & Carrasco, 2009), and even change the subjective appearance of objects (Abrams, Barbot, & Carrasco, 2010; Anton-Erxleben, Abrams, & Carrasco, 2010; Anton-Erxleben, Herrmann, & Carrasco, 2013; Carrasco, Ling, & Read, 2004; Störmer, McDonald, & Hillyard, 2009) in neurotypical observers (for reviews, see Anton-Erxleben & Carrasco, 2013; Carrasco, 2011, 2014; Carrasco & Barbot, 2015).
Our group of human, amblyopic adults and their age- and gender-matched controls monocularly performed an orientation discrimination task that is contingent on contrast sensitivity and for which the effects of both types of covert spatial attention have been well established in both neurotypical observers (Cameron et al., 2002; Carrasco et al., 2000; Herrmann et al., 2010; Ling & Carrasco, 2006a; Liu et al., 2005; Pestilli & Carrasco, 2005; Pestilli et al., 2009) and special populations, such as adults with autism spectrum disorder (Grubb, Behrmann, Egan, Minshew, Carrasco, et al., 2013; Grubb, Behrmann, Egan, Minshew, Heeger et al., 2013). Importantly, we equated task performance in the neutral attention condition between all observers by adjusting stimulus contrast. This method has been used with other special populations with suboptimal vision; e.g., the elderly and individuals with Alzheimer's and Parkinson's disease (Amick, Cronin-Golomb, & Gilmore, 2003; Laudate et al., 2012). We compare task performance—accuracy and reaction times (RTs)—between both eyes of the amblyopes as well as to the “matched” eyes of controls as both the amblyopic and even the fellow eye have been shown to possess degraded visual abilities compared to neurotypical eyes (Chatzistefanou et al., 2005; Ho et al., 2005; Koskela, 1986; Leguire et al., 1990).
Because in most visual tasks discriminability drops (for reviews, see Carrasco & Barbot, 2015; Strasburger, Rentschler, & Jüttner, 2011) and speed of visual processing increases (Carrasco, McElree, Denisova, & Giordano, 2003) as stimuli are placed at increasing eccentricities, many studies of covert attention place stimuli at isoeccentric locations to equate task performance across stimulus locations (e.g., Cameron et al., 2002; Carrasco & McElree, 2001; Eckstein, 1998; Palmer, Verghese, & Pavel, 2000). However, there are also reliable and pronounced differences in performance accuracy (Abrams, Nizam, & Carrasco, 2012; Carrasco, Talgar, & Cameron, 2001; Corbett & Carrasco, 2011; Fuller & Carrasco, 2009; Rovamo, Virsu, Laurinen, & Hyvärinen, 1982) and speed of processing (Carrasco, Giordano, & McElree, 2004) around the visual field even when eccentricity is held constant. These stereotypical “performance fields” and their underlying neural correlates (Liu, Heeger, & Carrasco, 2006) include both a significant horizontal–vertical anisotropy (HVA; better performance along the horizontal compared to the vertical meridian, often driven by worse performance at the upper vertical meridian) and vertical meridian asymmetry (VMA; better performance in the lower visual field compared to the upper visual field along the vertical meridian). Exogenous attention improves performance to a similar extent across these isoeccentric locations (Cameron et al., 2002; Carrasco et al., 2001; Talgar & Carrasco, 2002). By placing the stimuli along the cardinal axes in Experiment 1, we investigated whether amblyopes demonstrate the canonical performance fields that have reliably been shown on a wide variety of visual tasks in neurotypical observers and whether the effect of exogenous attention changes as a function of location.
Experiment 1: Exogenous attention
Methods
Observers
Fourteen amblyopic adults (11 female; M age = 31.4 ± 11.9 years) and 14 age- and gender-matched control observers (11 female; M age = 30.8 ± 11.6 years) with normal or corrected-to-normal vision participated in this experiment (Table 1). All amblyopic observers were clinically diagnosed through medical examination by Dr. Theodore Smith, an ophthalmologist at the NYU Langone Medical Center, or by providing a verified medical record from an eye exam conducted within the last year by their personal eye doctor. Three potential amblyopic observers were excluded as they did not fulfill our inclusion criteria; they were unable to perform the task above chance with at least one of their eyes or could not fixate appropriately. All experimental procedures were approved by the University Committee on Activities Involving Human Subjects at New York University and were in agreement with the Declaration of Helsinki. All observers (except for author M. R., who participated as a control observer) were naive to the experimental hypotheses and signed written consent to participate in the study.
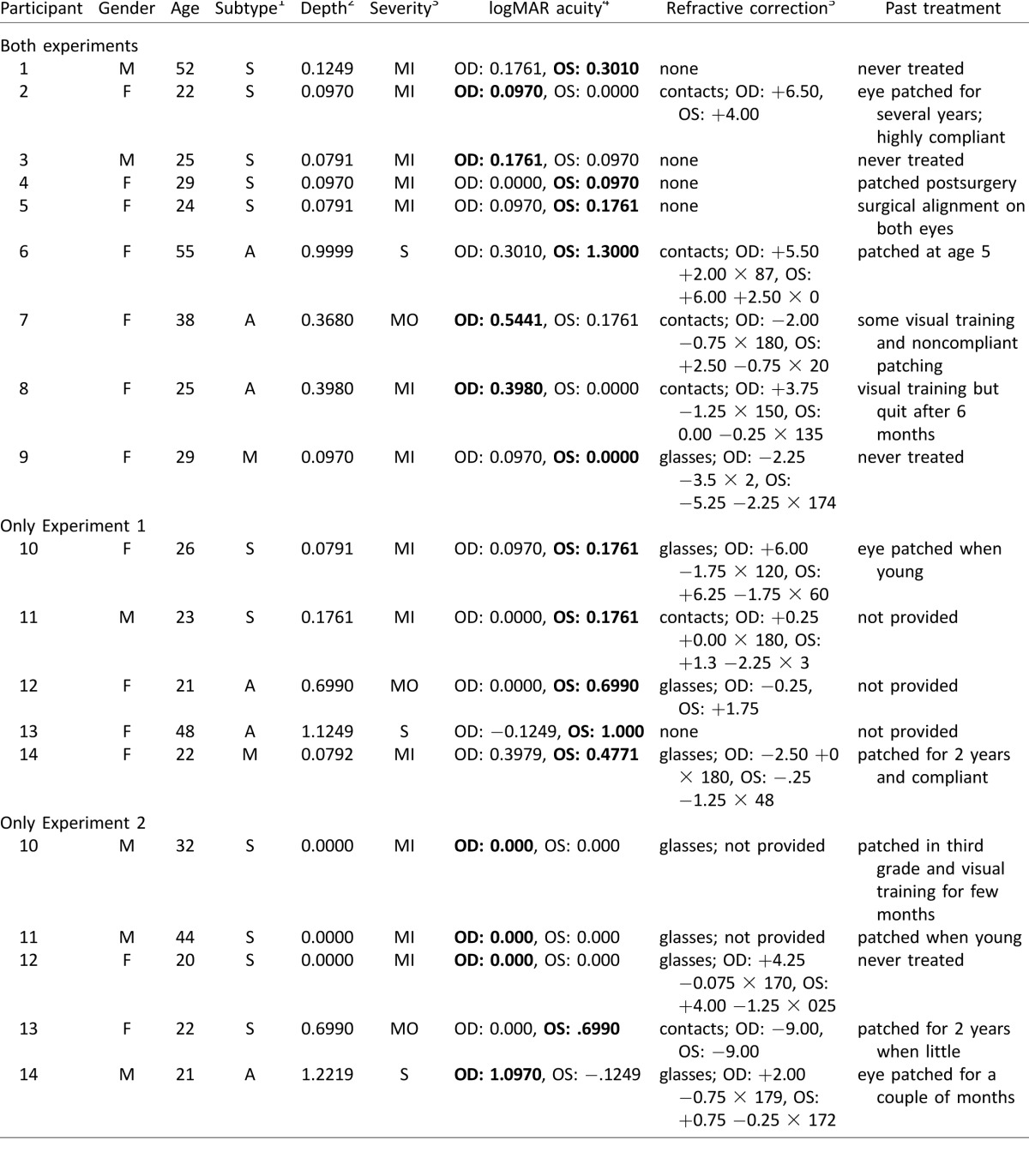
Table 1.
