ABSTRACT
Zinc finger antiviral protein (ZAP) is a host factor that specifically inhibits the replication of certain viruses. There are two ZAP isoforms arising from alternative splicing, which differ only at the C termini. It was recently reported that the long isoform (ZAPL) promotes proteasomal degradation of influenza A virus (IAV) proteins PA and PB2 through the C-terminal poly(ADP-ribose) polymerase (PARP) domain, which is missing in the short form (ZAPS), and that this antiviral activity is antagonized by the viral protein PB1. Here, we report that ZAP inhibits IAV protein expression in a PARP domain-independent manner. Overexpression of ZAPS inhibited the expression of PA, PB2, and neuraminidase (NA), and downregulation of the endogenous ZAPS enhanced their expression. We show that ZAPS inhibited PB2 protein expression by reducing the encoding viral mRNA levels and repressing its translation. However, downregulation of ZAPS only modestly enhanced the early stage of viral replication. We provide evidence showing that the antiviral activity of ZAPS is antagonized by the viral protein NS1. A recombinant IAV carrying an NS1 mutant that lost the ZAPS-antagonizing activity replicated better in ZAPS-deficient cells. We further provide evidence suggesting that NS1 antagonizes ZAPS by inhibiting its binding to target mRNA. These results uncover a distinct mechanism underlying the interactions between ZAP and IAV.
IMPORTANCE ZAP is a host antiviral factor that has been extensively reported to inhibit the replication of certain viruses by repressing the translation and promoting the degradation of the viral mRNAs. There are two ZAP isoforms, ZAPL and ZAPS. ZAPL was recently reported to promote IAV protein degradation through the PARP domain. Whether ZAPS, which lacks the PARP domain, inhibits IAV and the underlying mechanisms remained to be determined. Here, we show that ZAPS posttranscriptionally inhibits IAV protein expression. This antiviral activity of ZAP is antagonized by the viral protein NS1. The fact that ZAP uses two distinct mechanisms to inhibit IAV infection and that the virus evolved different antagonists suggests an important role of ZAP in the host effort to control IAV infection and the importance of the threat of ZAP to the virus. The results reported here help us to comprehensively understand the interactions between ZAP and IAV.
KEYWORDS: Influenza A virus, NS1, PB2, virus-host interactions, zinc finger antiviral protein
INTRODUCTION
Zinc finger antiviral protein (ZAP) is a type I interferon (IFN)-inducible host factor (1–3) that inhibits the replication of certain viruses, including Sindbis virus (SINV) and Ross River virus (4), Ebola virus and Marburg virus (5), murine leukemia virus (MLV) (6), human immunodeficiency virus type 1 (HIV-1) (7), and hepatitis B virus (HBV) (8). ZAP does not seem to be a general antiviral factor; it does not inhibit the replication of some viruses, such as herpes simplex virus I and yellow fever virus (4).
There are two isoforms of ZAP arising from alternative splicing, which differ only at the C termini (9). In the N-terminal domain of ZAP, there are four CCCH-type zinc finger motifs. The N-terminal domain of 254 amino acids, which includes these zinc finger motifs, in fusion with the zeocin resistance gene product displayed antiviral activity comparable to that of the full-length protein against at least some viruses (6). In the C-terminal domain of the long isoform (ZAPL), there is a poly(ADP-ribose) polymerase (PARP) domain, which is missing in the short isoform (ZAPS). Most of the previously reported studies on the antiviral activities of ZAP and the underlying mechanisms were performed with ZAPS (4, 6, 7, 10–15). Nonetheless, given that ZAPL contains all the sequences of ZAPS, it would be reasonable to expect that ZAPL shares these antiviral activities and mechanisms with ZAPS. In this report, ZAP refers to both ZAPL and ZAPS.
ZAPS binds directly to specific viral mRNAs (10) and represses the translation of target mRNA by interfering with the assembly of the translation initiation complex (14). Furthermore, ZAPS recruits the cellular mRNA degradation machinery to promote target mRNA decay (7). ZAPS recruits the deadenylase PARN to remove the poly(A) tail and subsequently recruits a 3′-5′ exoribonuclease complex, the RNA exosome, to degrade the RNA body from the 3′ end. ZAPS also recruits the decapping complex Dcp1a/Dcp2 through its cofactor p72, a DEAD box RNA helicase, to remove the cap structure of target mRNA (7, 12). Removal of the cap structure exposes the RNA body to the 5′-3′ exoribonuclease Xrn1 for degradation (7, 12).
Whether a virus is sensitive to ZAPS seems to be determined by the presence of a ZAP-responsive element (ZRE) in the viral mRNA. The ZRE of MLV was mapped to the 3′ untranslated region (UTR), SINV to multiple fragments (10), Ebola virus to the L domain (5), HIV-1 to the 5′ UTRs of multiply spliced mRNAs (7), and HBV to the terminal redundant region of pregenomic RNA (8). No conserved sequences have been identified in these ZREs, and all the ZREs so far identified are more than 400 nucleotides long. Structural analysis of the RNA-binding domain of ZAP, the N-terminal domain, implied that ZAP may recognize a tertiary structure of the target RNA (13). The presence of a ZRE in the viral mRNA does not guarantee sensitivity of a virus to ZAPS. We previously reported that ZAPS inhibited murine gammaherpesvirus 68 open reading frame 64 (ORF64) expression but had little effect on the lytic replication of the virus because ZAPS was antagonized by the viral protein RTA (15). Given the lack of predictable target sequences in viruses and possible antagonism by viral proteins, whether a virus is sensitive to ZAPS can only be tested experimentally.
Influenza A virus (IAV) is a member of the orthomyxovirus family. The viral genome is composed of eight negative-sense RNA segments, which typically encode 11 viral proteins (16). On the surfaces of the virion particles are the receptor-binding protein hemagglutinin (HA), which mediates virus entry, and the glycoside hydrolase enzyme neuraminidase (NA), which is important for mature-virion release. M2 protein, encoded by the M segment, is also on the surface, forming ion channels. Another protein encoded by the M segment, M1, is a matrix protein that sustains the virion structure. Transcription of viral mRNAs and replication of viral RNAs (vRNAs) rely on the RNA-dependent RNA polymerase complex, which is composed of polymerase acidic protein (PA) and basic proteins 1 and 2 (PB1 and PB2). The nucleoprotein (NP) encapsidates the viral RNAs and is packaged into vRNP. Other major viral proteins include nuclear export protein (NEP) (also known as NS2) and nonstructural protein 1 (NS1), both of which are encoded by the NS segment. The proapoptotic protein PB1-F2 is encoded by the PB1 segment (16–18).
IAV infection induces type I IFN production and subsequent expression of interferon-stimulated genes (ISGs), such as 2′-5′ oligoadenylate synthetase (OAS) and protein kinase R (PKR) (19). To establish effective infection, IAV has developed mechanisms to counteract the immune responses (19). NS1 is an important player for the virus in counteracting host immune responses by a variety of mechanisms (20). NS1 limits IAV infection-induced RIG-I-mediated IFN production by inhibiting the E3 ligase activity of TRIM25 (21). Moreover, NS1 inhibits the antiviral activities of some ISGs: it inhibits OAS/RNase L in a manner dependent on its RNA-binding activity (22) and PKR by directly binding to the protein (23, 24). NS1 has also been reported to enhance viral replication by modulating host factors. For example, NS1 binds to p85β, the regulatory subunit of phosphatidylinositol 3-kinase (PI3K), and activates the PI3K pathway, which is important for efficient viral replication (25). NS1 specifically increases translation initiation of the viral mRNAs, but not nonviral mRNAs, possibly through interactions with eIF4G and PABP (26–28).
It was recently reported that ZAPL promotes the ubiquitination of IAV PA and PB2 proteins in a PARP domain-dependent manner, which leads to the degradation of the target viral proteins (29). This antiviral activity of ZAPL is antagonized by the viral protein PB1 (29). In the present study, we show that ZAP inhibits the expression of the IAV proteins PA, PB2, and NA in a PARP domain-independent manner. We further provide evidence showing that this antiviral activity of ZAP is antagonized by the viral protein NS1.
