Abstract
Psychopharmacological research, if properly designed, may offer insight into both timing and area of effect, increasing our understanding of the brain's neurotransmitter systems. For that purpose, the acute influence of the selective serotonin reuptake inhibitor citalopram (30 mg) and the acetylcholinesterase inhibitor galantamine (8 mg) was repeatedly measured in 12 healthy young volunteers with resting state functional magnetic resonance imaging (RS‐fMRI). Eighteen RS‐fMRI scans were acquired per subject during this randomized, double blind, placebo‐controlled, crossover study. Within‐group comparisons of voxelwise functional connectivity with 10 functional networks were examined (P < 0.05, FWE‐corrected) using a non‐parametric multivariate approach with cerebrospinal fluid, white matter, heart rate, and baseline measurements as covariates. Although both compounds did not change cognitive performance on several tests, significant effects were found on connectivity with multiple resting state networks. Serotonergic stimulation primarily reduced connectivity with the sensorimotor network and structures that are related to self‐referential mechanisms, whereas galantamine affected networks and regions that are more involved in learning, memory, and visual perception and processing. These results are consistent with the serotonergic and cholinergic trajectories and their functional relevance. In addition, this study demonstrates the power of using repeated measures after drug administration, which offers the chance to explore both combined and time specific effects. Hum Brain Mapp 38:308–325, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: resting state fMRI, functional connectivity, psychopharmacology, SSRI, serotonin, AChEI, acetylcholine, citalopram, galantamine, resting state network
INTRODUCTION
Drugs acting on serotonin (5‐hydroxytryptamine; 5‐HT) and acetylcholine (ACh) are known for their regulating influence on behavior and cognition. Selective serotonin reuptake inhibitors (SSRIs) are accepted for their mood altering properties and usually prescribed to treat depression and anxiety disorders [Carr and Lucki, 2011; Jacobs and Azmitia, 1992]. Acetylcholinesterase inhibitors (AChEIs) are found to be beneficial in neurodegenerative disorders [Alzheimer's disease (AD), dementia with Lewy bodies and Parkinson's disease] due to their effect on attention, learning, and memory [Perry et al., 1999; Soreq and Seidman, 2001].
The brain's serotonergic axonal pathways originate in the midbrain's medial and dorsal raphe nuclei. In the central nervous system (CNS), a particularly high density of 5‐HT receptors is observed in the cerebral cortex, limbic structures, basal ganglia and brain stem regions [Daubert and Condron, 2010; Tork, 1990]. For ACh, the major source is the basal forebrain, with fibers diffusing to the cortex, amygdala, and hippocampus [Benarroch, 2010]. The finding that specific neurotransmitters like 5‐HT and ACh also act as neuromodulators, has led to the formation of distributed computational network models [Baxter et al., 1999; Doya, 2002; Marder and Thirumalai, 2002]. Consequently, studies of cholinergic or serotonergic drug effects also need to consider their extensive modulatory effects [Bargmann, 2012]. This is possible with resting state functional magnetic resonance imaging (RS‐fMRI) in the context of pharmacological stimulation [Fox and Raichle, 2007; Khalili‐Mahani et al., 2014].
Evidence is growing on the sensitivity of resting state networks, consisting of regions with coherent blood‐oxygen‐level‐dependent fluctuations, to pharmacological challenges [Cole et al., 2013; Khalili‐Mahani et al., 2015, 2012; Klumpers et al., 2012; Niesters et al., 2012]. These networks have consistently been found in healthy and clinical conditions, and are related to specific functions of the brain (i.e., motor, auditory, visual, emotional, and executive function) [Beckmann et al., 2005; Damoiseaux et al., 2006; Seeley et al., 2007; Smith et al., 2009]. Disruptions of functional networks have been demonstrated in both depressed and demented patients, especially for the default mode network (DMN) [Hafkemeijer et al., 2012; Sundermann et al., 2014; Wang et al., 2006]. Several studies point to normalization of DMN connectivity in depression after SSRI administration [McCabe and Mishor, 2011; McCabe et al., 2011; Van de Ven et al., 2013; Van Wingen et al., 2014]. Yet, there is also proof of more extensive effects of SSRIs on brain connectivity [Klaassens et al., 2015; Schaefer et al., 2014]. In AD patients, cholinergic stimulation induces alterations in connectivity for DMN regions [Goveas et al., 2011; Li et al., 2012; Solé‐Padullés et al., 2013], as well as networks involved in attention, control, and salience processing [Wang et al., 2014].
Characteristically, neuromodulators support the processing of sensory information, coordination of motor output, and higher order cognitive functioning [Foehring and Lorenzon, 1999; Gu, 2002; Hasselmo, 1995]. In line with the diverse and widespread patterns of effect of both transmitters we investigated the direct influence of the SSRI citalopram 30 mg and the AChEI galantamine 8 mg on various brain networks. Both RS‐fMRI and functional (cognitive and neuroendocrine) responses were examined in 12 healthy young volunteers in a repeated measures fashion. Galantamine was hypothesized to mainly affect connectivity with brain structures that are involved in learning and memory mechanisms. Based on our previous study with the SSRI sertraline 75 mg, we expected to see widespread decreases in connectivity immediately after citalopram administration in the absence of cognitive change.
METHOD
Subjects
Twelve healthy young volunteers (mean age 22.1 ± 2.7, range 18–27; gender ratio 1:1, BMI 21–28 kg/m2) were recruited to participate in the study. All subjects underwent a thorough medical screening at the Centre for Human Drug Research (CHDR) to investigate whether they met the inclusion and exclusion criteria. They had a normal history of physical and mental health and were able to refrain from using nicotine and caffeine during study days. Exclusion criteria included positive drug or alcohol screen on study days, regular excessive consumption of alcohol (>4 units/day), caffeine (>6 units/day) or cigarettes (>5 cigarettes/day), use of concomitant medication 2 weeks prior to study participation, and involvement in an investigational drug trial 3 months prior to administration. The study was approved by the medical ethics committee of the Leiden University Medical Center (LUMC) and the scientific review board of the CHDR. Written informed consent was obtained from each subject prior to study participation.
Study Design
This was a single center, double blind, placebo‐controlled, crossover study with citalopram 30 mg and galantamine 8 mg. Each subject received citalopram, galantamine and placebo on three different occasions with a washout period in between of at least 7 days. Citalopram has an average time point of maximum concentration (T max) of 2–4 h, with a half‐life (T ½) of 36 h. For galantamine, T max = 1–2 h and T ½ = 7–8 h. To correct for the different pharmacokinetic (PK) profiles of the compounds, citalopram 20 mg was administered at T = 0 h, followed by a second dose of 10 mg at T = 1 h (if the first dose was tolerated and subjects did not become too nauseous). Galantamine was given as a single 8 mg dose at T = 2 h. Blinding was maintained by concomitant administration of double‐dummy placebo's at all three time points. All subjects also received an unblinded dose of granisetron 2 mg at T = −0.5 h, to prevent the most common drug‐induced adverse effects of nausea and vomiting.
Six RS‐fMRI scans were acquired during study days, two at baseline and four after administering citalopram, galantamine or placebo (at T = 2.5, 3.5, 4.5, and 6 h post dosing). Each scan was followed by performance of computerized cognitive tasks (taken twice at baseline) on the NeuroCart® test battery, developed by the CHDR for quantifying pharmacological effects on the CNS [Dumont et al., 2005; Gijsman et al., 2002; Liem‐Moolenaar et al., 2011]. By including multiple measurements during the T max interval, this repeated measures profile increases the statistical power of the analysis and allows for identification of time related effects, associated with changing serum concentrations. Nine blood samples were taken during the course of the day to define the PK profile of citalopram, citalopram's active metabolite desmethylcitalopram, galantamine, and concentrations of cortisol and prolactin [Jacobs et al., 2010b; Umegaki et al., 2009]. An overview of the study design is provided in Figure 1.
Figure 1.
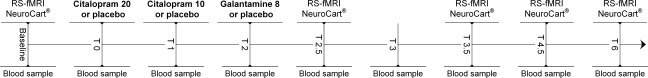
Schematic overview of a study day. Each subject received citalopram, galantamine, and placebo on three different days. At baseline, two RS‐fMRI scan were acquired, followed by the NeuroCart® CNS test battery. After drug administration, four more RS‐fMRI scans were acquired at time points T = 2.5, 3.5, 4.5, and 6 h post dosing, each time followed by the NeuroCart® test battery. During the day, nine blood samples were taken to measure the concentrations of citalopram, desmethylcitalopram, galantamine, cortisol, and prolactin. On each study day there were three moments of administration. The second administration only took place when subjects tolerated the first dose well (did not vomit or feel too nauseous): Galantamine study day: T = 0) placebo T = 1) placebo T = 2) galantamine 8 mg. Citalopram study day: T = 0) citalopram 20 mg T = 1) citalopram 10 mg T = 2) placebo. Placebo study day: T = 0) placebo T = 1) placebo T = 2) placebo.
Blood Sampling
Pharmacokinetics
Blood samples were collected in 4 mL EDTA plasma tubes at baseline and 1, 2, 2.5, 3, 3.5, 4.5, and 6 h post dosing, centrifuged (2,000g for 10 min) and stored at −40°C until analysis with liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). PK parameters for citalopram, galantamine and citalopram's active metabolite desmethylcitalopram were calculated using a non‐compartmental analysis. Maximum plasma concentrations (C max) and time of C max (T max) were obtained directly from the plasma concentration data. The area under the plasma concentration versus time curve was calculated from time zero to the time of the last quantifiable measured plasma concentration, which is equal to the last blood sample of the study day (AUC0‐last). The calculated PK parameters were not used for further analysis but investigated to validate the choice of time points of measurements.