Clinical characteristics of individuals with amblyopia. Notes: 1S = strabismic, A = anisometropic, M = mixed; note that all mixed type amblyopes were both strabismic and anisometropic. 2Depth = (LogMAR VA amblyopic eye − LogMAR VA fellow eye) as calculated in Popple and Levi (2008). 3MI = mild, MO = moderate, S = severe; according to LogMAR VAcc (maximally corrected acuity) in amblyopic eye (table 3, Colenbrander, 2002). 4In VAcc if wears corrective lenses or VAsc (sans correction) if does not wear corrective lenses; oculus dextrus (OD) = right eye, oculus sinister (OS) = left eye; amblyopic eye in bold. 5All provided prescriptions are listed; some eye charts were missing values.
Apparatus and setup
Observers were tested in the same dimly lit, sound-attenuated room for both experiments. Stimuli were programmed on an Apple iMac MC413LL/A 21.5-in. desktop (3.06 GHz Intel Core 2 Duo) using MATLAB (MathWorks, Natick, MA) in conjunction with the MGL toolbox (http://gru.brain.riken.jp/mgl). They were presented at a viewing distance of 57 cm on a 21-in. IBM P260 CRT monitor (1280 × 960 pixel resolution, 90 Hz refresh rate), which had been calibrated and linearized using a Photo Research (Chatworth, CA) PR-650 SpectraScan Colorimeter. Observers performed the experiments using a forehead and chin rest that was affixed to the table to ensure head stabilization. Eye movements were monitored using an EyeLink 1000 Desktop Mount eye tracker (SR Research, Ontario, Canada); fixation breaks (eye movements ≥1° from the center of the fixation cross) during the trial sequence were considered fixation break trials and excluded from the analyses. Overall, we analyzed ∼93% of the data: 94% for control and 93% for amblyopes (amblyopic eye: 91%; fellow eye: 94%).
Stimuli
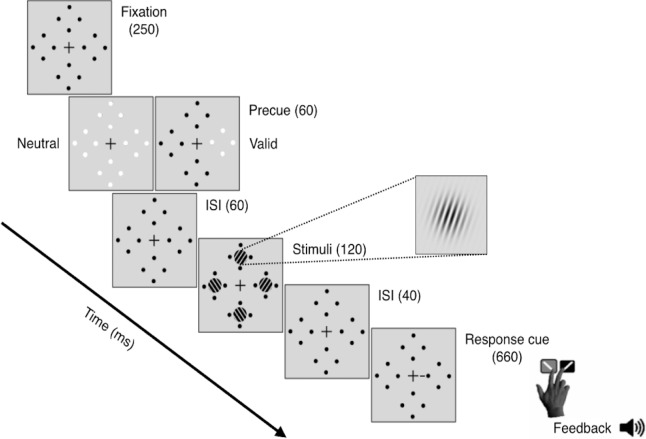
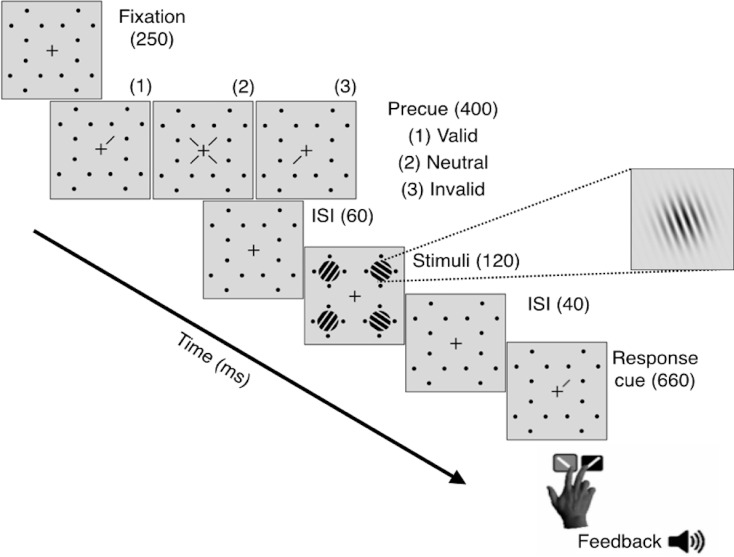
Observers were asked to fixate on a black, centrally placed cross (0.5° across) throughout the trial (Figure 1). Four placeholders—each comprised of four black dots (0.05° radius) concentrically arranged around the location of an upcoming Gabor patch stimulus (with 0.5° separation to prevent masking)—were constantly presented on the screen to reduce location uncertainty. The target and three distractor stimuli were all 3.2° in diameter, 4 c/° Gabor patches (contrast-defined sinusoidal gratings embedded in a Gaussian envelope, σ = 0.46°) randomly and independently tilted either ±20° from vertical, centered at 6.4° eccentricity along the cardinal axes and with the same mean luminance as the uniform gray background. To manipulate exogenous attention, either one (valid peripheral precue) or all four (neutral precue) placeholders grew in size (to 0.16° radius) and underwent a brief color change from black to white. The response cue (a 0.8° line placed 0.3° from the central fixation cross) indicated the target location by pointing to one placeholder (matching the peripheral precue location) and eliminated location uncertainty at the response time for both conditions.
Figure 1.
Trial sequence for Experiment 1.
Procedure
Observers performed the same 1-hr experimental procedure twice, once with each eye while the other was patched. On Day 1, amblyopes used their fellow, i.e., nonamblyopic, eye to ensure that they were able to see the stimuli and learn the task. The eyes of each control observer were arbitrarily labeled as amblyopic or fellow to match the sides of their amblyopic counterpart, i.e., if the left eye was amblyopic, we labeled the control observer's left eye as amblyopic. At the beginning of each experimental session, observers completed practice blocks (24 trials each, 100% stimulus contrast) until they could perform the task reliably above chance. When needed, we gave the amblyopes more training trials. At the end of the practice blocks, the amblyopes (amblyopic eye: M = 50%, SD = 31%; fellow eye: M = 50%, SD = 29%) still needed a higher contrast (p = 0.07) than the controls (“amblyopic eye”: M = 26%, SD = 30%; “fellow eye”: M = 33%, SD = 36%). Then, all observers underwent a staircase procedure (neutral cues only) to obtain their individual stimulus contrast threshold yielding 80% accuracy as both groups showed large individual variability. For each individual, the contrast of all Gabor patch stimuli was initially set at this contrast threshold value. Stimulus contrast was held constant throughout each block of the main experiment but automatically adjusted between blocks when necessary to maintain overall performance level. Observers completed ∼16 experimental blocks of 48 trials each—as many as possible across the two hour-long sessions—for a total of ∼768 trials: ∼96 trials for each one of the four target locations in each attention condition.
Task and trial sequence
Observers performed a two-alternative, forced-choice (2AFC) orientation discrimination task monocularly while exogenous spatial attention was manipulated via presentation of either a valid peripheral (50% of trials) or a neutral, distributed (50% of trials) precue (Figure 1). In every trial, observers were encouraged to respond as accurately as possible without time stress. In addition to the mandatory 1000-ms intertrial interval, trial initiation was contingent upon central fixation. After 250 ms, the valid or neutral precue was presented for 60 ms, followed by a brief interstimulus interval (ISI) of 60 ms. The 120-ms stimulus onset asynchrony between precue onset and stimulus was designed to optimize the attentional effects of the exogenous cue and prevent any voluntary deployment of attention (Carrasco, 2011; Muller & Rabbitt, 1989; Nakayama & Mackeben, 1989). After the interval, the target and distractor Gabor patches appeared simultaneously inside the placeholders for 120 ms. There was a brief 40-ms ISI between display offset and the response cue. An auditory tone indicated the beginning of the 5000-ms response window, in which observers had to report the target orientation (clockwise or counterclockwise relative to vertical) using one of two keyboard presses (“1” for clockwise, “2” for counterclockwise) with their right hand. Observer response terminated the response window. Auditory feedback was provided at the end of each trial, and visual feedback indicating observers' accuracy and number of fixation breaks was presented at the end of each block.
Results
Overall performance
Overall performance accuracy (percentage correct) was our primary dependent variable and median RT was our secondary dependent variable; we analyzed the median values because RTs do not follow a normal distribution (Whelan, 2008). We averaged performance accuracy and median RT for each experimental condition as well the mean stimulus contrast (one value per block) across all eight blocks. As some observers' amblyopic eyes were on the left and others were on the right, the data were labeled according to nasal/temporal coordinates. Finally, averages for each observer group (amblyopes and controls) were calculated separately for each eye.