RESULTS
ZAPS inhibits the expression of IAV proteins PA, PB2, and NA.
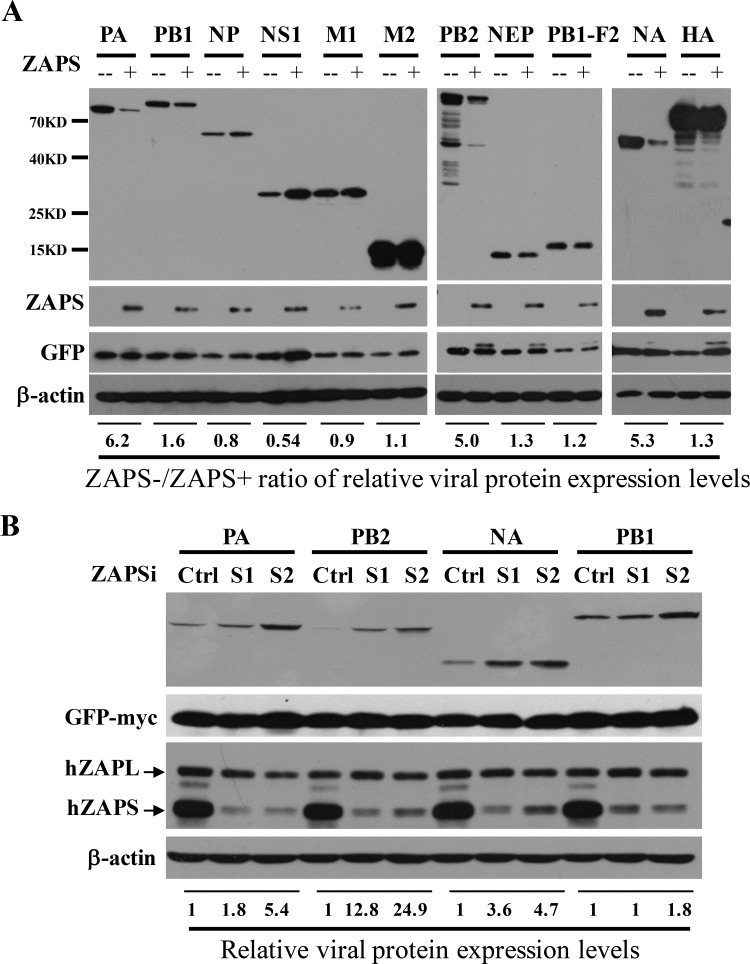
To explore whether ZAP interacts with IAV in a PARP domain-independent manner, we set out to analyze whether ZAPS inhibits the expression of the viral proteins. The expression levels of 11 viral proteins from IAV strain A/WSN/1933 (H1N1) were analyzed in the absence or presence of ectopic expression of human ZAPS (hZAPS) in HEK293 cells. The data showed that ZAPS significantly reduced the protein levels of PA, PB2, and NA (Fig. 1A). In comparison, the levels of the other IAV proteins were little affected (Fig. 1A). To substantiate the inhibitory activity of ZAPS against the expression of PA, PB2, and NA, the endogenous ZAPS was downregulated in HeLa cells using two small interfering RNAs (siRNAs), and the effects on the viral protein levels were evaluated. ZAPS was specifically and effectively downregulated by the siRNAs (Fig. 1B). Downregulation of ZAPS increased the levels of PA, PB2, and NA proteins (Fig. 1B). In comparison, downregulation of ZAPS had only a marginal effect on the protein levels of PB1 (Fig. 1B). These results suggested that expression of ZAPS reduced the protein levels of PA, PB2, and NA at an endogenous level or upon overexpression.
FIG 1.
ZAPS inhibits the expression of IAV proteins PA, PB2, and NA. (A) A plasmid expressing the Flag-tagged viral protein indicated was transiently transfected into HEK293 cells with (+) or without (−) a plasmid expressing myc-tagged human ZAPS. A plasmid expressing myc-tagged green fluorescent protein (GFP) was included to serve as a control for transfection efficiency and sample handling. At 48 h posttransfection, the cells were lysed, and the lysates were subjected to Western blotting analyses. The relative band intensities of the viral proteins were quantified with Image J software and normalized to the band intensities of GFP-myc. The ratio of the viral protein level in the absence of ZAPS (ZAPS−) to that in the presence of ZAPS (ZAPS+) was calculated. (B) HeLa cells were transfected with a control siRNA (Ctrl) or siRNAs targeting ZAPS (S1 and S2), together with a plasmid expressing the viral protein indicated. At 24 h posttransfection, protein expression levels were analyzed by Western blotting. The relative band intensities of the viral proteins were quantified with Image J software and normalized to the band intensities of GFP-myc. The relative expression level of the viral protein in the control cells was set as 1. The data presented are representative of the results of two independent experiments.
ZAPS reduces PB2 mRNA levels and represses its translation.
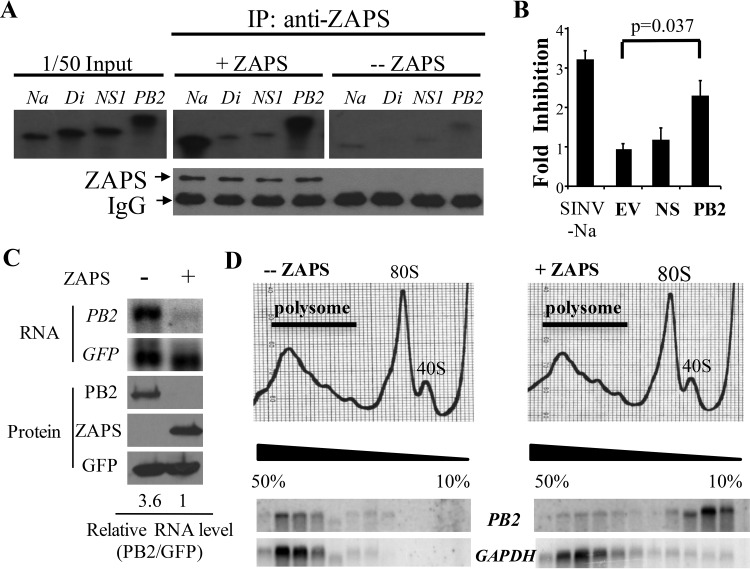
We next investigated the mechanisms by which ZAPS inhibits the expression of the viral proteins. Assuming that ZAPS inhibits the expression of PA, PB2, and NA by the same mechanism, we chose to focus on PB2. We previously reported that ZAPS inhibits target mRNA expression by binding to the mRNA, repressing the translation and/or promoting the degradation of the target mRNA. We first analyzed whether ZAPS binds to PB2 RNA using an in vitro binding assay (10). An RNA fragment derived from SINV that has been shown to bind ZAPS, Na, was used as a positive control, and a fragment from SINV that does not bind ZAPS, Di, was used as a negative control (10). Indeed, ZAPS specifically bound to PB2 RNA (Fig. 2A). In contrast, ZAPS did not bind to NS1 RNA (Fig. 2A), consistent with the above-mentioned results showing that ZAPS did not inhibit NS1 protein expression. To further demonstrate that PB2 is the target of ZAPS, the sense sequence of PB2 was cloned into a luciferase reporter downstream of the coding sequence of luciferase. Note that in this reporter, PB2 protein is not expected to be expressed. The reporter was transfected into HEK293TRex-hZAPS cells, which express human ZAPS in a tetracycline-inducible manner (7). The sensitivity of the reporter to ZAPS was indicated by the fold inhibition, which was calculated as the luciferase activity in mock-treated cells divided by that in the tetracycline-treated cells. As expected, cloning of the PB2 sequence rendered the reporter sensitive to ZAPS (Fig. 2B). In contrast, cloning of the NS sequence into the reporter had little effect (Fig. 2B).
FIG 2.
