ABSTRACT
Neuraminidase (NA) is a sialidase expressed on the surface of influenza A viruses that releases progeny viruses from the surface of infected cells and prevents viruses becoming trapped in mucus. It is a homotetramer, with each monomer consisting of a transmembrane region, a stalk, and a globular head with sialidase activity. We recently characterized two swine viruses of the pandemic H1N1 lineage, A/swine/Virginia/1814-1/2012 (pH1N1low-1) and A/swine/Virginia/1814-2/2012 (pH1N1low-2), with almost undetectable NA enzymatic activity compared to that of the highly homologous A/swine/Pennsylvania/2436/2012 (pH1N1-1) and A/swine/Minnesota/2499/2012 (pH1N1-2) viruses. pH1N1-1 transmitted to aerosol contact ferrets, but pH1N1low-1 did not. The aim of this study was to identify the molecular determinants associated with low NA activity as potential markers of aerosol transmission. We identified the shared unique substitutions M19V, A232V, D248N, and I436V (N1 numbering) in pH1N1low-1 and pH1N1low-2. pH1N1low-1 also had the unique Y66D substitution in the stalk domain, where 66Y was highly conserved in N1 NAs. Restoration of 66Y was critical for the NA activity of pH1N1low-1 NA, although 19M or 248D in conjunction with 66Y was required to recover the level of activity to that of pH1N1 viruses. Studies of NA stability and molecular modeling revealed that 66Y likely stabilized the NA homotetramer. Therefore, 66Y in the stalk domain of N1 NA was critical for the stability of the NA tetramer and, subsequently, for NA enzymatic activity.
IMPORTANCE Neuraminidase (NA) is a sialidase that is one of the major surface glycoproteins of influenza A viruses and the target for the influenza drugs oseltamivir and zanamivir. NA is important as it releases progeny viruses from the surface of infected cells and prevents viruses becoming trapped in mucus. Mutations in the globular head domain that decrease enzymatic activity but confer resistance to NA inhibitors have been characterized; however, the importance of specific mutations in the stalk domain is unknown. We identified 66Y (N1 numbering), a highly conserved amino acid that was critical for the stability of the NA tetramer and, subsequently, for NA enzymatic activity.
KEYWORDS: influenza, neuraminidase, stalk
INTRODUCTION
Influenza A viruses express two major glycoproteins, hemagglutinin (HA) and neuraminidase (NA), on their surface. The classical role of HA is to attach to sialic acids on the surface of cells to initiate infection, while the classical role of NA is to release progeny virions from infected cells by enzymatically cleaving sialic acids bound to HA. Due to their opposing roles, a balance between the binding avidity of HA and the enzymatic activity of NA is important, particularly for viral transmission between human hosts. This is evident in that pandemic H1N1 2009 viruses, which can readily transmit between human hosts and show a functional balance between HA and NA activities, while putative swine precursor viruses that do not transmit do not show such an HA/NA balance (1, 2). NA enzymatic activity has also been linked to transmission, as viruses with low NA activity do not transmit by respiratory droplets (3, 4). These viruses with reduced NA activity were significantly inhibited by mucus, which is a likely explanation for the reduced transmission efficiency observed. Respiratory mucus contains a large number of sialic acid moieties which are present on the large, heavily sialylated mucin proteins that give mucus its viscoelastic properties (reviewed in reference 5). Therefore, in the respiratory tract of a potential host, influenza viruses bind to sialic acids on mucins via HA. NA enzymatic activity is important here to facilitate viral penetration of mucus and reach the epithelial cells below (3, 4, 6). Detailing the molecular and/or phenotypic markers of NA activity may, therefore, provide insights into viruses more or less likely to transmit between species.
The NA protein is a type II integral membrane protein that is presented on the viral surface as homotetrameric spikes. An NA monomer consists of a transmembrane domain (TMD) and a globular catalytic domain, connected by a stalk. Mutations that affect NA enzymatic activity have been characterized, particularly in viruses passaged with NA inhibitors such as oseltamivir and zanamivir. These mutations were located in the head domain of NA, either in the active site or proximal to it (framework mutations). Both types of mutations could alter the shape of the active site, thereby interfering with the activity of the enzyme (7–10).
We recently characterized two naturally occurring pandemic H1N1 viruses that were isolated in swine displaying influenza-like illness: A/swine/Virginia/1814-1/2012 and A/swine/Virginia/1814-2/2012 (pH1N1low-1 and -2, respectively). These viruses expressed NAs with almost undetectable enzymatic activity, did not transmit via respiratory droplets in the ferret model of influenza virus transmission, and were significantly inhibited by human mucus (4). The aim of this study was to further understand the phenotype of these viruses by understanding how the structure of the NA proteins expressed by these viruses contributed to the extremely low enzymatic activity observed and to define the molecular determinants of reduced transmissibility.
RESULTS
Amino acid changes in the neuraminidase of pH1N1low viruses were rare or unique.
