ABSTRACT
Human TIM and TAM family proteins were recently found to serve as phosphatidylserine (PS) receptors which promote infections by many different viruses, including dengue virus, West Nile virus, Ebola virus, Marburg virus, and Zika virus. In the present study, we provide substantial evidence demonstrating that TIM-1 is important for efficient infection by hepatitis C virus (HCV). The knockdown of TIM-1 expression significantly reduced HCV infection but not HCV RNA replication. Likewise, TIM-1 knockout in Huh-7.5 cells remarkably lowered HCV cell attachment and subsequent HCV infection. More significantly, the impairment of HCV infection in the TIM-1 knockout cells could be restored completely by ectopic expression of TIM-1 but not TIM-3 or TIM-4. Additionally, HCV infection and cell attachment were inhibited by PS but not by phosphatidylcholine (PC), demonstrating that TIM-1-mediated enhancement of HCV infection is PS dependent. The exposure of PS on the HCV envelope was confirmed by immunoprecipitation of HCV particles with a PS-specific monoclonal antibody. Collectively, these findings demonstrate that TIM-1 promotes HCV infection by serving as an attachment receptor for binding to PS exposed on the HCV envelope.
IMPORTANCE TIM family proteins were recently found to enhance infections by many different viruses, including several members of the Flaviviridae family. However, their importance in HCV infection has not previously been examined experimentally. The TIM family proteins include three members in humans: TIM-1, TIM-3, and TIM-4. The findings derived from our studies demonstrate that TIM-1, but not TIM-3 or TIM-4, promotes HCV infection by functioning as an HCV attachment factor. Knockout of the TIM-1 gene resulted in a remarkable reduction of HCV cell attachment and infection. PS-containing liposomes blocked HCV cell attachment and subsequent HCV infection. HCV particles could also be precipitated with a PS-specific monoclonal antibody. These findings suggest that TIM-1 and its binding ligand, PS, may serve as novel targets for antiviral intervention.
KEYWORDS: hepatitis C virus, TIM-1, attachment, infection, receptor, TIM-3, TIM-4
INTRODUCTION
Hepatitis C virus (HCV) is an enveloped RNA virus containing a 9.6-kb single-stranded RNA genome of positive polarity (1). It is the prototype member of the Hepacivirus genus in the Flaviviridae family (2, 3). The viral RNA genome consists of a long open reading frame (ORF), encoding a single polyprotein, and untranslated regions (UTRs) at both the 5′ and 3′ ends. Upon translation, the viral polyprotein precursor is cleaved by cellular peptidases and the viral NS2/NS3 metalloprotease and NS3/4A serine protease into 10 individual structural and nonstructural (NS) proteins, designated core (C), envelope proteins 1 and 2 (E1 and E2), p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B (4). The structural proteins C, E1, and E2 are essential for the formation of HCV particles (5). The NS3 to NS5B proteins are the minimal set of viral proteins required for HCV RNA replication, although all NS proteins play indispensable roles in HCV morphogenesis (6–9). The 5′ and 3′ UTRs contain cis-acting RNA elements that are important for HCV RNA replication (10, 11). In addition, cellular components, such as microRNAs, proteins, and lipids, also play critical roles at various steps of the HCV life cycle (12–15).
HCV is a hepatotropic virus primarily infecting human hepatocytes. Its infection requires multiple cell surface receptors, including but not limited to heparan sulfate proteoglycans (HSPGs), CD81, claudin, occludin, SR-BI, and the low-density lipoprotein receptor (LDLR) (16, 17). Our previous studies demonstrated that the cellular protein apolipoprotein E (apoE) is incorporated on the HCV envelope and mediates virion attachment to target cells by binding to the cell surface HSPGs, mainly the syndecan-1 (SDC1) proteoglycan (18–20). HCV E2 interacts with other cell surface receptors/coreceptors, such as CD81, claudin, occludin, SR-BI, and LDLR, which all play crucial roles in HCV cell entry at postattachment steps (21–28). Additionally, several other cellular proteins and pathways were found to be important for efficient HCV infection in cell culture, including epidermal growth factor receptor (EGFR), EphA2, Niemann-Pick C1 (NPC1L1), phosphatidylinositol 3-kinase (PI3K)–Akt, and CIDEB (29–32). However, the underlying molecular mechanisms of these different cellular proteins and pathways in the promotion of HCV entry and uncoating remain largely unknown.
In recent years, human T cell immunoglobulin and mucin domain-containing (TIM) proteins were found to enhance infections by various RNA viruses, including hepatitis A virus (HAV), dengue virus (DENV), Ebola virus (EBOV), Marburg virus (MARV), retrovirus, and Zika virus (ZIKV) (33–39). Human TIM family proteins (TIM-1, TIM-3, and TIM-4) specifically recognize phosphatidylserine (PS), a phospholipid component of viral envelopes and eukaryotic membranes (40). Other PS recognition receptors include Tyrol3, Axl, and MERTK (TAM family proteins), which indirectly interact with PS through the bridging molecules Gas6 and ProS (36, 41, 42). Axl has been reported to promote infections by EBOV, MARV, and ZIKV (33, 35, 42, 43). However, the roles of TIM and TAM family proteins in HCV infection have not previously been examined experimentally. In the present study, we demonstrate that TIM-1, but not TIM-3 or TIM-4, enhances the attachment and infection of HCV through an interaction with PS exposed on the HCV envelope.
RESULTS
Silencing of TIM-1 expression reduced HCV infection but not replication.