ZAPS posttranscriptionally inhibits PB2 expression. (A) (Top) Radiolabeled RNA fragments were prepared by in vitro transcription and incubated with ZAPS that was immobilized on Sepharose resin. The RNA bound to ZAPS was analyzed by electrophoresis on a urea polyacrylamide gel, followed by radioautography. (Bottom) Western blotting confirmed that equivalent amounts of ZAPS were immobilized on the resins. Na, a SINV-derived fragment serving as a positive control; Di, a SINV-derived fragment serving as a negative control; +ZAPS, lysate of 293TRex-hZAPS cells treated with tetracycline to induce ZAPS expression; –ZAPS, lysates of 293TRex-hZAPS cells without tetracycline treatment. IP, immunoprecipitation. (B) The sense sequences of NS and PB2 were cloned into the firefly luciferase reporter pGl3-Luc-linker. The plasmids were transfected into 293TRex-hZAPS cells together with a Renilla luciferase-expressing control reporter. At 6 h posttransfection, cells were mock treated or treated with tetracycline to induce ZAPS expression. At 48 h posttransfection, the cells were lysed and luciferase activities were measured. Firefly luciferase activity was normalized to Renilla luciferase activity. The fold inhibition was calculated as normalized luciferase activity in mock-treated cells divided by that in tetracycline-treated cells. The data presented are means and standard deviations (SD) of the results of three independent experiments. EV, empty vector (pGl3-Luc-linker); SINV-Na, pGL3-Luc reporter containing an Na fragment as a positive control. (C and D) A plasmid expressing triple-Flag-tagged PB2 was transfected into HEK293 cells with an empty vector (–ZAPS) or a plasmid expressing ZAPS (+ZAPS). A plasmid expressing GFP-myc was included to serve as a control. At 48 h posttransfection, cycloheximide was added to stop translation, and the cells were lysed. (C) An aliquot of the lysate was analyzed by Northern blotting for PB2 RNA levels (top) or subjected to Western blotting (bottom). The relative RNA levels of PB2 were quantified with a phosphorimager and normalized to the RNA levels of GFP. The relative RNA level of PB2 in the cells expressing ZAPS was set as 1. (D) (Top) The rest of the lysate was applied to sucrose continuous-gradient centrifugation, followed by polysome-profiling analysis. (Bottom) PB2 mRNA levels in each fraction were measured by Northern blotting. The data presented are representative of the results of two independent experiments.
We next analyzed the effect of ZAPS overexpression on the levels of PB2 RNA. A plasmid expressing PB2 was transfected into HEK293 cells with or without ZAPS, and PB2 mRNA levels were measured by Northern blotting. The data showed that ZAPS significantly reduced the PB2 mRNA level, as well as the protein level (Fig. 2C). To test whether ZAPS also represses the translation of PB2, polysome-profiling assays were performed. Consistent with our previous results (14), ZAPS did not change the polysome distribution pattern of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA, indicating that ZAPS did not affect global mRNA translation (Fig. 2D). In the presence of ZAPS, most PB2 mRNA was excluded from the polysome fractions (Fig. 2D). Taken together, these results indicate that ZAPS reduces the mRNA levels of PB2 and represses its translation.
ZAP inhibits IAV replication only at an early stage.
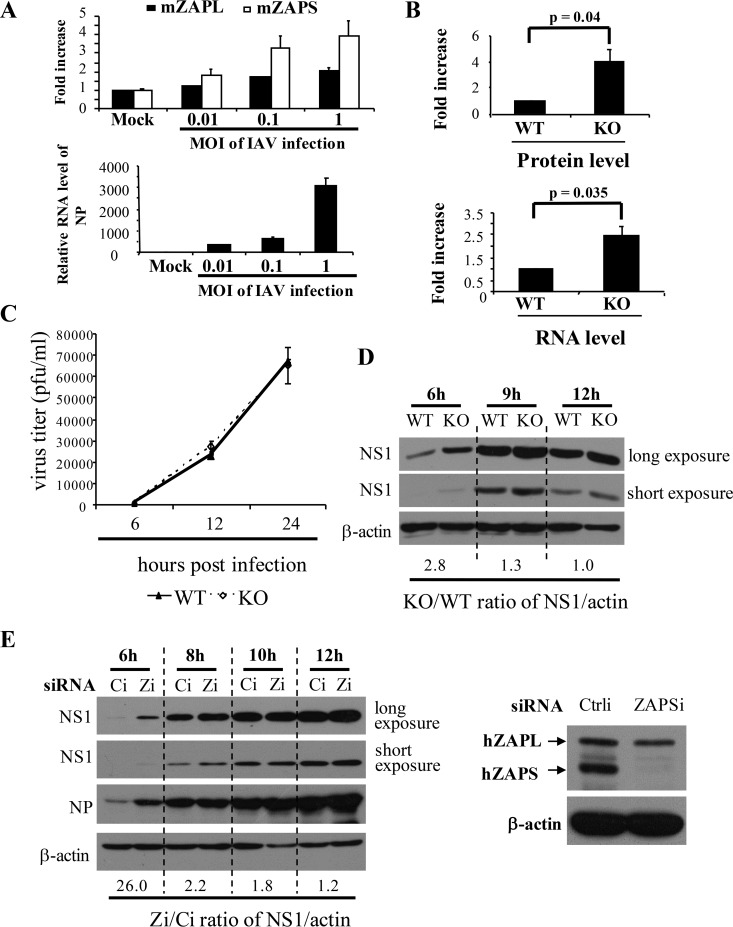
PA and PB2 play essential roles as components of the polymerase complex in the transcription and replication of the viral RNAs. We therefore asked whether ZAPS inhibits the replication of IAV. We first used mouse embryonic fibroblasts (MEFs) isolated from wild-type and ZAP knockout mice to evaluate the antiviral activity of endogenous ZAP. In the knockout cells, the second exon, which encodes a fragment of the N-terminal domain of ZAP protein, was deleted, so that neither the ZAPS nor the ZAPL protein could be expressed (30). The basal mRNA levels of ZAPS and ZAPL were relatively low in wild-type MEFs, and IAV infection upregulated their expression (Fig. 3A). Consistent with previously reported results (31), IAV infection induced higher levels of ZAPS expression than ZAPL (Fig. 3A). To test whether the endogenous ZAP in the MEFs posttranscriptionally inhibits IAV protein expression, a ZAPS-sensitive reporter, PB2-luc, in which the coding sequence of the viral protein was fused in frame with that of firefly luciferase, was transfected into the MEFs. In the knockout cells, both the protein and mRNA levels of the reporter were indeed increased (Fig. 3B). To determine the effect of ZAP knockout on the replication of IAV, the MEFs were challenged with IAV at a multiplicity of infection (MOI) of 0.1, and the titers of the progeny virus in the culture supernatants were measured by plaque assays in MDCK cells at various time points to monitor virus replication. Surprisingly, little difference was observed in the knockout and wild-type cells (Fig. 3C). We then analyzed the early stage of viral replication in these cells. The cells were infected with the virus at an MOI of 1, and viral replication was monitored by measuring the protein levels of NS1, which is newly synthesized during viral replication. The data showed that at 6 h postinfection, higher levels of NS1 protein were detected in the knockout cells; the ratio of the NS1 protein level in ZAP knockout cells to that in wild-type cells was about 2.8 (Fig. 3D). This ratio decreased over time (Fig. 3D); the NS1 protein levels were comparable in the two types of cells at 12 h postinfection (Fig. 3D). These results suggested that ZAP knockout enhanced viral replication at an early stage. To substantiate this notion and to evaluate the role of ZAPS in IAV replication, ZAPS was specifically downregulated with an siRNA in HeLa cells (Fig. 3E). IAV replication in these cells was monitored by measuring the protein levels of NS1 and NP. Unlike NS1, NP is from both viral replication and the incoming virions. The data showed that downregulation of ZAPS led to increased NS1 and NP levels at 6 h postinfection compared with those in the control cells (Fig. 3E), indicating that the virus replicated better in the ZAPS-downregulated cells at this time point. However, the difference in the NS1 protein levels in the cells with and without ZAPS downregulation dropped over time (Fig. 3E). Taken together, these results indicate that the endogenous ZAPS modestly inhibited IAV replication at a very early stage. However, ZAP failed to significantly inhibit the long-term replication of IAV.
