Abstract
Hydrophobic inner mitochondrial membrane proteins with internal targeting signals, such as the metabolite carriers, use the carrier translocase (TIM22 complex) for transport into the inner membrane. Defects in this transport pathway have been associated with neurodegenerative disorders. While the TIM22 complex is well studied in baker's yeast, very little is known about the mammalian TIM22 complex. Using immunoprecipitation, we purified the human carrier translocase and identified a mitochondrial inner membrane protein TIM29 as a novel component, specific to metazoa. We show that TIM29 is a constituent of the 440 kDa TIM22 complex and interacts with oxidized TIM22. Our analyses demonstrate that TIM29 is required for the structural integrity of the TIM22 complex and for import of substrate proteins by the carrier translocase.
Keywords: carrier translocase, membrane protein, mitochondria, protein translocation, TIM22, TIM29
Abbreviations
BSA, bovine serum albumin
Dic1, dicarboxylate carrier
IMS, intermembrane space
PiC, phosphate carrier
PMSF, phenylmethylsulfonylfluoride
TOM, translocase of the outer membrane
The human mitochondrial proteome consists of 1500 different proteins. Most mitochondrial proteins are translated on cytosolic ribosomes and are transported via different routes to their respective compartment within the mitochondrion 1, 2. The translocase of the outer membrane (TOM) serves as a central entry gate for the transport of mitochondrial proteins into mitochondria 3, 4. After crossing the outer membrane, transport pathways diverge into several routes, depending on specific signals within the amino acid sequence. Two translocases in the inner membrane mediate the transport of proteins across or into the inner membrane. Presequence‐containing proteins are transported with the help of the translocase of the inner membrane, TIM23 5. Substrates of the second translocase, the TIM22 complex, are hydrophobic multi‐spanning proteins, which are targeted with the help of internal targeting signals 6. The inner membrane of mitochondria is rich in an evolutionarily conserved family of multi‐spanning membrane proteins that catalyze the selective transfer of metabolites across the lipid bilayer, referred to as carrier proteins 7. The ADP/ATP carrier (AAC), the phosphate carrier (PiC), and the dicarboxylate carrier (Dic1) have been found to be typical substrates of the TIM22 complex 8, 9. The carrier proteins, are usually built of six transmembrane spans connected by short, matrix exposed, loops 10. Carrier proteins lack predicted targeting signals but apparently contain multiple internal signals within the mature polypeptide chain 11, 12. Additional substrates of the TIM22 complex in yeast are the central subunits of the inner membrane protein translocases, Tim23, Tim22, and Tim17 5, 7.
The TIM22 complex is built of a membrane and a peripheral module that have been best studied in Saccharomyces cerevisiae 13. The membrane module of the 300 kDa S. cerevisiae carrier translocase consists of the four integral membrane proteins, Tim22, Tim54, Tim18, and Sdh3 14, 15, 16, 17, 18. Tim22 is the central, pore‐forming, subunit of the complex 16, 19. Tim54, Tim18, and Sdh3, are currently thought to support assembly and stability of the complex 15, 17. Sdh3, which is related to Tim18, is also a major component of the succinate dehydrogenase complex 18. The human TIM22 displays 40% homology with the yeast Tim22 20, 21. Human and yeast Tim22 possess cysteine residues in the oxidized state, which are important for TIM22 complex assembly 20, 22, 23. In the case of the human protein, four cysteine residues are oxidized, likely forming disulfide bonds 20. Clear homologs of Tim54 or Tim18 have not been identified in mammals and there is no evidence that the Sdh3 homolog, SDHC, interacts with the TIM22 complex.
In yeast, the small Tim proteins, Tim9, Tim10, and Tim12 participate in the transport of metabolite carriers into the inner membrane 11, 24, 25. Three molecules of Tim9 and three molecules of Tim10 form a soluble 70 kDa complex 26, 27, 28. The peripheral module of the TIM22 complex is formed when one molecule of Tim10 is substituted by one molecule of Tim12 29 which associates with the membrane integral module through the intermembrane space domain of Tim54 30. The family of small Tim proteins comprises of two further proteins, Tim8 and Tim13 31. These proteins have been shown to effect the import of certain noncarrier proteins into the inner membrane, such as Tim23 32, 33, 34. In mammals, six members of the family of small Tims are expressed. One homolog each of Tim9 and Tim13 (human TIM9 and TIM13) and two homologs each of Tim8 (DDP1 and DDP2) and Tim10 (TIM10A and TIM10B) have been described in 21, 35. TIM10B was further found to act as a functional homolog of yeast Tim12 36. Mutations in TIM8 (DDP1, the deafness‐dystonia peptide) cause the Mohr–Tranebjaerg syndrome, a progressive neurodegenerative disorder characterized by sensorineural hearing loss, dystonia, mental retardation, and blindness 31, 37.