Neuroendocrine variables
Blood samples were also obtained to determine cortisol and prolactin concentrations. Serum samples were taken in a 3.5 mL gel tube at baseline (twice) and 1, 2, 2.5, 3.5, 4.5, and 6 h post dosing, centrifuged (2,000g for 10 min) and stored at −40°C until analysis. Serum concentrations were quantitatively determined with electrochemiluminescence immunoassay. Cortisol and prolactin concentrations were subsequently used for statistical analysis using a mixed effects model with treatment, time, visit and treatment by time as fixed effects, subject, subject by treatment, and subject by time as random effects and the average of the period baseline (pre‐dose) values as covariate (SAS for Windows V9.4; SAS Institute, Inc., Cary, NC).
NeuroCart® Test Battery
Each RS‐fMRI scan was followed by functional CNS measures outside the scanner using the computerized NeuroCart® test battery measuring alertness, mood, and calmness [Visual Analogue Scales (VAS) Bond & Lader], nausea (VAS Nausea), vigilance and visual motor performance (Adaptive Tracking task), reaction time (Simple Reaction Time task), attention, short‐term memory, psychomotor speed, task switching, and inhibition (Symbol Digit Substitution Test and Stroop task), working memory (N‐back task) and memory imprinting and retrieval (Visual Verbal Learning Test) [Bond and Lader, 1974; Borland and Nicholson, 1984; Laeng et al., 2005; Lezak, 2004; Lim et al., 2008; Norris, 1971; Rogers et al., 2004; Stroop, 1935; Wechsler, 1981]. The Visual Verbal Learning Test was only performed once during each day (at 3 and 4 h post dosing) as the test itself consists of different trials (imprinting and retrieval). Duration of each series of NeuroCart® brain function tests was approximately 20 min. To minimize learning effects, training for the NeuroCart® tasks occurred during the screening visit within 3 weeks prior to the first study day.
Analysis
All within period repeatedly measured CNS endpoints were analyzed using a mixed effects model with treatment, time, visit and treatment by time as fixed effects, subject, subject by treatment and subject by time as random effects and the average of the period baseline (pre‐dose) values as covariate (SAS for Windows V9.4; SAS Institute, Inc., Cary, NC). As data of the Simple Reaction Time task were not normally distributed, these data were log‐transformed before analysis and back transformed after analysis. The data of the Visual Verbal Learning test were analyzed using a mixed effects model with treatment and visit as fixed effects and subject as random effect. Treatment effects were considered significant at P < 0.05 (uncorrected).
Imaging
Scanning was performed at the LUMC on a Philips 3.0 Tesla Achieva MRI scanner (Philips Medical System, Best, The Netherlands) using a 32‐channel head coil. During the RS‐fMRI scans, all subjects were asked to close their eyes while staying awake. They were also instructed not to move their head during the scan. Instructions were given prior to each scan on all study days. T1‐weighted anatomical images were acquired once per visit. To facilitate registration to the anatomical image, each RS‐fMRI scan was followed by a high‐resolution T2*‐weighted echo‐planar scan. Duration was approximately 8 min for the RS‐fMRI scan, 5 min for the anatomical scan and 30 sec for the high‐resolution scan. Heart rate signals were recorded during each scan.
RS‐fMRI data were obtained with T2*‐weighted echo‐planar imaging (EPI) with the following scan parameters: 220 whole brain volumes, repetition time (TR) = 2,180 ms; echo time (TE) = 30 ms; flip angle = 85°; field‐of‐view (FOV) = 220 × 220 × 130 mm; in‐plane voxel resolution = 3.44 × 3.44 mm, slice thickness = 3.44 mm, including 10% interslice gap. The next parameters were used to collect T1‐weighted anatomical images: TR = 9.1 ms; TE = 4.6 ms; flip angle = 8°; FOV = 224 × 177 × 168 mm; in‐plane voxel resolution = 1.17 × 1.17 mm; slice thickness = 1.2 mm. Parameters of high‐resolution T2*‐weighted EPI scans were set to: TR = 2,200 ms; TE = 30 ms; flip angle = 80°; FOV = 220 × 220 × 168 mm; in‐plane voxel resolution = 1.96 × 1.96 mm; slice thickness = 2.0 mm.
Analysis
All analyses were performed using the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL, Oxford, United Kingdom) version 5.0.7 [Jenkinson et al., 2012; Smith et al., 2004; Woolrich et al., 2009]. Each individual functional EPI image was inspected, brain‐extracted and corrected for geometrical displacements due to head movement with linear (affine) image registration. Images were spatially smoothed with a 6 mm full‐width half‐maximum Gaussian kernel and co‐registered with the brain extracted high resolution T2*‐weighted EPI scans (with 6 degrees of freedom) and T1 weighted images (using the Boundary‐Based‐Registration method) [Greve and Fischl, 2009; Smith, 2002]. The T1‐weighted scans were non‐linearly registered to the MNI 152 standard space (the Montreal Neurological Institute, Montreal, QC, Canada) using FMRIB's Nonlinear Image Registration Tool. Registration parameters were estimated on non‐smoothed data to transform fMRI scans into standard space. Automatic Removal of Motion Artifacts based on Independent Component Analysis (ICA‐AROMA vs0.3‐beta) was used to detect and remove motion related artifacts. ICA decomposes the data into independent components that are either noise related or pertain to functional networks. ICA‐AROMA attempts to identify noise components by investigating its temporal and spatial properties and removes these components from the data that are classified as motion related. Registration was thereafter applied on the denoised functional data with registration parameters as derived from non‐smoothed data. As recommended, high pass temporal filtering (with a high pass filter of 150 sec) was applied after denoising the fMRI data with ICA‐AROMA [Pruim et al., 2015a, 2015b].
RS‐fMRI networks were thereafter extracted from each individual denoised RS‐fMRI dataset (12 subjects × 3 days × 6 scans = 216 datasets) applying a dual regression analysis [Beckmann et al., 2009; Filippini et al., 2009] based on 10 predefined standard network templates as used in our previous research [Klaassens et al., 2015]. Confound regressors of time series from white matter (measured from the center of the corpus callosum) and cerebrospinal fluid (measured from the center of lateral ventricles) were included in this analysis to account for non‐neuronal signal fluctuations [Birn, 2012]. The 10 standard templates have previously been identified using a data‐driven approach [Smith et al., 2009] and comprise the following networks: three visual networks (consisting of medial, occipital pole, and lateral visual areas), DMN (medial parietal (precuneus and posterior cingulate), bilateral inferior–lateral–parietal and ventromedial frontal cortex), cerebellar network, sensorimotor network (supplementary motor area, sensorimotor cortex, and secondary somatosensory cortex), auditory network (superior temporal gyrus, Heschl's gyrus, and posterior insular), executive control network (medial–frontal areas, including anterior cingulate and paracingulate) and two frontoparietal networks (frontoparietal areas left and right). With the dual regression method, spatial maps representing voxel‐to‐network connectivity were estimated for each dataset separately in two stages for use in within‐group comparisons. First, the weighted network maps were simultaneously used in a spatial regression into each dataset. This stage generated 12 time series per dataset that describe the average temporal course of signal fluctuations of the 10 networks plus 2 confound regressors (cerebrospinal fluid and white matter). Next, this combination of time series was entered in a temporal regression into the same dataset. This resulted in a spatial map per network per dataset with regression coefficients referring to the weight of each voxel being associated with the characteristic signal change of a specific network. The higher the value of the coefficient, the stronger the connectivity of this voxel with a given network. These individual statistical maps were subsequently used for higher level analysis.
To infer treatment effects of citalopram and galantamine versus placebo across time as well as for each time point separately we used non‐parametric combination (NPC) as provided by FSL's Permutation Analysis for Linear Models tool (PALM vs65‐alpha) [Pesarin, 1990; Winkler et al., 2016]. NPC is a multivariate method that offers the possibility to combine data of separate, possibly non‐independent tests, such as our multiple time points, and investigate the presence of joint effects across time points, in a test that has fewer assumptions and is more powerful than repeated‐measurements analysis of variance (ANOVA) or multivariate analysis of variance (MANOVA). NPC testing was used in two phases to estimate for each network whether connectivity was significantly different on drug relative to placebo days. First, tests were performed for each time point using 5,000 synchronized permutations. More specifically, to investigate changes in voxelwise functional connectivity with each of the 10 functional networks, four t‐tests (drug vs. placebo) were performed for all post‐dose time points (T = 2.5, 3.5, 4.5, and 6 h), with average heart rate (beats/m) per RS‐fMRI scan as confound regressor [Khalili‐Mahani et al., 2013]. The average of the two baseline RS‐fMRI scans was used as covariate as well, by adding the coefficient spatial map as a voxel‐dependent regressor in the model. Second, tests for the four time points were combined non‐parametrically via NPC using Fisher's combining function [Fisher, 1932] and the same set of synchronized permutations as mentioned above. Threshold‐free cluster enhancement was applied to the tests at each time point and after the combination, and the resulting voxelwise statistical maps were corrected for the familywise error rate using the distribution of the maximum statistic [Smith and Nichols, 2009; Winkler et al., 2014]. Voxels were considered significant at P‐values <0.05, corrected.