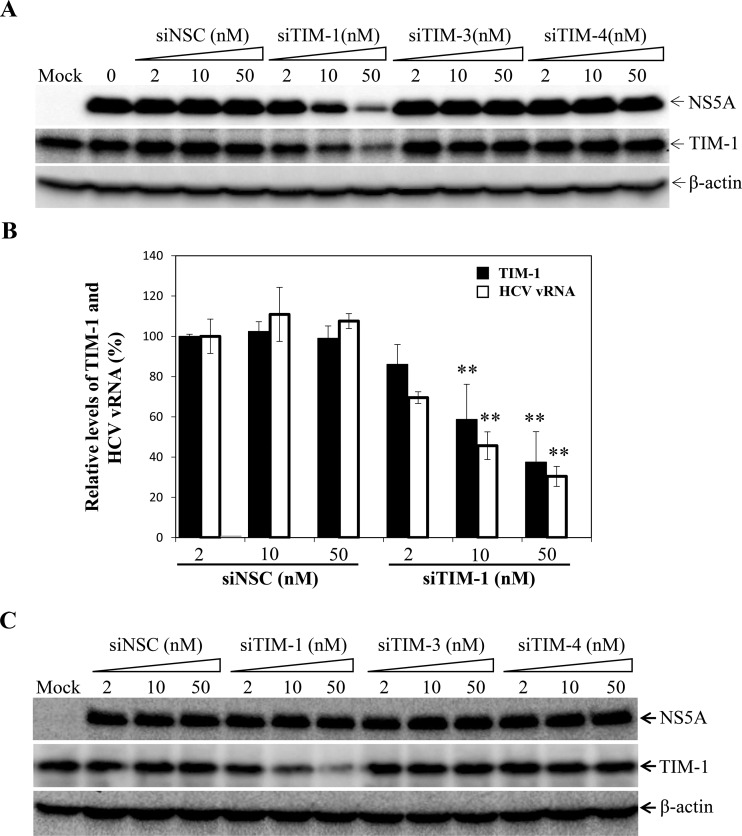
To determine a possible role of TIM family proteins in HCV infection, we initially determined the effects of TIM-1-, TIM-3-, and TIM-4-specific small interfering RNAs (siRNAs) on HCV infection. A mix of 4 siRNAs targeting different regions of TIM-1, -3, and -4 mRNAs was transfected into Huh-7.5 cells. At 48 h posttransfection (p.t.), cells were infected with HCV at a multiplicity of infection (MOI) of 10. At 24 h postinfection (p.i.) (single-cycle HCV growth assay) (44), Huh-7.5 cells were lysed in RIPA buffer. The levels of HCV NS5A were determined by Western blotting using an NS5A-specific monoclonal antibody. As anticipated, TIM-1-specific siRNAs silenced TIM-1 expression in a dose-dependent manner. TIM-1 siRNAs lowered TIM-1 expression by 7.5%, 53%, and 68% at 2, 10, and 50 nM, respectively (Fig. 1A). HCV infection was remarkably inhibited by silencing of TIM-1 expression, as determined by decreased levels of NS5A protein. The levels of NS5A were decreased by 8%, 45%, and 64% at TIM-1 siRNA concentrations of 2, 10, and 50 nM, respectively (Fig. 1A). However, nonspecific control (NSC), TIM-3, and TIM-4 siRNAs had no significant effects on HCV infection, based on similar levels of NS5A in the siRNA-transfected cells (Fig. 1A). It should be noted that TIM-3 and TIM-4 are not expressed to levels detectable by Western blotting in Huh-7.5 cells (data not shown). The levels of positive-strand HCV RNA in the NSC and TIM-1 siRNA-transfected Huh-7.5 cells were also quantified by quantitative reverse transcription-PCR (qRT-PCR). Similar to the NS5A decreases, TIM-1 siRNAs resulted in reductions of the level of positive-strand HCV RNA, by 30%, 54%, and 70% at siRNA concentrations of 2 nM, 10 nM, and 50 nM, respectively (Fig. 1B). To examine whether silencing of TIM family protein expression affects HCV replication, a stable Huh-7.5 cell line harboring a replicating subgenomic RNA of HCV JFH1 was used. Similar to that observed in Huh-7.5 cells, TIM-1 siRNAs lowered TIM-1 expression by up to 73% at 50 nM siRNA. However, silencing of TIM-1 gene expression did not affect the replication of HCV replicon RNA, as determined by similar levels of NS5A in cells transfected with NSC siRNA and TIM1-, TIM3-, and TIM4-specific siRNAs (Fig. 1C). These results demonstrate that TIM-1 is important for HCV infection but not replication.
FIG 1.
Effects of siRNA-induced knockdown of TIM-1, TIM-3, and TIM-4 expression on HCV infection. Huh-7.5 cells in 12-well cell culture plates were transfected with various concentrations (2, 10, and 50 nM) of a nonspecific control siRNA (siNSC) or SmartPool siRNAs specific to the TIM-1 (siTIM-1), TIM-3 (siTIM-3), or TIM-4 (siTIM-4) gene by use of the RNAiMax reagent. At 48 h p.t., Huh-7.5 cells were infected with HCV at an MOI of 10 at 37°C for 2 h. Upon removal of unbound HCV by extensive washing with PBS, the HCV-infected cells were incubated with fresh DMEM. At 24 h p.i., cell lysates were collected and used for quantification of HCV NS5A, TIM-1, TIM-3, and TIM-4 by Western blotting using specific antibodies. β-Actin was used as an internal control. (A) Detection of HCV NS5A and TIM-1 by Western blotting. A total of 35 μg of cell lysate was loaded into a 10% SDS-PAGE gel. Upon electrophoresis, proteins were transferred to a PVDF membrane. HCV NS5A, TIM-1, and β-actin were detected by Western blotting using monoclonal antibodies. (B) Quantification of positive-strand HCV RNA in TIM-1 siRNA-transfected cells by qRT-PCR. Mean values and standard deviations (SD) were derived from three independent experiments and were converted to percentages of the control level (the level of HCV RNA without siRNA transfection, considered to be 100%). (C) Effects of TIM-1, TIM-3, and TIM-4 gene silencing on HCV RNA replication. siRNA transfection was done in the same way as that described above, except that a stable Huh-7.5 cell line containing a subgenomic RNA of HCV JFH1 was used. At 48 h p.t., siRNA-transfected cells were lysed, and the levels of HCV NS5A and TIM-1 in cell lysates were determined by Western blotting using monoclonal antibodies, with β-actin as a control. Naive Huh-7.5 cells were used as a mock control. siRNAs and their concentrations (nanomolar) are highlighted at the top. NS5A, TIM-1, and β-actin are indicated on the right. **, P < 0.01.
Knockout of TIM-1 but not TIM-4 impaired HCV cell attachment and infection.
Previous studies suggested that both TIM-1 and TIM-4 promote the entry of many enveloped viruses, including DENV (36, 37). However, our results obtained by siRNA-mediated silencing of TIM family gene expression showed that only TIM-1 is efficiently used for HCV infection (Fig. 1). To confirm the above findings, we sought to make TIM-1 and TIM-4 knockout Huh-7.5 cell lines by using clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9-mediated gene editing technology. Recombinant lentiviruses expressing TIM-1 or TIM-4 single guide RNAs (sgRNAs) were constructed and used to transduce Huh-7.5 cells. Upon selection with puromycin, individual cell clones were amplified and screened by Western blotting and genomic DNA sequence analysis. The TIM-1 knockout cell clone contains a single-nucleotide thymidine (T) deletion within the sgRNA target region (Fig. 2A). Consequently, TIM-1 was not expressed as determined by Western blotting (Fig. 2B). The TIM-4 knockout cell line has a thymidine insertion in the middle of the sgRNA target region (Fig. 2C). However, TIM-4 was not detectable even in the parent Huh-7.5 cells (data not shown), suggesting that it is not efficiently expressed. The TIM-4-specific antibody worked in Western blots, as shown by detection of ectopically expressed TIM-4 (see Fig. 6). These specific gene knockout cell lines were used for the subsequent HCV infection and attachment experiments.
FIG 2.
Construction of TIM-1 and TIM-4 knockout Huh-7.5 cell lines. Huh-7.5 cells were transduced with a lentivirus expressing CRISPR/Cas9 and TIM-1 or TIM-4 sgRNA. Upon selection with puromycin, stable cell clones were picked up and amplified. Genomic DNA was extracted by use of a Qiagen DNA isolation kit. TIM-1 and TIM-4 DNA fragments were amplified by PCR, using specific primers flanking the sgRNA target regions. PCR DNA products were subjected to DNA sequence analysis. (A) Confirmation of a TIM-1 knockout Huh-7.5 cell line by DNA sequencing. A single deletion of a T nucleotide (bold italics) was found within the TIM-1 sgRNA target sequence (−1). (B) Validation of TIM-1 knockout by Western blotting using a TIM-1-specific monoclonal antibody. (C) Confirmation of TIM-4 knockout by DNA sequence analysis. There is a single-nucleotide T insertion (bold italics) in the middle of the sgRNA target sequence (+1).