In summary, despite the conservation of the small Tim proteins in mammals, the membrane module of the carrier translocate is not well conserved. We speculate that, besides the channel forming subunit TIM22, constituents of the human TIM22 complex have been substituted by mammalian specific subunits during evolution. In order to identify novel subunits of the human TIM22 complex, we used immunoprecipitation studies, combined with a quantitative mass spectrometric approach. We found TIM29 (C19orf52) to be a specific binding partner of TIM22. TIM29 is a constituent of the 440 kDa human TIM22 complex. Depletion of TIM29 affects carrier protein translocation and concomitantly causes a significant decrease in cell growth. Thus, the human carrier translocase displays a composition that differs from its yeast counterpart. We conclude that adaptation of the translocation machinery to the more complex physiology of human mitochondria required novel translocase constituents to facilitate protein transport and membrane insertion.
Materials and methods
Cell culture
Human embryonic kidney cell lines (HEK293T‐Flp‐In™ T‐Rex™) were cultured in DMEM, supplemented with 10% (v/v) heat‐inactivated fetal bovine serum (Biochrom, Berlin, Germany) and 2 mm l‐glutamine and incubated at 37 °C with 5% CO2. For SILAC analysis, cells were grown for five passages on DMEM medium lacking arginine and lysine, supplemented with 10% (v/v) dialyzed fetal bovine serum, 600 mg·L−1 proline, 42 mg·L−1 arginine hydrochloride (13C6, 15N4‐arginine in ‘heavy’ media), and 146 mg·L−1 lysine hydrochloride (13C6, 15N2‐lysine in ‘heavy’ media) (Cambridge Isotope Laboratories, Tewksbury, MA, USA) 38.
For the overexpression of HisTIM22 variants, 2.0 × 106 cells were seeded into 10 cm culture dishes in high glucose medium (4500 mg·L−1). At 24 h after seeding, the medium was replaced with low‐glucose (1000 mg·L−1) DMEM medium, and cells were cultured for a further 24 h. Next, the medium was changed to galactose medium and the cells were transfected with pcDNA3.1 Zeo+ ‐containing HisTIM22, HisTIM22‐C69S, HisTIM22‐C138S, and HisTIM22‐C160S 20 using GeneJuice Transfection Reagent, according to the manufacturer's recommendation (EMD Millipore, Billerica, MA, USA). Cells were harvested 24 h after transfection.
For analysis of cell viability, 20 μg·mL−1 3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazoliumbromid (MTT) was added to cells in standard growth medium. After 90 min of incubation, the medium was removed and cells containing blue precipitates were dissolved in 4 mm HCl, 0.1% NP‐40 in isopropanol. Absorbance was measured at 570 nm in a 96‐well plate reader.
Antibodies
Antibodies used in this study are polyclonal antibodies raised in rabbit or purchased (anti‐TIM22 (Proteintech, Rosemont, IL, USA); anti‐c19orf52 (Proteintech); anti‐TIM13 (Proteintech); anti‐TIM9 (Proteintech); anti‐SLC25A6 (Proteintech); anti‐ATPB (Abcam, Cambridge, UK)).
Mitochondrial preparation and fractionation
Cells were harvested in PBS and washed using isolation buffer (300 mm Trehalose; 10 mm KCl; 10 mm HEPES, pH 7.4; 1 mm EDTA; 0.5 mm PMSF). Cells in isolation buffer were homogenized on ice using a Potter S Homogenizer (Sartorius, Goettingen, Germany). Cell debris was removed by centrifugation at 800 g for 10 min. Mitochondria were collected by centrifugation at 11 000 g for 10 min. Freshly isolated mitochondria were washed in isolation buffer and protein concentrations were determined by Bradford analysis using bovine serum albumin (BSA) as a standard.
Membrane integration of proteins was determined by incubation of mitochondria or total cell extracts with 100 mm Na2CO3 (pH 10.8 or 11.5), followed by centrifugation for 30 min at 100 000 g, 4 °C. Submitochondrial localization was analyzed by a protease protection assay as previously described in 39. Briefly, mitochondrial membranes were either osmotically stabilized in SEM buffer (250 mm sucrose, 1 mm EDTA, and 10 mm MOPS, pH 7.2), subjected to EM buffer (1 mm EDTA and 10 mm MOPS, pH 7.2), to rupture the outer mitochondrial membrane, or sonicated to disrupt mitochondrial membranes. These treatments were carried out in the presence or absence of proteinase K. After incubation for 10 min on ice, reactions were stopped by the addition of 1 mm PMSF.
Blue native PAGE
Mitochondria were solubilized in buffer (1% digitonin, 20 mm Tris/HCl, pH 7.4, 0.1 mm EDTA, 50 mm NaCl, 10% (w/v) glycerol, and 1 mm PMSF) to a final concentration of 1 μg·μL−1 for 30 min at 4 °C. Lysates were cleared by centrifugation at 14 000 g for 10 min at 4 °C and 10× loading dye was added (5% Coomassie brilliant blue G‐250, 500 mm 6‐aminohexanoic acid, and 100 mm Bis‐Tris pH 7.0). Samples were loaded onto 4–13% polyacrylamide gradient gels and separated as described in 40.