Within group:
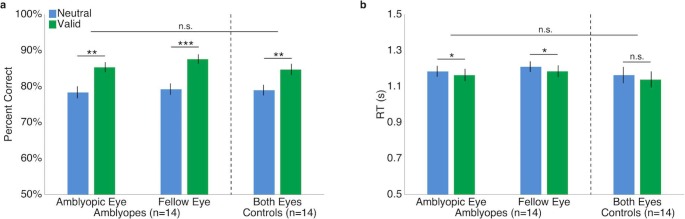
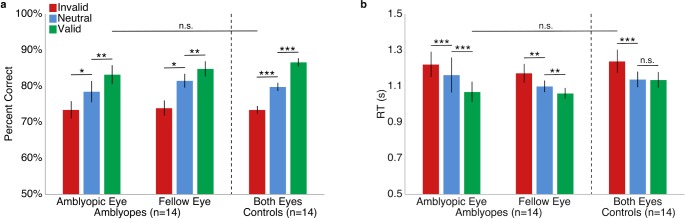
Repeated-measure (cue condition × eye) ANOVAs of accuracy within each group revealed no significant two-way interactions. There was a significant main effect of cue, amblyopes: F(1, 13) = 38.6, p < 0.001; controls: F(1, 13) = 20, p = 0.001, but not eye, amblyopes: F(1, 13) = 1.5, p > 0.1; controls: F(1, 13) < 1. Each group demonstrated significantly higher accuracy in the valid than the neutral cueing conditions; i.e., they exhibited the classic benefit of exogenous attention. Critically, Figure 2a illustrates that the same pattern of results was found for the amblyopic and the fellow eyes of the amblyopes. Note that we collapsed accuracy across eyes for the control group as they were arbitrarily labeled and the effects were the same.
Figure 2.
Performance in Experiment 1: Exogenous attention. (a) Accuracy. (b) RTs. For illustration purposes only as the eyes of control observers were arbitrarily labeled amblyopic or fellow, average performance for both eyes is plotted for controls but separately for each eye of the amblyopes. Error bars are ±1 SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
We performed the same statistical tests with average median RT measured relative to the display onset for correct trials. For the amblyopes, neither the two-way interaction nor the main effect of eye was significant, but there was a significant main effect of cue, with faster responses for valid than neutral cue trials, F(1, 13) = 9.1, p = 0.01 (Figure 2b). For controls, neither the interaction nor the main effects were significant. In sum, we ruled out any speed–accuracy trade-off.
Between groups:
To evaluate whether the magnitude of the effects significantly differed between the amblyopes and their age- and gender-matched controls, we conducted a three-way mixed design ANOVA on accuracy and another on RT. For both dependent variables, neither the interactions nor the main effect of group was significant (all ps > 0.1), indicating no difference between groups (Figure 2).
Effect of target location and performance fields
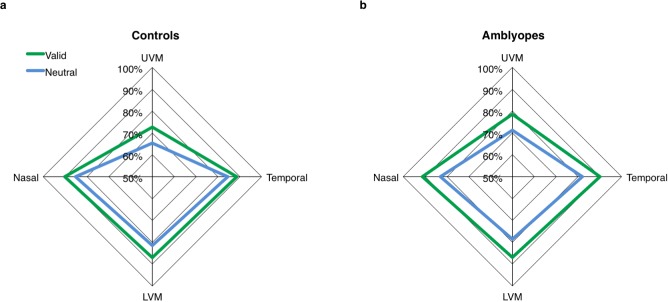
To evaluate whether amblyopes possess canonical performance fields, i.e., better performance at the horizontal than vertical meridians (HVA) and at the lower than the upper region of the vertical meridian (VMA), we tested whether both asymmetries significantly differed from zero (one-sample t tests). We averaged the data at the temporal and nasal locations to get overall percentage correct along the horizontal meridian (HM) and along the vertical meridian (VM). To calculate the observers' HVA, we subtracted performance along the VM from the HM, and to calculate their VMA, we subtracted performance along the upper minus the lower region.
In the amblyopes, we found significant main effects of target location in terms of both accuracy, F(3, 39) = 6.6, p = 0.001, and median RT, F(3, 39) = 3.3, p = 0.03, when collapsing across cue condition and eye (because the interactions were not significant). In controls, there was a main effect of target location on accuracy, F(3, 39) = 15, p < 0.001, but not median RT (F < 1), ruling out any speed–accuracy trade-offs. Paired sample t tests of the extents of the VMA and HVA for accuracy found that they did not differ between the two eyes of the amblyopes or controls (all ps > 0.05). When collapsing across their eyes, the amblyopes exhibited both a significant HVA, t(13) = 3.6, p = 0.003, and VMA, t(13) = 2.5, p = 0.03 (Figure 3b). As expected, the controls also demonstrated a significant HVA, t(13) = 4.3, p < 0.001, and VMA, t(13) = 4.5, p < 0.001 (Figure 3a). A two-way mixed design ANOVA of the HVA revealed that both main effects of eye and group were not significant (both ps > 0.1), but there was a significant interaction, F(1, 26) = 5.3, p = 0.03, because spuriously the eye effect (based on arbitrary labeling one eye “amblyopic” and the other “fellow”) was less pronounced for amblyopes than controls. A similar two-way mixed design ANOVA of VMA values revealed neither the main effects of eye and group nor their interaction to be significant (all ps > 0.1).
Figure 3.
Performance fields in Experiment 1: Exogenous attention. Performance accuracy (percentage correct) in the valid and neutral cue conditions plotted as a function of target location. For illustration purposes, we plot the average across the amblyopic and fellow eyes for both the controls (a) and amblyopes (b).
In terms of RT collapsed across eyes, one-sample t tests found that both groups exhibited a significant HVA, amblyopes: t(13) = −2.4, p = 0.03; controls: t(13) = −2.3, p = 0.04, but not VMA (both ps > 0.1). Two-way mixed design ANOVAs of both the HVA and VMA revealed no significant main effects of eye or group or interactions. Further, the exogenous valid attention cue significantly improved accuracy and sped up RTs to a similar degree at all target locations in both eyes of both groups (all cue × location interactions: p > 0.1).
Effects of amblyopia depth and severity
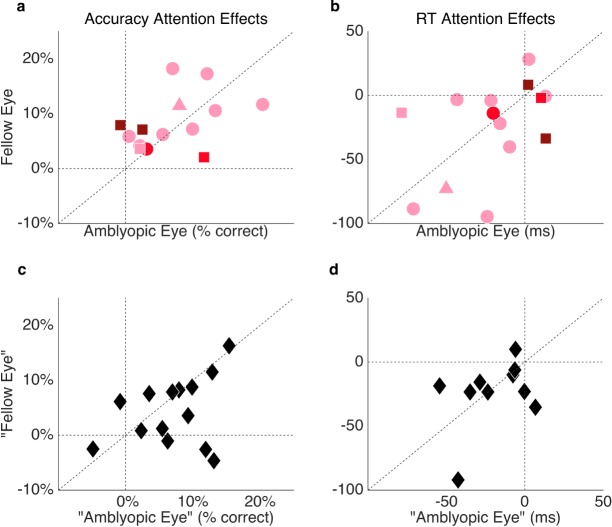
As an exploratory analysis, we conducted correlational analyses to investigate potential relationships between the depth and severity of amblyopia and (a) the magnitude of the exogenous attention benefit, (b) overall RTs. An individual's amblyopic depth—a quantifiable measure of the extent of interocular differences in acuity—was calculated as LogMAR acuity in the amblyopic eye minus that in the fellow eye. Amblyopic severity was equivalent to LogMAR acuity within the amblyopic eye. When assessing their amblyopic and fellow eyes separately, neither an amblyopic observer's depth nor severity (Figure 4) significantly correlated with the magnitude of his or her attention benefit on accuracy (all ps > 0.1; Figure 4a) or RT (all ps > 0.1; Figure 4b). The magnitude of the attention effect on accuracy or RT did not correlate between eyes of the control observers (both ps > 0.1; Figure 4c, d).
Figure 4.
Individual exogenous attention effects (valid − neutral cue condition) by eye in terms of accuracy (a, c) and RT (b, d) for the amblyopic participants (a, b), according to amblyopic severity (light pink = mild, red = moderate, maroon = severe) and subtype (circles = strabismic, square = anisometropic, triangle = mixed), and control participants (c, d; black diamonds). Note that one outlier control observer was excluded from panel d for illustration purposes only (RT for one eye: +215.40).