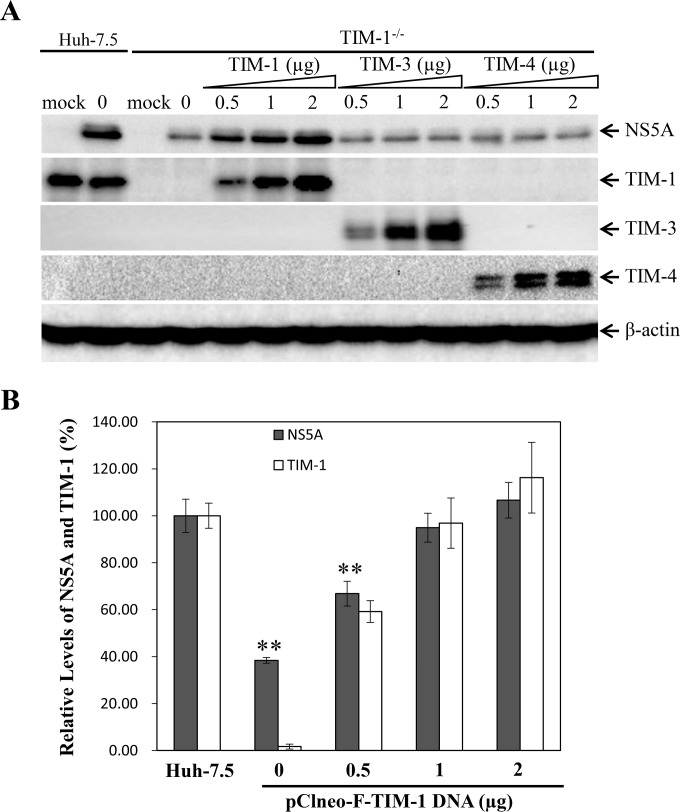
FIG 6.
Restoration of impaired HCV infection in TIM-1 knockout Huh-7.5 cells by ectopic expression of TIM-1 but not TIM-3 or TIM-4. TIM-1−/− cells in 12-well cell culture plates were transfected with various amounts (0, 0.5, 1, and 2 μg) of a TIM-1-, TIM-3-, or TIM-4-expressing vector by use of Lipofectamine 2000 reagent. The total amount of DNA was kept constant (2 μg) by use of the vector DNA. At 48 h p.t., cells were infected with HCV at an MOI of 10 at 37°C for 2 h. At 24 h p.i., cell lysates were collected for detection of HCV NS5A, TIM-1, TIM-3, and TIM-4 by Western blotting using specific antibodies. β-Actin was used as an internal control. (A) Detection of NS5A, TIM-1, TIM-3, and TIM-4 by Western blotting. (B) Correlation of HCV infection with TIM-1 expression. Average data obtained from three independent experiments performed as described above were plotted to correlate the levels of NS5A (solid bars) with those of ectopically expressed TIM-1 (open bars). The levels of NS5A and TIM-1 in parent Huh-7.5 cells were considered to be 100%. Mock, uninfected cells; 0, no DNA transfection. **, P < 0.01.
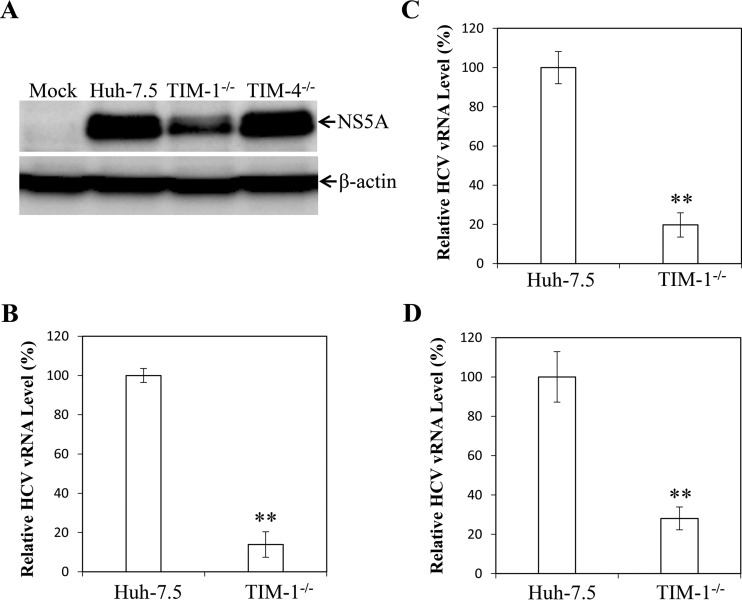
To determine the effects of TIM-1 and TIM-4 knockouts on HCV infection, the parent Huh-7.5, TIM-1 knockout, and TIM-4 knockout cells were used for single-cycle HCV growth assays. At 24 h p.i., cells were lysed and used for NS5A detection by Western blotting. TIM-1 knockout resulted in reductions of NS5A protein and positive-strand HCV RNA of 70% and 86%, respectively, in the HCV-infected cells compared to the parent Huh-7.5 cells (Fig. 3A and B). In contrast, TIM-4 knockout did not affect HCV infection (Fig. 3A), consistent with the findings derived from siRNA-mediated silencing of TIM-4 gene expression (Fig. 1). Next, we examined the mechanism of action of TIM-1 in HCV entry. As described above, silencing of TIM-1 expression significantly impaired HCV infection but did not affect HCV replication. The question of whether TIM-1 plays a role in HCV cell attachment and/or entry then arose. To determine the effect of TIM-1 knockout on HCV cell attachment, the parent and TIM-1 knockout Huh-7.5 cells were incubated with HCV at 37°C for 1 h (Fig. 3C) or on ice for 2 h (Fig. 3D). The amount of cell-attached HCV was determined by qRT-PCR quantification of the HCV virion RNA (vRNA) level. Strikingly, TIM-1 knockout lowered HCV cell attachment by 30% to 80% (Fig. 3C and D), similar to the reductions of NS5A and positive-strand HCV RNA in infected cells (Fig. 3A and B). To further validate the findings obtained from HCV single-cycle growth assays, we performed a multiple-cycle HCV growth experiment. Huh-7.5 and TIM-1−/− cells were infected with HCV at a lower MOI. The levels of HCV NS5A and positive-strand RNA were determined in the infected cells at different time points (24 h, 48 h, and 72 h) postinfection. Similar to the HCV single-cycle growth results, the levels of both NS5A and vRNA were 3 to 5 times lower at each time point in the TIM-1 knockout cells than in the parent Huh-7.5 cell line (Fig. 4). To exclude the possible alteration of key HCV receptor/coreceptor expression in the TIM-1 knockout cells, we determined the levels of CD81, claudin-1, occludin, SR-BI, and SDC1 expression by Western blotting. The results show that these key HCV receptors were expressed similarly in the TIM-1 knockout cells and the parent Huh-7.5 cells (Fig. 5). Taken together, these results demonstrate that TIM-1 is an important HCV attachment factor on the surfaces of hepatocytes.
FIG 3.