FIG 3.
ZAPS inhibits IAV replication only at an early stage. (A) (Top) Wild-type MEFs were infected with IAV at the MOIs indicated for 1 h. At 12 h postinfection, total RNA was isolated. The mRNA levels of ZAPL, ZAPS, and IAV NP were determined by real-time PCR using GAPDH mRNA as an internal control. The fold increase in ZAPS and ZAPL mRNA levels was calculated as the relative RNA level in the cells infected with IAV divided by that in mock-treated cells. (Bottom) The NP mRNA levels confirmed that the cells were infected with increasing amounts of IAV. The data presented are means and SD of the results of two independent experiments. mZAPL and mZAPS, murine ZAPL and ZAPS, respectively. (B) (Top) Wild-type (WT) and ZAP knockout (KO) MEFs were transfected with a plasmid expressing PB2-luc, together with a control Renilla luciferase reporter. At 36 h posttransfection, the cells were lysed and luciferase activities were measured. Firefly luciferase activity was normalized to Renilla luciferase activity. The fold increase in protein levels was calculated as normalized luciferase activity in KO cells divided by that in WT cells. (Bottom) The cytoplasmic RNA was isolated, and reporter RNA levels were determined by real-time PCR. The firefly reporter RNA level was normalized to the Renilla mRNA level. The fold increase in the RNA levels was calculated as the normalized mRNA level in KO cells divided by that in WT cells. The data presented are the means and SD of the results of three independent experiments. (C) MEFs were infected with IAV at an MOI of 0.1 for 2 h. At the time points indicated, virus titers were determined. The data presented are the means ± SD of two independent measurements, representative of the results of three independent experiments. (D) MEFs were infected with IAV at an MOI of 1 for 1 h. At the time points indicated, NS1 expression levels were analyzed by Western blotting. The relative band intensities of NS1 were quantified with Image J software and normalized to the band intensities of β-actin. The ratio of the NS1 expression level in KO cells to that in WT cells was calculated. (E) (Left) HeLa cells were transfected with a control siRNA (Ci) or an siRNA targeting ZAPS (Zi). At 24 h posttransfection, the cells were infected with IAV. At the time points indicated, the cells were lysed and viral protein expression levels were detected by Western blotting. The ratio of the NS1 expression level in cells transfected with Zi to that in the control cells was calculated as described above. The data presented are representative of the results of three independent experiments. (Right) Western blotting confirmed that ZAPS was specifically and effectively downregulated.
Overexpression of NS1 and NP suppresses the antiviral activity of ZAPS.
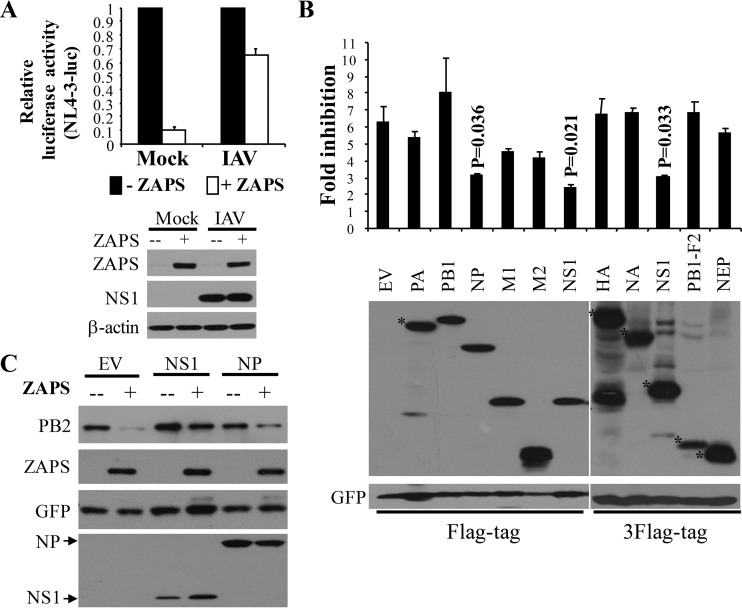
Based on the above-mentioned results, we speculated that the antiviral activity of ZAPS might be antagonized by the virus at a later stage of replication, when viral proteins were fully expressed. To test whether IAV expresses ZAPS antagonists, ZAPS-expressing cells were infected with IAV and then challenged with NL4-3luc, an HIV-1 vector that is inhibited by ZAPS (7). IAV infection generally inhibited the expression of NL4-3luc with or without ZAPS (data not shown), presumably due to the toxicity of viral infection to the cells. Nonetheless, IAV infection dramatically relieved ZAPS inhibition of NL4-3luc expression (Fig. 4A). Notably, IAV infection had little effect on the induced expression of ZAPS (Fig. 4A). These results supported our hypothesis that IAV might encode ZAPS antagonists. To identify ZAPS antagonists, IAV proteins were screened for the ability to relieve ZAPS inhibition of PB2-luc expression. The data showed that NS1 and NP displayed significant ZAPS-antagonizing activity, while the others had little effect, although all the viral proteins were expressed at comparable levels (Fig. 4B). To further demonstrate the ZAPS-antagonizing activities of NS1 and NP, we analyzed the effects of their overexpression on ZAPS inhibition of PB2-luc protein expression by Western blotting. Indeed, NS1 almost abolished ZAPS activity and NP modestly reduced ZAPS activity under these conditions (Fig. 4C).
FIG 4.
Identification of NS1 and NP as candidate ZAPS antagonists. (A) (Top) 293TRex-hZAPS cells were infected with IAV at an MOI of 0.1 for 2 h, followed by challenge with the vesicular stomatitis virus G protein (VSV-G)-pseudotyped HIV-1 vector NL4-3luc. The cells were mock treated or treated with tetracycline to induce ZAPS expression. At 24 h postinfection with NL4-3luc, the cells were lysed, luciferase activity was measured, and the expression levels of ZAPS and NS1 were detected by Western blotting. The relative luciferase activity in mock-treated cells was set as 1. The data presented are means and SD of three independent experiments. (Bottom) The expression of ZAPS was confirmed. NS1 protein expression was used to demonstrate IAV infection. (B) (Top) 293TRex-hZAPS cells were transfected with plasmids expressing the indicated Flag-tagged or triple-Flag-tagged IAV proteins, together with the PB2-luc reporter and a Renilla luciferase control reporter. At 6 h posttransfection, cells were mock treated or treated with tetracycline to induce ZAPS expression. At 48 h posttransfection, the cells were lysed and luciferase activities were measured. The fold inhibition was calculated as described in the legend to Fig. 2B. (Bottom) The expression levels of the viral proteins were analyzed by Western blotting. The data presented are means and SD of the results of three independent experiments. The positions of the IAV proteins are indicated by asterisks. (C) A plasmid expressing Flag-tagged PB2-luc was transfected into 293TRex-hZAPS cells with a plasmid expressing each indicated IAV protein. A plasmid expressing GFP-myc was used to serve as a control. At 6 h posttransfection, cells were mock treated or treated with tetracycline to induce ZAPS expression. At 48 h posttransfection, the cells were lysed and protein expression levels were analyzed by Western blotting.
NS1 antagonizes ZAPS by reducing its RNA-binding activity.