Experiment 2: Endogenous attention
In Experiment 1, we demonstrated that the benefit of inflexible, involuntary exogenous attention remains functionally intact in human amblyopic adults. Can amblyopes also flexibly and voluntarily deploy their covert spatial attention according to task demands? The two types of attention are subserved by highly interactive and partially overlapping yet distinct neural substrates; the neural basis of exogenous attention is hypothesized to primarily be subcortical, and the top-down modulatory signals of endogenous attention are thought to originate from within a distributed dorsal frontoparietal network (for reviews, see Corbetta, Patel, & Shulman, 2008; Patel et al., 2015; Petersen & Posner, 2012; Serences & Kastner, 2014). Furthermore, neurophysiology and neuroimaging studies suggest that the neural anomalies of amblyopia intensify as one moves progressively further along the dorsal and ventral streams (for reviews, see Asper et al., 2000b; Joly & Franko, 2014; Kiorpes, 2006; Levi, 2006).
In Experiment 2, we investigated whether endogenous spatial attention remains intact in human amblyopic adults by employing essentially the same task as was used in Experiment 1. Both the benefits at the attended location and the concomitant cost at the unattended location on similar orientation discrimination tasks have been well established in both neurotypical observers (Herrmann et al., 2010; Ling & Carrasco, 2006a, 2006b; Pestilli et al., 2009) and other special populations (Grubb, Behrmann, Egan, Minshew, Carrasco et al., 2013; Grubb, Behrmann, Egan, Minshew, Heeger et al., 2013). Eliminating (as best as possible) differences in stimulus parameters, experimental conditions, and task demands enabled us to directly compare the magnitudes of the endogenous and exogenous attention benefits on performance accuracy and RT within the same observers.
Methods
Observers
Fourteen amblyopic adults (nine female; M age = 31.3 ± 11.6 years) and 14 age- and gender-matched control observers (nine female; M age = 30.6 ± 11.2 years) with normal or corrected-to-normal vision participated in this experiment (Table 1). Nine amblyopes and two control observers had participated in Experiment 1. Three potential amblyopic observers were excluded as they did not fulfill our inclusion criteria (same as in Experiment 1).
Apparatus and setup
These were identical to Experiment 1.
Stimuli
These were identical to Experiment 1 except for the locations of the placeholders, Gabor patch stimuli, and the precue (Figure 5). Given that we had already established that amblyopes show canonical performance fields and to ensure that performance would not change across locations, we moved the stimuli away from the cardinal axes to the diagonals, at which performance does not differ across locations (Abrams et al., 2012; Carrasco, Giordano et al., 2004; Corbett & Carrasco, 2011). To manipulate endogenous spatial attention, we presented a central precue—either a single 0.8° line or four 0.2° lines (all 0.13° thick)—0.3° from the center of the fixation cross, which pointed to one or all (neutral, distributed condition) of the possible target locations. The response cue indicated the target location by pointing to one placeholder (that matched the single central precue for valid trials and mismatched for invalid trials) and eliminated location uncertainty at the response time for all conditions.
Figure 5.
Trial sequence for Experiment 2: Endogenous attention.
Procedure
The procedure was identical to Experiment 1 except that the number of trials per block was increased to accommodate three (neutral, valid, and invalid) rather than two (neutral and valid) cueing conditions. Observers completed ∼16 blocks of 60 trials each—as many as possible across the two hour-long sessions—for a total of ∼960 trials: ∼576 trials in the valid cue condition (60% of all trials) and ∼192 trials each in the invalid (20% of all trials) and neutral cue (20% of all trials) conditions. The eye (amblyopic or fellow) with which control observers ran on Day 1 was counterbalanced across observers. We implemented real-time trial replacement: When observers broke fixation during the trial sequence, the trial would immediately abort, and the text, “Please fixate,” would appear at the center of the screen. The cancelled trials were added to the end of the block. At the end of the practice blocks, the amblyope observers still needed a higher contrast in their amblyopic (M = 47%, SD = 32%) than their fellow eye (M = 32%, SD = 20%, p = 0.07). Unsurprisingly, control observers required similar amounts of contrast in their arbitrarily matched “amblyopic” (M = 33%, SD = 29%) and “fellow” eyes (M = 34%, SD = 33%).
Task and trial sequence
Observers performed the same 2AFC orientation discrimination task monocularly while endogenous spatial attention was manipulated via presentation of either a single (80% of all trials, of which 75% of trials were valid and 25% trials were invalid) or distributed central precue (20% of all trials). The sequence was the same as in Experiment 1 except that the precue duration was 400 ms to ensure that all observers had ample time to voluntarily deploy their endogenous attention (Liu, Stevens, & Carrasco, 2007; Muller & Rabbitt, 1989; Nakayama & Mackeben, 1989).
Results
Overall performance
Within groups:
Repeated-measure (cue condition × eye) ANOVAs of accuracy within each group revealed no significant two-way interactions. Note that whenever Mauchly's test indicated that the assumption of sphericity had been violated, degrees of freedom were adjusted using the Huynh-Feldt correction. In both groups, there was a significant main effect of cue, amblyopes: F(1.2, 16) = 17, p = 0.001; controls: F(2, 26) = 50, p < 0.001, but not eye (amblyopes: F < 1; controls: F < 1; Figure 6a). Accuracy was always significantly higher in the valid than the neutral cue conditions (both groups p < 0.001), which in turn were higher than for the invalid cue conditions (amblyopes: p < 0.05; controls: p < 0.001). A significant main effect of cue was found for both the amblyopic and fellow eyes of the amblyopes when considered separately (both ps < 0.001).
Figure 6.
Performance in Experiment 2: Endogenous attention. (a) Percent accuracy. (b) RTs. In both plots, for illustration purposes only, as the eyes of control subjects were arbitrarily labeled amblyopic or fellow, average performance for both eyes is plotted for controls but separately for each eye of the amblyopes. Error bars are ±1 SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
To rule out a speed–accuracy trade-off, we performed the same statistical tests with average median RT. For the amblyopes, neither the two-way interaction nor the main effect of eye was significant, but there was a significant main effect of cue, F(1.4, 18) = 56, p < 0.001 (Figure 6b). RTs were significantly faster in the valid than the neutral cue condition (p < 0.001), which in turn were faster than for the invalid cue condition (p < 0.001). Thus, whether collapsing across eyes or considering each eye separately, the amblyopes demonstrated a significant benefit in accuracy and RT when they deployed their endogenous attention to the correct target location and a significant cost when deploying it to the incorrect target location. In controls, the main effects of eye (F < 1); cue, F(1, 13) = 4.0, p = 0.07; and their interaction, F(1, 13) = 1.4, p > 0.1, were not significant.
Between groups:
To evaluate whether the magnitude of the effects significantly differed between the amblyopes and their age- and gender-matched controls, we conducted a three-way mixed design ANOVA on accuracy and RT. Neither of the three-way interactions nor main effects of group were significant (all ps > 0.1); accuracy (Figure 6a) and RTs (Figure 6b) did not significantly differ between the groups.
Effects of amblyopia depth and severity
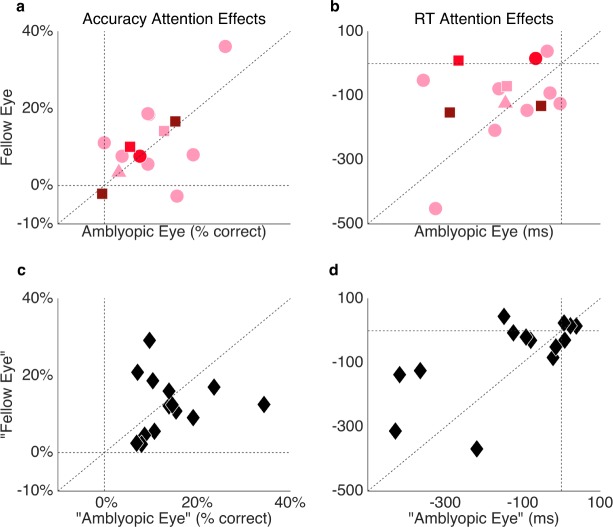
Neither the depth nor severity of amblyopia significantly correlated with the magnitude of the endogenous attention benefit or cost within the amblyopic observers in terms of overall accuracy or RT, either when comparing attention effects in the amblyopic and fellow eyes separately (Figure 7a, b) or when collapsing across the eyes (all ps > 0.1). Further, the depth or severity of amblyopia did not significantly correlate with overall RT (collapsed across locations and cue conditions), either when comparing the effects in the amblyopic and fellow eyes separately or when collapsing across eyes (all ps > 0.1). Finally, there were no significant correlations between either the depth or severity of amblyopia and required stimulus contrast (all ps > 0.1). The magnitude of the attention effect on accuracy or RT did not correlate between eyes of the control observers (both ps > 0.1; Figure 7c, d).
Figure 7.
Individual endogenous attention effects (valid − invalid cue condition) by eye in terms of accuracy (a, c) and RT (b, d) for the amblyopic participants (a, b), according to amblyopic severity (light pink = mild, red = moderate, maroon = severe) and subtype (circles = strabismic, square = anisometropic, triangle = mixed) and control participants (c, d; black diamonds).