Impairment of HCV infection and cell attachment by TIM-1 knockout. Parent (Huh-7.5) and TIM-1 (TIM-1−/−) or TIM-4 (TIM-4−/−) knockout Huh-7.5 cells in 12-well cell culture plates were infected with HCV at an MOI of 10 at 37°C for 2 h. Unbound HCV was removed by extensive washing with 1× PBS. The HCV-infected cells were incubated with fresh DMEM at 37°C for 24 h. (A) Cell lysates were collected for detection of NS5A by Western blotting. (B) Total RNAs in the HCV-infected cells were extracted with TRIzol reagent and used for quantification of positive-strand HCV RNA by qRT-PCR. (C and D) The HCV attachment assay was carried out by incubation of HCV with parent Huh-7.5 or TIM-1−/− cells at an MOI of 10 at 37°C for 1 h (C) or on ice (4°C) for 2 h (D). Unbound HCV was removed by extensive washing with 1× PBS. Total RNAs were extracted with TRIzol reagent. The vRNA levels of cell-bound HCV were quantified by a real-time qRT-PCR method. The relative levels of HCV vRNA (average values derived from three experiments) are shown as percentages of the control level (the HCV vRNA level in parent Huh-7.5 cells, considered to be 100%). **, P < 0.01.
FIG 4.
Effect of TIM-1 knockout on multiple-cycle growth of HCV. Parent and TIM-1 (TIM-1−/−) knockout Huh-7.5 cells in 12-well cell culture plates were infected with HCV at an MOI of 1 at 37°C for 2 h. Upon extensive washing with 1× PBS, the HCV-infected cells were incubated with fresh DMEM. (A) At different time points (24 h, 48 h, and 72 h) postinfection, cell lysates were collected for detection of NS5A by Western blotting. (B) Total RNAs in HCV-infected cells at 24 h, 48 h, and 72 h p.i. were extracted with TRIzol reagent. The levels of positive-strand HCV RNA were quantified by qRT-PCR. The relative levels of HCV vRNA (average values derived from three experiments) were converted to percentages of the control level (the HCV vRNA level in parent Huh-7.5 cells, considered to be 100%). *, P < 0.05; **, P < 0.01.
FIG 5.
Detection of key HCV receptors in TIM-1 knockout Huh-7.5 cells. Samples of 35 μg lysate from parent Huh-7.5 and TIM-1 knockout cells were loaded into 10% SDS-PAGE gels for electrophoresis analysis. Upon transfer to PVDF membranes, CD81, claudin-1, occludin (OCLD), SDC1, and SR-BI were detected by Western blotting using specific individual antibodies and an ECL staining kit. β-Actin was used as a control to normalize protein loads.
Restoration of HCV infection in TIM-1 knockout cells by ectopic expression of TIM-1 but not TIM-3 or TIM-4.
To exclude possible off-target effects of CRISPR/Cas9-mediated TIM-1 knockout on HCV infection, we determined whether ectopic TIM-1 expression would restore the impaired HCV infection in the TIM-1 knockout Huh-7.5 cells. Additionally, we sought to ascertain any roles of TIM-3 and TIM-4 in HCV infection, as Huh-7.5 cells do not express detectable levels of TIM-3 and TIM-4. The TIM-1-expressing vector pClneo-F-TIM-1 (45) was transfected into the TIM-1 knockout Huh-7.5 cells. At 48 h p.t., cells were infected with HCV at an MOI of 10 for 2 h. After 24 h of incubation in fresh Dulbecco's modified Eagle's medium (DMEM), the HCV-infected cells were lysed. The levels of TIM-1, TIM-3, TIM-4, and HCV NS5A were determined by Western blotting. As shown in Fig. 6A, TIM-1, TIM-3, and TIM-4 were all efficiently expressed in the TIM-1 knockout cells. Transfection with 1 μg pClneo-F-TIM-1 DNA resulted in TIM-1 expression equivalent to that in parent Huh-7.5 cells. The TIM-1 level was 1.3-fold higher than that in parent Huh-7.5 cells when 2 μg DNA was transfected into the TIM-1 knockout cells (Fig. 6). More importantly, ectopic expression of TIM-1 fully restored HCV infection. In contrast, ectopic expression of TIM-3 and TIM-4 had no effect on HCV infection (Fig. 6A). Overexpression (2 μg pClneo-F-TIM-1 DNA) of TIM-1 further enhanced HCV infection, by 131% compared to the HCV infection efficiency in the parent Huh-7.5 cells (Fig. 6). The complete restoration of the defective HCV infection in TIM-1 knockout cells by ectopic TIM-1 expression demonstrates that TIM-1 is an important cellular factor promoting HCV infection.
Inhibition of HCV infection by PS- but not PC-containing liposomes.
It is known that TIM family proteins specifically recognize the ligand PS exposed on the surfaces of apoptotic cells (40). It is thought that TIM-1 and TIM-4 promote virus infection by specific recognition of PS molecules on virus envelopes (36, 37). To determine the mode of action of TIM-1 in HCV infection, an HCV single-cycle growth assay was used to determine if PS-containing liposomes could inhibit HCV infection. Huh-7.5 cells were infected with HCV in the presence or absence of PS-, phosphatidylcholine (PC)-, or PS-PC-containing liposomes. Interestingly, PS-containing liposomes blocked HCV infection in a dose-dependent manner. Similarly, liposomes containing both PS and PC completely inhibited HCV infection at 100 μM. In contrast, PC-containing liposomes did not significantly affect HCV infection (Fig. 7A). Next, we carried out an HCV attachment experiment to determine whether PS would block HCV cell attachment. Interestingly, PS-containing liposomes also blocked HCV attachment in a dose-dependent manner, resulting in reductions of HCV attachment of up to 90% (Fig. 7B). To provide direct evidence of the exposure of PS on the HCV envelope, we sought to precipitate HCV particles by using a PS-specific monoclonal antibody. An HCV E2-specific monoclonal antibody (CBH5) was used as a positive control, whereas a normal mouse IgG was used as a negative control. Compared to the amount of input HCV, anti-PS and anti-E2 monoclonal antibodies pulled down 40% and 60% of HCV particles, respectively (Fig. 8). These results indicate that PS is exposed on the envelopes of HCV particles. Taken together, our findings suggest that the TIM-1-mediated enhancement of HCV attachment and entry occurs through binding to the ligand PS exposed on the viral envelope, similar to its mechanism of action in the promotion of infection by other enveloped viruses.
FIG 7.
(A) Inhibition of HCV infection by PS-containing liposomes. PS-, PC-, and PS-PC-containing liposomes were prepared according to a previously described method (37). Huh-7.5 cells were infected with HCV at an MOI of 10 in the presence of various concentrations (0, 11.1, 33.3, and 100 μM) of liposomes at 37°C for 2 h. At 24 h p.i., cell lysates were collected for detection of HCV NS5A by Western blotting using an NS5A-specific monoclonal antibody (9E10). (B) Blockade of HCV cell attachment by PS-containing liposomes. Huh-7.5 cells were incubated with HCV in the presence of various concentrations (0, 11.1, 33.3, and 100 μM) of liposomes on ice (4°C) for 2 h. Unbound HCV was removed by washing with 1× PBS three times. Total RNAs were extracted with TRIzol reagent and used for quantification of HCV vRNA by qRT-PCR. The relative levels of HCV vRNA (average values derived from three experiments) are shown as percentages of the control level (the HCV vRNA level in parent Huh-7.5 cells, considered to be 100%). *, P < 0.05; **, P < 0.01.
FIG 8.
Immunoprecipitation of HCV particles by PS- and HCV E2-specific monoclonal antibodies. One milligram of protein G agarose beads was coated with 20 μg of anti-PS, anti-E2 (CBH5) (55), or normal mouse IgG (nmIgG) at 4°C overnight. HCV (200 μl) was then incubated with antibody-coated protein G agarose beads at 4°C overnight. HCV-bound beads were washed three times with 150 mM NaCl. Ten micrograms of Zika virus (ZIKV) RNA-containing total cellular RNA was added to HCV-bound agarose beads, followed by RNA extraction with TRIzol-LS reagent (Invitrogen). The levels of both HCV and ZIKV vRNAs were quantified by qRT-PCR. The levels of ZIKV vRNA were used for normalization of HCV vRNA between anti-PS, CBH5, and nmIgG. The relative levels of HCV vRNA were calculated as percentages of the control level, with 200 μl of input HCV giving a level of 100%.
