ABSTRACT
Pigs are considered a mixing vessel for the generation of novel pandemic influenza A viruses through reassortment because of their susceptibility to both avian and human influenza viruses. However, experiments to understand reassortment in pigs in detail have been limited because experiments with regular-sized pigs are difficult to perform. Miniature pigs have been used as an experimental animal model, but they are still large and require relatively large cages for housing. The microminipig is one of the smallest miniature pigs used for experiments. Introduced in 2010, microminipigs weigh around 10 kg at an early stage of maturity (6 to 7 months old) and are easy to handle. To evaluate the microminipig as an animal model for influenza A virus infection, we compared the receptor distribution of 10-week-old male pigs (Yorkshire Large White) and microminipigs. We found that both animals have SAα2,3Gal and SAα2,6Gal in their respiratory tracts, with similar distributions of both receptor types. We further found that the sensitivity of microminipigs to influenza A viruses was the same as that of larger miniature pigs. Our findings indicate that the microminipig could serve as a novel model animal for influenza A virus infection.
IMPORTANCE The microminipig is one of the smallest miniature pigs in the world and is used as an experimental animal model for life science research. In this study, we evaluated the microminipig as a novel animal model for influenza A virus infection. The distribution of influenza virus receptors in the respiratory tract of the microminipig was similar to that of the pig, and the sensitivity of microminipigs to influenza A viruses was the same as that of miniature pigs. Our findings suggest that microminipigs represent a novel animal model for influenza A virus infection.
KEYWORDS: animal models, influenza, microminipig
INTRODUCTION
Pigs are considered a mixing vessel for the generation of novel pandemic influenza A viruses through reassortment because of their susceptibility to both avian and human influenza viruses (1, 2). In 2009, the swine-origin H1N1 pandemic influenza virus likely emerged from reassortment in pigs (3, 4). However, experiments to study reassortment events in pigs in detail have been limited because such studies with regular-sized pigs are difficult to do. In place of regular-sized pigs, researchers have used miniature pigs as an experimental animal model. For example, we used miniature pigs to analyze influenza virus sensitivity (5, 6); however, even these animals were cumbersome and hard to use. Furthermore, they require large animal housing facilities and physical strength to maneuver. In the present study, we assessed the usability of the microminipig as a novel experimental animal model for influenza virus infection. The microminipig was introduced in 2010 by Fuji Micra, Inc. (Shizuoka, Japan), as one of the smallest miniature pigs for experimental use (7). The body weight of 6- to 7-month-old microminipigs (an early stage of maturation) is approximately 10 kg (cf. the Göttingen miniature pig [14 kg] or the Landrace pig [100 kg]) and the growth of male microminipig plateaus at ∼20 kg (cf. miniature pig [40 kg] or pig [350 kg]) when they reach at least 18 months old (7, 8). Microminipigs are easy to handle and have been successfully used as an experimental animal model for life science research (8–17) and toxicological studies (18, 19). However, there are no reports of using microminipigs as an animal model for infectious diseases. For infection experiments, animals must be isolated and, given the compact size of microminipigs, it is easy to keep them in isolation. In this study, we evaluated microminipigs as a novel animal model for influenza A virus infection.
RESULTS
Effect of housing microminipigs in an isolation cage on their growth.
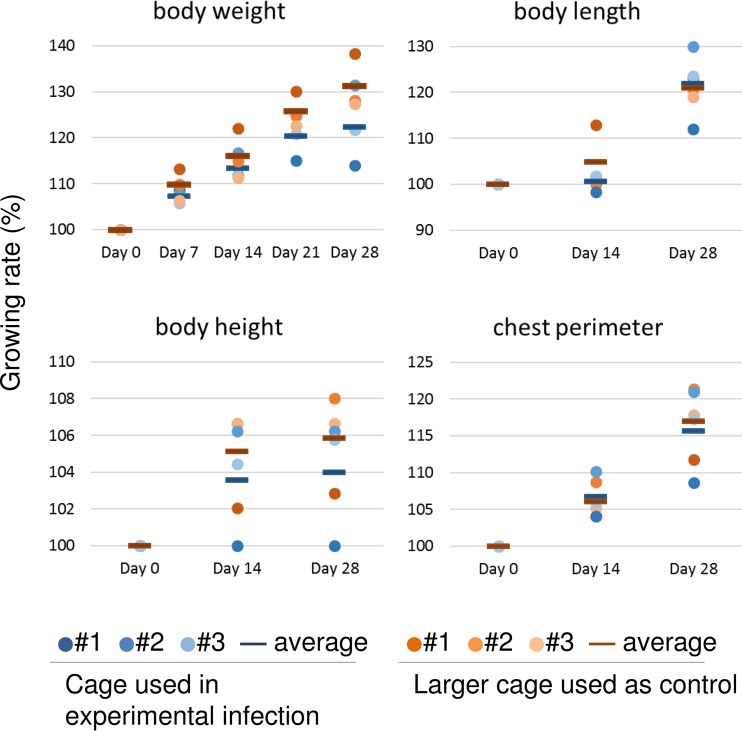
For our experimental infection studies with microminipigs, we designed an isolation cage (housing space, 55.0 cm by 27.5 cm by 37.5 cm; Fig. 1A). To assess the effect of this cage on the growth of the microminipigs, six 8-week-old male microminipigs were divided into two groups and housed in either three isolation cages (n = 3) or three larger cages (n = 3) (housing space, 80.0 cm by 60.0 cm by 75.0 cm) and monitored for 28 days (to 12 weeks old). We found no significant differences between microminipigs housed in isolation cages and those housed in larger cages in terms of their body weight, body length, body height, or chest perimeter (Fig. 2). Blood parameters and organ size were also not significantly different between the two groups of microminipigs (data not shown). Histological analysis of liver, spleen, kidney, heart, lung, adrenal gland, thyroid, thymus, pituitary, and skin also showed no differences between these microminipigs (data not shown). Therefore, we concluded that this isolation cage could be used to house 8- to 12-week-old microminipigs for at least 28 days without any detrimental effects on their health.