Comparing effects of exogenous and endogenous attention
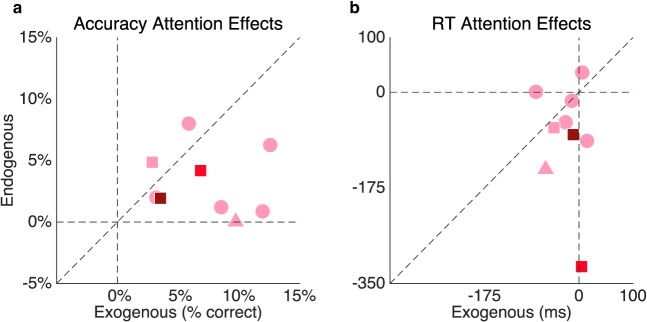
Required stimulus contrast significantly correlated for the nine amblyopic participants who participated in both Experiments 1 and 2 for the amblyopic eye, r(7) = 0.79, p = 0.01; the fellow eye, r(7) = 0.66, p = 0.05; or averaged across both eyes, r(7) = 0.92, p < 0.001. A two-way ANOVA of type of attention (exogenous or endogenous) × eye (amblyopic or fellow) revealed that, for the nine amblyopic observers who participated in both experiments, the magnitude of the exogenous attention benefit was significantly higher than that of endogenous attention, F(1, 8) = 6.1, p = 0.04 (Figure 8a), with no significant difference between the eyes (F < 1) and no significant interaction, F(1, 8) = 4.6, p = 0.06. The corresponding RT analysis revealed that the endogenous attention cue sped up average RTs significantly more than the exogenous attention cue, F(1, 8) = 6.1, p = 0.04 (Figure 8b), regardless of eye (F < 1) with no significant interaction (F < 1).
Figure 8.
Endogenous versus exogenous attentional effect (valid − neutral cue condition for both experiments) within the same amblyopic participants in terms of (a) accuracy and (b) RT, according to their severity (light pink = mild, red = moderate, maroon = severe) and subtype (circles = strabismic, square = anisometropic, triangle = mixed). For illustration purposes, the average across both eyes is plotted.
Discussion
Overall performance and attention effects
This is the first study to directly operationalize, manipulate, and measure both exogenous (Experiment 1) and endogenous (Experiment 2) attention in a group of human, adult amblyopes and their age- and gender-matched controls. Observers were peripherally or centrally cued to attend either one or all possible target locations while monocularly performing a 2AFC orientation discrimination task that is contingent upon contrast sensitivity and for which the effects of both types of covert spatial attention have been well established in both neurotypical observers (Cameron et al., 2002; Carrasco et al., 2000; Dosher & Lu, 2000a, 2000b; Herrmann et al., 2010; Ling & Carrasco, 2006a, 2006b; Liu et al., 2005; Lu & Dosher, 1998, 2000; Pestilli & Carrasco, 2005; Pestilli et al., 2009) and other special populations (Grubb, Behrmann, Egan, Minshew, Carrasco et al., 2013; Grubb, Behrmann, Egan, Minshew, Heeger et al., 2013). In both experiments, performance accuracy in the neutral cue condition did not significantly differ between the eyes within each group or across matched eyes between groups. Thus, task difficulty was well equated across observers by adjusting stimulus contrast between blocks.
In both experiments, both amblyopes and controls demonstrated significant benefits of exogenous and endogenous attention, i.e., increased accuracy in the discrimination task for the valid cued trials compared to neutral. Critically, the magnitudes of these attention benefits were highly similar between the amblyopic and fellow eyes within the amblyopes and when compared to the matched eyes of visually intact controls. Moreover, in Experiment 2, the amblyopes demonstrated a significant cost of deploying their endogenous attention to the incorrect target, i.e., reduced accuracy in the discrimination task for the invalidly cued trials compared to neutral, to the same extent in both of their eyes and to the same degree as visually intact controls. Moreover, these accuracy effects were accompanied by changes in RT, our secondary dependent variable. For both groups (and both eyes in each group), responses were faster for valid than neutral trials, which in turn were faster than for invalid trials. Thus, both attention manipulations improved accuracy and sped RT. In summary, we provide the first psychophysical evidence that both exogenous and endogenous covert spatial attention are functionally intact in human amblyopic adults. These findings are in agreement with those showing that attentional cues improve accuracy and reduce RTs in a motion discrimination task in amblyopic nonhuman primates (Kiorpes, Pham, & Carrasco, 2013).
This study clearly demonstrates that selective visuospatial attention—as assessed by our particular low-level discrimination task and static stimuli—remains functionally intact in amblyopia. However, as attention is not a unitary concept, we cannot conclude that amblyopes possess no deficits in all forms of attention. Indeed, if we were to systematically manipulate attention and test amblyopes on a series of tasks, attention-related deficits may manifest further along the visual pathways or for higher level cognitive functions. For instance, in tasks using crowded and dynamic stimulus displays thought to involve higher level forms of attention, e.g., attentional tracking of multiple object displays (Ho et al., 2006) and the attentional blink paradigm (Popple & Levi, 2008).
Further, we do not argue that the underlying neural mechanisms of attention are the same in amblyopes and neurotypical adults. Future research will establish if the reduced attentional modulation found for contrast detection (Hou et al., 2016) is also present for the discrimination task we employed here. We encourage future studies investigating these and other aspects of attention with neurotypical and special populations to take advantage of precise terminology and well-established experimental protocols, such as those employed in this study, which allow experimenters to reliably isolate, manipulate, and measure particular types of attention.
Visual performance fields
We also demonstrated, for the first time, that amblyopes possess canonical performance fields; in Experiment 1, task performance in both groups was better (to an equal extent) at both locations along the HM compared to the two locations along the VM. Both groups were also significantly better in the lower visual field location compared to the upper visual field location along the VM. Furthermore, the benefit of exogenous attention did not differ as a function of target location, thus preserving the shape of the performance fields. This finding is consistent with previous studies with neurotypical observers (Talgar & Carrasco, 2002).
Amblyopic severity and depth
By definition, amblyopia is a visual disorder that encompasses a wide range of underlying etiologies and resultant perceptual (dis)abilities. It has been shown that there are slight, but reliable, differences in the perceptual abilities of amblyopes depending on their subtype and severity, particularly correlating with the depth of their abnormal binocular functioning (McKee et al., 2003). The results of both experiments revealed that, regardless of whether the observers suffered from strabismic, anisometropic, or mixed amblyopia or whether the severity of their amblyopia was mild, moderate, or severe, the magnitudes of their exogenous and endogenous attention benefits (both in terms of performance accuracy and RT) as well as their endogenous attention costs were virtually indistinguishable. Moreover, the correlations suggest that the depth or severity of their amblyopia was not predictive of the magnitudes of their exogenous and endogenous benefits or endogenous cost. We note we had a limited and uneven number of observers of each amblyopic subtype and severity (a majority of our observers possessed mild or moderate strabismic amblyopia) and that, by design, the attention effects could only improve 20%. Thus, it remains to be seen whether any significant differences in attentional ability exist among the three subtypes.
Conclusion
Encouragingly, our study demonstrates that despite their impaired visual systems, amblyopes are able to significantly improve the quality of their visual perception with the deployment of spatial attention to the same degree as neurotypical individuals. Interestingly, it appears that the visual attention benefit in discrimination accuracy may be even greater when its deployment is reflexive and involuntary rather than voluntary, i.e., requiring additional cognitive effort and further depleting the limited energetic resources of an already handicapped visual system. These findings have important theoretical implications for basic science research studies and practical implications for clinical visual training protocols.
Supplementary Material
Acknowledgments
This work was supported by NIH (RO1-EY016200) to MC and the NSF Graduate Research Fellowship Program under Grant No. DGE 1342536 to MR. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. The authors would like to thank the members of the Carrasco lab for constructive comments on the manuscript. The authors have no conflict of interest to declare. MR, LK, and MC designed the study. MR conducted data collection and analyses and helped with observer recruitment. RC recruited amblyopic observers and RTS verified their diagnoses. MR and MC wrote the manuscript.
Commercial relationships: none.
Corresponding authors: Mariel Roberts; Marisa Carrasco.
Email: mariel.roberts@nyu.edu; marisa.carrasco@nyu.edu.
Address: Department of Psychology, New York University, New York, NY, USA.
Footnotes
Tripathy and Levi (2008) found that the deficit in tracking ability of the amblyopic eye was on the order of about 15% relative to the fellow eye.