DISCUSSION
The TIM gene family is composed of eight members (TIM-1 to TIM-8) in mice and three members (TIM-1, TIM-3, and TIM-4) in humans. The TIM proteins were initially found to play an important role in the regulation of host immune responses by specific recognition of the ligand PS exposed on the surfaces of apoptotic cells (40, 46). Now several studies have demonstrated that both TIM-1 and TIM-4 can also promote infections by a number of different viruses, including but not limited to flaviviruses, filoviruses, arenaviruses, alphaviruses, and retroviruses, through apoptotic mimicry (35–39, 47). In the present study, we obtained substantial evidence demonstrating that TIM-1 is also an important entry factor for HCV infection. The siRNA-mediated silencing of TIM-1 expression reduced HCV infection in a dose-dependent manner, by about 70% (Fig. 1). Likewise, CRISPR/Cas9-induced knockout of the TIM-1 gene also remarkably lowered HCV infection, similarly to the siRNA-mediated knockdown of TIM-1 expression (Fig. 3 and 4). More significantly, the impaired HCV infection in the TIM-1-deficient cells could be rescued fully by ectopic expression of TIM-1 but not by TIM-3 or TIM-4 expression, excluding any possible CRISPR/Cas9- and sgRNA-induced off-target effects (Fig. 6). The defective HCV infection in the TIM-1 knockout cells was also observed in other TIM-1 knockout Huh-7.5 cell lines (data not shown). Collectively, these findings demonstrate that TIM-1 promotes HCV infection.
The findings obtained from our present study also suggest that TIM-1 plays an important role in HCV attachment to the surfaces of hepatocytes. TIM-1 gene knockout resulted in a striking reduction of HCV attachment to cells (Fig. 3C and D), consistent with the impairment of HCV infection (Fig. 3A and B). Additionally, knockdown of TIM-1 expression did not affect HCV RNA replication (Fig. 1B). More importantly, TIM-1 gene knockout did not affect the expression of SDC1, which is a major HCV attachment receptor as demonstrated by our earlier work (20), nor was the expression of other key HCV receptors/coreceptors, such as CD81, claudin-1, occludin, and SR-BI, affected in the TIM-1 knockout cells, as they were expressed similarly to those in the parent Huh-7.5 cells (Fig. 5). Therefore, the defect of HCV infection in the TIM-1 knockout cells was most likely the result of an impairment of HCV cell attachment. Thus, TIM-1 can be considered another HCV attachment receptor, besides SDC1. TIM-1 and TIM-4 were previously found to be important attachment factors for other enveloped viruses when ectopically expressed in TIM-1- or TIM-4-deficient cells (38, 47). A direct interaction between TIM-1/TIM-4 and DENV was actually confirmed using a pulldown assay with soluble TIM-1-Fc and TIM-4-Fc (36). In general, it is believed that TIM-1 and TIM-4 promote infections by many different viruses through their specific recognition of PS exposed on the envelopes of viral particles (36–38, 47). The data from the present study also show that HCV infection and attachment were efficiently inhibited by PS-containing liposomes (Fig. 7), suggesting that TIM-1-mediated enhancement of HCV infection is PS dependent. This mechanism of action was further supported by immunoprecipitation of HCV particles with a PS-specific monoclonal antibody (Fig. 8). Collectively, our findings suggest that TIM-1 promotes HCV infection via direct binding to PS exposed on the HCV envelope.
The TIM family proteins contain four structurally distinct domains: an N-terminal immunoglobulin-like (IgV) domain, a stalk-like mucin domain of variable length and O/N-linked glycosylation, a single transmembrane domain, and a cytoplasmic region. The IgV domains of all TIM proteins possess a conserved pocket for PS binding, which facilitates the engulfment of apoptotic cells (40). However, only TIM-1 and TIM-4, not TIM-3, were found to promote efficient entry of various enveloped viruses (36, 37). Similarly, our study demonstrated that ectopic expression of TIM-3 in the TIM-1 knockout Huh-7.5 cells did not have a significant impact on HCV infection (Fig. 6), confirming previous findings that TIM-3 does not promote virus entry (36, 37). Structurally, TIM-3 has a much shorter mucin stalk than those of TIM-1 and TIM-4 (40), which may explain why TIM-3 does not promote virus infection. This interpretation is further supported by the finding that truncated TIM-1 mutants with mucin stalk lengths similar to that for TIM-3 were used less efficiently than wild-type TIM-1 in infections by several other enveloped viruses (37). However, it is not clear why ectopic expression of TIM-4 in the TIM-1 knockout Huh-7.5 cells failed to promote HCV infection (Fig. 6). TIM-4 has been shown to promote the entry of diverse viruses, including DENV (36, 37). Ectopic expression of TIM-4 could completely restore infection of DENV in TIM-1 knockout Huh-7.5 cells, similarly to ectopically expressed TIM-1 (L. Qiao, N. Qi, and G. Luo, unpublished results), suggesting that TIM-4 is fully functional when expressed ectopically. It is possible that the biochemical compositions of viral envelopes and the usages of different viral receptors influence the binding of PS to TIM-4. Consistent with this argument, the efficiency of virus entry mediated by TIM-1 and other PS-binding receptors was found to vary significantly among different viruses (37). It is known that HCV also uses the cellular protein apolipoprotein E for its attachment to the cell surface HSPGs (18, 48). Future investigations are warranted to determine whether the apolipoprotein(s) incorporated into the HCV envelope and/or HCV binding to its key receptors serves as a physical hindrance to TIM-4 access. Another question arose as to whether TIM-1 plays any role in viral pathogenesis in vivo. Epidemiological studies suggested that the TIM-1 gene is highly polymorphic and is associated with natural HCV clearance (49, 50). The physiological significance of TIM-1 in HCV infection in vivo awaits further determinations using humanized mouse models (51, 52).
In conclusion, the results originated from this study demonstrate that TIM-1 plays an important role in HCV cell attachment and infection and promotes HCV entry through a specific interaction with the ligand PS exposed on the HCV envelope. Therefore, TIM-1 and/or its binding partner, PS, may be considered a novel target for antiviral intervention against HCV and other enveloped viruses (53).
MATERIALS AND METHODS
Cell culture and virus.
Cells of the human hepatoma cell line Huh-7.5 and the lentivirus packaging cell line HEK293T were grown in DMEM supplemented with 10% fetal bovine serum (FBS), 0.1 mM nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 incubator. A cell culture-adapted HCV strain of genotype 2a (JFH1) was grown in Huh-7.5 cells as previously described (7).
Antibodies.