FIG 1.
Images of the cage and the isolator. (A) New isolation cage produced for this study of 8- to 12-week-old microminipigs. (B) Negative-pressured isolator containing the isolation cage with a microminipig (1.46 kg) inside. (C) Negative-pressured isolator with a hatch door with HEPA filters (blue and round) closed.
FIG 2.
Growth rate of microminipigs housed in isolation cages or larger cages. Six 8-week-old male microminipigs were divided into two groups and housed individually in either three isolation cages (n = 3) or three larger cages (n = 3) and monitored for 28 days (to 12 weeks old). Increases in body weight on days 0, 7, 14, 21, and 28, and in body length, body height, and chest perimeter on days 0, 14, and 28 are shown. Each data point represents an individual animal. Bars show the average for each group. There were no significant differences between microminipigs housed in isolation cages and those housed in larger cages.
Distribution of sialic acids in the respiratory tract of a regular-sized pig and a microminipig.
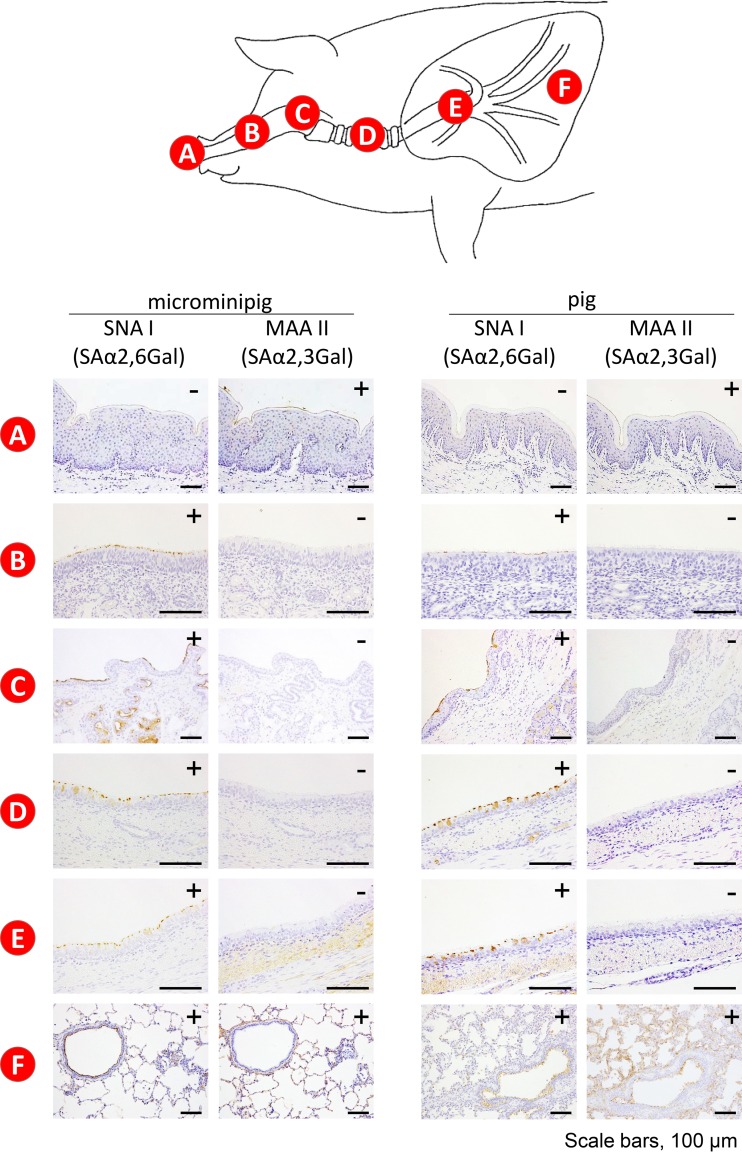
Pigs are considered a mixing vessel for the generation of human-avian reassortants because they express receptors for both human and avian influenza viruses in their airways (sialic acid linked to galactose by an α2,6 linkage [SAα2,6Gal] and an α2,3 linkage [SAα2,3Gal], respectively [1, 2]). We therefore first compared the receptor distribution of a 10-week-old male microminipig to that of a regular-sized pig of the same age (Yorkshire Large White). Maackia amurenis lectin II (MAA II), which is specific for SAα2,3Gal, reacted with the epithelial cells only in the nasal vestibule of the microminipig; in contrast, Sambucus nigra lectin I (SNA I), which is specific for SAα2,6Gal, did not react with the epithelial cells in the nasal vestibule but did react with cells in the deep portion of the nasal turbinate of the microminipig (Fig. 3A and B). These reaction patterns were similar in a regular pig (Fig. 3A and B). In the pharynx, trachea, and bronchus, SNA I strongly reacted with the epithelial cells of both pigs but MAA II did not (Fig. 3C, D, and E). Both SNA I and MAA II reacted with the epithelial cells in the lungs of both pigs (Fig. 3F). The reaction of SNA I was much stronger than that of MAA II under the same lectin staining condition. We extended the color development time to increase the intensity of the possibly weak reaction signals of MAA II. Nevertheless, MAA II signals were detected only in the lung (Fig. 3F). Therefore, there appears to be more SAα2,6Gal than SAα2,3Gal in the lungs of these animals. Our results indicate that the regular-sized pig and the microminipig have both SAα2,3Gal and SAα2,6Gal in their respiratory tracts and that the distribution of these receptors in the microminipig is identical to that in the regular-sized pig.