To identify the amino acids likely responsible for reduced NA activity, we compared the NA amino acid sequences of the viruses with low NA activity (pH1N1low-1 and -2) to those of two closely related viruses displaying robust NA activity (pH1N1-1 and -2). There were four shared amino acid changes, at positions 19, 232, 248, and 436, that were common to pH1N1low NAs compared to pH1N1 NAs. The amino acids at these positions in pH1N1low NAs were V, V, N, and V, respectively, while in pH1N1 NAs they were M or T, A, D, and I, respectively. A change at amino acid 66 (Y in pH1N1 NAs and D in pH1N1low-1) was unique to the pH1N1low-1 NA, and changes at amino acids 62 and 382 (V and G in pH1N1 NAs and A and E in pH1N1low-2 NA, respectively) were unique to the pH1N1low-2 NA. Amino acid 19 was located in the transmembrane domain (TMD) (11, 12); amino acids 62 and 66 were located in the stalk domain; and the remaining amino acids were located in the globular head, outside the active site (Fig. 1A and B). We analyzed the frequency of amino acid identities at these sites using the Influenza Research Database (http://www.fludb.org) and found that the consensus amino acids at positions 19, 232, 248, and 436 were the same in human and swine pH1N1 viruses and matched the identities of the amino acids in the pH1N1-1 and -2 viruses. The amino acids at these positions in pH1N1low-1 and -2 viruses did not match the consensus in human or swine pH1N1 viruses in the database (Table 1). Further, the amino acids at 19, 232, 248, and 436 in the pH1N1low-1 and -2 viruses were relatively rare in human pH1N1 viruses in the database, at 0.07%, 0.43%, 10.7%, and 0.59% prevalences, respectively. They were also similarly rare in other swine pH1N1 viruses, at 0%, 3.2%, 14.1%, and 2.5% prevalences, respectively (Table 1). D was present in pH1N1low-1 at position 66, whereas Y was at this position in pH1N1 viruses. No other N1 viruses contained D at this position. Further, amino acid 66 was highly conserved, as Y was the amino acid at this location in all but one of the viruses in the database. A and E were present at positions 62 and 382 in pH1N1low-2, respectively, while V and G were at these positions in pH1N1 viruses, respectively. V and G were highly conserved at positions 62 and 382, respectively, in both swine and human viruses, at 99.4% and 98.7% prevalences, respectively. No other N1 viruses in the database contained A at position 62, and the prevalences of 382E were relatively low, at 0.6% in human viruses and 0.5% in swine viruses (Table 1). Therefore, the naturally occurring substitutions in the NAs of the pH1N1low viruses were either rare or unique in comparison to those in other N1 NAs.
FIG 1.
Locations of mutations in the neuraminidase tetramer. (A) Location of mutations over the full length of the neuraminidase tetramer (side view). Mutations are shown in only one monomer. Horizontal black lines indicate the transmembrane region. (B) Top view of the neuraminidase tetramer, showing the locations of the mutations. (C and D) The amino acids in pH1N1-1 (C) and pH1N1low-1 (D) at position 19, in the transmembrane domain, were modeled, showing their orientation within the tetramer. (E and F) The amino acids in pH1N1-1 (E) and pH1N1low-1 (F) at position 66, in the stalk domain, were modeled, showing their orientation within the tetramer.
TABLE 1.
Frequency of amino acid identities in the neuraminidases of pandemic H1N1 (pH1N1) viruses in the Influenza Research Database that differed between pH1N1low and pH1N1 virusesa
| Amino acid(s) in pH1N1low-1 and -2 | Amino acid(s) in pH1N1-1 and -2 | Amino acid no. | Consensus in human viruses or swine viruses | No. of amino acids at these positions in human pH1N1 viruses (n = 7,913) or swine pH1N1 viruses (n = 396)b |
|---|---|---|---|---|
| V | M (pH1N1-1), T (pH1N1-2) | 19 | M | M = 7,872, I = 18, L = 17, V = 6 |
| M | M = 395, T = 1 | |||
| V | A | 232 | A | A = 7,869, V = 34, T = 10 |
| A | A = 378, T = 4, V = 13 | |||
| N | D | 248 | D | D = 7,058, N = 848, G = 2, H = 1, V = 1 |
| D | D = 335, N = 56, G = 4 | |||
| V | I | 436 | I | I = 7,863, V = 47, T = 2, L = 1 |
| I | I = 385, V = 10, T = 1 | |||
| pH1N1low-1 | ||||
| D | Y | 66 | Y | Y = 7,909, F = 1 |
| Y | Y = 396 | |||
| pH1N1low-2 | ||||
| A | V | 62 | V | V = 7,869, I = 39, L = 3 |
| V | V = 391, I = 5 | |||
| E | G | 382 | G | G = 7,836, E = 44, R = 29, A = 1 |
| G | G = 383, R = 11, E = 2 |
Boldface and lightface characters represent human and swine virus data, respectively.
The amino acid identities and frequencies in this column do not include data from the pH1N1 and pH1N1low viruses studied here.
Amino acid changes in pH1N1low neuraminidases important for enzymatic activity.
To determine which of the mutations described above were responsible for the reduction in NA activity in pH1N1low viruses, we performed site-directed mutagenesis on the NA gene of pH1N1low-1, reverting each substitution to that in the pH1N1-1 virus. We then expressed these proteins in Baby hamster kidney (BHK) cells and measured the activities of the expressed NA proteins using 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUNANA) as a substrate. NA activities were normalized as a percentage of the activity of pH1N1-1 NA. Prior to mutagenesis, the enzymatic activity of the pH1N1low-1 NA was 2.43 ± 0.42% of the activity of the pH1N1-1 NA.