An HCV NS3-specific monoclonal antibody (Mab15) was produced in the lab as described previously (44). An NS5A monoclonal antibody (9E10) was provided by Tim Tellinghuisen. A β-actin monoclonal antibody (AC15) was purchased from Sigma-Aldrich. Human TIM-1 and TIM-3 monoclonal antibodies (MAB1750 and AF2365) were purchased from R&D Systems. A human claudin-1 monoclonal antibody and occludin- and SR-BI-specific rabbit polyclonal antibodies were purchased from Invitrogen. Human TIM-4 (G-6; sc-390805), CD81 (5A6; sc-23962), and syndecan-1 (DL-101; sc-12765) monoclonal antibodies, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (sc-2005) and rabbit anti-goat IgG (sc-2922) secondary antibodies, and normal mouse IgG were purchased from Santa Cruz. An HRP-conjugated goat anti-rabbit antibody (7074) was purchased from Cell Signaling. An anti-phosphatidylserine (PS) monoclonal antibody (clone 1H6) was purchased from EMD Millipore.
Silencing of TIM family gene expression.
TIM-1-, TIM-3-, and TIM-4-specific small interfering RNAs (siRNAs) (SmartPool siRNAs) and nonspecific control (NSC) siRNA were synthesized by Dharmacon. Cell culture plates (12- and 24-well plates) were coated with 50 μg/ml of collagen (Roche). Huh-7.5 cells were transfected with siRNAs at various concentrations (0, 2, 10, and 50 nM) by using RNAiMax transfection reagent (Invitrogen) as described previously (54). At 48 h p.t., the expression of TIM-1, TIM-3, and TIM-4 was determined by Western blotting using specific antibodies, with the β-actin housekeeping protein used as an internal control.
Plasmid DNA construction.
TIM-1- and TIM-4-specific sgRNAs were designed by use of the website of Feng Zhang (http://crispr.mit.edu/). Oligonucleotide primers for construction of sgRNA expression vectors were synthesized by IDT. The vector for TIM-1 sgRNA expression was constructed with primers TIM-1/F (5′-CACCGCACACGCTATAAGCTATTGG-3′) and TIM-1/R (5′-AAACCCAATAGCTTATAGCGTGTGC-3′). The primers used for construction of the TIM-4 sgRNA vector were TIM-4/F (5′-CACCGGTGCCCCTACTCCGGTTGCA-3′) and TIM-4/R (5′-AAACTGCAACCGGAGTAGGGGCACC-3′). Oligonucleotide primers (100 nmol [each]) were initially treated with T4 polynucleotide kinase and then annealed in T4 ligation buffer (New England BioLabs). The annealed oligonucleotides were inserted into the BsmBI-digested LentiCRISPR v2 vector (Addgene) by using T4 DNA ligase. The ligation mixture was transformed into Stbl3 competent cells (Transgen). Plasmid DNAs were prepared using a miniprep DNA isolation kit (Qiagen) and were confirmed by DNA sequence analysis. The TIM-1-expressing vector pClneo-F-TIM-1 was kindly provided by Shan-Lu Liu (45). To express TIM-3, the TIM-3 cDNA (RDC0670; R&D Systems) was digested with HindIII and SmaI and inserted into the pShuttle-CMV vector (Addgene) digested with HindIII and EcoRV. The TIM-4 expression vector pCMV6-entry-TIM-4 was constructed by inserting TIM-4 cDNA into the pCMV6-entry vector. TIM-4 cDNA (RDC1370) was purchased from R&D Systems. TIM-4 cDNA was amplified by a PCR using primers 5′-GCCGCGATCGCCATGTCGAAAGAACCT-3′ and 5′-CGTACGCGTGGCGCGCCTTAGAGG-3′. The TIM-4 PCR DNA fragment was digested with the AsiSI and MluI restriction enzymes and cloned into the pCMV6-entry vector, which was also cut with both AsiSI and MluI.
Generation of TIM-1 and TIM-4 knockout Huh-7.5 cell lines by CRISPR/Cas9-mediated genome editing.
HEK293T cells seeded in 10-cm dishes were transfected with a mixture of LentiCRISPR/Cas9-sgRNA plasmid DNA, psPAX2 (Addgene), and pCMV-VSV-G (Addgene) by use of Lipofectamine 2000 reagent (Invitrogen). At 72 h p.t., the recombinant lentiviruses, expressing specific CRISPR/Cas9 and sgRNAs in the supernatant, were collected and used for transduction of Huh-7.5 cells. TIM-1 and TIM-4 knockout cell clones were selected with 2 μg/ml of puromycin in cell culture medium for 2 to 3 weeks. Individual cell clones were transferred to 24-well cell culture plates for amplification and screening. Genomic DNAs of different cell clones were extracted using a DNeasy Blood & Tissue DNA isolation kit (Qiagen) and were subject to PCR amplification of TIM-1 and TIM-4 DNA fragments flanking the sgRNA target sites. The TIM-1 DNA fragment was amplified by a PCR using two specific primers (forward primer, 5′-GCAGGTCCATCTGTCACACT-3′; and reverse primer, 5′-AACAGGACTTACGGGAACCTC-3′). The TIM-4 DNA fragment was amplified using primers TIM-4-F (5′-GGAGTAAGCAAGGCACATCA-3′) and TIM-4-R (5′-CGAGTTCCCTTTCCACCTTCC-3′). The TIM-1 and TIM-4 PCR products were purified by 1% agarose gel electrophoresis and DNA extraction using a QIAquick gel extraction kit (Qiagen). TIM-1 and TIM-4 gene knockouts were confirmed by DNA sequence analysis.
HCV attachment and infection assays.
Parent Huh-7.5 cells (Huh-7.5 WT) and TIM-1 knockout (TIM-1−/−) Huh-7.5 cells were seeded in 12-well cell culture plates. Cells were incubated with HCV at a multiplicity of infection (MOI) of 10 at 37°C for 1 h (attachment assay). The unbound HCV was removed by washing with 1× phosphate-buffered saline (PBS) three times. Total RNA was extracted with TRIzol reagent (Invitrogen) and used for quantification of HCV virion RNA (vRNA) by a quantitative reverse transcription-PCR (qRT-PCR) method. HCV infection was carried out by incubation of HCV with cells at 37°C for 2 h. The HCV-infected cells were washed with PBS and incubated with fresh medium at 37°C for 24 h. The HCV-infected cells were lysed in protease inhibitor cocktail-containing RIPA buffer for detection of HCV NS5A by Western blotting, using β-actin as an internal control, as described previously (44).
HCV RNA extraction and quantification by qRT-PCR.
Total RNA in the HCV-infected or HCV-bound cells was extracted with TRIzol reagent. The level of positive-strand HCV RNA was determined by qRT-PCR, using a TaqMan RNA-to-CT-1-Step kit (Applied Biosystems) and specific primers and probe in a StepOnePlus real-time PCR system (Applied Biosystems). The oligonucleotide primers 2aF (5′-ATGGCGTTAGTATGAGTGTCG-3′) and 2aR (5′-CGGGCATAGAGTGGGTTTATC-3′) are complementary to the HCV 5′UTR sequence. The probe (5′-FAM-AGAGCCATA-ZEN-GTGGTCTG-3′) contains a 5′ 6-carboxyfluorescein (FAM) label and an internal ZEN quencher (IDT). The cellular glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control and was quantified using a mixture of primers and probe from Life Technology. The in vitro T7 transcript of the HCV JFH1 RNA genome was used as a standard. One-step real-time qRT-PCR was carried out using the following program: 48°C for 15 min and 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min.
Western blot analysis.