FIG 3.
Distribution of SAα2,6Gal and SAα2,3Gal oligosaccharides in the respiratory tract of a microminipig and a regular-sized pig. Sections containing SAα2,6Gal and SAα2,3Gal receptors were reacted with the linkage-specific lectins SNA I and MAA II, respectively. (A) Nasal vestibule. (B) Nasal turbinate. (C) Larynx. (D) Trachea. (E) Bronchus. (F) Lung. MAA II reacted with the epithelial cells only in the nasal vestibule of both pigs; in contrast, SNA I did not react with the epithelial cells in the nasal vestibule (A). MAA II did not react with epithelial cells in the nasal turbinate, but SNA I did react with cells in the deep portion of the nasal turbinates of both pigs (B). In the pharynx, trachea, and bronchus, SNA I strongly reacted with the epithelial cells of both pigs but MAA II did not (C, D, and E). Both SNA I and MAA II reacted with the bronchioepithelial cells, and type 1 and type 2 pneumocytes in the lungs of both pigs (F).
Clinical signs and virus shedding in influenza virus-infected microminipigs.
Twelve 9- to 10-week-old male microminipigs were intramuscularly anesthetized and intranasally inoculated with 107 PFU of A/California/04/2009 (CA04; pdmH1N1) (n = 6) or A/Indiana/10/2011 (IN10; H3N2v) (n = 6) virus. None of the infected microminipigs showed any clinical signs or changes in body weight or temperature (data not shown). All of the CA04- and IN10-infected microminipigs continued to shed viruses from their nasal cavity during the experimental period, until day 6 (Table 1). Our previous data from CA04-infected miniature pigs indicated that they shed virus from their nasal cavity until day 7; these miniature pigs also did not show any clinical signs (5). Thus, the clinical signs and virus shedding from the nasal cavity of microminipigs mirrored those of larger miniature pigs.
TABLE 1.
Virus titers in nasal swabs from infected microminipigsa
| Day | Virus titer (log10 PFU/ml) in swab samples collected from animals infected with: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA04 (H1N1) |
IN10 (H3N2) |
|||||||||||
| 1 | 2 | 3 | 5 | 7 | 8 | 11 | 12 | 13 | 14 | 15 | 16 | |
| 1 | 6.89 | 6.83 | 6.68 | 5.91 | 6.73 | 5.92 | 6.49 | 6.92 | 7.48 | 6.81 | 5.98 | 5.69 |
| 3 | 5.85 | 8.00 | 4.69 | 4.95 | 6.65 | 5.68 | 5.43 | 6.52 | 5.73 | 5.52 | 5.45 | 5.49 |
| 5 | NA | NA | NA | 4.63 | 4.99 | 4.76 | NA | NA | NA | 4.62 | 4.41 | 4.38 |
| 6 | NA | NA | NA | 2.40 | 4.17 | 3.00 | NA | NA | NA | 2.11 | 1.81 | 2.02 |
Microminipigs were intranasally infected with 107 PFU (1 ml) of virus. Nasal swabs were collected every other day for virus titration. NA, not applicable (animals were euthanized on day 3 postinfection). Animal identification numbers are indicated in each column subheading. Detection limit, 1.0 log10 PFU/ml.