We first reverted the NA substitutions common to both pH1N1low viruses individually, at positions 19, 232, 248, and 436. We hypothesized that these were most likely the reason for the shared phenotype. Individually, these mutants did not significantly impact NA activity. We next tested all possible combinations of dual and triple mutants, and, again, no significant improvement of NA activity was observed. Surprisingly, a quadruple mutant that contained all four mutations had minimal impact on NA activity (Fig. 2A). Since these four common mutations were not responsible for the low activity of pH1N1low-1 NA, we turned to position 66, the remaining mutation in the pH1N1low-1 NA that distinguished it from the pH1N1 NAs. Reverting this mutation alone significantly increased activity to 21.94 ± 0.838% of pH1N1-1 NA activity (P < 0.01; Fig. 2A). Reverting 66 in addition to the other four mutations restored the enzymatic activity of pH1N1low-1 NA to 90.62 ± 6.46% of pH1N1-1 NA activity (P < 0.01; Fig. 2A). Therefore, 66 was a critical amino acid for the activity of this NA, although the presence of that amino acid alone was not enough to rescue the activity of the pH1N1low-1 NA.
FIG 2.
Mutagenesis of pH1N1low neuraminidases revealed the importance of amino acid 66. (A) Mutagenesis of the pH1N1low-1 neuraminidase (NA) showed that 66Y was required for the rescue of NA enzymatic activity. Relative to the enzymatic activity of pH1N1-1 NA, 66Y alone rescued 21.94 ± 0.838% of activity and all 5 mutations rescued 90.62 ± 6.46% of activity. 66Y with 19M, 232A, 248D, or 436I rescued 66.64 ± 4.81%, 37.04 ± 3.06%, 63.32 ± 3.23%, or 29.75 ± 3.02% of activity, respectively. (B) Mutagenesis of the pH1N1low-2 NA revealed that 62V or 382G did not appreciably increase NA activity, while the quadruple mutant containing 19M, 232A, 248D, and 436I rescued 95.97 ± 7.39% of activity relative to that seen with pH1N1-1 NA. Data points represent the results of three independent experiments. Statistical significance was determined based on comparisons to the wild-type NA pH1N1low-1 protein. **, P < 0.01.
We next constructed pH1N1low-1 NA double mutants containing 66Y in conjunction with 19M, 232A, 248D, or 436I to determine the minimum mutations needed to restore activity. The 232A/66Y and 436I/66Y mutants did not significantly increase the NA activity of the 66Y mutant, at 37.04 ± 3.06% and 29.75 ± 3.02% of pH1N1-1 NA activity, respectively. However, 19M/66Y and 248D/66Y mutants increased the NA activity to 66.64 ± 4.81% (P < 0.01) and 63.32 ± 3.23% (P < 0.01) of pH1N1-1 NA activity, respectively (Fig. 2A). As amino acid 19 was located in the NA TMD, we considered the possibility that interactions between the HA and NA TMDs may have an impact on NA tetramer stability. To examine this, we coexpressed the pH1N1low-1 HA with the pH1N1low-1 NAs containing 19M, 19M/66Y, 248D/66Y, or 66Y. We found that HA did not affect the activity of any of these NA proteins (data not shown). Therefore, it did not appear that HA-NA interactions at the TMD affected NA activity. Overall, 66Y in conjunction with either 19M or 248D was important for the activity of the pH1N1low-1 NA.
We next performed mutagenesis on the pH1N1low-2 NA, which had 66Y and 29.87 ± 1.06% of the activity seen with pH1N1-1 NA. This activity was similar to that of the pH1N1low-1 NA with the 66Y reversion, as expected. The pH1N1low-2 NA contained two unique mutations at amino acids 62 and 382. These mutations did not appear to be important for NA activity, as their reversion did not have a significant effect on NA activity, at 32.46 ± 1.2% and 15.51 ± 1.03% compared to pH1N1-1 NA activity, respectively (Fig. 2B). Reversion of all four common substitutions in the pH1N1low-2 NA to 19M, 232A, 248D, and 436I restored NA activity to 95.97 ± 7.39% of that of the pH1N1-1 NA (P < 0.01) (Fig. 2B). Collectively, these data show that 66Y was critical for NA enzymatic activity but was not itself sufficient to fully restore activity.
Neuraminidase protein stability is altered in pH1N1low-1 compared to pH1N1-1.
We were interested in determining why the identified mutations had such a dramatic effect on NA enzymatic activity, particularly as they were not in the active site of the enzyme or, in the case of 19 and 66, even in the same domain as the active site. We speculated that protein expression or stability might have been affected. To begin to investigate these possibilities, we examined the NA proteins expressed on BHK cells by SDS-PAGE/Western blotting (Fig. 3A). We analyzed the intensity of the NA bands in relation to that of pH1N1-1 NA, which was normalized to a value of 1 (Fig. 3B). Interestingly, the intensity of the pH1N1low-1 NA band was less than half the intensities of the pH1N1-1 NA and pH1N1low-2 NA bands (0.380 ± 0.082, 1, and 1.274 ± 0.497, respectively; Fig. 3). As the pH1N1low-1 NA was the only one containing 66D, this was likely the reason for the reduced band intensity. In agreement with the enzymatic activity data, 66Y in the pH1N1low-1 NA markedly increased band intensity to 1.264 ± 0.171, as did 66Y in conjunction with 19M, 232A, 248D, and 436I (1.17 ± 0.323; Fig. 3). Reversion to 19M, 232A, 248D, or 436I individually did not rescue the NA band intensity (0.600 ± 0.242, 0.681 ± 0.183, 0.618 ± 0.074, or 0.639 ± 0.180, respectively; Fig. 3). The intensity of the pH1N1low-2 NA band, at 1.274 ± 0.479, was similar to that of pH1N1-1, and the 62V or 382G mutations did not appreciably affect the mean band intensity (1.024 ± 0.143 and 0.929 ± 0.698, respectively; Fig. 3). Overall, these results show that the accumulation of pH1N1low-1 NA in cells was increased following the introduction of 66Y.