Affinity purification
Anti‐TIM22, anti‐C19orf52, or control serum was coupled to Protein A‐sepharose (GE Healthcare, Chicago, IL, USA) by chemical crosslinking with dimethyl pimelimidate. Isolated mitochondria were solubilized in buffer (20 mm Tris/HCl, pH 7.4; 100 mm NaCl; 0.5 mm EDTA; 10% (w/v) glycerol; 1 mm phenylmethylsulfonylfluoride (PMSF); and 1% digitonin) to a final concentration of 1 μg·μL−1 and incubated at 4 °C for 30 min. Lysates were cleared by centrifugation at 14 000 g for 10 min at 4 °C. The supernatants were added to the antibody‐coupled Protein A and incubated for 90 min at 4 °C with mild agitation. Unbound material was collected and affinity resins were washed extensively with buffer containing 0.3% digitonin. Bound proteins were eluted with 0.1 m glycine (pH 2.8) and subsequently analyzed by SDS/PAGE or BN‐PAGE.
For affinity purification of HisTIM22, HisTIM22‐C69S, HisTIM22‐C138S, and HisTIM22‐C160S, HEK293 cells (1.5 mg protein) were solubilized in digitonin‐containing buffer (1% (w/v) digitonin, 10% (v/v) glycerol, 20 mm Tris/HCl, 100 mm NaCl, 20 mm imidazole, 50 mm IAA, and 1 mm PMSF, pH 7.4) for 20 min at 4 °C. After a clarifying spin (20 000 ×g for 20 min at 4 °C), the supernatant was transferred to Ni‐NTA column (Qiagen, Hilden, Germany) and incubated for 1 h at 4 °C. Next, the column was washed twice with washing buffer (20 mm Tris/HCl, 100 mm NaCl, and 20 mm imidazole, pH 7.4). Bound protein complexes were eluted with Laemmli buffer, heated for 15 min at 65 °C and centrifuged for 3 min at 300 g. Samples were subjected to SDS/PAGE, followed by western blotting.
In vitro synthesis of precursor proteins and import into isolated mitochondria
ANT3 was amplified by PCR from pCMV‐Entry‐SLC25A6 (OriGene, Rockville, MD, USA) using primers containing the SP6 promoter site. [35S]‐labeled precursor proteins were synthesized in vitro with the TNT SP6 Quick Coupled Transcription/Translation system (Promega, Madison, WI, USA) using the respective RNA. Import reactions into freshly isolated human mitochondria were performed at 37 °C in import buffer (250 mm sucrose, 20 mm Hepes/KOH, 80 mm potassium acetate, 5 mm magnesium acetate, pH 7.4) in the presence of 2.25 mm ATP, 2.25 mm NADH, 15 mm sodium succinate, and 15 mm malate. The membrane potential was dissipated by the addition of 32 μm antimycin A, 80 μm oligomycin, and 4 μm valinomycin. Mitochondria were washed with SEM buffer (250 mm sucrose, 20 mm MOPS/KOH, and 1 mm EDTA, pH 7.2) and analyzed on BN‐PAGE in conjunction with autoradiography.
Microscopy
Human HeLa cells were chemically fixed with prewarmed (37 °C) 4% formaldehyde for 10 min. Cells were permeabilized with 0.5% Triton X‐100 and blocked with 5% BSA in PBS for 5 min. Subsequently, the cells were incubated with affinity purified anti‐C19orf52 serum and monoclonal mouse anti‐ATPB antibodies, which detect the beta subunit of the mitochondrial ATP synthase (Abcam). After some washing steps, the secondary antibodies (anti‐rabbit Star635P (Abberior, Goettingen, Germany) and anti‐mouse OregonGreen488 (Molecular Probes, ThermoFisher, Waltham, MA, USA)) were applied for 1 h at room temperature. Following further washing steps, the cells were embedded in Mowiol. Confocal images were recorded with a Leica SP8 confocal microscope (Leica Microsystems, Wetzlar, Germany). Z‐image series were taken and maximum projections of the stacks are displayed.
Mass spectrometric analysis
Mass spectrometric analyses of proteins isolated with antibodies against TIM22 were performed after SILAC‐labeling of HEK293T cells (see above). Coaffinity precipitation with TIM22 antibodies was performed with solubilized mitochondria derived from cells with ‘heavy’ labeling. Control IgG isolations were performed using cells with ‘light’ labeling. Coaffinity purified proteins were eluted from beads with equal amounts of Laemmli buffer, mixed in a 1 : 1 ratio, and proteins were separated by SDS/PAGE. Proteins were analyzed by mass spectrometry and quantified exactly as described in 41. Table S1 shows detected mitochondrial proteins with at least two identified peptides.
siRNA‐mediated knockdown
The ON‐TARGETplus SMARTpool, comprising of four siRNA targeting the C19ORF52 transcript (cat. #L‐015520‐02‐0020), and the corresponding ON‐TARGETplus nontargeting SMARTpool (cat. #D‐001810‐10‐20) were purchased from GE Dharmacon (Lafayette, CO, USA) . An siRNA targeting TIM22 (GUG‐AGG‐AGC‐AGA‐AGA‐UGA) and the corresponding nontargeting duplexes were purchased from Eurogentec (Liege, Belgium). A concentration of approximately 500 000 cells/25 cm2 flask were transfected with 16 nM siRNA. Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) in OptiMEM‐I medium (Gibco, ThermoFisher, Waltham, MA, USA) was used for transfection, according to the manufacturer's instructions. Cells were transfected for 72 h and used for subsequent assays.