Virus replication.
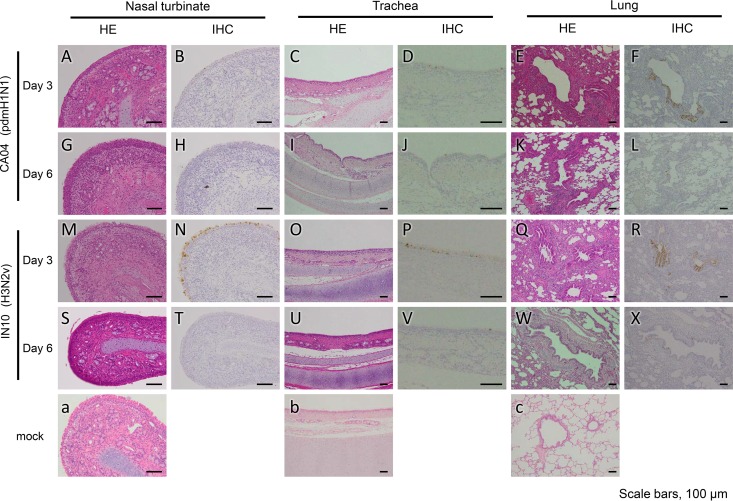
On days 3 (n = 3) and 6 (n = 3) after infection, microminipigs were euthanized, and their organs were collected for virological and pathological examinations. Although no clinical signs were observed, both viruses replicated efficiently in the respiratory organs and tonsils but not in the other tested organs (heart, liver, spleen, kidneys, duodenum, and rectum) of microminipigs (Table 2). On pathological examination, rhinitis and tracheitis with moderate inflammation were found on days 3 and 6 postinfection with CA04 and IN10 (Fig. 4A, C, G, I, M, O, S, and U) compared to a mock-infected animal (Fig. 4a and b). Both of the lungs on day 3 postinfection with CA04 or IN10 showed moderate inflammation as bronchitis, bronchiolitis, and/or alveolitis (Fig. 4E and Q); the inflammation was slightly milder on day 6 postinfection with either virus (Fig. 4K and W) compared to a mock-infected animal (Fig. 4C). Immunohistochemistry analyses revealed that viral antigens were mainly detected in the epithelial cells of the nasal turbinate, trachea, and lungs of the CA04- or IN10-infected microminipigs on day 3 postinfection (Fig. 4B, D, F, N, P, and R) and that the number of antigen-positive cells decreased on day 6 postinfection (Fig. 4H, J, L, T, V, and X). These results are consistent with those from our previous study with CA04-infected miniature pigs (5). Therefore, the sensitivity of microminipigs to influenza A viruses appears to be the same as that of larger miniature pigs.
TABLE 2.
Virus titers in organs of infected microminipigsa
| Organ site | Virus titer (log10 PFU/g) in organs collected from animals infected with: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA04 (H1N1) |
IN10 (H3N2) |
|||||||||||
| Day 3 |
Day 6 |
Day 3 |
Day 6 |
|||||||||
| 1 | 2 | 3 | 5 | 7 | 8 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Lung (left cranial cranial) | 8.11 | 7.07 | 8.15 | –b | 2.70 | – | 5.79 | 7.03 | 7.10 | – | – | – |
| Lung (left cranial caudal) | 7.63 | 6.49 | 6.64 | – | – | – | 6.56 | 7.14 | 7.72 | – | – | – |
| Lung (left caudal) | 7.10 | 6.01 | 4.79 | – | – | – | 5.65 | 6.59 | 6.99 | – | – | – |
| Lung (right cranial) | 8.93 | 7.15 | 4.90 | – | – | – | 3.10 | 5.43 | 8.90 | – | – | – |
| Lung (right middle) | 8.08 | 7.18 | 6.93 | – | 2.30 | – | 6.99 | 7.28 | 8.04 | – | – | – |
| Lung (right caudal) | 6.92 | 5.88 | 5.88 | – | – | – | 2.70 | 6.69 | 7.49 | – | – | – |
| Lung (right accessory) | 7.30 | 6.80 | 5.87 | – | – | – | – | 5.80 | 6.24 | – | – | – |
| Bronchus (left) | 6.63 | 6.05 | 5.48 | – | – | – | 5.48 | 7.24 | 6.78 | – | 2.70 | – |
| Bronchus (right) | 7.52 | 5.97 | 5.03 | – | – | – | 6.32 | 7.29 | 6.44 | – | – | – |
| Trachea | 5.13 | 5.07 | 5.16 | – | – | – | 6.73 | 5.77 | 5.56 | – | – | – |
| Nasal turbinate (left) | 5.58 | 6.87 | 5.48 | – | – | – | 6.45 | 6.11 | 6.34 | 2.90 | – | – |
| Nasal turbinate (right) | 5.09 | 6.20 | 4.92 | – | – | – | 6.27 | 6.57 | 4.92 | – | – | – |
| Tonsil (left) | 4.98 | 3.00 | 3.00 | – | – | – | 2.62 | 3.40 | 2.00 | 3.21 | – | 2.15 |
| Tonsil (right) | 6.70 | 4.15 | 2.42 | – | – | – | – | 2.82 | 3.40 | – | – | 2.78 |
Microminipigs were intranasally infected with 107 PFU (1 ml) of virus. Animal identification numbers are indicated in each column subheading.