FIG 3.
66Y increased protein accumulation in transfected baby hamster kidney cells. (A) The intensity of the neuraminidase (NA) band of pH1N1-1 was greater than that of pH1N1low-1 but similar to that of pH1N1low-2. Introduction of 19M, 232A, 248D, or 436I into pH1N1low-1 NA did not restore the band intensity to that of pH1N1-1, but the introduction of 66Y alone or all five mutations rescued band intensity. Introduction of 62V or 382G into pH1N1low-2 NA did not increase band intensity. Introduction of 19M, 232A, 248D, and 436I into pH1N1low-2 NA increased band intensity. (B) The band intensities were normalized to that of pH1N1-1. Data in panel B represent the means ± standard errors of the results of n = 3 transfections. NA was detected using an anti-HA tag antibody, and β-actin was used as loading control.
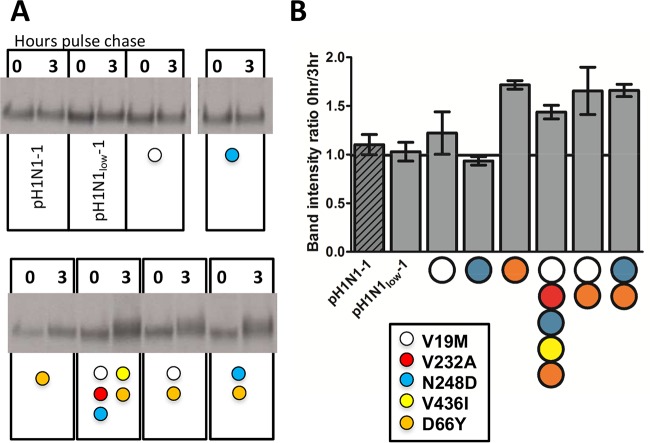
We next performed pulse-chase experiments to study NA protein stability (Fig. 4). We compared the intensities of the NA band at 0 and 3 h postchase and expressed them as a ratio (Fig. 4B). The band intensity ratios were similar between pH1N1-1 NA, pH1N1low-1 NA, and pH1N1low-1 NA containing 19M or 248D (ratios = 1.102 ± 0.103, 1.029 ± 0.09, and 1.221 ± 0.218 or 0.935 ± 0.043, respectively; Fig. 4B). In those pH1N1low-1 NAs containing 66Y, the band intensity ratios were greater (66Y = 1.716 ± 0.044; 5 mutations = 1.437 ± 0.07; 19M and 66Y = 1.655 ± 0.243; 248D and 66Y = 1.659 ± 0.063) (Fig. 4B). These data indicate that 66Y was important for the stability of the NA tetramer.
FIG 4.
66Y increased the stability of pH1N1low-1 neuraminidase, as measured by pulse-chase. The intensities of the neuraminidase (NA) bands at 0 and 3 h postchase (A) were compared and expressed in a bar graph (B). The intensities of the bands produced by pH1N1low-1 NA containing 66Y at 3 h postchase were greater than those seen at 0 h postchase. The intensity of bands produced by pH1N1low-1 NA containing 19M or 248D only at 3 h postchase were similar to those seen at 0 h postchase. Data in panel B represent the means ± standard errors of the results of n = 3 transfections.
Infectivity in the presence of human mucus was increased by 66Y.
As we previously observed that pH1N1low-1 could not penetrate human mucus as effectively as pH1N1-1, we were interested to determine if any of the mutations could rescue this phenotype and facilitate infection in the presence of human mucus (4). To study this, we constructed 6+2 reverse genetics (rg) viruses in an A/WSN/1933 (H1N1) background. These viruses contained six genes from A/WSN/1933 (H1N1) and the HA and NA genes of the viruses studied here. We overlaid Madin-Darby canine kidney (MDCK) cell monolayers with human mucus prior to addition of the rg viruses and then removed the inocula and incubated the monolayers for 7 h. In the presence of mucus, the infectivity of rg pH1N1low-1 was less than that of rg pH1N1-1 (0.03 ± 0.02% and 0.16 ± 0.06% cells infected, respectively; Fig. 5A and B and 6). All of the other rg viruses infected a significantly greater percentage of cells than rg pH1N1low-1 or rg pH1N1-1 (rg 19M and 66Y = 1.92 ± 0.15% cells infected, P < 0.001; rg 66Y and 248D = 2.48 ± 0.17%, P < 0.001; rg 66Y = 0.85 ± 0.13%, P < 0.01; rg pH1N1low-1 HA/pH1N1-1 NA = 0.80 ± 0.09%, P < 0.01) (Fig. 6). Of all the viruses, rg 19M and 66Y and rg 66Y and 248D infected the greatest percentage of cells in the presence of mucus. These NA mutants also showed the greatest NA enzymatic activity, therefore demonstrating that increasing NA activity increases the capacity of viruses to penetrate mucus, as previously shown (3, 4, 6, 13–16). It should be noted that enzymatic activity was studied in the context of tetramer stability. Due to the interdependence of tetramer stability and enzymatic activity, there is no robust method available with which to evaluate the relative contributions of NA activity and stability to mucus penetration. Overall, these data confirm our earlier results obtained using cell-expressed NA proteins, showing that they are functional in viruses and impact the viral phenotype.
FIG 5.