Miscellaneous
Standard methods were used for SDS/PAGE and western blotting of proteins onto polyvinylidene fluoride membranes (EMD Millipore). Antigen–antibody complexes were detected by HRP‐coupled secondary antibodies and enhanced chemiluminescence detection on X‐ray films (GE Healthcare). Cell fractionation was carried out essentially as described in 20.
Results and Discussion
The mitochondrial protein TIM29 is a novel interaction partner of TIM22
Little is known about the molecular composition of the membrane module of the mammalian TIM22 complex. The affinity isolation of core components of the mitochondrial protein translocases has successfully revealed new components in yeast and so we used a similar strategy to identify novel components of the human TIM22 complex. Isolated mitochondria from HEK293T cells were solubilized and TIM22 was immunoprecipitated using TIM22‐specific antibodies bound to IgG‐Sepharose. TIM22 was efficiently isolated from mitochondria by the TIM22 antibody, but not recovered in the mock control. In contrast, the small Tim protein, TIM13, which is part of the soluble small Tim complex of the intermembrane space, was not detected in the eluate fraction, confirming the specificity of the isolation (Fig. 1A).
Figure 1.

Proteomic analysis identifies association of Tim22 with Tim29, the previously uncharacterized protein C19orf52. (A) Immunoprecipitation of TIM22 from digitonin solubilized HEK293T mitochondria using TIM22 antiserum or mock control beads (control column). Eluates were analyzed by SDS/PAGE, followed by western blotting. Total 1%; Eluate 100%. (B) Mitochondria were isolated after stable isotope labeling of cells, solubilized using digitonin, and TIM22 was immunoprecipitated. Ratio of peptides/proteins enriched in TIM22 affinity isolation (‘heavy’, H) versus isolation using IgG‐Sepharose (‘light’, L) is shown in log scale. The relative ranking of copurified mitochondrial proteins in both ‘heavy’ and ‘light’ samples are given on the x‐axis. Specifically enriched proteins belonging to the TIM complex are listed. For the entire mitochondrial protein list, see Table S1. (C) Primary sequence of TIM29. Gray shading indicates putative presequence and boxed area depicts the predicted transmembrane segment. (D) HeLa cells were immunolabeled using TIM29 antiserum and antibodies against the beta subunit of the F1FO‐ATP synthase (ATPB) to highlight the mitochondria. Scale bar, 10 μm.
In order to identify interaction partners of TIM22 in the immunoprecipitate, we used a quantitative mass spectrometry approach. Therefore, cells in two cell cultures were differentially labeled by stable isotope labeling with amino acids in cell culture (SILAC). Solubilized mitochondria from both cell cultures were subjected to TIM22 immunoprecipitation or to mock‐immunoprecipitation using IgG‐Sepharose as a control. Differential labeling allowed a quantitative analysis of proteins enriched by TIM22 immunoprecipitation compared to the control. Together with the bait, TIM22, there was a specific enrichment of the peripherally associated small Tim proteins, TIM10A and TIM10B (Fig. 1B and Table S1). Mass spectrometric analysis also identified a previously not characterized protein, annotated as C19orf52 (Fig. 1B and Table S1). Based on our following results, C19orf52 was termed TIM29, as a novel component of the TIM22 complex and in accordance with its predicted molecular weight of 29 kDa.
Sequence analysis revealed that TIM29 is conserved in metazoan, but no yeast homolog could be identified. The primary sequence of TIM29 displays a single predicted transmembrane span and a predicted mitochondrial targeting presequence (Fig. 1C, Fig. S1) 42. To confirm mitochondrial localization of TIM29, we analyzed its cellular localization by immunofluorescence microscopy using an antibody specifically directed against TIM29. The TIM29 signal largely colocalized with that of the beta subunit of the mitochondrial F1Fo ATP synthase (Fig. 1D). This further supports that TIM29 predominantly localizes to mitochondria.
TIM29 is a constituent of the carrier translocase
To define the submitochondrial localization of TIM29, we assessed accessibility of TIM29 to externally added protease. Proteins of the outer mitochondrial membrane, such as TOM70, were accessible to protease digestion in mitochondria. In contrast, TIM29 remained protected by the outer membrane. Upon disruption of the outer mitochondrial membrane by hypo‐osmotic swelling, the intermembrane space (IMS) becomes accessible to protease treatment. Under these conditions, the IMS protein, COA6, the inner membrane protein, TIM23, and TIM29 were all digested by protease. Matrix localized proteins, such as LETM1 and TIM44, only became accessible upon the solubilization of all mitochondrial membranes with Triton‐X100 (Fig. 2A). These data indicate that the epitope of TIM29, recognized by the antibody, resides within the intermembrane space.