References
- Abrams J., Barbot A., & Carrasco M.. (2010). Voluntary attention increases perceived spatial frequency. Attention, Perception, & Psychophysics, 72 (6), 1510–1521, doi:10.3758/APP.72.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams J., Nizam A., & Carrasco M.. (2012). Isoeccentric locations are not equivalent: The extent of the vertical meridian asymmetry. Vision Research, 52 (1), 70–78, doi:10.1016/j.visres.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amick M. M., Cronin-Golomb A., & Gilmore G. C.. (2003). Visual processing of rapidly presented stimuli is normalized in Parkinson's disease when proximal stimulus strength is enhanced. Vision Research, 43 (26), 2827–2835. [DOI] [PubMed] [Google Scholar]
- Anderson S. J., Holliday I. E., & Harding G. F.. (1999). Assessment of cortical dysfunction in human strabismic amblyopia using magnetoencephalography (MEG). Vision Research, 39 (9), 1723–1738. [DOI] [PubMed] [Google Scholar]
- Anderson S. J., & Swettenham J. B.. (2006). Neuroimaging in human amblyopia. Strabismus, 14 (1), 21–35. [DOI] [PubMed] [Google Scholar]
- Anton-Erxleben K., Abrams J., & Carrasco M.. (2010). Evaluating comparative and equality judgments in contrast perception: Attention alters appearance. Journal of Vision, 10 (11): 6, 1–22, doi:10.1167/10.11.6 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton-Erxleben K., & Carrasco M.. (2013). Attentional enhancement of spatial resolution: Linking behavioural and neurophysiological evidence. Nature Reviews Neuroscience, 14 (3), 188–200, doi:10.1038/nrn3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton-Erxleben K., Herrmann K., & Carrasco M.. (2013). Independent effects of adaptation and attention on perceived speed. Psychological Science, 24 (2), 150–159, doi:10.1177/0956797612449178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asper L., Crewther D., & Crewther S. G.. (2000a). Strabismic amblyopia. Part 1. Psychophysics. Clinical and Experimental Optometry, 83 (2), 49–58. [DOI] [PubMed] [Google Scholar]
- Asper L., Crewther D., & Crewther S. G.. (2000b). Strabismic amblyopia. Part 2. Neural processing. Clinical and Experimental Optometry, 83 (4), 200–211. [DOI] [PubMed] [Google Scholar]
- Baker D. H., Meese T. S., & Hess R. F.. (2008). Contrast masking in strabismic amblyopia: Attenuation, noise, interocular suppression and binocular summation. Vision Research, 48 (15), 1625–1640, doi:10.1016/j.visres.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Baker D. H., Simard M., Saint-Amour D., & Hess R. F.. (2015). Steady-state contrast response functions provide a sensitive and objective index of amblyopic deficits. Investigative Ophthalmology & Visual Science, 56 (2), 1208–1216. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankó É. M., Körtvélyes J., Németh J., & Vidnyánszky Z.. (2014). Amblyopic deficit beyond the fovea: Delayed and variable single-trial ERP response latencies, but unaltered amplitudes. Investigative Ophthalmology & Visual Science, 55 (2), 1109–1117. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Barnes G. R., Hess R. F., Dumoulin S. O., Achtman R. L., & Pike G. B.. (2001). The cortical deficit in humans with strabismic amblyopia. Journal of Physiology, 533 (1), 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H., Zhang B., Tao X., Harwerth R. S., Smith E. L., III, & Chino Y. M.. (2011). Neuronal responses in visual area V2 (V2) of macaque monkeys with strabismic amblyopia. Cerebral Cortex, 21 (9), 2033–2045, doi:10.1093/cercor/bhq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron E. L., Tai J. C., & Carrasco M.. (2002). Covert attention affects the psychometric function of contrast sensitivity. Vision Research, 42 (8), 949–967. [DOI] [PubMed] [Google Scholar]
- Carrasco M. (2011). Visual attention: The past 25 years. Vision Research, 51 (13), 1484–1525, doi:10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. (2014). Spatial covert attention: Perceptual modulation. Nobre K. & Kastner S.. (Eds.), The Oxford Handbook of Attention (pp 183–230). Oxford, UK: Oxford University Press. [Google Scholar]
- Carrasco M., & Barbot A.. (2015). How attention affects spatial resolution. Cold Spring Harbor Symposia on Quantitative Biology, 79, 149–160, doi:10.1101/sqb.2014.79.024687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M., Giordano A. M., & McElree B.. (2004). Temporal performance fields: Visual and attentional factors. Vision Research, 44 (12), 1351–1365, doi:10.1016/j.visres.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Carrasco M., Giordano A. M., & McElree B.. (2006). Attention speeds processing across eccentricity: Feature and conjunction searches. Vision Research, 46 (13), 2028–2040, doi:10.1016/j.visres.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M., Ling S., & Read S.. (2004). Attention alters appearance. Nature Neuroscience, 7 (3), 308–313, doi:10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M., Loula F., & Ho Y. X.. (2006). How attention enhances spatial resolution: Evidence from selective adaptation to spatial frequency. Perception & Psychophysics, 68 (6), 1004–1012. [DOI] [PubMed] [Google Scholar]
- Carrasco M., & McElree B.. (2001). Covert attention accelerates the rate of visual information processing. Proceedings of the National Academy of Sciences, USA, 98 (9), 5363–5367, doi:10.1073/pnas.081074098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M., McElree B., Denisova K., & Giordano A. M.. (2003). Speed of visual processing increases with eccentricity. Nature Neuroscience, 6 (7), 699–670, doi:10.1038/nn1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M., Penpeci-Talgar C., & Eckstein M.. (2000). Spatial covert attention increases contrast sensitivity across the CSF: Support for signal enhancement. Vision Research, 40 (10–12), 1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M., Talgar C. P., & Cameron E. L.. (2001). Characterizing visual performance fields: Effects of transient covert attention, spatial frequency, eccentricity, task and set size. Spatial Vision, 15 (1), 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M., Williams P. E., & Yeshurun Y.. (2002). Covert attention increases spatial resolution with or without masks: Support for signal enhancement. Journal of Vision, 2 (6): 4, 467–479, doi:10.1167/2.6.4 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Carrasco M., & Yeshurun Y.. (2009). Covert attention effects on spatial resolution. Progress in Brain Research, 176, 65–86, doi:10.1016/s0079-6123(09)17605-7. [DOI] [PubMed] [Google Scholar]
- Chatzistefanou K. I., Theodossiadis G. P., Damanakis A. G., Ladas I. D., Moschos M. N., & Chimonidou E.. (2005). Contrast sensitivity in amblyopia: The fellow eye of untreated and successfully treated amblyopes. Journal of American Association for Pediatric Ophthalmology and Strabismus, 9 (5), 468–474. [DOI] [PubMed] [Google Scholar]
- Choi M. Y., Lee K.-M., Hwang J.-M., Choi D. G., Lee D. S., Park K. H., & Yu Y. S.. (2001). Comparison between anisometropic and strabismic amblyopia using functional magnetic resonance imaging. British Journal of Ophthalmology, 85 (9), 1052–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colenbrander A. (2002). Visual standards: Aspects and ranges of vision loss with emphasis on population surveys. Paper presented at the Report prepared for the International Council of Ophthalmology at the 29th International Congress of Ophthalmology, Sydney, Australia. [Google Scholar]
- Conner I. P., Odom J. V., Schwartz T. L., & Mendola J. D.. (2007). Monocular activation of V1 and V2 in amblyopic adults measured with functional magnetic resonance imaging. Journal of American Association for Pediatric Ophthalmology and Strabismus, 11 (4), 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett J. E., & Carrasco M.. (2011). Visual performance fields: Frames of reference. PLoS One, 6 (9), e24470, doi:10.1371/journal.pone.0024470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Patel G., & Shulman G. L.. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58 (3), 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W. (1998). Critical periods and amblyopia. Archives of Ophthalmology, 116 (4), 502–505. [DOI] [PubMed] [Google Scholar]
- de Zarate B. R., & Tejedor J.. (2007). Current concepts in the management of amblyopia. Clincal Ophthalmology, 1 (4), 403–414. [PMC free article] [PubMed] [Google Scholar]
- Dosher B. A., & Lu Z.-L.. (2000a). Mechanisms of perceptual attention in precuing of location. Vision Research, 40 (10), 1269–1292. [DOI] [PubMed] [Google Scholar]