66Y facilitated infection in the presence of human mucus. Reverse genetics (rg) analysis of 6+2 viruses on an A/WSN/33 (H1N1) background and containing the hemagglutinin (HA) and neuraminidase (NA) genes of pH1N1low-1 and the D66Y mutation in NA showed increased infectivity of Madin-Darby canine kidney (MDCK) cells in the presence of human mucus compared to rg pH1N1low-1 (A, C, G, and H). The infectivity of rg pH1N1low-1 was also less than that of pH1N1-1 in the presence of mucus (panels A and B, respectively). In the absence of mucus, the infectivities of these viruses were similar to those of each other and to that of the rg pH1N1low-1 virus (D, E, F, J, and K). The infectivity of rg pH1N1low-1 was also less than the infectivity of the rg virus containing the HA of pH1N1low-1 and the NA of pH1N1-1 in the presence of mucus (panels A and I, respectively) but similar in the absence of mucus (panels D and L, respectively). Scale bars, 100 μm. Blue, DAPI nuclear stain; green, influenza nucleoprotein. Images are representative of the results of three replicates of each experimental condition.
FIG 6.
66Y alone or with 19M or 248D increased viral infectivity in the presence of mucus. Reverse genetics (rg) analysis of 6+2 viruses on an A/WSN/33 (H1N1) background and containing the HA and NA of pH1N1low-1 and the D66Y mutation in NA showed increased infectivity of Madin-Darby canine kidney (MDCK) cells in the presence of human mucus compared to rg pH1N1low-1. Viruses with D66Y and V19M or N248D infected the greatest percentage of cells. Viruses containing D66Y alone or the rg virus containing the HA of pH1N1low-1 and the NA of pH1N1-1 infected similar percentages of cells but infected fewer cells than viruses containing 66Y and either 19M or 248D. **, P < 0.01; ***, P < 0.001 (compared to the mean infectivity of rg pH1N1low-1 or pH1N1-1). Three monolayers were inoculated with each virus in the presence of human mucus. Each data point represents the mean percentage of cells infected in four representative fields.
Interestingly, rg pH1N1low-1 HA/pH1N1-1 NA infected a significantly greater percentage of cells than rg pH1N1-1, despite these viruses sharing the same NA. We previously showed that the HA binding avidity of pH1N1low-1 HA is significantly greater than that of pH1N1-1 HA (4). This result indicates that the functional balance of HA/NA is important in mucus penetration.
DISCUSSION
A functional balance between HA and NA, the main surface glycoproteins of influenza virus, is important for the transmissibility of pandemic H1N1 viruses via respiratory droplets in humans (2). However, viruses lacking this functional balance appear to exist in swine, suggesting the lack of selective pressure for a functional balance in this host. We have characterized two swine-isolated pH1N1 viruses, pH1N1low-1 and -2, that showed a dramatic difference in the functionality of HA and NA, in that the enzymatic activity of NA was almost undetectable whereas the HA binding affinity for α2,6-linked sialic acids was greater than that of the highly similar viruses pH1N1-1 and -2. While both pH1N1-1 and pH1N1low-1 could transmit by direct contact in the ferret model of human influenza virus transmission, only pH1N1-1 could transmit via respiratory droplets. pH1N1low-1 was also inhibited by respiratory mucus to a much greater degree than pH1N1-1, which likely contributed to the lack of respiratory droplet transmission (4).
The goal of this study was to understand why the NA proteins of pH1N1low viruses displayed such low enzymatic activity and, in doing so, identify possible molecular markers associated with optimal transmission. Sequence comparisons showed four mutations common to pH1N1low viruses compared to pH1N1 viruses, at positions 19, 232, 248, and 436. Amino acid 19 was located in the TMD, while 232, 248, and 436 were in the globular head domain. An additional mutation at position 66 was unique to pH1N1low-1 (N1 numbering; Fig. 1A). We found that amino acid 66 in the stalk domain was critical for enzymatic activity. However, reversion of the amino acid at position 66 in pH1N1low-1 to that in pH1N1 viruses (D66Y) alone was sufficient to rescue only approximately 25% of enzymatic activity. Reversion of amino acid 19 or 248 in conjunction with amino acid 66 was required to restore the majority of NA enzymatic activity (Fig. 2). Therefore, an amino acid in the stalk domain of NA, distal from the enzymatic active site, was a critical determinant of the enzymatic activity of the protein. Amino acids both within the NA active site (catalytic) and outside it (framework) that can alter the shape of the active site of NA and thus enzymatic activity have been characterized in the context of NA inhibitor resistance (reviewed in references 7, 8, and 9). Almost all of the mutations that conferred resistance to inhibitors also greatly decreased NA enzymatic activity, which in turn can affect viral transmissibility. For example, Q136K was observed following serial passaging of a pH1N1 virus in vitro in the presence of increasing concentrations of zanamivir. Q136K was located in the periphery of the neuraminic acid binding site and reduced NA activity and NA protein expression while conferring resistance to zanamivir (17). Q136K also decreased viral replication in vitro and resulted in attenuation in the guinea pig model of transmission (17). These characteristics are similar to those we observed in viruses containing 66D.
NA is expressed on the surface of virions as homotetramers, and, while each monomer contains an enzymatic active site within the globular head domain, the enzyme is active only in the tetrameric form, even in the absence of the stalk domain and TMD (18–21). While the globular head domains themselves can form tetramers, the NA TMD is required for tetramerization when the stalk domain is present to stabilize the N terminus of the stalk (22). Interactions between the TMDs of NA monomers are also very important for the assembly of NA tetramers, such that there is evidence of coevolution of the TMD and the globular head domains (22–24). A M19V mutation was recently observed in a WSN virus containing the NA ectodomain of WSN and the TMD of a more contemporary pH1N1 virus. In this setting, M19V was one of the mutations that rescued the temperature-dependent replication efficiency of this virus. M19V facilitated the proper folding of NA at 37°C compared to 33°C. This facilitated viral budding from infected cells and therefore increased viral titers (23). Here we also observed the importance of amino acid 19 for the activity of the pH1N1low-1 NA, although 66Y was also necessary. This suggests that amino acid 19 was important for the stability of pH1N1low-1 NA, which in turn increased the enzymatic activity of the tetramer.