The protein concentrations in cell lysates were determined using a protein assay reagent (Bio-Rad). Thirty-five micrograms of total protein for each sample was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane by use of a semidry blotter (Bio-Rad). After blocking with 5% nonfat milk, the membrane was incubated first with primary antibodies specific to TIM-1, TIM-3, TIM-4, NS5A, and β-actin. Cellular β-actin was used as an internal control. A horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit secondary antibody was used for protein staining, which was amplified using an enhanced chemiluminescence (ECL) kit (Pierce). Protein images were obtained with a Chemi-Doc MP imaging system (Bio-Rad).
Ectopic expression of TIM family proteins in TIM-1 knockout Huh-7.5 cells.
To ectopically express TIM-1, TIM-3, and TIM-4, pClneo-F-TIM-1, pShuttle-CMV-TIM-3, and pCMV6-entry-TIM-4 were individually transfected into TIM-1 knockout (TIM-1−/−) Huh-7.5 cells by use of Lipofectamine 2000 reagent (Invitrogen). The pShuttle-CMV vector was used as a negative control. At 48 h p.t., cells were infected with HCV at an MOI of 10 for 2 h. The HCV-infected cells were washed with PBS and incubated with fresh DMEM at 37°C for 24 h. The levels of TIM-1, TIM-3, TIM-4, HCV NS5A, and the housekeeping protein β-actin were detected by Western blotting using specific antibodies.
Liposome blocking assay.
1,2-Diacyl-sn-glycero-3-phospho-l-serine (PS) and 1,2-diacyl-sn-glycero-3-phosphocholine (PC) were purchased from Sigma. Liposomes were prepared as described previously (37). A liposome blocking assay of HCV infection was carried out by incubating HCV with Huh-7.5 cells in the presence of various concentrations (0, 11.1, 33.3, and 100 μM) of PS, PC, or a PS-PC mixture. At 24 h p.i., cell lysates were collected for detection of NS5A by Western blotting. To determine the effect of PS-containing liposomes on HCV attachment, Huh-7.5 cells were incubated on ice for 2 h in the presence of various concentrations of liposomes. Total RNAs were extracted with TRIzol reagent. The levels of cell-bound HCV vRNA were quantified by qRT-PCR.
HCV immunoprecipitation.
Protein G-conjugated agarose beads (Life Technology) were coated with 20 μg of a monoclonal antibody specific to phosphatidylserine or HCV E2 (CBH5) (55) or a normal mouse IgG in a final volume of 500 μl at 4°C overnight. The antibody-coated protein G agarose beads were then incubated with 200 μl of HCV at 4°C overnight. Immunoprecipitated HCV was washed with 150 mM NaCl three times. HCV vRNA was extracted by use of TRIzol-LS reagent (Invitrogen). Zika virus RNA was added as a control for HCV vRNA extraction and quantification. The vRNA levels of immunoprecipitated HCV were quantified by a real-time qRT-PCR method.
Statistical analysis.
Graphical representation and statistical analyses were performed by use of Prism5 software (GraphPad Software). Results are shown as means ± standard deviations (SD) of the data obtained from at least three independent experiments. Comparisons between samples were done using the paired two-tailed t test. P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
We thank Charlie Rice (Rockefeller University), Timothy Tellinghuisen (The Scripps Research Institute, FL), and Shan-Lu Liu (OSU) for providing the Huh-7.5 cell line, the NS5A monoclonal antibody 9E10, and the TIM1-expressing vector pClneo-F-TIM1, respectively. We also thank Kyung-Don Kang for help with data quantification and for technical assistance.
This work was supported by the Natural Science Foundation of China (NSFC grant 81130082), NIH grants AI097318 and AI091953, the National Basic Research Program of China (grant 2012CB518900 of the 973 Program), and the University of Alabama at Birmingham (UAB) Center For AIDS Research (CFAR), an NIH-funded program (grant P30 AI027767).
REFERENCES
- 1.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 2.Robertson B, Myers G, Howard C, Brettin T, Bukh J, Gaschen B, Gojobori T, Maertens G, Mizokami M, Nainan O, Netesov S, Nishioka K, Shin IT, Simmonds P, Smith D, Stuyver L, Weiner A. 1998. Classification, nomenclature, and database development for hepatitis C virus (HCV) and related viruses: proposals for standardization. International Committee on Virus Taxonomy. Arch Virol 143:2493–2503. [DOI] [PubMed] [Google Scholar]
- 3.Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. 2014. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology 59:318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tellinghuisen TL, Evans MJ, von Hahn T, You S, Rice CM. 2007. Studying hepatitis C virus: making the best of a bad virus. J Virol 81:8853–8867. doi: 10.1128/JVI.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumert TF, Ito S, Wong DT, Liang TJ. 1998. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J Virol 72:3827–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J, Luo G. 2012. Cell culture-adaptive mutations promote viral protein-protein interactions and morphogenesis of infectious hepatitis C virus. J Virol 86:8987–8997. doi: 10.1128/JVI.00004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jirasko V, Montserret R, Lee JY, Gouttenoire J, Moradpour D, Penin F, Bartenschlager R. 2010. Structural and functional studies of nonstructural protein 2 of the hepatitis C virus reveal its key role as organizer of virion assembly. PLoS Pathog 6:e1001233. doi: 10.1371/journal.ppat.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi M, Ma Y, Yates J, Lemon SM. 2007. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J Virol 81:629–638. doi: 10.1128/JVI.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frolov I, McBride MS, Rice CM. 1998. cis-Acting RNA elements required for replication of bovine viral diarrhea virus-hepatitis C virus 5′ nontranslated region chimeras. RNA 4:1418–1435. doi: 10.1017/S1355838298981031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagan SM, Chahal J, Sarnow P. 2015. cis-Acting RNA elements in the hepatitis C virus RNA genome. Virus Res 206:90–98. doi: 10.1016/j.virusres.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. 2005. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science 309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 13.Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. 2007. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol 9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 14.Norman KL, Sarnow P. 2010. Modulation of hepatitis C virus RNA abundance and the isoprenoid biosynthesis pathway by microRNA miR-122 involves distinct mechanisms. J Virol 84:666–670. doi: 10.1128/JVI.01156-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, Landthaler M, Landgraf P, Kan S, Lindenbach BD, Chien M, Weir DB, Russo JJ, Ju J, Brownstein MJ, Sheridan R, Sander C, Zavolan M, Tuschl T, Rice CM. 2007. Cellular cofactors affecting hepatitis C virus infection and replication. Proc Natl Acad Sci U S A 104:12884–12889. doi: 10.1073/pnas.0704894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubuisson J, Helle F, Cocquerel L. 2008. Early steps of the hepatitis C virus life cycle. Cell Microbiol 10:821–827. doi: 10.1111/j.1462-5822.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 17.Zeisel MB, Fofana I, Fafi-Kremer S, Baumert TF. 2011. Hepatitis C virus entry into hepatocytes: molecular mechanisms and targets for antiviral therapies. J Hepatol 54:566–576. doi: 10.1016/j.jhep.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Jiang J, Cun W, Wu X, Shi Q, Tang H, Luo G. 2012. Hepatitis C virus attachment mediated by apolipoprotein E binding to cell surface heparan sulfate. J Virol 86:7256–7267. doi: 10.1128/JVI.07222-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J, Luo G. 2009. Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J Virol 83:12680–12691. doi: 10.1128/JVI.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Q, Jiang J, Luo G. 2013. Syndecan-1 serves as the major receptor for attachment of hepatitis C virus to the surfaces of hepatocytes. J Virol 87:6866–6875. doi: 10.1128/JVI.03475-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. 