–, Virus was not detected (detection limit, 1.0 log10 PFU/ml). Virus was also not detected at the following sites: heart, liver, spleen, kidneys, duodenum, and rectum.
FIG 4.
Pathological examination of the nasal turbinate, trachea, and lungs of infected microminipigs. Nasal turbinate, trachea, and lung tissue of microminipigs on days 3 and 6 postinfection with CA04 (A to L), IN10 (M to X) or mock-infected (a to c). Rhinitis and tracheitis with moderate inflammation were detected on days 3 and 6 postinfection with CA04 and IN10 (A, C, G, I, M, O, S, and U). Both of the lungs on day 3 postinfection with CA04 or IN10 showed moderate inflammation as bronchitis, bronchiolitis, and/or alveolitis (E and Q); the inflammation was slightly milder on day 6 postinfection with either virus (K and W). Immunohistochemistry revealed that viral antigens were mainly detected in the epithelial cells of the nasal turbinate, trachea, and lungs of the CA04- or IN10-infected microminipigs on day 3 postinfection (B, D, F, N, P, and R) and that the number of antigen-positive cells decreased on day 6 postinfection with either virus (H, J, L, T, V, and X). HE, hematoxylin and eosin staining; IHC, immunohistochemistry.
DISCUSSION
To evaluate microminipigs as a novel animal model for influenza A virus infection, it is important to compare the susceptibility to influenza A viruses between microminipigs and miniature pigs. In this study, we found that microminipigs are as susceptible to influenza A viruses as are miniature pigs (Fig. 4, Tables 1 and 2) (5). These results thus indicate that microminipigs have the same properties as regular-sized pigs and miniature pigs with respect to the replication of influenza A viruses.
The distribution of sialic acid in the respiratory tract of pigs varies across published reports. We have reported that pig trachea contains SAα2,3Gal and SAα2,6Gal oligosaccharides (1, 20), whereas other groups have reported that SAα2,6Gal oligosaccharides predominates in the trachea (21–24), although SAα2,3Gal oligosaccharides are also found. This discrepancy may originate from a difference in the source of the SAα2,3Gal- and SAα2,6Gal-specific lectins used or in the staining procedures used. In the present study, we used animals of the same age and sex, and we used the same staining procedure to exclude variability. Under these conditions, we found that the distribution of receptors between the regular-sized pig and the microminipig was identical (Fig. 3).
The biggest advantage of microminipigs as a model animal of infection is their body size. For this study, we produced a new, smaller isolation cage that can be placed in a separate isolator and is suitable for use in experiments with highly pathogenic agents. Although a larger cage is needed for adult male microminipigs (∼20 kg of body weight) (8), our isolation cage is suitable for experiments with 8- to 12-week-old microminipigs.
A comparison of the genomic sequence of the microminipig with the genomic database for pigs did not detect any clear, substantial genomic variance (25). The distribution of influenza virus receptors in the respiratory tract and the sensitivity of microminipigs to influenza A viruses were the same as those of regular-sized pigs and miniature pigs. Therefore, we can conclude that microminipigs would be a suitable alternative animal model for the study of influenza A virus infection.
MATERIALS AND METHODS
Animals.
Microminipigs, free from Mycoplasma, Toxoplasma, Erysipelothrix rhusiopathiae, Actinobacillus, Bordetella bronchiseptica, Pasteurella, Haemophilus parasuis, suid herpesvirus 1, porcine reproductive and respiratory syndrome virus, and a regular-sized pig (Yorkshire Large White) for this study were bred at the Swine and Poultry Research Center, Shizuoka Prefectural Research Institute of Animal Industry. The research protocol used to assess the microminipigs is in accordance with the Regulations for Animal Care of the University of Tokyo and the Guidelines for Proper Conduct of Animal Experiments by the Science Council of Japan, the representative organization of the Japanese Scientist Community (2006), and was approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo (approval PA13-59) and by the Animal Care and Use Committee of Shizuoka Prefectural Research Institute of Animal Industry, Swine and Poultry Research Center.