As only the crystal structure of the globular head domain of NA has been solved, we can only estimate the location of amino acids 19 and 66 using predictive modeling. On the basis of the modeled full-length structure, amino acid 19 was located in the transmembrane domain. 19M may form a hydrophobic core and help in the stability of the tetramer by keeping together the monomers anchored in the membrane. Mutation of this amino acid to V may disrupt this interaction. Although V is also hydrophobic, it is substantially smaller than M (Fig. 1C and D). Amino acid 66 is located in the stalk of the NA protein and was also likely to affect the tetramerization of the protein (Fig. 1E and F). Y is an aromatic/hydrophobic amino acid, and in the homotetrameric helical bundle of the stalk, four of these coming together might form a hydrophobic core, helping the protein remain a tetramer. D, on the other hand, has a negatively charged side chain, and four of them coming together might lead to disruption of the stalk. Amino acid 248, a framework residue located in the NA globular head domain, was also important for rescuing the enzymatic activity of pH1N1low-1 NA. While framework mutations can alter the shape of the active site, this is unlikely to be the case here, as the pH1N1low-1 NA double mutant 19M and 66Y displayed enzymatic activity similar to that seen with 248D and 66Y. Therefore, it is more likely that amino acid 248 increased the enzymatic activity of this NA by increasing the stability of the NA oligomer. This may have been due to N being likely to undergo posttranslational modification, which may have disrupted enzymatic activity.
Overall, NA activity correlated with viral infectivity in the presence of mucus (Fig. 5 and 6), although there was one exception, the rg pH1N1low-1 HA/pH1N1-1 NA 6+2 virus, which showed infectivity similar to that of the D66Y single mutant despite containing the most enzymatically active NA (Fig. 6). It was also interesting that the infectivity of this virus was significantly greater than that of the rg pH1N1-1 HA/NA 6+2 virus, despite the two viruses containing the same NA. Because the binding affinity of the pH1N1low-1 HA was significantly greater than that of the pH1N1-1 HA, this result indicates that the HA/NA functional balance was important for mucus penetration.
In summary, we have shown that a single amino acid in the NA stalk domain at position 66 can have a profound impact on NA enzymatic activity. The highly conserved nature of this amino acid in N1 NAs attests to its importance. The location of this amino acid is therefore important with respect to protein stability and/or oligomerization. Further, we have also shown that the functional balance of HA and NA may be an important determinant of viral infectivity in the presence of human mucus and that this correlates to mammalian transmissibility.
MATERIALS AND METHODS
Viruses and cells.
The viruses studied here were isolated from nasal swabs obtained from pigs in commercial swine herds in the United States that showed symptoms of influenza-like illness, as described previously (4). A/swine/Virginia/1814-1/2012 (H1N1) (pH1N1low-1) and A/swine/Virginia/1814-2/2012 (H1N1) (pH1N1low-2) were isolated from pigs in the same herd and had minimal NA activity. A/swine/Pennsylvania/2436/2012 (H1N1) (pH1N1-1) and A/swine/Minnesota/2499/2012 (H1N1) (pH1N1-2) were genetically similar to the pH1N1low-1 and −2 viruses but displayed greater NA activity. Viruses were propagated in swine testes epithelial (STE) cells (ATCC, Manassas, VA) with 1 μg/ml tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK) trypsin, and viral titers were determined using Madin-Darby canine kidney (MDCK) cells (ATCC). MDCK and STE cells were maintained in minimal essential medium (MEM) supplemented with 10% (vol/vol) fetal bovine serum (Thermo Scientific, Waltham, MA). Viruses were diluted in infection medium (MEM supplemented with 5% [vol/vol] bovine serum albumin [BSA]) (Sigma, St. Louis, MO). Baby hamster kidney (BHK) cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 5% (vol/vol) fetal bovine serum (Thermo Scientific).
Cloning of NA genes.
The NA genes of pH1N1low-1, pH1N1low-2, and pH1N1-1 were cloned into the multicloning site (MCS) of the pCAGGS vector. An HA epitope tag (YPYDVPDYA) was also included at the C terminus of these proteins. Mutagenesis was performed using QuikChange Multi or QuikChange XL site-directed mutagenesis kits and specific primers per the instructions of the manufacturer (Agilent Technologies, Santa Clara, CA).
Cell transfection and protein expression.
BHK cells were transfected with the respective NA gene-containing pCAGGS plasmids by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), per the manufacturer's instructions. For comparison of different proteins, BHK cells were transfected with equal quantities of the respective pCAGGS plasmids (0.5 μg) in a 12-well plate, in parallel. Cells were harvested approximately 36 h posttransfection. For the subsequent NA enzyme activity assay, cells were lysed with nondenaturing NA enzyme buffer containing 0.5% (vol/vol) Triton X-100. NA activity levels of the cell lysates were measured as an endpoint after 30 min of incubation at 37°C in a buffer containing 33 mM 2-(N-morpholino) ethanesulfonic acid hydrate and 4 mM CaCl2 (pH 6.5), using 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUNANA; Sigma, St. Louis, MO) as a substrate.
Immunoprecipitation of NA protein.