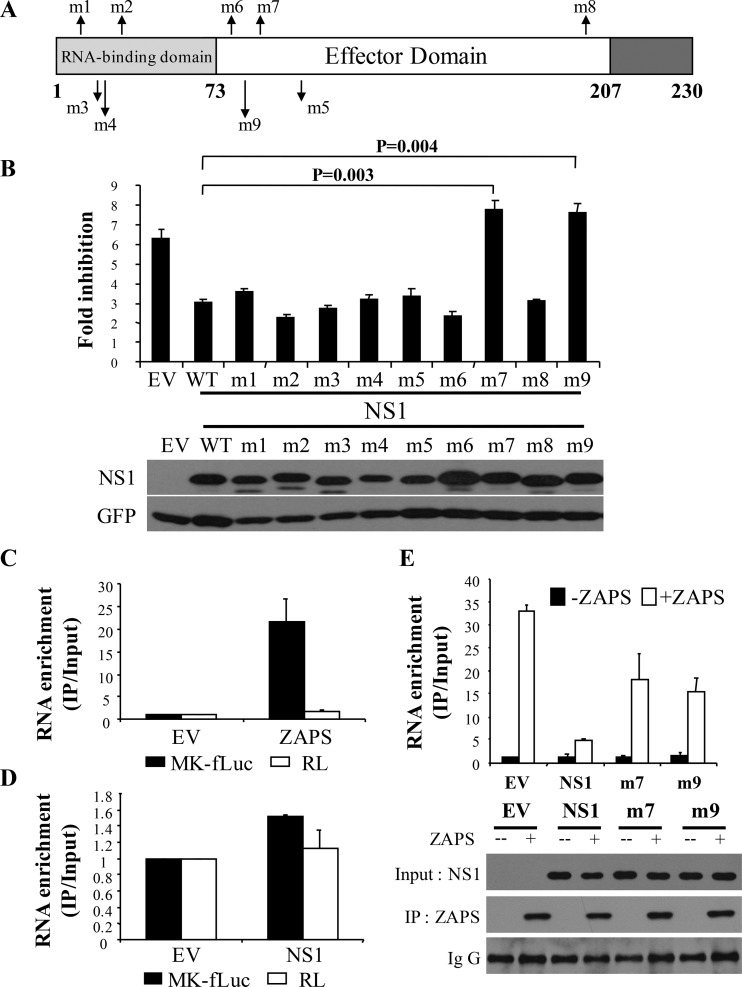
NS1 displayed much stronger ZAPS-antagonizing activity than NP (Fig. 4C). In addition, NS1 has been extensively studied (32). We thus focused on exploring the mechanism by which NS1 antagonizes ZAPS. We set out to search for NS1 mutants that failed to antagonize ZAPS. NS1 protein is composed of three domains: the N-terminal RNA-binding domain; a disordered C-terminal tail with about 20 amino acids; and an effector domain in the middle, which has been reported to mediate interactions with host proteins (20) (Fig. 5A). Based on the functions of the residues reported in the literature, nine NS1 mutants were constructed (Fig. 5A). The functions of these residues include those critical for RNA binding, important for counteracting IFN production, and those involved in interactions with the translational initiation complex (Table 1). These mutants were tested for the ability to antagonize the antiviral activity of ZAPS against PB2-luc. Among the nine mutants, seven retained ZAPS-antagonizing activity and two, NS1-m7 and NS1-m9, lost the activity (Fig. 5B).
FIG 5.
Identification of NS1 mutants that fail to antagonize ZAPS. (A) Schematic representation of NS1 mutants. (B) (Top) 293TRex-hZAPS cells were transfected with a plasmid expressing NS1 or the indicated NS1 mutants, together with the PB2-luc reporter and Renilla luciferase control reporter. At 6 h posttransfection, cells were mock treated or treated with tetracycline to induce ZAPS expression. At 48 h posttransfection, the cells were lysed and luciferase activities were measured. The fold inhibition was calculated as described in the legend to Fig. 2B. (Bottom) The expression levels of the NS1 mutants were measured by Western blotting. The data presented are means and SD of three independent experiments. (C and D) HEK293 cells were transfected with a plasmid expressing Flag-tagged NZAP or NS1, together with the ZAP-responsive reporter MK-fLuc and a control Renilla reporter (RL). At 48 h posttransfection, NZAP (C) or NS1 (D) was immunoprecipitated, and the amount of associated RNA was measured by real-time PCR. RNA enrichment was calculated as the amount of the precipitated reporter RNA divided by the amount of the RNA in the cell lysate. The relative RNA level nonspecifically associated in the lysate of empty-vector-transfected cells was set as 1. The data presented are means and SD of the results of two independent experiments. (E) (Top) HEK293 cells were transfected with a plasmid expressing NZAP and the MK-fLuc reporter, together with an empty vector or a plasmid expressing NS1 or an NS1 mutant. At 48 h posttransfection, NZAP was immunoprecipitated, and the amount of associated RNA was measured by real-time PCR. RNA enrichment was calculated as described for panels C and D. (Bottom) Western blotting confirmed that equivalent amounts of ZAPS were immunoprecipitated and that comparable levels of NS1 and the mutants were expressed.
TABLE 1.
Summary of NS1 mutants
| Name | Mutation | Function(s) of residuesa | References |
|---|---|---|---|
| m1 | D34ER35E | Conserved residues in a region involved in interaction with dsRNA | 33, 34 |
| m2 | R44EG45ER46E | Conserved residues in a region involved in interaction with dsRNA | 33, 34 |
| m3 | R38AK41A | Critical for dsRNA binding | 34, 35 |
| m4 | S42R | Involved in antagonizing host IFN response through NF-κB and IRF-3 pathways | 35, 36 |
| m5 | I123AM124A | Required for inhibiting PKR activity and regulating viral RNA synthesis | 37 |
| m6 | Y89A | Required for interaction with p85 beta and activation of PI3K | 25 |
| m7 | P107AK108AQ109AK110A | In a region mediating interaction with eIF4G | 27, 38 |
| m8 | R200A | Highly conserved residues on protein surface with functions unknown | 38 |
| m9 | E96AE97A | Required for interaction with Trim25 to block IFN response | 21 |
dsRNA, double-stranded RNA.
The NS1-m7 and NS1-m9 mutants were utilized to probe the mechanism by which NS1 antagonizes ZAPS. RNA binding is the first step for ZAPS to inhibit target mRNA expression. We thus analyzed the effect of NS1 expression on ZAPS binding to the target RNA using the RNA immunoprecipitation assay we previously reported (15). The N-terminal domain of ZAPS, which is the major RNA-binding domain of the protein, and a ZAP-responsive reporter, MK-fLuc (10), were coexpressed in HEK293 cells, together with NS1 or the NS1 mutants. ZAPS was immunoprecipitated, and the amount of reporter RNA coprecipitated was measured. The RNA-binding activity of ZAPS was indicated by the relative amount of coprecipitated reporter RNA out of the total reporter RNA in the cell lysate. The data showed that immunoprecipitation of ZAPS coprecipitated a considerable amount of the Mk-fLuc reporter RNA, but not the control reporter RNA (Fig. 5C), and that NS1 itself did not specifically bind to the reporter RNA (Fig. 5D). Expression of the wild-type NS1 reduced the RNA-binding activity of ZAPS by about 7-fold (Fig. 5E, top). In comparison, expression of the NS1 mutants reduced the RNA-binding activity of ZAPS by only about 2-fold (Fig. 5E, top). Western blot analysis revealed that comparable levels of NS1 and its mutants were expressed and that equivalent amounts of ZAPS were immunoprecipitated (Fig. 5E, bottom). Collectively, these results suggest that NS1 antagonizes ZAPS by inhibiting its binding to target RNA.
A recombinant IAV carrying NS1-m7 replicates better in ZAPS-deficient cells.