- Dosher B. A., & Lu Z.-L.. (2000b). Noise exclusion in spatial attention. Psychological Science, 11 (2), 139–146. [DOI] [PubMed] [Google Scholar]
- Eckstein M. P. (1998). The lower visual search efficiency for conjunctions is due to noise and not serial attentional processing. Psychological Science, 9 (2), 111–118. [Google Scholar]
- El-Shamayleh Y., Kiorpes L., Kohn A., & Movshon J. A.. (2010). Visual motion processing by neurons in area MT of macaque monkeys with experimental amblyopia. Journal of Neuroscience, 30 (36), 12198–12209, doi:10.1523/JNEUROSCI.3055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F., & Norcia A. M.. (2011). Impaired visual decision-making in individuals with amblyopia. Journal of Vision, 11 (14): 6, 1–10, doi:10.1167/11.14.6 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S., & Carrasco M.. (2009). Perceptual consequences of visual performance fields: The case of the line motion illusion. Journal of Vision, 9 (4): 13, 1–17, doi:10.1167/9.4.13 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A. M., McElree B., & Carrasco M.. (2009). On the automaticity and flexibility of covert attention: A speed-accuracy trade-off analysis. Journal of Vision, 9 (3): 30, 1–10, doi:10.1167/9.3.30 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golla H., Ignashchenkova A., Haarmeier T., & Thier P.. (2004). Improvement of visual acuity by spatial cueing: A comparative study in human and non-human primates. Vision Research, 44 (13), 1589–1600. [DOI] [PubMed] [Google Scholar]
- Goodyear B. G., Nicolle D. A., Humphrey G. K., & Menon R. S.. (2000). BOLD fMRI response of early visual areas to perceived contrast in human amblyopia. Journal of Neurophysiology, 84 (4), 1907–1913. [DOI] [PubMed] [Google Scholar]
- Grubb M. A., Behrmann M., Egan R., Minshew N. J., Carrasco M., & Heeger D. J.. (2013). Endogenous spatial attention: Evidence for intact functioning in adults with autism. Autism Research, 6 (2), 108–118, doi:10.1002/aur.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb M. A., Behrmann M., Egan R., Minshew N. J., Heeger D. J., & Carrasco M.. (2013). Exogenous spatial attention: Evidence for intact functioning in adults with autism spectrum disorder. Journal of Vision, 13 (14): 9, 1–13, doi:10.1167/13.14.9 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K., Montaser-Kouhsari L., Carrasco M., & Heeger D. J.. (2010). When size matters: Attention affects performance by contrast or response gain. Nature Neuroscience, 13 (12), 1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R. F., & Field D. J.. (1994). Is the spatial deficit in strabismic amblyopia due to loss of cells or an uncalibrated disarray of cells? Vision Research, 34 (24), 3397–3406. [DOI] [PubMed] [Google Scholar]
- Hess R. F., & Thompson B.. (2015). Amblyopia and the binocular approach to its therapy. Vision Research, 114, 4–16, doi:10.1016/j.visres.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Ho C. S., & Giaschi D. E.. (2009). Low- and high-level motion perception deficits in anisometropic and strabismic amblyopia: Evidence from fMRI. Vision Research, 49 (24), 2891–2901, doi:10.1016/j.visres.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Ho C. S., Giaschi D. E., Boden C., Dougherty R., Cline R., & Lyons C.. (2005). Deficient motion perception in the fellow eye of amblyopic children. Vision Research, 45 (12), 1615–1627. [DOI] [PubMed] [Google Scholar]
- Ho C. S., Paul P. S., Asirvatham A., Cavanagh P., Cline R., & Giaschi D. E.. (2006). Abnormal spatial selection and tracking in children with amblyopia. Vision Research, 46 (19), 3274–3283, doi:10.1016/j.visres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Hou C., Kim Y. J., Lai X. J., & Verghese P.. (2016). Degraded attentional modulation of cortical neural populations in strabismic amblyopia. Journal of Vision, 16 (3): 16, 1–16, doi:10.1167/16.3.16 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Tao L., Zhou Y., & Lu Z.-L.. (2007). Treated amblyopes remain deficient in spatial vision: A contrast sensitivity and external noise study. Vision Research, 47 (1), 22–34. [DOI] [PubMed] [Google Scholar]
- Huang C.-B., Zhou J., Lu Z.-L., Feng L., & Zhou Y.. (2009). Binocular combination in anisometropic amblyopia. Journal of Vision, 9 (3): 17, 1–16, doi:10.1167/9.3.17 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-B., Zhou J., Lu Z.-L., & Zhou Y.. (2011). Deficient binocular combination reveals mechanisms of anisometropic amblyopia: Signal attenuation and interocular inhibition. Journal of Vision, 11( 6): 4, 1–17, doi:10.1167/11.6.4 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvarinen J., Hyvarinen L., & Linnankoski I.. (1981). Modification of parietal association cortex and functional blindness after binocular deprivation in young monkeys. Experimental Brain Research, 42 (1), 1–8. [DOI] [PubMed] [Google Scholar]
- Imamura K., Richter H., Fischer H., Lennerstrand G., Franzen O., Rydberg A.,… Langstrom B.. (1997). Reduced activity in the extrastriate visual cortex of individuals with strabismic amblyopia. Neuroscience Letters, 225 (3), 173–176. [DOI] [PubMed] [Google Scholar]
- Joly O., & Franko E.. (2014). Neuroimaging of amblyopia and binocular vision: A review. Front Integrative Neuroscience, 8 (62), 1–10, doi:10.3389/fnint.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. M., Levi D. M., & Bedell H. E.. (1984). Central and peripheral contrast sensitivity in amblyopia with varying field size. Documenta Ophthalmologica, 58 (4), 351–373. [DOI] [PubMed] [Google Scholar]
- Kiorpes L. (2006). Visual processing in amblyopia: Animal studies. Strabismus, 14 (1), 3–10, doi:10.1080/09273970500536193. [DOI] [PubMed] [Google Scholar]
- Kiorpes L., Kiper D. C., O'Keefe L. P., Cavanaugh J. R., & Movshon J. A.. (1998). Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. Journal of Neuroscience, 18 (16), 6411–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L., & McKee S. P.. (1999). Neural mechanisms underlying amblyopia. Current Opinion in Neurobiology, 9 (4), 480–486. [DOI] [PubMed] [Google Scholar]
- Kiorpes L., & Movshon J. A.. (1996). Amblyopia: A developmental disorder of the central visual pathways. Cold Spring Harbor Symposia on Quantitative Biology, 61, 39–48. [PubMed] [Google Scholar]
- Kiorpes L., & Movshon J. A.. (2004). Neural limitations on visual development in primates. Chalupa L. M. & Werner J. S.. (Eds.), The visual neurosciences (pp 159–173). Cambridge, MA: MIT Press. [Google Scholar]
- Kiorpes L., Pham A., & Carrasco M.. (2013). Attention improves visual performance in amblyopic macaque monkeys. Journal of Vision, 13 (9): 469, doi:10.1167/13.9.469 [Abstract] [Google Scholar]
- Koskela P. U. (1986). Contrast sensitivity in amblyopia II. Changes during pleoptic treatment. Acta Ophthalmologica, 64 (5), 563–569. [DOI] [PubMed] [Google Scholar]
- Laudate T. M., Neargarder S., Dunne T. E., Sullivan K. D., Joshi P., Gilmore G. C.,… Cronin-Golomb A.. (2012). Bingo! Externally supported performance intervention for deficient visual search in normal aging, Parkinson's disease, and Alzheimer's disease. Aging Neuropsychology and Cognition, 19 (1–2), 102–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leguire L. E., Rogers G. L., & Bremer D. L.. (1990). Amblyopia: The normal eye is not normal. Journal of Pediatric Ophthalmology and Strabismus, 27 (1), 32–38. [DOI] [PubMed] [Google Scholar]
- Lerner Y., Hendler T., Malach R., Harel M., Leiba H., Stolovitch C., & Pianka P.. (2006). Selective fovea-related deprived activation in retinotopic and high-order visual cortex of human amblyopes. NeuroImage, 33 (1), 169–179, doi:10.1016/j.neuroimage.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Levi D. M. (2006). Visual processing in amblyopia: Human studies. Strabismus, 14 (1), 11–19, doi:10.1080/09273970500536243. [DOI] [PubMed] [Google Scholar]
- Levi D. M. (2013). Linking assumptions in amblyopia. Visual Neuroscience, 30 (5–6), 277–287, doi:10.1017/S0952523813000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D. M., & Carkeet A. D.. (1993). Amblyopia: A consequence of abnormal visual development. Simons K. (Ed.), Early visual development: Normal and abnormal (pp. 391–408). New York, NY: Oxford University Press. [Google Scholar]
- Levi D. M., Klein S. A., & Sharma V.. (1999). Position jitter and undersampling in pattern perception. Vision Research, 39 (3), 445–465. [DOI] [PubMed] [Google Scholar]