BHK cells were transfected with pCAGGS plasmids containing the respective NA genes using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), per the manufacturer's instructions. At 24 h posttransfection, cells were maintained in culture for 30 min in methionine (Met)- and cysteine (Cys)-free medium and then labeled for 15 min with 100 μCi of [35S]-labeled Promix (Amersham Pharmacia Biotech, Piscataway, NJ) medium lacking cysteine and methionine. Cells were washed with phosphate-buffered saline (PBS) and chased for 3 h in 3 ml DMEM (2 mM Met, 2 mM Cys, 20 mM HEPES buffer). Samples were lysed with ice-cold radioimmunoprecipitation assay (RIPA) buffer containing 0.15 M NaCl, 9.25 mg/ml Iodoacetamide (Sigma), and Complete protease inhibitor tablets (Roche). Lysates were clarified by centrifugation at 40,000 × g in an Optima L-90K ultracentrifuge (Beckman Coulter) at 4°C. Supernatant was incubated overnight (18 to 22 h) at 4°C with 8 μl of a mouse monoclonal antibody that binds the HA tag of NA proteins at a dilution of 1:200 (anti-HA H3663; Sigma-Aldrich). Immune complexes were adsorbed to protein G-Sepharose 4 Fast Flow (GE Healthcare) for 1 h at 4°C. Samples were washed three times with RIPA buffer containing 0.3 M NaCl, three times with RIPA buffer containing 0.15 M NaCl, and once with 50 mM Tris buffer (0.25 Mm EDTA, 0.15 M NaCl, pH 7.4). The samples were resuspended in 50 μl of sample buffer (Nupage) and 12% β-mercaptoethanol. The samples were then boiled for 5 min, centrifuged at high speed for 1 min, and fractionated on 12% NuPAGE Bis-Tris polyacrylamide-SDS gels (Invitrogen). The gels were dried (model 583 gel dryer; Bio-Rad) and visualized after exposure on an autoradiography film (Kodak).
Modeling of mutant NA proteins.
The biopolymer module of the molecular modeling program MOE (Molecular Operating Environment, Version 2011.10) was used to model the mutant proteins. The high-resolution crystal structure of NA from pandemic strain A/California/07/2009 (H1N1) was used as the template structure (PDB identifier [ID] 4B7R).
Generation of viruses by reverse genetics.
The viruses generated by reverse genetics in this study were based on an eight-plasmid reverse genetics system (25). The HA and NA genes were derived from pH1N1low or pH1N1-1 viruses, while the remaining genes were from A/WSN/33 (H1N1). Reverse genetics viruses were passaged twice in MDCK cells.
Viral infection of MDCK cells in the presence of human mucus.
MDCK cell cultures were prepared on 16-well chamber slides (Thermo Scientific). Human mucus was obtained from normal human bronchial epithelial (NHBE) cell washes (Lonza). Human mucus was added to MDCK cell cultures in a total volume of 50 μl per well. Cells and mucus were incubated at 37°C (5% CO2) for 30 min prior to addition of a multiplicity of infection (MOI) of 1 in 50 μl of infection medium per well. Cultures were then incubated for 1 h at 37°C and then washed, and 200 μl of infection medium was then added to each well and cultures were incubated for 7 h at 37°C (5% CO2). Cells were then fixed, permeabilized, stained for DAPI (4′,6-diamidino-2-phenylindole) and influenza virus nucleoprotein, and imaged on a Nikon E800 fluorescence microscope. Infected cells in the four imaged fields that best represented the entire well were counted using NIS-Elements software (Nikon, Melville, NY). Images were taken at a magnification of ×200 using a Nikon E800 microscope (Nikon).
Data analysis and statistics.
Data collected were inputted and graphed using Graphpad Prism (Graphpad Software). Statistical analysis was performed using the Mann-Whitney test, with P values of <0.05 deemed statistically significant.
Accession number(s).
The sequences of the HA and NA genes of the viruses studied in the manuscript can be found on GenBank under the following accession numbers: for A/swine/Virginia/1814-1/2012 (H1N1) (pH1N1low-1), KP938849 (HA) and KP938850 (NA); for A/swine/Virginia/1814-2/2012 (H1N1) (pH1N1low-2), KP938853 (HA) and KP938854 (NA); for A/swine/Pennsylvania/2436/2012 (H1N1) (pH1N1-1), KP938851 (HA) and KP938852 (NA); and for A/swine/Minnesota/2499/2012 (H1N1) (pH1N1-2), KP938855 (HA) and KP938856 (NA).
ACKNOWLEDGMENTS
We thank Elena Govorkova for helpful discussions and advice and Victoria Frohlich and Jennifer Peters for their expert help and advice with imaging.
This study was funded by the National Institute of Allergy and Infectious Diseases and the National Institutes of Health under contract no. HHSN266200700005C and HHSN272201400006C.