RESULTS
Pharmacokinetics
The time to reach maximum plasma concentrations (T max) was highly variable for both citalopram and galantamine (see Fig. 2 for individual and median PK time profiles). Maximum plasma concentrations of citalopram were reached between 1.93 and 6 h after the first dose (mean T max: 2.99 ± 1.18), and between 2.48 and 6.05 h (mean T max: 4.92 ± 1.33) for desmethylcitalopram. C max for citalopram was between 20.4 and 42.4 ng/mL (mean C max: 35.8 ± 6.34) and between 1.45 and 4.7 ng/mL (mean C max: 2.95 ± 1.07) for desmethylcitalopram. AUC0‐last was between 86.8 and 186 ng*h/mL (mean AUC0‐last: 146 ± 25.2) for citalopram and between 5.43 and 18.6 ng*h/mL (mean AUC0‐last: 11.7 ± 4.78) for desmethylcitalopram. Maximum plasma concentrations of galantamine were reached between 0.5 and 4 h (mean T max: 2.67 ± 1.11). Consequently, maximum concentrations were reached between 2.5 and 6 h post zero point (mean T max: 4.67 ± 1.11). C max for galantamine was between 25.6 and 61.4 ng/mL (mean C max: 40.7 ± 10.4). AUC0‐last was between 49.1 and 152 ng*h/mL (mean AUC0‐last: 95.1 ± 27.7).
Figure 2.
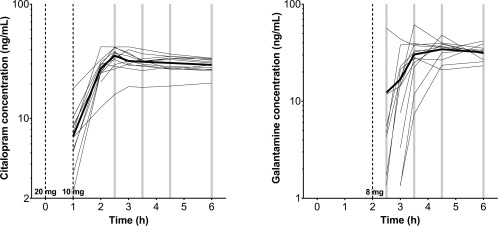
Median (bold line) and individual (thin lines) pharmacokinetic profiles for citalopram (left) and galantamine (right) concentrations in nanograms per milliliter on semi‐log scale. Gray bars illustrate moments of RS‐fMRI acquisition post drug administration. Observations below limit of quantification were dismissed.
Cortisol and Prolactin
As shown in Figure 3a,b, concentrations of cortisol and prolactin increased after citalopram, relative to placebo (P < 0.01). There was no significant treatment effect of galantamine on either neuroendocrine hormone concentration.
Figure 3.
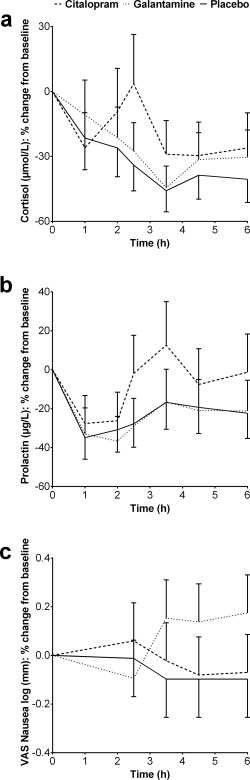
Least squares means percent change from baseline profiles of cortisol and prolactin concentrations and nausea as measured with the Visual Analogue Scales (with standard errors of the mean as error bars).
NeuroCart® Test Battery
There were no significant treatment effects of citalopram and galantamine on measures of cognitive performance. Compared with placebo, galantamine increased the level of nausea as measured with the VAS Nausea (P < 0.05). Citalopram did not cause significant nausea (see Fig. 3c). The effects of citalopram and galantamine on all cognitive and subjective NeuroCart® measures are summarized in the Supporting Information.
Imaging
Citalopram: combined test
Combining the data of all post‐dose time points, there was a decrease in connectivity after administering citalopram compared with placebo (Fig. 4a) between (1) the sensorimotor network and the pre‐ and postcentral gyri, supplementary motor area (SMA), precuneus, posterior and anterior cingulate cortex (PCC/ACC), medial prefrontal cortex and cerebellum, and (2) the right frontoparietal network and brain stem.
Figure 4.
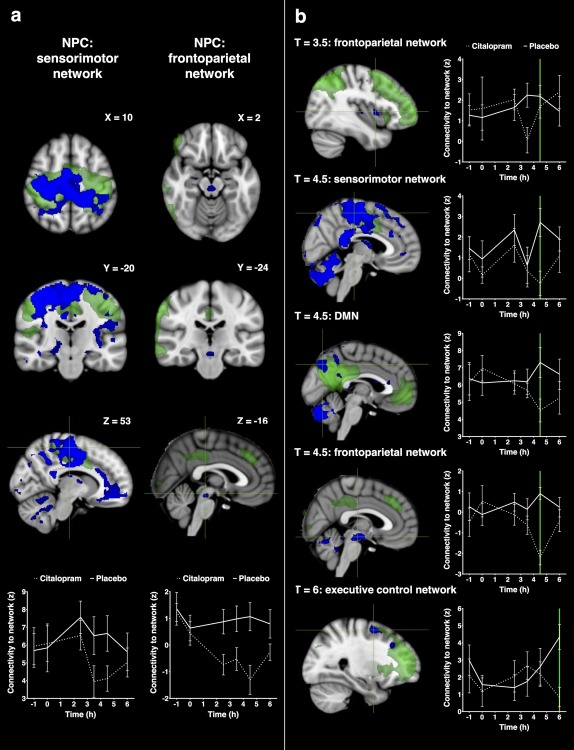
Statistical maps of citalopram induced decreases in functional connectivity. Networks are shown in green with decreases in connectivity with the network in blue (at P < 0.05, corrected). Figure (a) shows significant alterations in connectivity for all time points post dosing combined (with coordinates in mm). Figure (b) shows significant alterations in connectivity for each time point separately. Plots visualize the corresponding average time profiles of changes in functional connectivity for citalopram (dotted line) and placebo (continuous line) conditions (z‐values with standard errors of the mean as error bars). Coronal and axial slices are displayed in radiological convention (left = right). [Color figure can be viewed at http://wileyonlinelibrary.com.]
Citalopram: partial tests
Time specific effects of citalopram compared with placebo were explored by investigating changes in connectivity for each partial test (each time point post dosing) that contributed to the combined test (see Fig. 4b).
At T = 2.5 h after citalopram administration there were no significant changes in connectivity.
At T = 3.5 h after citalopram administration there was a decrease in connectivity between the right frontoparietal network and the insula and Heschl's gyrus.
At T = 4.5 h after citalopram administration there was a decrease in connectivity between (1) the default mode network and the precuneus, PCC, ACC, cerebellum, and left temporal lobe, (2) the sensorimotor network and the pre‐ and postcentral gyri, SMA, precuneus, PCC, ACC, medial prefrontal cortex, planum temporale, and Heschl's gyrus, and (3) the right frontoparietal network and brain stem.
At T = 6 h after citalopram administration there was a decrease in connectivity between the executive control network and the middle and superior frontal gyrus.
Specifications of citalopram's combined and partial effects (sizes of significant regions and peak z‐values) are provided in Table 1.
Table 1.
Overview of significant decreases in functional connectivity after citalopram as estimated with threshold‐free cluster enhancement (P < 0.05, corrected)
| Network | NPC/T | Region (Harvard–Oxford) | z* | x | y | z | # voxels | |
|---|---|---|---|---|---|---|---|---|
| Sensorimotor network | NPC | L/R/M | ACC, PCC, precuneus, SMA, post‐ and precentral gyrus, medial, and orbital frontal cortex | 5.23 | −22 | 50 | −16 | 27,308 |
| L/R/M | Cerebellum | 4.28 | 0 | −72 | −30 | 1,696 | ||
| L/M | Lateral occipital cortex, inferior, and superior division | 4.20 | −14 | −92 | 20 | 585 | ||
| R | Cerebellum and temporal occipital fusiform cortex | 3.92 | 36 | −78 | −22 | 530 | ||
| R/M | Occipital pole and lingual gyrus | 3.28 | 8 | −96 | −6 | 92 | ||
| L | Superior frontal gyrus | 3.25 | −18 | 32 | 28 | 91 | ||
| Sensorimotor network | T = 4.5 | L/R/M | ACC, PCC, precuneus, SMA, brain stem, post‐ and precentral gyrus, orbital frontal cortex and cerebellum, lateral occipital cortex, inferior, and superior division | 5.03 | −14 | −92 | 20 | 46,242 |
| L | Insular cortex, temporal, and frontal opercular cortex | 4.91 | −38 | 16 | −4 | 498 | ||
| M | Thalamus | 4.72 | 2 | −12 | 18 | 212 | ||
| Frontoparietal network right | NPC | M | Brain stem | 4.42 | 2 | −26 | −16 | 55 |
| Frontoparietal network right | T = 3.5 | R | Insular and central opercular cortex | 5.22 | 40 | 4 | 4 | 41 |
| R | Insular cortex and Heschl's gyrus | 4.53 | 40 | −16 | 4 | 11 | ||
| Frontoparietal network right | T = 4.5 | L/R/M | Brain stem and cerebellum | 4.43 | 8 | −44 | −18 | 1,655 |
| L | Frontal orbital cortex | 3.72 | −26 | 8 | −14 | 45 | ||
| L | Parietal opercular cortex | 3.78 | −32 | −44 | 28 | 34 | ||
| Default mode network | T = 4.5 | L/R | Cerebellum | 5.14 | −22 | −78 | −24 | 5,374 |
| L/M | Precuneus, PCC, hippocampus, temporal, and supramarginal gyrus | 4.56 | −36 | −58 | 28 | 2,407 | ||
| L | Lateral occipital cortex, inferior, and superior division | 4.40 | −28 | −82 | 8 | 134 | ||
| Executive control network | T = 6 | R | Precentral gyrus, inferior, and middle frontal gyrus | 4.63 | 42 | 16 | 20 | 302 |
| R | Superior and middle frontal gyrus | 4.48 | 28 | 2 | 58 | 213 | ||
| R | Lateral occipital cortex, inferior, and superior division | 3.91 | 56 | −70 | −4 | 187 | ||
| R | Inferior temporal gyrus | 4.07 | 54 | −44 | −24 | 37 | ||
| R | Parietal operculum cortex | 5.21 | 36 | −36 | 20 | 19 | ||
| R | Precentral gyrus | 4.18 | 42 | 0 | 32 | 15 |
Abbreviations: L, left; R, right; M, midline; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; SMA, supplementary motor area. Voxel dimension = 2 mm × 2 mm × 2 mm (voxel volume 0.008 mL). * = standardized z‐value of the uncorrected peak Fisher‐ (NPC) or t‐statistic (partial tests) within regions.