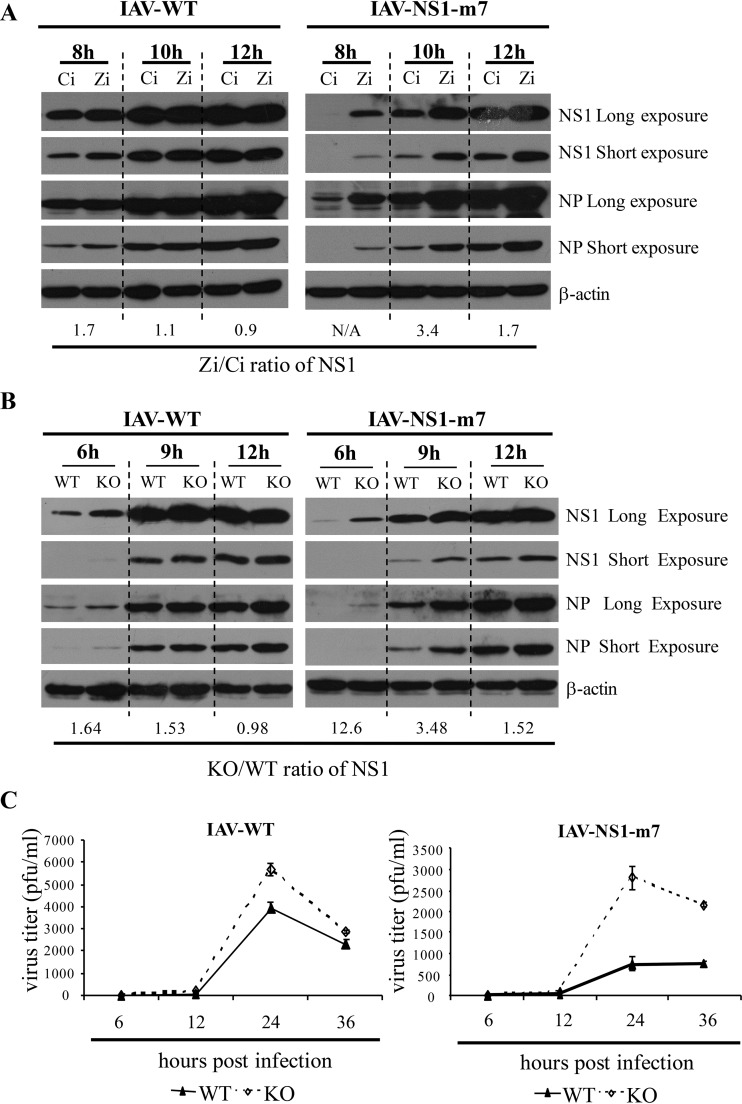
To further demonstrate the role of NS1 antagonism of ZAPS, we tried to produce recombinant IAV carrying NS1-m7 or NS1-m9. While the virus carrying NS1-m9 failed to replicate, NS1-m7 supported sustained viral replication, although the production efficiency and replication ability of the mutant virus were lower than those of the wild-type virus (data not shown). We first analyzed the early-stage viral replication in HeLa cells as described above. Downregulation of ZAPS led to modestly increased NS1 and NP levels at 8 h postinfection for the wild-type virus (Fig. 6A). In comparison, for the mutant virus, the increase in the NS1 and NP levels was much more dramatic at this time point (Fig. 6A). At 10 h postinfection, while the NS1 levels were comparable for the wild-type virus, the difference in the NS1 levels was still obvious for the mutant virus (Fig. 6A). Similar results were observed in MEFs (Fig. 6B). We next analyzed the long-term replication of the virus in MEFs. The data showed that the long-term replication curves of the wild-type virus were similar in the wild-type and ZAP knockout cells (Fig. 6C). In contrast, replication of the mutant virus was significantly increased in the knockout cells (Fig. 6C). Collectively, these results indicate that ZAP inhibits IAV replication at an endogenous level and that NS1 antagonizes ZAP.
FIG 6.
A recombinant IAV containing a mutant NS1 that fails to antagonize ZAPS replicates better in ZAPS-deficient cells. (A) HeLa cells were transfected with a control siRNA (Ci) or an siRNA targeting ZAPS (Zi). At 24 h posttransfection, the cells were infected with wild-type IAV (IAV-WT) or the mutant IAV containing NS1-m7 (IAV-NS1-m7). At the time points indicated, the cells were lysed and viral protein expression levels were detected by Western blotting. The ratio of the NS1 expression level in the cells transfected with Zi to that in the control cells was calculated as described in the legend to Fig. 3E. N/A, the NS1 protein level in the control cells was below detection limit. (B) MEFs were infected with the indicated IAVs at an MOI of 1 for 1 h. The viral protein expression levels were measured at the time points indicated. The NS1 ratio in KO and WT cells was calculated as described in the legend to Fig. 3D. The data presented are representative of the results of two independent experiments. (C) MEFs were infected with the indicated IAVs at an MOI of 0.01 for 2 h. The virus titers in the culture supernatants at the time points indicated were determined. The data presented are means ± SD of three independent measurements representative of the results of three independent experiments.
DISCUSSION
IAV infection induces an IFN-mediated immune response (19). To establish effective infection, the virus needs to develop mechanisms to counteract the immune responses. IAV infection of MEFs upregulated the expression of both ZAPS and ZAPL (Fig. 3A). ZAPL inhibits the replication of IAV by promoting proteasomal degradation of PB2 and PA (29). This antiviral activity of ZAPL is antagonized by PB1. Here, we provide evidence showing that ZAPS posttranscriptionally inhibits IAV protein expression. This antiviral activity of ZAPS is antagonized by NS1. The fact that ZAP uses two distinct mechanisms to inhibit IAV replication and that both are antagonized by the virus-encoded proteins suggests an important role of ZAP in the efforts of the host to control IAV infection and the importance of the threat from ZAP to the virus.
In this study, the antiviral activity of endogenous ZAP against IAV replication was examined in both HeLa cells and MEFs. Downregulation of endogenous ZAPS in HeLa cells and knockout of ZAP in MEFs both led to higher viral protein levels at an early stage of viral replication (Fig. 3). However, the difference decreased over time. We provide evidence suggesting that the antiviral activity of ZAP was antagonized by NS1 (Fig. 4). Overexpressed NP also displayed ZAPS-antagonizing activity (Fig. 4). Further investigation is needed to confirm this activity and to explore its role in the interactions between ZAP and IAV.
IAV NS1 is an RNA-binding protein that plays multiple roles in viral replication and in counteracting the host immune responses (20, 32). Here, we provide evidence suggesting that NS1 antagonizes ZAP by reducing its binding to the target RNA. How NS1 reduced the RNA binding activity of ZAP is not yet clear. We screened nine NS1 mutants (Table 1). Residues mutated in NS1-m1 and -m2 are critical for the RNA-binding activity of NS1 (33). NS1-m3 has been reported to have reduced RNA-binding activity (33, 34). These mutants retained the ZAPS-antagonizing activity (Fig. 5B), suggesting that the RNA-binding activity of NS1 is not required for its antagonizing activity. Moreover, NS1 did not specifically bind the target RNA of ZAPS (Fig. 5D). These results suggest that NS1 did not antagonize ZAPS by competing with ZAPS for binding target RNA. NS1-m3 has also been reported to have compromised activity to inhibit the production or action of IFN (34–36). Residues mutated in NS1-m4 play important roles in suppressing IFN induction and activation of the NF-κB and IRF-3 pathways (36). NS1-m5 does not bind to PKR and cannot antagonize it (37). The residue mutated in NS1-m6 is required for NS1 binding to p85β and the activation of PI3K signaling (25). These mutants all retained the ZAPS-antagonizing activity, suggesting that the above-mentioned processes are not involved in the NS1 antagonism of ZAPS.
Two NS1 mutants, NS1-m7 and NS1-m9, that lost ZAPS-antagonizing activity were identified. The residues mutated in NS1-m7 reside in a region involved in NS1 interaction with eIF4G, but the functions of these residues are not known (27, 38). NS1-m9 has been reported to lose the ability to suppress the E3 ligase activity of TRIM25 (21). Our preliminary results suggest that TRIM25-mediated ubiquitination of ZAPS is critical for its antiviral activity (X. Zheng and G. Gao, unpublished results). NS1 suppressed TRIM25-mediated ubiquitination of ZAP, but NS1-m7 and NS1-m9 failed to do so (Q. Tang and G. Gao, unpublished results). These results suggest that NS1 might antagonize ZAPS through TRIM25. In-depth understanding of the mechanisms by which NS1 antagonizes ZAPS awaits further investigation.
In summary, here, we report that ZAPS inhibits IAV replication at an early stage of infection, at least partially through posttranscriptional inhibition of the expression of PA, PB2, and NA. This antiviral activity of ZAP is antagonized by the virus-encoded NS1.
MATERIALS AND METHODS
Plasmids and antibodies.