Figure 2.
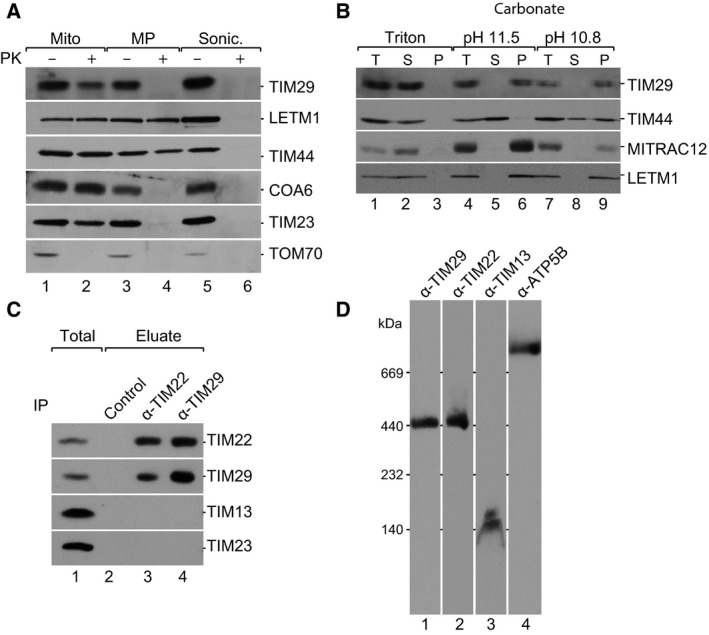
TIM29 is a mitochondrial inner membrane protein, which forms a stable complex with TIM22. (A) Submitochondrial localization analysis using mitochondria or mitoplasts from HEK293T cells. Mito., mitochondria; MP, mitoplasts; Sonic., sonication; PK, proteinase K. Samples were analyzed by SDS/PAGE and western blotting. (B) Mitochondria were subjected to carbonate extraction at indicated pH or detergent lysis, followed by differential centrifugation; pellet, P; supernatant, S; total, T. Samples were analyzed by SDS/PAGE and western blotting. (C) Isolated mitochondria from HEK293T cells were solubilized in digitonin and both TIM22 and TIM29 were immunoprecipitated. Protein A‐Sepharose beads were used for the control column. Eluates were analyzed by SDS/PAGE, followed by western blotting using antiserum for the indicated proteins. Total 1%, Eluate 100%. (D) Isolated mitochondria from HEK293T cells were solubilized in digitonin and subjected to BN‐PAGE analysis (4–13% gradient), followed by western blotting and immunodetection.
In order to analyze whether the TIM29 protein is an integral membrane protein, a carbonate extraction experiment was performed. Most peripheral proteins are extracted from mitochondrial membranes into the supernatant already at pH 10.8, whereas proteins strongly interacting with the membrane, such as TIM44, are only extracted at pH 11.5. TIM29 was resistant to carbonate extraction at pH 11.5, indicating that it is an integral membrane protein, like MITRAC12 or LETM1 (Fig. 2B). In summary, TIM29 is an integral inner mitochondrial membrane protein, partially exposed to the intermembrane space.
As TIM29 was identified by mass spectrometry upon the isolation of TIM22, we further analyzed its association with the carrier translocase. To this end, we used immunoprecipitation to isolate TIM22 from solubilized mitochondrial membranes and detected TIM29 in the eluate (Fig. 2C). In contrast, the soluble protein, TIM13, and the core component of the presequence translocase, TIM23, were not found in the eluate, confirming the specificity of the isolation. In the reverse experiment, TIM29 specifically coisolated TIM22. The specific TIM22–TIM29 interaction was further confirmed using a cell line expressing TIM22 fused N‐terminally to 10 histidine residues (His‐tag) (Fig. 4A).
Figure 4.

Disulfide bridge formation in TIM22 promotes integration of TIM29 into the mature TIM22 complex. (A) HEK293T cells expressing HisTIM22 were solubilized in digitonin‐containing buffer. The protein complexes were isolated via affinity chromatography. Load: 3%; eluate: 100%. (B) HEK293 cells expressing HISTIM22 and HISTIM22 mutants lacking cysteine residues were fractionated and analyzed by SDS/PAGE and western blotting. T, Total cell extract; Cyto., cytoplasm; Mito., mitochondria. The mitochondrial proteins, TIM29, TIM23, NDUFS1, and ALR‐1 were used as mitochondrial controls and Hsp70 was used as a cytosolic marker. (C) Carbonate extraction of cell extracts of HEK293T expressing HisTIM22‐C69S, HisTIM22‐C138S, or HisTIM22‐C160S at pH 10.8. After differential centrifugation, samples were analyzed by SDS/PAGE and western blotting; pellet, P; supernatant, S; total, T. (D) Cells expressing the indicated HisTIM22 construct were subjected to affinity chromatograpy as in (A). Load: 2%; eluate: 100%.