Galantamine: combined test
Combining the data of all post‐dose time points, there was an increase in connectivity after administering galantamine compared with placebo (Fig. 5a) between visual network 2 (occipital pole) and the left and right hippocampus, precuneus, thalamus, fusiform gyrus, precentral and superior frontal gyrus, PCC, and cerebellum.
Figure 5.
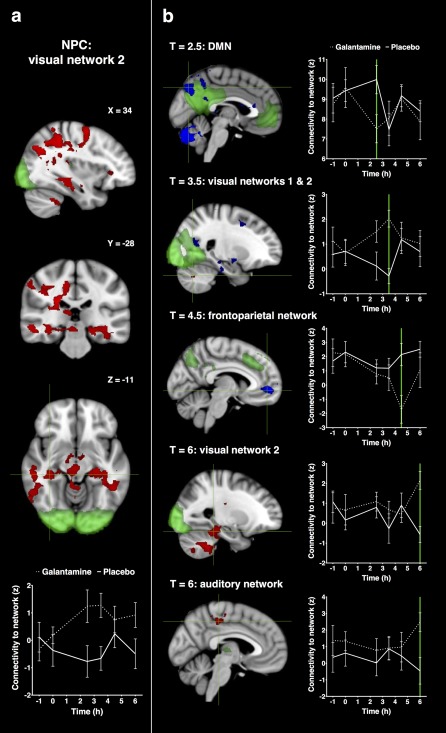
Statistical maps of galantamine induced increases and decreases in functional connectivity. Networks are shown in green with increases in connectivity with the network in red and decreases in connectivity in blue (at P < 0.05, corrected). Figure (a) shows significant alterations in connectivity for all time points post dosing combined (with coordinates in mm). Figure (b) shows significant alterations in connectivity for each time point separately. Plots visualize the corresponding average time profiles of changes in functional connectivity for galantamine (dotted line) and placebo (continuous line) conditions (z‐values with standard errors of the mean as error bars). Coronal and axial slices are displayed in radiological convention (left = right). [Color figure can be viewed at http://wileyonlinelibrary.com.]
Galantamine: partial tests
Time specific effects of galantamine compared with placebo were explored by investigating changes in connectivity for each partial test (each time point post dosing) that contributed to the combined test (see Fig. 5b).
At T = 2.5 h after galantamine administration there was a decrease in connectivity between the default mode network and precuneus, PCC, and calcarine cortex.
At T = 3.5 h after galantamine administration there was (1) a decrease in connectivity between visual network 1 (medial visual areas) and the right hippocampus, PCC, and ACC, and (2) an increase in connectivity between visual network 2 (occipital pole) and the cerebellum.
At T = 4.5 h after galantamine administration there was a decrease in connectivity between the left frontoparietal network and the medial prefrontal cortex, precuneus, PCC, and ACC.
At T = 6 h after galantamine administration there was an increase in connectivity between (1) visual network 2 (occipital pole) and the hippocampus, brain stem, cerebellum and fusiform cortex, and (2) the auditory network and PCC, precuneus, and pre‐ and postcentral gyri.
Specifications of galantamine's combined and partial effects (sizes of significant regions and peak z‐values) are provided in Table 2.
Table 2.
Overview of significant decreases (↓) and increases (↑) in functional connectivity after galantamine as estimated with threshold‐free cluster enhancement (P < 0.05, corrected)
| Network | NPC/T | Region (Harvard–Oxford) | z* | x | y | z | # voxels | |
|---|---|---|---|---|---|---|---|---|
| Visual network 2 (↑) | NPC | L/R/M | Hippocampus, thalamus, precuneus, PCC, lateral occipital cortex, brain stem, fusiform gyrus, superior frontal gyrus, precentral gyrus, and cerebellum | 4.84 | 2 | −62 | −26 | 10,765 |
| L/M | Cerebellum and brain stem | 4.21 | 8 | −48 | −44 | 1,249 | ||
| M | Precuneus and PCC | 3.10 | −8 | −40 | 54 | 147 | ||
| R | Frontal orbital cortex | 4.09 | 34 | 32 | 0 | 31 | ||
| R | Inferior frontal gyrus | 3.56 | 48 | 14 | 14 | 25 | ||
| R | Frontal operculum cortex | 3.40 | 50 | 10 | −2 | 16 | ||
| Default mode network (↓) | T = 2.5 | R/M | Precuneus, PCC, and calcarine cortex | 4.36 | 10 | −58 | 26 | 210 |
| R | Lateral occipital cortex, superior division | 4.34 | 40 | −72 | 28 | 74 | ||
| M | Lingual gyrus | 3.70 | 4 | −54 | 2 | 15 | ||
| Visual network 1 (↓) | T = 3.5 | M | ACC and paracingulate gyrus | 4.41 | 8 | 22 | 18 | 246 |
| R | Precuneus and PCC | 3.64 | 14 | −56 | 16 | 210 | ||
| R | Superior temporal gyrus, Heschl's gyrus, and planum polare | 4.06 | 48 | −26 | 0 | 105 | ||
| R/M | ACC, paracingulate gyrus, superior, and inferior frontal gyrus | 3.65 | 14 | 8 | 38 | 93 | ||
| L | Lingual gyrus, parahippocampal gyrus | 3.46 | −18 | −46 | −12 | 91 | ||
| R | Pallidum, amygala, and putamen | 4.54 | 18 | 2 | −8 | 76 | ||
| Visual network 2 (↑) | T = 3.5 | R | Cerebellum | 4.79 | 24 | −66 | −36 | 14 |
| Visual network 2 (↑) | T = 6 | L/R/M |
Hippocampus, parahippocampal gyrus, cerebellum, brain stem, temporal occipitalfusiform cortex and inferior temporal gyrus |
5.01 | 8 | −48 | −44 | 4,876 |
| R | Lateral occipital cortex, superior division | 4.24 | 28 | −62 | 52 | 677 | ||
| R | Precentral gyrus, superior, and middle frontal gyrus | 3.85 | 32 | −8 | 38 | 470 | ||
| R | PCC, precuneus, and precentral gyrus | 4.32 | 14 | −24 | 44 | 372 | ||
| L | Precuneus and lateral occipital cortex, superior division | 3.66 | −18 | −68 | 52 | 152 | ||
| R | Lateral occipital cortex, superior division | 3.62 | 42 | −76 | 30 | 146 | ||
| Frontoparietal network left (↓) | T = 4.5 | L/R/M | Frontal medial cortex and ACC | 5.25 | −2 | 52 | −2 | 630 |
| R | Precuneus and PCC | 4.12 | 16 | −50 | 12 | 110 | ||
| R | Parahippocampal gyrus, posterior division | 4.53 | 12 | −30 | −16 | 44 | ||
| R | Temporal occipital fusiform cortex and lingual gyrus | 3.44 | 36 | −42 | −10 | 14 | ||
| Auditory network (↑) | T = 6 | L/M | PCC, precuneus, and precentral gyrus | 4.83 | −4 | −32 | 48 | 188 |
| L | Postcentral gyrus | 4.65 | −46 | −28 | 50 | 23 | ||
Abbreviations: L, left; R, right; M, midline; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex. Voxel dimension = 2 mm × 2 mm × 2 mm (voxel volume 0.008 mL). * = standardized z‐value of the uncorrected peak Fisher‐ (NPC) or t‐statistic (partial tests) within regions.
DISCUSSION
Single‐dose SSRI and AChEI administration is usually not sufficient to alter cognitive and behavioral states in depression or dementia [Burke et al., 2002; Dumont et al., 2005; Lanctot et al., 2003; Repantis et al., 2010; Wagner et al., 2004]. Pharmacological research and development is therefore often restricted to clinical trials that last for weeks or even months. However, considering the acute elevations of synaptic neurotransmitters, it is expected that changes will already take place on a neural level, well before this results in improved performance and clinical outcome. In our study, both agents altered resting state functional connectivity within our time frame of measurements. The results of our study replicate the finding that SSRIs can have an immediate and widespread diminishing impact on interactions of the healthy neural system [Klaassens et al., 2015; Murphy et al., 2009; Schaefer et al., 2014]. In conjunction with other SSRIs, citalopram had clear neuroendocrine effects [Seifritz et al., 1996], but did not induce cognitive or subjective changes as measured with the NeuroCart® battery. Network effects of galantamine were more discrete and variable over time. The relatively low dose and highly variable PK properties of this drug in our study and an unexpected delay in onset of galantamine's T max, which may in hindsight be related to a food interaction with lunch, may have obscured the detection of more subtle fMRI effects and time‐related changes. Galantamine increased nausea but did not alter cognitive or behavioral states.