The firefly luciferase reporters pGL3-Luc-linker, pFL-MK-fLuc, and pNL4-3luc and the Renilla luciferase control reporter pRL-TK have been described previously (6, 10, 39). The expression vector pcDNA4-myc-HisB was modified from pcDNA4/TO/myc-HisB (Invitrogen) by deleting the Tet operon sequence. The IAV sequences were derived from strain A/WSN/1933 (H1N1) (40). To construct the reporter expressing PB2-luc, the coding sequence of PB2 was cloned in frame with the firefly luciferase coding sequence into pcDNA4-myc-HisB. The sequences of the NS and PB2 segments were cloned into pGL3-Luc-linker downstream of the firefly luciferase coding sequence to test their ability to render the reporter sensitive to ZAP. To construct plasmids for in vitro transcription of IAV segments, the NS1 and PB2 sequences were cloned into pBluescript (10). To express Flag-tagged or triple-Flag-tagged IAV proteins, the coding sequences were PCR amplified and cloned into pcDNA4-Flag or pcDNA4-3Flag, which were modified from pcDNA4-HisB-myc. The coding sequence of HA was cloned into pCAGGS-3Flag (pCAGGS was kindly provided by George F. Gao). The mutations of NS1 were generated by overlapping PCR and cloned into the expression vector pCMV-HA-Flag (11) or pcDNA4-myc.
To express myc-tagged ZAPS, the coding sequence was cloned into pcDNA4/TO/myc-HisB (Invitrogen). To express the N-terminal 254 amino acids of ZAP with 2 Flag-tags (NZAP-2Flag), the coding sequence of the zeocin resistance gene in pcDNA4-TO/myc-NZAP-Zeo (7) was replaced with that of 2Flag-tag. The sequence of NZAP-2Flag was then cloned into pcDNA4-myc-HisB to generate pcDNA4-NZAP-2Flag.
Antibodies against human ZAP (PA5-31650) were obtained from Thermo Fisher. The antibody against NS1 (sc-130568) was obtained from Santa Cruz. The antibody against NP was kindly provided by Wenjun Liu of the Institute of Microbiology, Chinese Academy of Sciences (40).
Cells and viruses.
All cells were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (Gibco). HEK293TRex-hZAPS cells, which express human ZAPS in a tetracycline-inducible manner, have been described previously (7, 10). MEFs were isolated from 14- to 16-day-old mouse embryos. Plasmid transfection was performed using neofectin following the manufacturer's instructions (NeoBiolab).
To downregulate endogenous ZAPS expression, HeLa cells (ATCC) were seeded in 35-mm tissue culture dishes. The next day, the cells were transfected with 100 pmol of a control siRNA or siRNAs targeting ZAPS using Lipofectamine 2000 (Invitrogen). At 24 h posttransfection, the cells were transfected again with 100 pmol siRNAs together with the reporter plasmids using Lipofectamine 2000. Twenty-four hours later, the cells were analyzed. The target sequences of the siRNAs (Genepharma) against hZAPS were sihZAPS-1, 5′-AGGGTTTTGGTGAGAGATAATAT-3′, and sihZAPS-2, 5′-TTGTTTTGCTGTGGATTCTTTTT-3′.
IAVs were generated as described previously (41). The virus stock was grown in 10-day-old fertilized eggs for 72 h. The virus was harvested for titration on MDCK cells, as described previously (42).
Luciferase activities were measured using either the luciferase assay system for firefly luciferase or the Dual-luciferase Reporter Assay System for both firefly and Renilla luciferase (Promega).
Real-time PCR.
RNA was reverse transcribed in a 20-μl reaction mixture (RNA template, 5 pmol/ml reverse transcription primer, 0.5 mM each deoxynucleoside triphosphate [dNTP], 50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2, 5 mM dithiothreitol [DTT], and 1 μl reverse transcriptase). The RNA levels were measured by SYBR green real-time PCR (Tiangen) in a Rotor-gene 6000 (Corbett Life Science) with the following program: (i) 95°C for 10 min and (ii) 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s for 40 cycles. The primers for real-time PCR were as follows: mZAP FP, 5′-AGAATTACGAGTTAAGCTTTCAAGGGATGA-3′; mZAPL RP, 5′-CGCGTGAAATAGGAACATCTCAGTCTTGTT-3′; mZAPS RP, 5′-AAACCCAAGGCCTTGTATCTGTTACACAAA-3′; mGAPDH FP, 5′-CAACAGCAACTCCCACTCTTC-3′; mGAPDH RP, 5′-GGTCCAGGGTTTCTTACTCCTT-3′; PB2 FP, 5′-GAGCAGGGACAAACTTTATGGAGTAA-3′; PB2 RP, 5′-ATGCCACCATCAGAGGAGAAATTTT-3′; FL-MK-fLuc FP, 5′-TGAGGCACTGGGCAGGTGTC-3′; FL-MK-fLuc RP, 5′-ATGCAGTTGCTCTCCAGCGG-3′; RL FP, 5′-TGAGGCACTGGGCAGGTGTC-3′; and RL RP, 5′-ATGAAGGAGTCCAGCACGTTC-3′.
Polysome-profiling analysis.
A plasmid expressing triple-Flag-tagged PB2 was transfected into HEK293 cells (ATCC) together with a plasmid expressing ZAPS. At 48 h posttransfection, cycloheximide was added to the medium at a final concentration of 50 μg/ml to stop translation for 30 min. The cells were lysed with lysis buffer (300 mM KCl, 5 mM MgCl2, 10 mM HEPES [pH 7.4], 0.5% NP-40, and 100 μg/ml cycloheximide). The lysates were clarified, applied to a 10 to 50% sucrose continuous gradient, and centrifuged at 36,000 rpm for 3.5 h at 4°C. Twelve fractions, 1 ml each, were collected on ice. The absorbance at 254 nm was monitored and recorded to indicate the positions of ribosome subunits and polysomes. To extract RNA, 500 μl of each proteinase K-treated fraction was extracted twice with 500 μl phenol-chloroform-isoamyl alcohol (25:24:1) and once with 500 μl chloroform-isoamyl alcohol (49:1), followed by ethanol precipitation. The RNA was detected by Northern blotting.
Northern blotting.
RNA samples were separated by electrophoresis, transferred to a nylon membrane, and hybridized for 15 to 20 h with 32P-labeled probes prepared with the random-primer-labeling kit (Stratagene). The membrane was washed three times with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and once with 0.1% sodium dodecyl sulfate at 42°C, followed by exposure to X-ray films.
ACKNOWLEDGMENTS
We thank George F. Gao, Wenjun Liu, and Xin Ye of the Institute of Microbiology, Chinese Academy of Sciences, for providing invaluable materials, and we thank members of the laboratory of Wenjun Liu for technical assistance.