Cells and viruses.
Madin-Darby canine kidney (MDCK) cells were maintained in Eagle minimal essential medium (MEM) containing 5% newborn calf serum at 37°C in 5% CO2. Human pandemic influenza A(H1N1) virus A/California/04/2009 (pdmH1N1; CA04) (5) and swine-origin influenza A(H3N2) virus A/Indiana/10/2011 (H3N2v; IN10) (26) were propagated in MDCK cells with MEM containing 0.3% bovine serum albumin (BSA).
Plaque assay.
Viruses were diluted in MEM containing 0.3% BSA. Confluent monolayers of MDCK cells were washed with MEM containing 0.3% BSA, infected with diluted viruses, and incubated for 30 to 60 min at 37°C. After the virus inoculum was removed, the cells were washed with MEM containing 0.3% BSA and overlaid with a 1:1 mixture of 2× MEM–0.6% BSA and 2% agarose containing 1 μg of TPCK (tolylsulfonyl phenylalanyl chloromethyl ketone)-trypsin/ml. The plates were incubated at 37°C for 48 h before the virus plaques were counted.
Effect of housing in an isolation cage on the growth of microminipigs.
To assess the effect of housing microminipigs in our isolation cage, three 8-week-old male microminipigs were placed individually in three isolation cages (housing space, 55.0 cm by 27.5 cm by 37.5 cm, Showa Science; see Fig. 1A). For comparison, three 8-week-old male microminipigs were placed individually in three larger cages (housing space, 80.0 cm by 60.0 cm by 75.0 cm, Ishihara Co., Ltd.). The volume of this cage is 6.3 times larger than that of the isolation cage. The body weight of each microminipig was measured every 7 days. Body length, height, chest perimeter, and hematological parameters (blood cell count, differential leukocyte count, total protein, albumin level, and albumin-globulin ratio) were examined every 14 days. On day 28, all microminipigs were euthanized, and their organs (liver, spleen, kidney, heart, lung, adrenal gland, thyroid, thymus, pituitary, and skin) were collected and examined histologically. To compare these physiological measures from the same animal at different time points, we fitted a linear mixed-effects model to the data using the R package NLME; the time, the animal, and the interaction between these two factors were considered. Next, we built a contrast matrix to compare the strains in a pairwise fashion at the same time points using the R package PHIA. Because the comparisons were performed individually, the final P values were adjusted using Holm's method to account for multiple comparisons.
Experimental infection of microminipigs.
Eight- to nine-week-old male microminipigs (body weight, 2.2 to 4.1 kg), were transferred to a biosafety level 3 facility at the University of Tokyo. Each microminipig was moved into an isolation cage (Fig. 1A and B) and placed in a separated isolator (Showa Science; Fig. 1B and C). After 1 week of acclimatization, 12 of these microminipigs were intramuscularly anesthetized and intranasally inoculated with 107 PFU (1 ml) of CA04 (n = 6) or IN10 (n = 6). The body weight and temperature of each microminipig were measured every day. We observed the microminipigs twice a day, once in the morning and once in the evening, during the experiments to monitor their clinical symptoms. Nasal swabs were collected every other day for virus titration. Three microminipigs per group were euthanized on days 3 and 6 postinfection, and their organs were collected (lungs, bronchus, trachea, nasal turbinate, heart, liver, spleen, kidney, duodenum, rectum, and tonsil). Virus titers in these organs were determined by use of plaque assays in MDCK cells.
Pathological examination.
The excised respiratory and intestinal tract tissues were fixed in 4% paraformaldehyde phosphate (PFA) buffer solution for 48 h and processed for paraffin embedding. Nasal samples were immersed in EDTA solution for decalcification after being fixed in PFA. The paraffin blocks were cut into 3-μm-thick sections and mounted on silane-coated glass slides. To detect sialic acid linked to galactose by an α2,6 linkage (SAα2,6Gal) or an α2,3 linkage (SAα2,3Gal), the sections were pretreated with 0.05% trypsin (Difco Laboratories, Detroit, MI) at 37°C for 15 min and with 0.3% hydrogen peroxide at room temperature for 30 min. They were then incubated at 4°C overnight with biotin-conjugated Sambucus nigra lectin I (SNA I; EY Laboratories) for SAα2,6Gal or biotinylated-Maackia amurenis lectin II (MAA II; Vector Laboratories) for SAα2,3Gal. After being washed, the sections were then incubated with horseradish peroxidase-conjugated streptavidin and visualized by staining with 3,3′-diaminobenzidine (DAB). The sections were also stained using a standard hematoxylin-and-eosin procedure, and each serial section was processed for immunohistochemistry with a rabbit polyclonal antibody for type A influenza nucleoprotein antigen (prepared in our laboratory) that reacts comparably with both of the viruses used in this study. Specific antigen-antibody reactions were visualized by DAB staining using the Dako Envision system (Dako Cytomation).