Citalopram
In congruence with both task‐related [Bruhl and Herwig, 2009; Murphy et al., 2009] and resting‐state fMRI paradigms with SSRIs [Klaassens et al., 2015; Schaefer et al., 2014], citalopram rapidly lowered connectivity in several cortical and subcortical regions. This is consistent with the numerous afferent and efferent serotonergic fibers originating from the brain stem's raphe nuclei [Baumgarten and Grozdanovic, 1995]. Compared with our recent results on the SSRI sertraline [Klaassens et al., 2015], there was considerable overlap between the two SSRIs in direction (decreased connectivity) and regions (ACC, PCC, precuneus, prefrontal cortex, midbrain, and motor cortex) of effect, especially with respect to other pharmacological compounds that usually show more restricted responses [Khalili‐Mahani et al., 2012; Klumpers et al., 2012; Niesters et al., 2012]. Part of these findings is in line with RS‐fMRI studies in depressed patients who exhibit hyperconnectivity of cortical midline structures (ACC, PCC, precuneus, and medial prefrontal regions) that are related to emotion regulation and modulated by serotonin transmission [Kupfer et al., 2012; Sundermann et al., 2014]. It has been hypothesized that this increase in connectivity in depression is representative of disruptions in self‐consciousness and rumination of negative thoughts [Hamilton et al., 2011; Zhu et al., 2012]. An explanation of the overall inhibitory effect of acute SSRI exposure is the relative predominance of inhibitory 5‐HT1 versus stimulatory 5‐HT2 receptor subtypes [Peroutka and Snyder, 1979] that has been demonstrated throughout the cortex [Amargos‐Bosch et al., 2004; Barnes and Sharp, 1999; Celada et al., 2013; Lidow et al., 1989]. Most outstanding was the citalopram induced decrease in connectivity with the sensorimotor network, mainly due to alterations at T = 4.5 h. Citalopram also increased cortisol and prolactin levels, most noticeable at one time point as well (T = 2.5 h for cortisol and T = 3.5 h for prolactin). Although this took place before appearance of the largest alterations in connectivity, it postulates an apex in the pharmacodynamic effect of citalopram. Equal SSRI effects for the sensorimotor network (decreased connectivity with the sensorimotor region, supplementary motor area, precuneus, and cingulate cortex) have been found earlier [Klaassens et al., 2015]. The primary motor and somatosensory cortex are both characterized by a high 5‐HT axon density [Wilson and Molliver, 1991] and serotonin is recognized to be important for motor behavior in animals and humans [Geyer, 1996; Hindmarch, 1995]. This is demonstrated by enhanced motor area activity during improved motor performance after SSRI administration [Loubinoux et al., 2002a, 2002b]. The precuneus and cingulate cortex are presumed to support voluntary and complex motor control [Cavanna and Trimble, 2006; Shima and Tanji, 1998] and seem to play a central role in SSRI enhancement [Klaassens et al., 2015]. While the effect was more focal, connectivity between the midbrain and right fronto‐parietal network was decreased as well. This matches observations that acute blockade of serotonin reuptake activates 5‐HT1A autoreceptors in the midbrain's median and dorsal raphe nuclei [Briley and Moret, 1993; Daubert and Condron, 2010; Jacobs and Azmitia, 1992], in turn leading to reduced 5‐HT release in particularly the forebrain [Adell et al., 2002; Bel and Artigas, 1992].
Nevertheless, comparing effects of citalopram and sertraline, we did not find alterations in relation to exact identical functional networks. No differences have been found on the antidepressant efficacy of both SSRIs [Ekselius et al., 1997; Stahl, 2000], although sertraline induces more gastrointestinal side effects than citalopram [Ekselius et al., 1997; Stahl, 2000]. This corresponds to our finding that sertraline significantly increased the level of nausea, whereas this did not occur in our current study group. Citalopram is also known as the most selective SSRI; sertraline has more affinity for dopamine, noradrenaline, and σ‐receptors than citalopram [Carrasco and Sandner, 2005], which in turn modulate N‐methyl‐D‐aspartate and glutamate receptors as well [Urani et al., 2002]. Citalopram, on the other hand, has a high affinity on histamine H1 receptors [Carrasco and Sandner, 2005]. It is possible that these properties may account for differences in network changes between the two SSRIs [Cole et al., 2013; Villemagne et al., 1991]. However, it is yet to be established what the value is of specific network versus region effects in connectivity analyses. Considering the resemblance in direction and location of effect we presume that sertraline and citalopram induce quite comparable connectivity alterations.
Galantamine
The cholinergic system is mostly related to aging and aging related diseases, as cholinergic malfunction, especially in the hippocampus, cortex, the entorhinal area, the ventral striatum, and the basal forebrain, plays a key role in associated functional degeneration [Kasa et al., 1997; Schliebs and Arendt, 2011]. Combining fMRI data of all time points, we found an increase in connectivity with the visual network which was mostly associated with effects on T = 6 h. The medial and lateral cholinergic pathways, originating from Meynert's basal nucleus, supply a large portion of the brain and merge in the posterior occipital lobe [Selden et al., 1998]. In dementia and schizophrenia, it is hypothesized that cholinergic dysregulation is responsible for psychotic manifestations and AChEIs have been successfully used for treatment of visual hallucinations [Bentley et al., 2008; Sarter and Bruno, 1998]. ACh release in the primary visual cortex is increased during visual stimulation pointing to ACh as influencing visual processing and learning mechanisms [Dotigny et al., 2008; Kang et al., 2014]. It has been proposed that cholinergic enhancement facilitates bottom‐up visual attention and perception by increasing activity in the extrastriate cortex [Bentley et al., 2004, 2003]. More importantly, galantamine altered connectivity with areas that are highly relevant in learning and memory: the left and right hippocampus and thalamus. Changes in cholinergic markers such as choline acetyltransferase, acetylcholinesterase, and muscarinic and nicotinic acetylcholine receptor availability in hippocampal regions is typical for AD and normal aging [Schliebs and Arendt, 2011]. In patients with AD, hippocampal volume loss appears to slow down during treatment with donezepil [Hashimoto et al., 2005] and cholinergic enhancement even improved visual and verbal memory episodic memory and long‐term visual episodic recall in healthy young subjects, memory domains that are specifically related to hippocampal functioning [Gron et al., 2005]. Cholinergic treatment aided the processing of novel faces in AD patients, which was accompanied by normalization in the fusiform gyrus [Kircher et al., 2005; Rombouts et al., 2002], where we found connectivity changes as well. In addition, acute exposure to cholinergic stimulation increased activation in occipital and hippocampal regions of patients with AD and mild cognitive impairment (MCI) during a visual memory task [Goekoop et al., 2004, 2006]. The thalamus, considered to be a gate for sensory information, contains various nuclei that receive excitatory cholinergic input [McCance and Phillis, 1968], including the lateral geniculate nucleus that has feedback connections with the primary visual cortex [Phillis et al., 1967; Sillito et al., 2006]. Results for this network are compatible with previous studies [Furey et al., 2000; Murphy and Sillito, 1991], indicating that cholinergic enhancement benefits memory performance and visual stimulation orientation by selective perceptual processing.
Repeated Measures
Collecting multiple scans per day increases the power of the statistical test and decreases individual variability, which reduces the need for large sample sizes. The observed variation in connectivity on placebo days emphasizes the importance of a placebo‐controlled design with repeated measures, providing insight into potential diurnal fluctuations. Furthermore, it offers possibilities to investigate effects on different time points and relate these effects to other pharmacodynamic and PK profiles. NPC groups the data of all time points to test one joint null hypothesis without the necessity to explicitly model their dependence [Pesarin, 1990; Winkler et al., 2016], ending up with effects among them that are statistically most robust. In addition, univariate partial tests allow for inference per time point. For citalopram, it might have been sufficient to acquire scans at one time point (T = 4.5 h). However, it is largely impossible to predict beforehand at which specific moment we can expect the most stable and “real” drug effect, since the peak effect does not appear to coincide directly with the observed T max in plasma and neuroendocrine responses. Effects at other time points may not reach significance but still contribute to the net result. The combined outcome therefore tends to be more reliable and powerful in defining pharmacological effects that are variable over time, as it will grasp the strongest effects without the risk of missing out on important information [Fisher, 1932]. This does not imply that the partial (time specific) effects are meaningless. A decrease in connectivity at T = 4.5 h between the default mode network and precuneus, PCC and ACC is in line with earlier results [Klaassens et al., 2015; McCabe and Mishor, 2011; McCabe et al., 2011; Van de Ven et al., 2013; Van Wingen et al., 2014] and in agreement with opposite features in depression, which is characterized by increased connectivity of DMN components [Sundermann et al., 2014]. Especially the posterior part of the DMN, where citalopram effects were most prevalent, has been implicated in SSRI efficacy in depression [Greicius et al., 2007; Li et al., 2013]. Furthermore, consistent with an increased cerebellar‐DMN connectivity in depression [Sundermann et al., 2014], citalopram reduced connectivity between the DMN and cerebellum. The cerebellum is primarily known for its service in motor control, illustrating our findings for the sensorimotor network, but influences mood regulation as well [Schmahmann, 2004; Schmahmann and Sherman, 1998].