REFERENCES
- 1.Xu R, Zhu X, McBride R, Nycholat CM, Yu W, Paulson JC, Wilson IA. 2012. Functional balance of the hemagglutinin and neuraminidase activities accompanies the emergence of the 2009 H1N1 influenza pandemic. J Virol 86:9221–9232. doi: 10.1128/JVI.00697-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yen HL, Liang CH, Wu CY, Forrest HL, Ferguson A, Choy KT, Jones J, Wong DD, Cheung PP, Hsu CH, Li OT, Yuen KM, Chan RW, Poon LL, Chan MC, Nicholls JM, Krauss S, Wong CH, Guan Y, Webster RG, Webby RJ, Peiris M. 2011. Hemagglutinin-neuraminidase balance confers respiratory-droplet transmissibility of the pandemic H1N1 influenza virus in ferrets. Proc Natl Acad Sci U S A 108:14264–14269. doi: 10.1073/pnas.1111000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenkrantz D, Roberts KL, Shelton H, Lycett S, Barclay WS. 2013. The short stalk length of highly pathogenic avian influenza H5N1 virus neuraminidase limits transmission of pandemic H1N1 virus in ferrets. J Virol 87:10539–10551. doi: 10.1128/JVI.00967-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanin M, Marathe B, Wong SS, Yoon SW, Collin E, Oshansky C, Jones J, Hause B, Webby R. 2015. Pandemic swine H1N1 influenza viruses with almost undetectable neuraminidase activity are not transmitted via aerosols in ferrets and are inhibited by human mucus but not swine mucus. J Virol 89:5935–5948. doi: 10.1128/JVI.02537-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lillehoj EP, Kato K, Lu W, Kim KC. 2013. Cellular and molecular biology of airway mucins. Int Rev Cell Mol Biol 303:139–202. doi: 10.1016/B978-0-12-407697-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. 2004. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol 78:12665–12667. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferraris O, Lina B. 2008. Mutations of neuraminidase implicated in neuraminidase inhibitors resistance. J Clin Virol 41:13–19. doi: 10.1016/j.jcv.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Richard M, Deleage C, Barthelemy M, Lin YP, Hay A, Lina B, Ferraris O. 2008. Impact of influenza A virus neuraminidase mutations on the stability, activity, and sensibility of the neuraminidase to neuraminidase inhibitors. J Clin Virol 41:20–24. doi: 10.1016/j.jcv.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Richard M, Ferraris O, Erny A, Barthelemy M, Traversier A, Sabatier M, Hay A, Lin YP, Russell RJ, Lina B. 2011. Combinatorial effect of two framework mutations (E119V and I222L) in the neuraminidase active site of H3N2 influenza virus on resistance to oseltamivir. Antimicrob Agents Chemother 55:2942–2952. doi: 10.1128/AAC.01699-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolentino-Lopez L, Segura-Cabrera A, Reyes-Loyola P, Zimic M, Quiliano M, Briz V, Munoz-Fernandez A, Rodriguez-Perez M, Ilizaliturri-Flores I, Correa-Basurto J. 2013. Outside-binding site mutations modify the active site's shapes in neuraminidase from influenza A H1N1. Biopolymers 99:10–21. doi: 10.1002/bip.22130. [DOI] [PubMed] [Google Scholar]
- 11.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 12.Möller S, Croning MD, Apweiler R. 2001. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 17:646–653. doi: 10.1093/bioinformatics/17.7.646. [DOI] [PubMed] [Google Scholar]
- 13.Burnet FM. 1947. Enzymic action of influenza viruses on glandular mucin and on purified blood group substances. Aust J Sci 10:21. [PubMed] [Google Scholar]
- 14.Cohen M, Zhang XQ, Senaati HP, Chen HW, Varki NM, Schooley RT, Gagneux P. 2013. Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase. Virol J 10:321. doi: 10.1186/1743-422X-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohuchi M, Asaoka N, Sakai T, Ohuchi R. 2006. Roles of neuraminidase in the initial stage of influenza virus infection. Microbes Infect 8:1287–1293. doi: 10.1016/j.micinf.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Yang XL, Steukers L, Forier K, Xiong R, Braeckmans K, Van Reeth K, Nauwynck H. 2014. A beneficiary role for neuraminidase in influenza virus penetration through the respiratory mucus. PLoS One 9:e110026. doi: 10.1371/journal.pone.0110026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaminski MM, Ohnemus A, Staeheli P, Rubbenstroth D. 2013. Pandemic 2009 H1N1 influenza A virus carrying a Q136K mutation in the neuraminidase gene is resistant to zanamivir but exhibits reduced fitness in the guinea pig transmission model. J Virol 87:1912–1915. doi: 10.1128/JVI.02507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucher DJ, Kilbourne ED. 1972. A 2 (N2) neuraminidase of the X-7 influenza virus recombinant: determination of molecular size and subunit composition of the active unit. J Virol 10:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laver WG. 1978. Crystallization and peptide maps of neuraminidase “heads” from H2N2 and H3N2 influenza virus strains. Virology 86:78–87. doi: 10.1016/0042-6822(78)90009-0. [DOI] [PubMed] [Google Scholar]
- 20.Paterson RG, Lamb RA. 1990. Conversion of a class II integral membrane protein into a soluble and efficiently secreted protein: multiple intracellular and extracellular oligomeric and conformational forms. J Cell Biol 110:999–1011. doi: 10.1083/jcb.110.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varghese JN, Laver WG, Colman PM. 1983. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature 303:35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- 22.da Silva DV, Nordholm J, Madjo U, Pfeiffer A, Daniels R. 2013. Assembly of subtype 1 influenza neuraminidase is driven by both the transmembrane and head domains. J Biol Chem 288:644–653. doi: 10.1074/jbc.M112.424150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Silva DV, Nordholm J, Dou D, Wang H, Rossman JS, Daniels R. 2015. The influenza virus neuraminidase protein transmembrane and head domains have coevolved. J Virol 89:1094–1104. doi: 10.1128/JVI.02005-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nordholm J, da Silva DV, Damjanovic J, Dou D, Daniels R. 2013. Polar residues and their positional context dictate the transmembrane domain interactions of influenza A neuraminidases. J Biol Chem 288:10652–10660. doi: 10.1074/jbc.M112.440230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]