ACKNOWLEDGMENTS
We thank Susan Watson for editing the manuscript; Naomi Fujimoto, Yukihiko Sugita, Satoshi Fukuyama, and Makoto Yamashita for technical support; and Tokiko Watanabe for helpful discussions.
This research was supported by Strategic Basic Research Programs from the Japan Science and Technology Agency (JST); by Leading Advanced Projects for Medical Innovation (LEAP) from the Japan Agency for Medical Research and Development (AMED); and by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Science, Sports, and Technology (MEXT) of Japan (16H06429, 16K21723, and 16H06434).
REFERENCES
- 1.Ito T, Couceiro JN, Kelm S, Baum LG, Krauss S, Castrucci MR, Donatelli I, Kida H, Paulson JC, Webster RG, Kawaoka Y. 1998. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol 72:7367–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma W, Kahn RE, Richt JA. 2008. The pig as a mixing vessel for influenza viruses: human and veterinary implications. J Mol Genet Med 3:158–166. [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann G, Noda T, Kawaoka Y. 2009. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team, Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 5.Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Takahashi K, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K, Kawaoka Y. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe T, Kiso M, Fukuyama S, Nakajima N, Imai M, Yamada S, Murakami S, Yamayoshi S, Iwatsuki-Horimoto K, Sakoda Y, Takashita E, McBride R, Noda T, Hatta M, Imai H, Zhao D, Kishida N, Shirakura M, de Vries RP, Shichinohe S, Okamatsu M, Tamura T, Tomita Y, Fujimoto N, Goto K, Katsura H, Kawakami E, Ishikawa I, Watanabe S, Ito M, Sakai-Tagawa Y, Sugita Y, Uraki R, Yamaji R, Eisfeld AJ, Zhong G, Fan S, Ping J, Maher EA, Hanson A, Uchida Y, Saito T, Ozawa M, Neumann G, Kida H, Odagiri T, Paulson JC, Hasegawa H, Tashiro M, Kawaoka Y. 2013. Characterization of H7N9 influenza A viruses isolated from humans. Nature 501:551–555. doi: 10.1038/nature12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneko N, Itoh K, Sugiyama A, Izumi Y. 2011. Microminipig, a non-rodent experimental animal optimized for life science research: preface. J Pharmacol Sci 115:112–114. doi: 10.1254/jphs.10R16FM. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi H, Miyoshi N, Miura N, Fujiki M, Horiuchi M, Izumi Y, Miyajima H, Nagata R, Misumi K, Takeuchi T, Tanimoto A, Yoshida H. 2011. Microminipig, a non-rodent experimental animal optimized for life science research: novel atherosclerosis model induced by high fat and cholesterol diet. J Pharmacol Sci 115:115–121. doi: 10.1254/jphs.10R17FM. [DOI] [PubMed] [Google Scholar]
- 9.Sugiyama A, Nakamura Y, Akie Y, Saito H, Izumi Y, Yamazaki H, Kaneko N, Itoh K. 2011. Microminipig, a non-rodent experimental animal optimized for life science research: in vivo proarrhythmia models of drug-induced long QT syndrome: development of chronic atrioventricular block model of microminipig. J Pharmacol Sci 115:122–126. doi: 10.1254/jphs.10R21FM. [DOI] [PubMed] [Google Scholar]
- 10.Sakai C, Iwano S, Shimizu M, Onodera J, Uchida M, Sakurada E, Yamazaki Y, Asaoka Y, Imura N, Uno Y, Murayama N, Hayashi R, Yamazaki H, Miyamoto Y. 2016. Analysis of gene expression for microminipig liver transcriptomes using parallel long-read technology and short-read sequencing. Biopharm Drug Dispos 37:220–232. doi: 10.1002/bdd.2007. [DOI] [PubMed] [Google Scholar]
- 11.Akioka K, Kawaguchi H, Kitajima S, Miura N, Noguchi M, Horiuchi M, Miyoshi N, Tanimoto A. 2014. Investigation of necessity of sodium cholate and minimal required amount of cholesterol for dietary induction of atherosclerosis in microminipigs. In Vivo 28:81–90. [PubMed] [Google Scholar]
- 12.Miura N, Kawaguchi H, Nagasato T, Yamada T, Ito T, Izumi H, Shameshima H, Miyoshi N, Tanimoto A, Maruyama I. 2013. Coagulation activity and white thrombus formation in the microminipig. In Vivo 27:357–361. [PubMed] [Google Scholar]