In contrast to citalopram, the effects of galantamine were more focal, less related to a specific network or point in time and less uniform with regard to direction of effect. This heterogeneity in effect possibly reflects the large kinetic variability in this study. Although the variation in timing of T max did not clearly differ between citalopram and galantamine, the variance of galantamine's C max (ranging from 25.6 to 61.4 ng/mL) was high compared with citalopram and desmethylcitalopram. Since the combined effect mainly depended on the last time point it is possible that the impact of galantamine does not follow a time course that equals the PK profile or that effects might have become larger and more stable later in time. This is congruent with the unanticipated delay in onset of galantamine's T max in our study group, resulting in a less powerful aggregation of data, in which especially the value of measurements at T = 2.5 is questionable. Although galantamine is known for a T max of 1–2 h after dosing, the mean T max in our sample was 2.67 h (4.67 h after zero point), whereas citalopram, known for a T max between 2 and 4 h, did reach its maximum concentration at 2.99 h post dosing. Furthermore, the relatively low dose and variable kinetic time profile of galantamine might have contributed to the absence of a larger response on functional connectivity and neuroendocrine parameters. A larger sample size, a higher dose of galantamine (16–32 mg), and earlier drug administration might have reduced this variability in response. The outcomes of our partial tests reveal additional information beyond the combined approach as well. There is a tendency toward diminished DMN activity in normal aging, MCI and dementia, pointing to reduced integrity of structures that are vulnerable to atrophy, beta amyloid deposition and reduced glucose metabolism [Hafkemeijer et al., 2012]. Studies that are performed on the resting state fMRI response to cholinergic interventions are restricted to AD patients and mainly indicate an increase in connectivity with DMN areas [Goveas et al., 2011; Li et al., 2012; Solé‐Padullés et al., 2013]. In another study, no effect on the DMN was found in both APOE ε4 carriers and non‐carriers [Wang et al., 2014]. Acute exposure to cholinergic stimulation decreased DMN connectivity with the precuneus and occipital cortex in our study group at T = 2.5 h. This direction of effect might be the consequence of investigating cholinergic responses in healthy young adults instead of subjects with impaired cholinergic systems. It is possible that when neural cholinergic processes are still intact, ceiling effects may prevent further activation and excessive stimulation may actually impair optimal connectivity. Moreover, these studies all used AChEI treatment for several weeks, instead of our single‐dose administration. More research is needed to unravel differential cholinergic responses among specific populations and treatment designs.
Limitations
Agents that enhance the cholinergic and serotonergic system commonly elicit gastrointestinal adverse events, which is attributable to their peripheral influences [Gauthier, 2001; Trindade et al., 1998]. In order to prevent these adverse effects, we administered granisetron on both drug‐ and placebo study days before study drug administration. RS‐fMRI effects of selective 5‐HT3 receptor antagonists as granisetron are lacking, but need to be taken into consideration when interpreting the results [Jacobs and Azmitia, 1992]. However, intolerability to our intensive study procedures would have been undesirable [Jacobs et al., 2010a] and vomiting might have altered brain connectivity as well. To reduce nausea, we also decided to administer citalopram in two doses, and to skip the second dose in case of tolerability issues. For citalopram, our measures were adequate, and all subjects received both doses without significant nausea. For the same reasons, a relatively low dose of galantamine was chosen. However, increased nausea was present after administering galantamine compared with placebo, primarily at the end of the day, which may also have influenced some of the observed network effects but justifies our decision to limit the dose of galantamine. This also emphasizes the mismatch of our repeated measurements and galantamine's absorption rate. Despite our attempt to equalize the PK responses of citalopram and galantamine during the course of the day, both drugs did not reach their maximum concentration at the same time point, which hampers comparability. Currently, no accepted methods are available to include individual drug concentrations in the network analysis. Further, fMRI effects, especially in pharmacological research, are potentially the result of vasodilation, and hence to changes in neurovascular coupling instead of true neural activity [Rack‐Gomer and Liu, 2012; Wong et al., 2012]. Although SSRIs do not typically alter the hemodynamic response [Feczko et al., 2012], AChEIs could increase vessel tone by contraction of the smooth muscles of blood vessels [Rosengarten et al., 2009; Stephenson and Kolka, 1990]. Yet, there was no significant treatment effect of either drug on heart rate frequency, which minimizes the probability of cardiac artifacts. Besides, vessel dilation would more likely alter connections throughout the entire brain instead of inducing the network‐specific effects that we observed. Lastly, the observed changes in regional connectivity might partly be the result of drug‐induced reductions of neuronal activity in the BOLD signal leading to a reduced signal to noise ratio. Future studies with appropriate protocols are needed to specify these processes more accurately.
Summary
This study provides further support for RS‐fMRI as a sensitive method for investigating instant neural processes after pharmacological challenges. The results on the SSRI citalopram and AChEI galantamine identify their neuromodulating role in cognitive and sensory systems. Citalopram altered connectivity with networks and regions that are mostly implied in sensorimotor functioning and self‐reference, whereas the results of galantamine show acetylcholine's relation to visual processing and learning mechanisms. Our findings also encourage the use of repeated measurements after single‐dose administration, leading to a more powerful and reliable picture of pharmacological effects. Results may have been partially obscured by the variability of individual PK characteristics, which was larger than expected. A future challenge therefore is to develop appropriate statistical models (PK/PD‐modeling) to investigate concentration‐dependent modulation of resting state functional connectivity.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Erica S. Klaassen and Jasper Stevens (CHDR) are acknowledged for their contribution to statistical analyses.
Corrections added on 19 September 2016, after online publication.
REFERENCES
- Adell A, Celada P, Abellan MT, Artigas F (2002): Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Rev 39:154–180. [DOI] [PubMed] [Google Scholar]
- Amargos‐Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F (2004): Co‐expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb. Cortex 14:281–299. [DOI] [PubMed] [Google Scholar]
- Bargmann CI (2012): Beyond the connectome: How neuromodulators shape neural circuits. Bioessays 34:458–465. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T (1999): A review of central 5‐HT receptors and their function. Neuropharmacology 38:1083–1152. [DOI] [PubMed] [Google Scholar]
- Baumgarten HG, Grozdanovic Z (1995): Psychopharmacology of central serotonergic systems. Pharmacopsychiatry 28:73–79. [DOI] [PubMed] [Google Scholar]
- Baxter DA, Canavier CC, Clark JW, Byrne JH (1999): Computational model of the serotonergic modulation of sensory neurons in Aplysia. J. Neurophysiol 82:2914–2935. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005): Investigations into resting‐state connectivity using independent component analysis. Philos T Roy Soc B 360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Mackay CE, Filippini N, Smith SM (2009): Group comparison of resting‐state FMRI data using multi‐subject ICA and dual regression. OHBM. [Google Scholar]
- Bel N, Artigas F (1992): Fluvoxamine Preferentially Increases Extracellular 5‐Hydroxytryptamine in the Raphe Nuclei – an Invivo Microdialysis Study. Eur. J. Pharmacol 229:101–103. [DOI] [PubMed] [Google Scholar]
- Benarroch EE (2010): Acetylcholine in the cerebral cortex Effects and clinical implications. Neurology 75:659–665. [DOI] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ (2008): Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer's disease and health. Brain 131:409–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Husain M, Dolan RJ (2004): Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron 41:969–982. [DOI] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ (2003): Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. Neuroimage 20:58–70. [DOI] [PubMed] [Google Scholar]
- Birn RM (2012): The role of physiological noise in resting‐state functional connectivity. Neuroimage 62:864–870. [DOI] [PubMed] [Google Scholar]
- Bond A, Lader M (1974): Use of Analog Scales in Rating Subjective Feelings. Brit. J. Med. Psychol 47:211–218. [Google Scholar]
- Borland RG, Nicholson AN (1984): Visual Motor Coordination and Dynamic Visual‐Acuity. Brit. J. Clin. Pharmacol 18: S69–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley M, Moret C (1993): Neurobiological mechanisms involved in antidepressant therapies. Clin. Neuropharmacol 16:387–400. [DOI] [PubMed] [Google Scholar]
- Bruhl A, Herwig U (2009): Differential modulation of emotion processing by single dose serotonergic and noradrenergic antidepressants – a pharmaco‐fMRI study. Pharmacopsychiatry 42:214–214. [Google Scholar]
- Burke WJ, Gergel I, Bose A (2002): Fixed‐dose trial of the single isomer SSRI escitalopram in depressed outpatients. J Clin Psychiat 63:331–336. [DOI] [PubMed] [Google Scholar]