2002. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J 21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. 2007. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Yang W, Shen L, Turner JR, Coyne CB, Wang T. 2009. Tight junction proteins claudin-1 and occludin control hepatitis C virus entry and are downregulated during infection to prevent superinfection. J Virol 83:2011–2014. doi: 10.1128/JVI.01888-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, Abrignani S. 1998. Binding of hepatitis C virus to CD81. Science 282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 25.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. 2009. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. 1999. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci U S A 96:12766–12771. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haberstroh A, Schnober EK, Zeisel MB, Carolla P, Barth H, Blum HE, Cosset FL, Koutsoudakis G, Bartenschlager R, Union A, Depla E, Owsianka A, Patel AH, Schuster C, Stoll-Keller F, Doffoel M, Dreux M, Baumert TF. 2008. Neutralizing host responses in hepatitis C virus infection target viral entry at postbinding steps and membrane fusion. Gastroenterology 135:1719.e1–1728.e1. doi: 10.1053/j.gastro.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Sourisseau M, Michta ML, Zony C, Israelow B, Hopcraft SE, Narbus CM, Parra Martin A, Evans MJ. 2013. Temporal analysis of hepatitis C virus cell entry with occludin directed blocking antibodies. PLoS Pathog 9:e1003244. doi: 10.1371/journal.ppat.1003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, Royer C, Fischer B, Zahid MN, Lavillette D, Fresquet J, Cosset FL, Rothenberg SM, Pietschmann T, Patel AH, Pessaux P, Doffoel M, Raffelsberger W, Poch O, McKeating JA, Brino L, Baumert TF. 2011. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med 17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sainz B Jr, Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, Marsh KA, Yu X, Chayama K, Alrefai WA, Uprichard SL. 2012. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med 18:281–285. doi: 10.1038/nm.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Tian Y, Machida K, Lai MM, Luo G, Foung SK, Ou JH. 2012. Transient activation of the PI3K-AKT pathway by hepatitis C virus to enhance viral entry. J Biol Chem 287:41922–41930. doi: 10.1074/jbc.M112.414789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, Lee EM, Hammack C, Robotham JM, Basu M, Lang J, Brinton MA, Tang H. 2014. Cell death-inducing DFFA-like effector b is required for hepatitis C virus entry into hepatocytes. J Virol 88:8433–8444. doi: 10.1128/JVI.00081-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Despres P, Amara A, Yssel H, Misse D. 2015. Biology of Zika virus infection in human skin cells. J Virol 89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HY, Eyheramonho MB, Pichavant M, Gonzalez Cambaceres C, Matangkasombut P, Cervio G, Kuperman S, Moreiro R, Konduru K, Manangeeswaran M, Freeman GJ, Kaplan GG, DeKruyff RH, Umetsu DT, Rosenzweig SD. 2011. A polymorphism in TIM1 is associated with susceptibility to severe hepatitis A virus infection in humans. J Clin Invest 121:1111–1118. doi: 10.1172/JCI44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, Sandersfeld LM, Quinn K, Weller M, McCray PB Jr, Chiorini J, Maury W. 2011. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci U S A 108:8426–8431. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A. 2012. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jemielity S, Wang JJ, Chan YK, Ahmed AA, Li W, Monahan S, Bu X, Farzan M, Freeman GJ, Umetsu DT, Dekruyff RH, Choe H. 2013. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog 9:e1003232. doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller-Tank S, Kondratowicz AS, Davey RA, Rennert PD, Maury W. 2013. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J Virol 87:8327–8341. doi: 10.1128/JVI.01025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morizono K, Chen IS. 2014. Role of phosphatidylserine receptors in enveloped virus infection. J Virol 88:4275–4290. doi: 10.1128/JVI.03287-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. 2010. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev 235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morizono K, Xie Y, Olafsen T, Lee B, Dasgupta A, Wu AM, Chen IS. 2011. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe 9:286–298. doi: 10.1016/j.chom.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimojima M, Takada A, Ebihara H, Neumann G, Fujioka K, Irimura T, Jones S, Feldmann H, Kawaoka Y. 2006. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J Virol 80:10109–10116. doi: 10.1128/JVI.01157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brindley MA, Hunt CL, Kondratowicz AS, Bowman J, Sinn PL, McCray PB Jr, Quinn K, Weller ML, Chiorini JA, Maury W. 2011. Tyrosine kinase receptor Axl enhances entry of Zaire ebolavirus without direct interactions with the viral glycoprotein. Virology 415:83–94. doi: 10.1016/j.virol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai Z, Zhang C, Chang KS, Jiang J, Ahn BC, Wakita T, Liang TJ, Luo G. 2005. Robust production of infectious hepatitis C virus (HCV) from stably HCV cDNA-transfected human hepatoma cells. J Virol 79:13963–13973. doi: 10.1128/JVI.79.22.13963-13973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Ablan SD, Miao C, Zheng YM, Fuller MS, Rennert PD, Maury W, Johnson MC, Freed EO, Liu SL. 2014. TIM-family proteins inhibit HIV-1 release. Proc Natl Acad Sci U S A 111:E3699–E3707. doi: 10.1073/pnas.1404851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. 2007. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moller-Tank S, Maury W. 2014. Phosphatidylserine receptors: enhancers of enveloped virus entry and infection. Virology 468–470:565–580. doi: 10.1016/j.virol.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang J, Wu X, Tang H, Luo G. 2013. Apolipoprotein E mediates attachment of clinical hepatitis C virus to hepatocytes by binding to cell surface heparan sulfate proteoglycan receptors. PLoS One 8:e67982. doi: 10.1371/journal.pone.0067982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abad-Molina C, Garcia-Lozano JR, Montes-Cano MA, Torres-Cornejo A, Torrecillas F, Aguilar-Reina J, Romero-Gomez M, Lopez-Cortes LF, Nunez-Roldan A, Gonzalez-Escribano MF. 2012. HAVCR1 gene haplotypes and infection by different viral hepatitis C virus genotypes. Clin Vaccine Immunol 19:223–227. doi: 10.1128/CVI.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosbruger TL, Duggal P, Goedert JJ, Kirk GD, Hoots WK, Tobler LH, Busch M, Peters MG, Rosen HR, Thomas DL, Thio CL. 2010. Large-scale candidate gene analysis of spontaneous clearance of hepatitis C virus. J Infect Dis 201:1371–1380. doi: 10.1086/651606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ploss A, Rice CM. 2009. Towards a small animal model for hepatitis C. EMBO Rep 10:1220–1227. doi: 10.1038/embor.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Washburn ML, Bility MT, Zhang L, Kovalev GI, Buntzman A, Frelinger JA, Barry W, Ploss A, Rice CM, Su L. 2011. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology 140:1334–1344. doi: 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soares MM, King SW, Thorpe PE. 2008. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat Med 14:1357–1362. doi: 10.1038/nm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang KS, Jiang J, Cai Z, Luo G. 2007. Human apolipoprotein E is required for infectivity and production of hepatitis C virus in cell culture. J Virol 81:13783–13793. doi: 10.1128/JVI.01091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keck ZY, Xia J, Cai Z, Li TK, Owsianka AM, Patel AH, Luo G, Foung SK. 2007. Immunogenic and functional organization of hepatitis C virus (HCV) glycoprotein E2 on infectious HCV virions. J Virol 81:1043–1047. doi: 10.1128/JVI.01710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]