In yeast, blue native gel electrophoresis (BN‐PAGE) was used to visualize the 300 kDa Tim22 membrane module and the 70 kDa hexameric complexes of Tim8‐13 and Tim9‐10 19, 27, 30, 43. We therefore employed BN‐PAGE to determine whether human TIM22 and TIM29 could be detected in the same complex. Solubilized mitochondrial membranes were separated by blue native gel electrophoresis and antibodies directed against TIM22 detected a complex of 440 kDa, corresponding to the expected size of the carrier translocase 36. Antibodies against TIM13 detected the soluble module at a size of 140 kDa (Fig. 2D). In accordance with its interaction with TIM22, antibodies against TIM29 detected a complex of 440 kDa. These data establish TIM29 as a core constituent of the TIM22 complex in the inner mitochondrial membrane.
TIM29 is required for the structural integrity of TIM22
To define the molecular function of TIM29, we used RNA interference to reduce gene expression in HEK293T cells. We tested the impact of TIM29 knockdown on cell growth, and observed a reduction in cell number by approximately 50% after 72 h of knockdown (Fig. 3A). Analysis of the reduction of 3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazoliumbromid (MTT) to its formazan, by mitochondrial reductases, revealed a decrease in cellular viability by approximately 40% (Fig. 3B). Mitochondria were isolated from knockdown cells and from cells treated with nontargeting RNA, and separated on SDS/PAGE, followed by western blot. Quantification of TIM29 knockdown showed a decrease in the protein levels in mitochondria by 52% (Fig. 3C). Concomitantly, we observed a reduction of TIM22 by 33%, whereas other mitochondrial proteins, such as TIM13, and the presequence‐containing ATP5B, were not affected under these knockdown conditions (Fig. 3C). We also monitored potential substrates of the carrier pathway, such as TIM23, as well as the adenine nucleotide transporter ANT3. The steady‐state levels of these proteins were only marginally reduced.
Figure 3.

TIM29 depletion affects cell growth and destabilizes the carrier translocase complex. (A) HEK293T cells were treated with TIM29‐specific siRNA or nontargeting siRNA for 72 h. Cells were trypsinized and total cell counts were measured using a hemocytometer. Shown are the total cell numbers per well of a six‐well plate (n = 3, P < 0.025). (B) Cells were treated as in (A) and cell viability was measured by determining the reduction of MTT by mitochondrial reductases. Shown is the percentage compared to control (n = 3, P < 0.015). (C) Following knockdown, mitochondria were isolated and analyzed by SDS/PAGE, followed by western blotting and immunodetection using the indicated antibodies. Quantification of selected proteins revealed a reduction of TIM29 by 52%, TIM22 by 33%, TIM23 by 20%, and ATP5B by 24%. (D) Isolated mitochondria from cells treated with TIM29 siRNA or nontargeting siRNA were solubilized in 1% digitonin and resolved using BN‐PAGE (4–13% gradient), followed by western blotting and detection using the indicated antibodies. A longer exposure of TIM22 showing residual amounts of TIM22 complex in Tim29 knockdown mitochondria is shown (lanes 5 and 6). (E) HEK293T cells were treated with TIM22‐specific siRNA or nontargeting siRNA. Mitochondria were isolated and analyzed by SDS/PAGE, followed by western blotting and immunodetection using the indicated antibodies. Quantification of TIM22 knockdown samples compared to control; TIM22 31%, TIM29 21%, TIM13 85%, TIM9 83%, TIM21 102%, HSP60 160%, and ATP5B 73%.
To analyze the organization of the TIM22 complex, mitochondria isolated from knockdown and control cells were solubilized and protein complexes were analyzed using BN‐PAGE. Upon depletion of TIM29, the amount of the 440 kDa complex, detected by the TIM29 antibody, was below the level of detection (Fig. 3D). TIM29 knockdown also caused a reduction of the TIM22 complex, detected by the TIM22 antibody. In contrast, the F1Fo‐ATP synthase and the complex formed by ANT3 were not affected (Fig. 3D). These data confirm that TIM29 is a constituent of the TIM22 complex. In the reverse experiment, siRNA‐mediated knockdown was used to deplete TIM22. Isolated mitochondria from siTIM22‐treated cells and cells treated with nontargeting RNA were separated using SDS/PAGE and analyzed by western blotting. Protein levels of TIM22 were clearly reduced. Interestingly, TIM22 knockdown also caused a significant reduction in TIM29 protein levels, demonstrating an interdependence of these two proteins (Fig. 3E). As the presequence‐containing proteins, Tim21, ATB5B, and HSP60 were not affected by TIM22 depletion, we concluded that protein transport by the TIM23 complex was not affected under our conditions (Fig. 3E). Accordingly, the observed reduction of Tim29, which contains a predicted presequence, cannot be due to an indirect effect on TIM23. In summary, these data show that TIM29 is a structurally relevant constituent of the TIM22 complex.