- Carr GV, Lucki I (2011): The role of serotonin receptor subtypes in treating depression: A review of animal studies. Psychopharmacology (Berl.) 213:265–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco JL, Sandner C (2005): Clinical effects of pharmacological variations in selective serotonin reuptake inhibitors: An overview. Int. J. Clin. Pract 59:1428–1434. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129:564–583. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Artigas F (2013): Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Oei NYL, Both S, van Gerven JMA, Rombouts SARB (2013): Differential and distributed effects of dopamine neuromodulations on resting‐state network connectivity. Neuroimage 78:59–67. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert EA, Condron BG (2010): Serotonin: A regulator of neuronal morphology and circuitry. Trends Neurosci 33:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotigny F, Ben Amor AY, Burke M, Vaucher E (2008): Neuromodulatory role of acetylcholine in visually‐induced cortical activation: Behavioral and neuroanatomical correlates. Neuroscience 154:1607–1618. [DOI] [PubMed] [Google Scholar]
- Doya K (2002): Metalearning and neuromodulation. Neural Netw 15:495–506. [DOI] [PubMed] [Google Scholar]
- Dumont GJH, de Visser SJ, Cohen AF, van Gerven JMA (2005): Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. Brit J Clin Pharmaco 59:495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekselius L, von Knorring L, Eberhard G (1997): A double‐blind multicenter trial comparing sertraline and citalopram in patients with major depression treated in general practice. Int Clin Psychopharm 12:323–331. [DOI] [PubMed] [Google Scholar]
- Feczko E, Miezin FM, Constantino JN, Schlaggar BL, Petersen SE, Pruett JR (2012): The hemodynamic response in children with Simplex Autism. Dev. Cogn. Neurosci 2:396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE (2009): Distinct patterns of brain activity in young carriers of the APOE‐epsilon 4 allele. Proc. Natl. Acad. Sci. U.S.A 106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. (1932) Statistical methods for research workers. Edinburgh: Oliver and Boyd. [Google Scholar]
- Foehring RC, Lorenzon NM (1999): Neuromodulation, development and synaptic plasticity. Can. J. Exp. Psychol 53:45–61. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV (2000): Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science 290:2315–2319. [DOI] [PubMed] [Google Scholar]
- Gauthier S (2001): Cholinergic adverse effects of cholinesterase inhibitors in Alzheimer's disease ‐ Epidemiology and management. Drugs Aging 18:853–862. [DOI] [PubMed] [Google Scholar]
- Geyer MA (1996): Serotonergic functions in arousal and motor activity. Behav. Brain Res 73:31–35. [DOI] [PubMed] [Google Scholar]
- Gijsman HJ, van Gerven JMA, Verkes RJ, Schoemaker RC, Pieters MSM, Pennings EJM, Hessing TJ, Cohen AF (2002): Saccadic peak velocity and EEG as end‐points for a serotonergic challenge test. Hum Psychopharm Clin 17:83–89. [DOI] [PubMed] [Google Scholar]
- Goekoop R, Rombouts SARB, Jonker C, Hibbel A, Knol DL, Truyen L, Barkhof F Scheltens P (2004): Challenging the cholinergic system in mild cognitive impairment: A pharmacological fMRI study. Neuroimage 23:1450–1459. [DOI] [PubMed] [Google Scholar]
- Goekoop R, Scheltens P, Barkhof F, Rombouts SARB (2006): Cholinergic challenge in Alzheimer patients and mild cognitive impairment differentially affects hippocampal activation ‐ a pharmacological fMRI study. Brain 129:141–157. [DOI] [PubMed] [Google Scholar]
- Goveas JS, Xie CM, Ward BD, Wu ZL, Li WJ, Franczak M, Jones JL, Antuono PG, Li SJ (2011): Recovery of Hippocampal Network Connectivity Correlates with Cognitive Improvement in Mild Alzheimer's Disease Patients Treated with Donepezil Assessed by Resting‐State fMRI. J. Magn. Reson. Imaging 34:764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF (2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B (2009): Accurate and robust brain image alignment using boundary‐based registration. Neuroimage 48:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gron G, Kirstein M, Thielscher A, Riepe MW, Spitzer M (2005): Cholinergic enhancement of episodic memory in healthy young adults. Psychopharmacology (Berl.) 182:170–179. [DOI] [PubMed] [Google Scholar]
- Gu Q (2002): Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111:815–835. [DOI] [PubMed] [Google Scholar]
- Hafkemeijer A van der Grond J, Rombouts SARB (2012): Imaging the default mode network in aging and dementia. BBA‐Mol. Basis Dis 1822:431–441. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH (2011): Default‐mode and task‐positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol. Psychiatry 70:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E (2005): Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer's disease? Am J Psychiat 162:676–682. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME (1995): Neuromodulation and Cortical Function ‐ Modeling the Physiological‐Basis of Behavior. Behav. Brain Res 67:1–27. [DOI] [PubMed] [Google Scholar]
- Hindmarch I (1995): The Behavioral Toxicity of the Selective Serotonin Reuptake Inhibitors. Int Clin Psychopharm 9:13–17. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC (1992): Structure and function of the brain‐serotonin system. Physiol Rev 72:165–229. [DOI] [PubMed] [Google Scholar]
- Jacobs GE, Kamerling IMC, de Kam ML, DeRijk RH, van Pelt J, Zitman FG, van Gerven JMA (2010a): Enhanced tolerability of the 5‐hydroxytryptophane challenge test combined with granisetron. J Psychopharmacol 24:65–72. [DOI] [PubMed] [Google Scholar]
- Jacobs GE, van der Grond J, Teeuwisse WM, Langeveld TJC, van Pelt J, Verhagen JCM, de Kam ML, Cohen AF, Zitman FG, van Gerven JMA (2010b): Hypothalamic glutamate levels following serotonergic stimulation: A pilot study using 7‐Tesla magnetic resonance spectroscopy in healthy volunteers. Prog Neuro‐Psychopharmacol Biol Psychiatry 34:486–491. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): FSL. Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Kang JI, Huppe‐Gourgues F, Vaucher E (2014): Boosting visual cortex function and plasticity with acetylcholine to enhance visual perception. Front Syst Neurosci 8:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasa P, Rakonczay Z, Gulya K (1997): The cholinergic system in Alzheimer's disease. Prog Neurobiol 52:511–535. [DOI] [PubMed] [Google Scholar]
- Khalili‐Mahani N, Chang C, van Osch MJ, Veer IM, van Buchem MA, Dahan A, Beckmann CF van Gerven JMA, Rombouts SARB (2013): The impact of “physiological correction” on functional connectivity analysis of pharmacological resting state fMRI. Neuroimage 65:499–510. [DOI] [PubMed] [Google Scholar]
- Khalili‐Mahani N, Niesters M, van Osch MJ, Oitzl M, Veer I, de Rooij M, van Gerveng J, van Buchem MA, Beckmann CF, Rombouts SARB, Dahan A (2015): Ketamine interactions with biomarkers of stress: A randomized placebo‐controlled repeated measures resting‐state fMRI and PCASL pilot study in healthy men. Neuroimage 108:396–409. [DOI] [PubMed] [Google Scholar]
- Khalili‐Mahani N, van Osch MJ, de Rooij M, Beckmann CF, van Buchem MA, Dahan A van Gerven JM, Rombouts SARB (2014): Spatial heterogeneity of the relation between resting‐state connectivity and blood flow: An important consideration for pharmacological studies. Hum Brain Mapp 35:929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili‐Mahani N, Zoethout RMW, Beckmann CF, Baerends E, de Kam ML, Soeter RP, Dahan A, van Buchem MA van Gerven JMA, Rombouts SARB (2012): Effects of morphine and alcohol on functional brain connectivity during “resting state”: A placebo‐controlled crossover study in healthy young men. Hum Brain Mapp 33:1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher TTJ, Erb M, Grodd W, Leube DT (2005): Cortical activation during cholinesterase‐inhibitor treatment in Alzheimer disease ‐ Preliminary findings from a pharmaco‐fMRI study. Am J Geriat Psychiat 13:1006–1013. [DOI] [PubMed] [Google Scholar]
- Klaassens BL, van Gorsel HC, Khalili‐Mahani N, van der Grond J, Wyman BT, Whitcher B, Rombouts SA, van Gerven JM (2015): Single‐dose serotonergic stimulation shows widespread effects on functional brain connectivity. Neuroimage 122:440–450. [DOI] [PubMed] [Google Scholar]
- Klumpers LE, Cole DM, Khalili‐Mahani N, Soeter RP, te Beek ET, Rombouts SARB, van Gerven JMA (2012): Manipulating brain connectivity with delta(9)‐tetrahydrocannabinol: A pharmacological resting state FMRI study. Neuroimage 63:1701–1711. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML (2012): Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet 379:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeng B, Lag T, Brennen T (2005): Reduced stroop interference for opponent colors may be due to input factors: Evidence from individual differences and a neural network simulation. J Exp Psychol Human 31:438–452. [DOI] [PubMed] [Google Scholar]
- Lanctot KL, Herrmann N, Yau KK, Khan LR, Liu BA, Loulou MM, Einarson TR (2003): Efficacy and safety of cholinesterase inhibitors in Alzheimer's disease: A meta‐analysis. Can Med Assoc J 169:557–564. [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. (2004) Neuropsychological Assessment. New York: Oxford University Press. [Google Scholar]
- Li BJ, Liu L, Friston KJ, Shen H, Wang LB, Zeng LL, Hu DW (2013): A treatment‐resistant default mode subnetwork in major depression. Biol Psychiatry 74:48–54. [DOI] [PubMed] [Google Scholar]
- Li W, Antuono PG, Xie C, Chen G, Jones JL, Ward BD, Franczak MB, Goveas JS, Li SJ (2012): Changes in regional cerebral blood flow and functional connectivity in the cholinergic pathway associated with cognitive performance in subjects with mild Alzheimer's disease after 12‐week donepezil treatment. Neuroimage 60:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Goldmanrakic PS, Gallager DW, Rakic P (1989): Quantitative autoradiographic mapping of serotonin 5‐ht1 and 5‐ht2 receptors and uptake sites in the neocortex of the rhesus‐monkey. J Comp Neurol 280:27–42. [DOI] [PubMed] [Google Scholar]
- Liem‐Moolenaar M, de Boer P, Timmers M, Schoemaker RC, van Hasselt JGC, Schmidt S, van Gerven JMA (2011): Pharmacokinetic‐pharmacodynamic relationships of central nervous system effects of scopolamine in healthy subjects. Br J Clin Pharmacol 71:886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HK, Juh R, Pae CU, Lee BT, Yoo SS, Ryu SH, Kwak KR, Lee C, Lee CU (2008): Altered verbal working memory process in patients with Alzheimer's disease. Neuropsychobiology 57:181–187. [DOI] [PubMed] [Google Scholar]
- Loubinoux I, Pariente J, Boulanouar K, Carel C, Manelfe C, Rascol O, Celsis P, Chollet F (2002a): A single dose of the serotonin neurotransmission agonist paroxetine enhances motor output: Double‐blind, placebo‐controlled, fMRI study in healthy subjects. Neuroimage 15:26–36. [DOI] [PubMed] [Google Scholar]