- Levi D. M., Knill D. C., & Bavelier D.. (2015). Stereopsis and amblyopia: A mini-review. Vision Research, 114, 17–30, doi:10.1016/j.visres.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D. M., & Tripathy S. P.. (2006). Is the ability to identify deviations in multiple trajectories compromised by amblyopia? Journal of Vision, 6 (12): 3, 1367–1379, doi:10.1167/6.12.3 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Li R. W., Klein S. A., & Levi D. M.. (2008). Prolonged perceptual learning of positional acuity in adult amblyopia: Perceptual template retuning dynamics. Journal of Neuroscience, 28 (52), 14223–14229, doi:10.1523/JNEUROSCI.4271-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. W., & Levi D. M.. (2004). Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. Journal of Vision, 4 (6): 7, 476–487, doi:10.1167/4.6.7 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Li X., Dumoulin S. O., Mansouri B., & Hess R. F.. (2007). Cortical deficits in human amblyopia: Their regional distribution and their relationship to the contrast detection deficit. Investigative Ophthalmology & Visual Science, 48 (4), 1575–1591. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Ling S., & Carrasco M.. (2006a). Sustained and transient covert attention enhance the signal via different contrast response functions. Vision Research, 46 (8–9), 1210–1220, doi:10.1016/j.visres.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S., & Carrasco M.. (2006b). When sustained attention impairs perception. Nature Neuroscience, 9 (10), 1243–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Heeger D. J., & Carrasco M.. (2006). Neural correlates of the visual vertical meridian asymmetry. Journal of Vision, 6 (11): 12, 1294–1306, doi:10.1167/6.11.12 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Pestilli F., & Carrasco M.. (2005). Transient attention enhances perceptual performance and FMRI response in human visual cortex. Neuron, 45 (3), 469–477, doi:10.1016/j.neuron.2004.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Stevens S. T., & Carrasco M.. (2007). Comparing the time course and efficacy of spatial and feature-based attention. Vision Research, 47 (1), 108–113. [DOI] [PubMed] [Google Scholar]
- Lu Z.-L., & Dosher B. A.. (1998). External noise distinguishes attention mechanisms. Vision Research, 38 (9), 1183–1198. [DOI] [PubMed] [Google Scholar]
- Lu Z.-L., & Dosher B. A.. (2000). Spatial attention: Different mechanisms for central and peripheral temporal precues? Journal of Experimental Psychology: Human Perception and Performance, 26 (5), 1534–1548. [DOI] [PubMed] [Google Scholar]
- McKee S. P., Levi D. M., & Movshon J. A.. (2003). The pattern of visual deficits in amblyopia. Journal of Vision, 3 (5): 5, 380–405, doi:10.1167/3.5.5 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- McKee S. P., Levi D. M., Schor C. M., & Movshon J. A.. (2016). Saccadic latency in amblyopia. Journal of Vision, 16 (5): 3, 1–15, doi:10.1167/16.5.3 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna B., Pestilli F., & Carrasco M.. (2009). Attention trades off spatial acuity. Vision Research, 49 (7), 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckli L., Kiess S., Tonhausen N., Singer W., Goebel R., & Sireteanu R.. (2006). Cerebral correlates of impaired grating perception in individual, psychophysically assessed human amblyopes. Vision Research, 46 (4), 506–526, doi:10.1016/j.visres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Muller H. J., & Rabbitt P. M.. (1989). Reflexive and voluntary orienting of visual attention: Time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception and Performance, 15 (2), 315–330. [DOI] [PubMed] [Google Scholar]
- Nakayama K., & Mackeben M.. (1989). Sustained and transient components of focal visual attention. Vision Research, 29 (11), 1631–1647. [DOI] [PubMed] [Google Scholar]
- Palmer J., Verghese P., & Pavel M.. (2000). The psychophysics of visual search. Vision Research, 40 (10), 1227–1268. [DOI] [PubMed] [Google Scholar]
- Patel G. H., Yang D., Jamerson E. C., Snyder L. H., Corbetta M., & Ferrera V. P.. (2015). Functional evolution of new and expanded attention networks in humans. Proceedings of the National Academy of Sciences, USA, 112 (30), 9454–9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestilli F., & Carrasco M.. (2005). Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Research, 45 (14), 1867–1875, doi:10.1016/j.visres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Pestilli F., Ling S., & Carrasco M.. (2009). A population-coding model of attention's influence on contrast response: Estimating neural effects from psychophysical data. Vision Research, 49 (10), 1144–1153, doi:10.1016/j.visres.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S. E., & Posner M. I.. (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35, 73–89, doi:10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popple A. V., & Levi D. M.. (2008). The attentional blink in amblyopia. Journal of Vision, 8 (13): 12, 1–19, doi:10.1167/8.13.12 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Posner M. I. (1980). Orienting of attention. The Quarterly Journal of Experimental Psychology, 32 (1), 3–25, doi:10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Rovamo J., Virsu V., Laurinen P., & Hyvärinen L.. (1982). Resolution of gratings oriented along and across meridians in peripheral vision. Investigative Ophthalmology & Visual Science, 23 (5), 666–670. [PubMed] [Article] [PubMed] [Google Scholar]
- Secen J., Culham J., Ho C., & Giaschi D.. (2011). Neural correlates of the multiple-object tracking deficit in amblyopia. Vision Research, 51 (23–24), 2517–2527, doi:10.1016/j.visres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Serences J. T., & Kastner S.. (2014). A multi-level account of selective attention. The Oxford Handbook of Attention (pp 76–104). Oxford, UK: Oxford University Press. [Google Scholar]
- Sharma V., Levi D. M., & Klein S. A.. (2000). Undercounting features and missing features: Evidence for a high-level deficit in strabismic amblyopia. Nature Neuroscience, 3 (5), 496–501, doi:10.1038/74872. [DOI] [PubMed] [Google Scholar]
- Shooner C., Hallum L. E., Kumbhani R. D., Ziemba C. M., Garcia-Marin V., Kelly J. G.,… Kiorpes L.. (2015). Population representation of visual information in areas V1 and V2 of amblyopic macaques. Vision Research, 114, 56–67, doi:10.1016/j.visres.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich L. C., Jocson C. M., & Horton J. C.. (2012). Neuronal projections from V1 to V2 in amblyopia. Journal of Neuroscience, 32 (8), 2648–2656, doi:10.1523/JNEUROSCI.4799-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Störmer V. S., McDonald J. J., & Hillyard S. A.. (2009). Cross-modal cueing of attention alters appearance and early cortical processing of visual stimuli. Proceedings of the National Academy of Sciences, USA, 106 (52), 22456–22461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasburger H., Rentschler I., & Jüttner M.. (2011). Peripheral vision and pattern recognition: A review. Journal of Vision, 11 (5): 13, 1–82, doi:10.1167/11.5.13 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talgar C. P., & Carrasco M.. (2002). Vertical meridian asymmetry in spatial resolution: Visual and attentional factors. Psychonomic Bulletin and Review, 9 (4), 714–722. [DOI] [PubMed] [Google Scholar]
- Thompson B., Chung S. T., Kiorpes L., Ledgeway T., & McGraw P. V.. (2015). A window into visual cortex development and recovery of vision: Introduction to the Vision Research special issue on amblyopia. Vision Research, 114, 1–3, doi:10.1016/j.visres.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Tripathy S. P., & Levi D. M.. (2008). On the effective number of tracked trajectories in amblyopic human vision. Journal of Vision, 8 (4): 8, 1–22, doi:10.1167/8.4.8 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- von Noorden G. K. (1990). Binocular vision & ocular motility. St. Louis, MO: Mosby. [Google Scholar]
- von Noorden G. K., & Crawford M. L.. (1978). The sensitive period. Transactions of the Ophthalmological Societies of the United Kingdom, 99 (3), 442–446. [PubMed] [Google Scholar]
- Whelan R. (2008). Effective analysis of reaction time data. The Psychological Record, 58 (3), 475–482. [Google Scholar]
- Wong A. M. (2012). New concepts concerning the neural mechanisms of amblyopia and their clinical implications. Canadian Journal of Ophthalmology, 47 (5), 399–409, doi:10.1016/j.jcjo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Xu P., Lu Z.-L., Qiu Z., & Zhou Y.. (2006). Identify mechanisms of amblyopia in Gabor orientation identification with external noise. Vision Research, 46 (21), 3748–3760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.