This work was supported by grants to Guangxia Gao from the Ministry of Science and Technology of the People's Republic of China (973 Program grant number 2012CB910203) and the National Natural Science Foundation of China (grant number 81530066). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Ryman KD, Meier KC, Nangle EM, Ragsdale SL, Korneeva NL, Rhoads RE, MacDonald MR, Klimstra WB. 2005. Sindbis virus translation is inhibited by a PKR/RNase L-independent effector induced by alpha/beta interferon priming of dendritic cells. J Virol 79:1487–1499. doi: 10.1128/JVI.79.3.1487-1499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang N, Dong Q, Li J, Jangra RK, Fan M, Brasier AR, Lemon SM, Pfeffer LM, Li K. 2010. Viral induction of the zinc finger antiviral protein is IRF3-dependent but NF-kappaB-independent. J Biol Chem 285:6080–6090. doi: 10.1074/jbc.M109.054486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDonald MR, Machlin ES, Albin OR, Levy DE. 2007. The zinc finger antiviral protein acts synergistically with an interferon-induced factor for maximal activity against alphaviruses. J Virol 81:13509–13518. doi: 10.1128/JVI.00402-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bick MJ, Carroll JWN, Gao G, Goff SP, Rice CM, MacDonald MR. 2003. Expression of the zinc-finger antiviral protein inhibits alphavirus replication. J Virol 77:11555–11562. doi: 10.1128/JVI.77.21.11555-11562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller S, Moller P, Bick MJ, Wurr S, Becker S, Gunther S, Kummerer BM. 2007. Inhibition of filovirus replication by the zinc finger antiviral protein. J Virol 81:2391–2400. doi: 10.1128/JVI.01601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao G, Guo X, Goff SP. 2002. Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science 297:1703–1706. doi: 10.1126/science.1074276. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, Chen G, Lv F, Wang X, Ji X, Xu Y, Sun J, Wu L, Zheng YT, Gao G. 2011. Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation. Proc Natl Acad Sci U S A 108:15834–15839. doi: 10.1073/pnas.1101676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao R, Nie H, Cai D, Zhang J, Liu H, Yan R, Cuconati A, Block TM, Guo JT, Guo H. 2013. Inhibition of hepatitis B virus replication by the host zinc finger antiviral protein. PLoS Pathog 9:e1003494. doi: 10.1371/journal.ppat.1003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerns JA, Emerman M, Malik HS. 2008. Positive selection and increased antiviral activity associated with the PARP-containing isoform of human zinc-finger antiviral protein. PLoS Genet 4:e21. doi: 10.1371/journal.pgen.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo X, Carroll JW, MacDonald MR, Goff SP, Gao G. 2004. The zinc finger antiviral protein directly binds to specific viral mRNAs through the CCCH zinc finger motifs. J Virol 78:12781–12787. doi: 10.1128/JVI.78.23.12781-12787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo X, Ma J, Sun J, Gao G. 2007. The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. Proc Natl Acad Sci U S A 104:151–156. doi: 10.1073/pnas.0607063104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Guo X, Lv F, Xu Y, Gao G. 2008. p72 DEAD box RNA helicase is required for optimal function of the zinc-finger antiviral protein. Proc Natl Acad Sci U S A 105:4352–4357. doi: 10.1073/pnas.0712276105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Xu Y, Zhang K, Wang X, Sun J, Gao G, Liu Y. 2012. Structure of N-terminal domain of ZAP indicates how a zinc-finger protein recognizes complex RNA. Nat Struct Mol Biol 19:430–435. doi: 10.1038/nsmb.2243. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Wang X, Goff SP, Gao G. 2012. Translational repression precedes and is required for ZAP-mediated mRNA decay. EMBO J 31:4236–4246. doi: 10.1038/emboj.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xuan Y, Gong D, Qi J, Han C, Deng H, Gao G. 2013. ZAP inhibits murine gammaherpesvirus 68 ORF64 expression and is antagonized by RTA. J Virol 87:2735–2743. doi: 10.1128/JVI.03015-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das K, Aramini JM, Ma LC, Krug RM, Arnold E. 2010. Structures of influenza A proteins and insights into antiviral drug targets. Nat Struct Mol Biol 17:530–538. doi: 10.1038/nsmb.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krug RM, Aramini JM. 2009. Emerging antiviral targets for influenza A virus. Trends Pharmacol Sci 30:269–277. doi: 10.1016/j.tips.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina RA, Garcia-Sastre A. 2011. Influenza A viruses: new research developments. Nat Rev Microbiol 9:590–603. doi: 10.1038/nrmicro2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrhardt C, Seyer R, Hrincius ER, Eierhoff T, Wolff T, Ludwig S. 2010. Interplay between influenza A virus and the innate immune signaling. Microbes Infect 12:81–87. doi: 10.1016/j.micinf.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Hale BG, Randall RE, Ortin J, Jackson D. 2008. The multifunctional NS1 protein of influenza A viruses. J Gen Virol 89:2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- 21.Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan M, Inoue S, Jung JU, Garcia-Sastre A. 2009. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5:439–449. doi: 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min JY, Krug RM. 2006. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: inhibiting the 2′-5′ oligo(A) synthetase/RNase L pathway. Proc Natl Acad Sci U S A 103:7100–7105. doi: 10.1073/pnas.0602184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergmann M, Garcia-Sastre A, Carnero E, Pehamberger H, Wolff K, Palese P, Muster T. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J Virol 74:6203–6206. doi: 10.1128/JVI.74.13.6203-6206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Min JY, Krug RM, Sen GC. 2006. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology 349:13–21. doi: 10.1016/j.virol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Hale BG, Jackson D, Chen YH, Lamb RA, Randall RE. 2006. Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling. Proc Natl Acad Sci U S A 103:14194–14199. doi: 10.1073/pnas.0606109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de la Luna S, Fortes P, Beloso A, Ortin J. 1995. Influenza virus NS1 protein enhances the rate of translation initiation of viral mRNAs. J Virol 69:2427–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aragon T, de la Luna S, Novoa I, Carrasco L, Ortin J, Nieto A. 2000. Eukaryotic translation initiation factor 4GI is a cellular target for NS1 protein, a translational activator of influenza virus. Mol Cell Biol 20:6259–6268. doi: 10.1128/MCB.20.17.6259-6268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgui I, Aragon T, Ortin J, Nieto A. 2003. PABP1 and eIF4GI associate with influenza virus NS1 protein in viral mRNA translation initiation complexes. J Gen Virol 84:3263–3274. doi: 10.1099/vir.0.19487-0. [DOI] [PubMed] [Google Scholar]
- 29.Liu CH, Zhou L, Chen G, Krug RM. 2015. Battle between influenza A virus and a newly identified antiviral activity of the PARP-containing ZAPL protein. Proc Natl Acad Sci U S A 112:14048–14053. doi: 10.1073/pnas.1509745112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Li MM, Zhao J, Li S, MacDonald MR, Rice CM, Gao X, Gao G. 2016. Sindbis virus can exploit a host antiviral protein to evade immune surveillance. J Virol 90:10247–10258. doi: 10.1128/JVI.01487-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodier JL, Pereira GC, Cheung LE, Rose RJ, Kazazian HH Jr. 2015. The broad-spectrum antiviral protein ZAP restricts human retrotransposition. PLoS Genet 11:e1005252. doi: 10.1371/journal.pgen.1005252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krug RM. 2015. Functions of the influenza A virus NS1 protein in antiviral defense. Curr Opin Virol 12C:1–6. doi: 10.1016/j.coviro.2015.01.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin C, Khan JA, Swapna GV, Ertekin A, Krug RM, Tong L, Montelione GT. 2007. Conserved surface features form the double-stranded RNA binding site of non-structural protein 1 (NS1) from influenza A and B viruses. J Biol Chem 282:20584–20592. doi: 10.1074/jbc.M611619200. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Riedel K, Lynch P, Chien CY, Montelione GT, Krug RM. 1999. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA 5:195–205. doi: 10.1017/S1355838299981621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donelan NR, Basler CF, Garcia-Sastre A. 2003. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J Virol 77:13257–13266. doi: 10.1128/JVI.77.24.13257-13266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiao P, Tian G, Li Y, Deng G, Jiang Y, Liu C, Liu W, Bu Z, Kawaoka Y, Chen H. 2008. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J Virol 82:1146–1154. doi: 10.1128/JVI.01698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min JY, Li S, Sen GC, Krug RM. 2007. A site on the influenza A virus NS1 protein mediates both inhibition of PKR activation and temporal regulation of viral RNA synthesis. Virology 363:236–243. doi: 10.1016/j.virol.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 38.Darapaneni V, Prabhaker VK, Kukol A. 2009. Large-scale analysis of influenza A virus sequences reveals potential drug target sites of non-structural proteins. J Gen Virol 90:2124–2133. doi: 10.1099/vir.0.011270-0. [DOI] [PubMed] [Google Scholar]
- 39.Connor RI, Chen BK, Choe S, Landau NR. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 40.Yu M, Liu X, Cao S, Zhao Z, Zhang K, Xie Q, Chen C, Gao S, Bi Y, Sun L, Ye X, Gao GF, Liu W. 2012. Identification and characterization of three novel nuclear export signals in the influenza A virus nucleoprotein. J Virol 86:4970–4980. doi: 10.1128/JVI.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez DR, Donis R, Hoffmann E, Hobom G, Kawaoka Y. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A 96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huprikar J, Rabinowitz S. 1980. A simplified plaque assay for influenza viruses in Madin-Darby kidney (MDCK) cells. J Virol Methods 1:117–120. doi: 10.1016/0166-0934(80)90020-8. [DOI] [PubMed] [Google Scholar]