Oxidized TIM22 promotes TIM29 integration into the mature TIM22 complex
Given that TIM22 is present in the carrier translocase in an oxidized state in human cells, we aimed to assess whether its oxidation status affects the interaction with TIM29. To this end, we used HEK293T cells to overexpress His‐tagged TIM22 variants. Affinity purification revealed the interaction between HisTIM22 and TIM29 (Fig. 4A), in addition to native TIM22 20. Other mitochondrial proteins were not found in the eluate, demonstrating assay specificity. In the next step, this assay was used to determine the interaction of TIM29 with oxidation variants of TIM22. We overexpressed TIM22 mutants lacking cysteine residues involved in disulfide bond formation; HisTIM22‐C69S and HisTIM22‐C160S 20. We also expressed HisTIM22‐C138S, a mutant lacking the cysteine residue that does not participate in disulfide bond formation. Cell fractionation demonstrated that all three variants are mitochondrial localized (Fig. 4B). Carbonate extraction was used to verify the correct integration of TIM22 cysteine mutants into the mitochondrial inner membrane. All three mutant constructs resisted the carbonate extraction procedure, confirming membrane integration (Fig. 4C). TIM29 was recovered in the eluate of the HisTIM22‐C138S purification (Fig. 4D). In contrast, similar to native TIM22, the interaction between TIM29 and mutants HisTIM22‐C69S and HisTIM22‐C160S was abolished. (Fig. 4D). These analyses demonstrated the importance of disulfide bonds in TIM22 for TIM29 interaction. Accordingly, TIM29 can only be stably assembled with the oxidized TIM22.
Cell growth is impaired in TIM29 knockdown cells
The carrier translocase is required for the biogenesis of a number of essential proteins, including the large family of mitochondrial carrier proteins. Upon approximately 50% depletion of TIM29, we did not observe a strong defect in the steady‐state levels of ANT3, which was previously shown to be transported by the TIM22 pathway (Fig. 5A) 36. However, considering that mitochondrial proteins can display a half‐life that significantly exceeds the time of knockdown, the depletion of TIM29 might cause only a moderate delay in the biogenesis of ANT3, which is not apparent in steady‐state level analysis. Therefore, we tested the assembly of ANT3 in a kinetic assay. ANT3 was translated in vitro and labeled in the presence of [35S]Methionine. [35S]ANT3 was imported into isolated, energized mitochondria. Subsequently, solubilized mitochondrial protein complexes were separated on BN‐PAGE. Autoradiography revealed the time‐dependent formation of a 120 kDa complex (Fig. 5A), representing the complex detected earlier using ANT3‐specific antibodies at steady state (Fig. 3D). Complex formation was strictly dependent on the membrane potential (Δψ), as expected for carrier translocase substrates (Fig. 5A). Next, we analyzed if the assembly of imported ANT3 was affected by TIM29 depletion. [35S]ANT3 was imported into isolated TIM29 knockdown and control mitochondria. A significantly reduced formation of the 120 kDa complex was observed upon TIM29 depletion after 45 min, indicating that TIM29 is required for ANT3 biogenesis (Fig. 5B).
Figure 5.

TIM29 is required for import of the ADP/ATP carrier, ANT3 (A) Radiolabeled ANT3 was imported into isolated mitochondria from wild‐type HEK293T cells, for the indicated times, in the presence or absence of membrane potential (Δψ) at 37 °C. Samples were analyzed by BN‐PAGE and digital autoradiography. (B) Radiolabeled ANT3 was imported into mitochondria isolated from HEK293T cells transfected with nontargeting or TIM29‐specific siRNA. Import reactions were performed for 45 min in the presence or absence of Δψ at 37 °C. Samples were analyzed by BN‐PAGE and digital autoradiography. Asterisk, stage III import intermediate 45 accumulating in the absence of Δψ in the IMS.
Conclusion
The TIM22 complex is one of the major protein import machineries in the inner mitochondrial membrane. It is required for the translocation and integration of the large family of metabolite carrier proteins into the inner membrane. Although components of the peripheral module are highly conserved from yeast to mammals, only TIM22 of the membrane module is conserved 13. We followed the hypothesis that during evolution, metazoa have acquired new subunits of the TIM22 complex. In order to identify novel components of the TIM22 complex, we used an immunoprecipitation approach, combined with quantitative mass spectrometry analysis, to reveal TIM29 (C19orf52) to be a new constituent of the TIM22 complex. TIM29 is an integral inner membrane protein. Protein prediction confirmed the existence of a transmembrane domain following a presequence. From this it can be speculated that TIM29 is integrated with its N terminus facing the matrix and its C terminus facing the intermembrane space. TIM29 is a core subunit of the carrier translocase and requires oxidized TIM22 to be integrated into the mature TIM22 complex. Using BN‐PAGE, we detected TIM22 and TIM29 in the same complex at a molecular size of 440 kDa and depletion of either TIM22 or TIM29 significantly reduced the amount of this complex. Interestingly, knockdown of TIM29 reduced the steady‐state levels of TIM22 and vice versa, causing a dramatic loss of the TIM22 complex. As TIM22 levels on SDS/PAGE (Fig. 3C) are not as dramatically reduced as on BN‐PAGE (Fig. 3D), we would speculate that a pool of TIM22, which is not integrated into the TIM22 complex remains stable in the cell for a limited time. Unassembled protein might be aggregated, or migrates too fast to be resolved on BN‐PAGE. Our analyses show that import and assembly of the carrier protein ANT3 was significantly reduced upon TIM29 depletion. While this manuscript was in preparation, Kang et al. assessed TIM23 as a substrate and found this also requires TIM29 for its import and assembly 44. Using isolated TIM29 knockdown mitochondria, the study shows reduced amounts of TIM23 complex on BN‐PAGE and an impaired assembly of in vitro imported TIM23 into its complex. These findings establish TIM29 as a carrier translocase constituent required for protein translocation.