- Loubinoux I, Pariente J, Rascol O, Celsis P, Chollet F (2002b): Selective serotonin reuptake inhibitor paroxetine modulates motor behavior through practice. A double‐blind, placebo‐controlled, multi‐dose study in healthy subjects. Neuropsychologia 40:1815–1821. [DOI] [PubMed] [Google Scholar]
- Marder E, Thirumalai V (2002): Cellular, synaptic and network effects of neuromodulation. Neural Netw 15:479–493. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z (2011): Antidepressant medications reduce subcortical‐cortical resting‐state functional connectivity in healthy volunteers. Neuroimage 57:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Filippini N, Cowen PJ, Taylor MJ, Harmer CJ (2011): SSRI administration reduces resting state functional connectivity in dorso‐medial prefrontal cortex. Mol Psychiatry 16:592–594. [DOI] [PubMed] [Google Scholar]
- McCance I, Phillis JW RAW, (1968): Acetylcholine‐sensitivity of thalamic neurones ‐ its relationship to synaptic transmission. Br J Pharmacol 32:635–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM (1991): Cholinergic enhancement of direction selectivity in the visual‐cortex of the cat. Neuroscience 40:13–20. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, O'Sullivan U, Cowen PJ, Harmer CJ (2009): Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry 194:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters M, Khalili‐Mahani N, Martini C, Aarts L, van Gerven J, van Buchem MA, Dahan A, Rombouts S (2012): Effect of subanesthetic ketamine on intrinsic functional brain connectivity a placebo‐controlled functional magnetic resonance imaging study in healthy male volunteers. Anesthesiology 117:868–877. [DOI] [PubMed] [Google Scholar]
- Norris H (1971): The action of sedatives on brain stem oculomotor systems in man. Neuropharmacology 10:181–191. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Snyder SH (1979): Multiple serotonin receptors ‐ differential binding of [5‐hydroxytryptamine‐h‐3, [lysergic‐h‐3 acid diethylamide and [h‐3]spiroperidol. Mol Pharmacol 16:687–699. [PubMed] [Google Scholar]
- Perry E, Walker M, Grace J, Perry R (1999): Acetylcholine in mind: A neurotransmitter correlate of consciousness? Trends Neurosci 22:273–280. [DOI] [PubMed] [Google Scholar]
- Pesarin F (1990): On a nonparametric combination method for dependent permutation tests with applications. Psychother Psychosom 54:172–179. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Tebecis AK, York DH (1967): A study of cholinoceptive cells in the lateral geniculate nucleus. J Physiol 192:695–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, Buitelaar JK, Beckmann CF (2015a): Evaluation of ICA‐AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage 112:278–287. [DOI] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF (2015b): ICA‐AROMA: A robust ICA‐based strategy for removing motion artifacts from fMRI data. Neuroimage 112:267–277. [DOI] [PubMed] [Google Scholar]
- Rack‐Gomer AL, Liu TT (2012): Caffeine increases the temporal variability of resting‐state BOLD connectivity in the motor cortex. Neuroimage 59:2994–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repantis D, Laisney O, Heuser I (2010): Acetylcholinesterase inhibitors and memantine for neuroenhancement in healthy individuals: A systematic review. Pharmacol Res 61:473–481. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Fukuda M, Kato N (2004): Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neurosci Res 50:1–11. [DOI] [PubMed] [Google Scholar]
- Rombouts SARB, Barkhof F, van Meel CS, Scheltens P (2002): Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer's disease. J Neurol Neurosurg Psychol 73:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten B, Paulsen S, Burr O, Kaps M (2009): Neurovascular coupling in Alzheimer patients: Effect of acetylcholine‐esterase inhibitors. Neurobiol Aging 30:1918–1923. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP (1998): Cortical acetylcholine, reality distortion, schizophrenia, and Lewy Body Dementia: Too much or too little cortical acetylcholine? Brain Cogn 38:297–316. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Burmann I, Regenthal R, Arelin K, Barth C, Pampel A, Villringer A, Margulies DS, Sacher J (2014): Serotonergic modulation of intrinsic functional connectivity. Curr Biol 24:2314–2318. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T (2011): The cholinergic system in aging and neuronal degeneration. Behav Brain Res 221:555–563. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD (2004): Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychol Clin Neurosc 16:367–378. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC (1998): The cerebellar cognitive affective syndrome. Brain 121:561–579. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifritz E, Baumann P, Muller MJ, Annen O, Amey M, Hemmeter U, Hatzinger M, Chardon F, HolsboerTrachsler E (1996): Neuroendocrine effects of a 20‐mg citalopram infusion in healthy males ‐ A placebo‐controlled evaluation of citalopram as 5‐HT function probe. Neuropsychopharmacology 14:253–263. [DOI] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon‐Murayama N, Parrish TB, Mesulam MM (1998): Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 121:2249–2257. [DOI] [PubMed] [Google Scholar]
- Shima K, Tanji J (1998): Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282:1335–1338. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Cudeiro J, Jones HE (2006): Always returning: Feedback and sensory processing in visual cortex and thalamus. Trends Neurosci 29:307–316. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang YY, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Solé‐Padullés C, Bartres‐Faz D, Llado A, Bosch B, Pena‐Gomez C, Castellvi M, Rami L, Bargallo N, Sanchez‐Valle R, Molinuevo JL (2013): Donepezil treatment stabilizes functional connectivity during resting state and brain activity during memory encoding in Alzheimer's disease. J Clin Psychopharmacol 33:199–205. [DOI] [PubMed] [Google Scholar]
- Soreq H, Seidman S (2001): Acetylcholinesterase ‐ new roles for an old actor. Nat Rev Neurosci 2:294–302. [DOI] [PubMed] [Google Scholar]
- Stahl SM (2000): Placebo‐controlled comparison of the selective serotonin reuptake inhibitors citalopram and sertraline. Biol Psychiat 48:894–901. [DOI] [PubMed] [Google Scholar]
- Stephenson LA, Kolka MA (1990): Acetylcholinesterase inhibitor, pyridostigmine bromide, reduces skin blood‐flow in humans. Am J Physiol 258:R951–R957. [DOI] [PubMed] [Google Scholar]
- Stroop JR (1935): Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662. [Google Scholar]
- Sundermann B, Beverborg MOL, Pfleiderer B (2014): Toward literature‐based feature selection for diagnostic classification: A meta‐analysis of resting‐state fMRI in depression. Front Hum Neurosci 8:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tork I (1990): Anatomy of the serotonergic system. Ann N Y Acad Sci 600:9–33. [DOI] [PubMed] [Google Scholar]
- Trindade E, Menon D, Topfer LA, Coloma C (1998): Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: A meta‐analysis. Can Med Assoc J 159:1245–1252. [PMC free article] [PubMed] [Google Scholar]
- Umegaki H, Yamamoto A, Suzuki Y, Iguchi A (2009): Responses of hypothalamo‐pituitary‐adrenal axis to a cholinesterase inhibitor. Neuroreport 20:1366–1370. [DOI] [PubMed] [Google Scholar]
- Urani A, Romieu P, Portales‐Casamar E, Roman FJ, Maurice T (2002): The antidepressant‐like effect induced by the sigma(1) (sigma(1)) receptor agonist igmesine involves modulation of intracellular calcium mobilization. Psychopharmacology (Berl.) 163:26–35. [DOI] [PubMed] [Google Scholar]
- Van de Ven V, Wingen M, Kuypers KPC, Ramaekers JG, Formisano E (2013): Escitalopram decreases cross‐regional functional connectivity within the default‐mode network. PLoS One 8:e68355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wingen GA, Tendolkar I, Urner M, van Marle HJ, Denys D, Verkes RJ, Fernandez G (2014): Short‐term antidepressant administration reduces default mode and task‐positive network connectivity in healthy individuals during rest. Neuroimage 88:47–53. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Dannals RF, Sanchezroa PM, Ravert HT, Vazquez S, Wilson AA, Natarajan TK, Wong DF, Yanai K, Wagner HN (1991): Imaging histamine h1 receptors in the living human brain with carbon‐11‐pyrilamine. J Nucl Med 32:308–311. [PubMed] [Google Scholar]
- Wagner KD, Robb AS, Findling RL, Jin JQ, Gutierrez MM, Heydorn WE (2004): A randomized, placebo‐controlled trial of citalopram for the treatment of major depression in children and adolescents. Am J Psychiat 161:1079–1083. [DOI] [PubMed] [Google Scholar]
- Wang L, Day J, Roe CM, Brier MR, Thomas JB, Benzinger TL, Morris JC, Ances BM (2014): The effect of apoe epsilon 4 allele on cholinesterase inhibitors in patients with alzheimer disease evaluation of the feasibility of resting state functional connectivity magnetic resonance imaging. Alzheimer Dis Assoc Disord 28:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K (2006): Changes in hippocampal connectivity in the early stages of Alzheimer's disease: Evidence from resting state fMRI. Neuroimage 31:496–504. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981): The psychometric tradition ‐ developing the wechsler adult intelligence scale. Contemp Educ Psychol 6:82–85. [Google Scholar]
- Wilson MA, Molliver ME (1991): The organization of serotonergic projections to cerebral‐cortex in primates ‐ regional distribution of axon terminals. Neuroscience 44:537–553. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. Neuroimage 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Webster MA, Brooks JC, Tracey I, Smith SM, Nichols TE (2016): Non‐parametric combination and related permutation tests for neuroimaging. Hum Brain Mapp 37:1486–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O, Liu TT (2012): Anti‐correlated networks, global signal regression, and the effects of caffeine in resting‐state functional MRI. Neuroimage 63:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM (2009): Bayesian analysis of neuroimaging data in FSL. Neuroimage 45:S173–S186. [DOI] [PubMed] [Google Scholar]
- Zhu XL, Wang X, Xiao J, Liao J, Zhong MT, Wang W, Yao SQ (2012): Evidence of a dissociation pattern in resting‐state default mode network connectivity in first‐episode, treatment‐naive major depression patients. Biol Psychiatry 71:611–617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