- 13.Kawaguchi H, Yamada T, Miura N, Ayaori M, Uto-Kondo H, Ikegawa M, Noguchi M, Wang KY, Izumi H, Tanimoto A. 2014. Rapid development of atherosclerosis in the world's smallest microminipig fed a high-fat/high-cholesterol diet. J Atheroscler Thromb 21:186–203. doi: 10.5551/jat.21246. [DOI] [PubMed] [Google Scholar]
- 14.Noguchi M, Miura N, Ando T, Kubota C, Hobo S, Kawaguchi H, Tanimoto A. 2015. Profiles of reproductive hormone in the microminipig during the normal estrous cycle. In Vivo 29:17–22. [PubMed] [Google Scholar]
- 15.Miyoshi N, Horiuchi M, Inokuchi Y, Miyamoto Y, Miura N, Tokunaga S, Fujiki M, Izumi Y, Miyajima H, Nagata R, Misumi K, Takeuchi T, Tanimoto A, Yasuda N, Yoshida H, Kawaguchi H. 2010. Novel microminipig model of atherosclerosis by high fat and high cholesterol diet, established in Japan. In Vivo 24:671–680. [PubMed] [Google Scholar]
- 16.Takeishi K, Horiuchi M, Kawaguchi H, Deguchi Y, Izumi H, Arimura E, Kuchiiwa S, Tanimoto A, Takeuchi T. 2012. Acupuncture improves sleep conditions of minipigs representing diurnal animals through an anatomically similar point to the Acupoint (GV20) effective for humans. Evid Based Complement Alternat Med 2012:472982. doi: 10.1155/2012/472982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kangawa A, Otake M, Enya S, Yoshida T, Kangawa Y, Shibata M. 2016. Spermatogenesis in the microminipig. Toxicol Pathol 44:974–986. doi: 10.1177/0192623316654586. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa T, Takahashi Y, Kawaguchi H, Utsunomiya S, Miura N, Izumi H, Miyoshi N, Tanimoto A. 2013. A dermal phototoxicity study following intravenous infusion administration of ciprofloxacin hydrochloride in the novel microminipigs. Toxicol Pathol 41:109–113. doi: 10.1177/0192623312452489. [DOI] [PubMed] [Google Scholar]
- 19.Guruge KS, Noguchi M, Yoshioka K, Yamazaki E, Taniyasu S, Yoshioka M, Yamanaka N, Ikezawa M, Tanimura N, Sato M, Yamashita N, Kawaguchi H. 2016. Microminipigs as a new experimental animal model for toxicological studies: comparative pharmacokinetics of perfluoroalkyl acids. J Appl Toxicol 36:68–75. doi: 10.1002/jat.3145. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki Y, Ito T, Suzuki T, Holland RE Jr, Chambers TM, Kiso M, Ishida H, Kawaoka Y. 2000. Sialic acid species as a determinant of the host range of influenza A viruses. J Virol 74:11825–11831. doi: 10.1128/JVI.74.24.11825-11831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelli RK, Kuchipudi SV, White GA, Perez BB, Dunham SP, Chang KC. 2010. Comparative distribution of human and avian type sialic acid influenza receptors in the pig. BMC Vet Res 6:4. doi: 10.1186/1746-6148-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Poucke SG, Nicholls JM, Nauwynck HJ, Van Reeth K. 2010. Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution. Virol J 7:38. doi: 10.1186/1743-422X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sriwilaijaroen N, Kondo S, Yagi H, Takemae N, Saito T, Hiramatsu H, Kato K, Suzuki Y. 2011. N-glycans from porcine trachea and lung: predominant NeuAcα2-6Gal could be a selective pressure for influenza variants in favor of human-type receptor. PLoS One 6:e16302. doi: 10.1371/journal.pone.0016302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trebbien R, Larsen LE, Viuff BM. 2011. Distribution of sialic acid receptors and influenza A virus of avian and swine origin in experimentally infected pigs. Virol J 8:434. doi: 10.1186/1743-422X-8-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miura N, Kucho K, Noguchi M, Miyoshi N, Uchiumi T, Kawaguchi H, Tanimoto A. 2014. Comparison of the genomic sequence of the microminipig, a novel breed of swine, with the genomic database for conventional pig. In Vivo 28:1107–1111. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. 2012. Update: influenza A (H3N2)v transmission and guidelines—five states, 2011. MMWR Morb Mortal Wkly Rep 60:1741–1744. [PubMed] [Google Scholar]