Author contributions
PR designed the project; AC, HU, SJ designed experiments and evaluated the data; SC, FR, DJ, CL, IL, KC, JD, and DP performed experiments and analyzed data. JD, PR, AC, wrote the paper with the input of other authors.
Supporting information
Fig. S1. TIM29 is conserved among mammals and amphibians. Alignment of TIM29 homolog proteins from different species, using ClustalW with Blossum62 score matrix. Black shading, 100% similarity; dark gray, 80–100% similarity; gray, 60–80% similarity; light gray, 50–60% similarity. Shown in red are the predicted transmembrane segment and the predicted mitochondrial targeting sequence.
Table S1. Proteins enriched in SILAC‐based quantitative mass spectroscopy analysis of affinity‐purified TIM22.
Acknowledgements
We are grateful to Dr. S. Dennerlein for discussions and thank Annika Kühn for her help in MS analysis. This work was supported by the Deutsche Forschungsgemeinschaft, the SFB1190 (HU, SJ, and PR), the Cluster of Excellence and DFG Research Center Nanoscale Microscopy (SJ), the Boehringer Ingelheim Foundation (FR), the European Research Council ERC335080 (PR), the Max Planck Society (HU, SJ, and PR), the National Science Centre (NCN; 2016/20/S/NZ1/00423) (KC), and the Polish Ministerial Ideas Plus Program 000263 (AC). Collaboration of AC and PR is supported by Copernicus Award of the Foundation for Polish Science and Deutsche Forschungsgemeinschaft.
Edited by Lukas Alfons Huber
References
- 1. Neupert W and Herrmann JM (2007) Translocation of proteins into mitochondria. Annu Rev Biochem 76, 723–749. [DOI] [PubMed] [Google Scholar]
- 2. Dudek J, Rehling P and van der Laan M (2013) Mitochondrial protein import: common principles and physiological networks. Biochim Biophys Acta 1833, 274–285. [DOI] [PubMed] [Google Scholar]
- 3. Hill K, Model K, Ryan MT, Dietmeier K, Martin F, Wagner R and Pfanner N (1998) Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature 395, 516–521. [DOI] [PubMed] [Google Scholar]
- 4. Künkele K, Heins S, Dembowski M, Nargang F, Benz R, Thieffry M, Walz J, Lill R, Nussberger S and Neupert W (1998) The preprotein translocation channel of the outer membrane of mitochondria. Cell 93, 1009–1019. [DOI] [PubMed] [Google Scholar]
- 5. Chacinska A, Koehler CM, Milenkovic D, Lithgow T and Pfanner N (2009) Importing mitochondrial proteins: machineries and mechanisms. Cell 138, 628–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chacinska A and Rehling P (2004) Moving proteins across and in outer and inner membranes of mitochondria. Biochem Soc Trans 32, 774–776. [DOI] [PubMed] [Google Scholar]
- 7. Rehling P, Brandner K and Pfanner N (2004) Mitochondrial import and the twin‐pore translocase. Nat Rev Mol Cell Biol 5, 519–530. [DOI] [PubMed] [Google Scholar]
- 8. Smagula C and Douglas M (1988) Mitochondrial import of the ADP/ATP carrier protein in Saccharomyces cerevisiae. Sequences required for receptor binding and membrane translocation. J Biol Chem 263, 6783–6790. [PubMed] [Google Scholar]
- 9. Pfanner N, Hoeben P, Tropschug M and Neupert W (1987) The carboxyl‐terminal two‐thirds of the ADP/ATP carrier polypeptide contains sufficient information to direct translocation into mitochondria. J Biol Chem 262, 14851–14854. [PubMed] [Google Scholar]
- 10. Nelson D, Felix C and Swanson J (1998) Highly conserved charge‐pair networks in the mitochondrial carrier family. J Mol Biol 277, 285–308. [DOI] [PubMed] [Google Scholar]
- 11. Pfanner N and Neupert W (1987) Distinct steps in the import of ADP/ATP carrier into mitochondria. J Biol Chem 262, 7528–7536. [PubMed] [Google Scholar]
- 12. Brandner K, Rehling P and Truscott KN (2005) The carboxyl‐terminal third of the dicarboxylate carrier is crucial for productive association with the inner membrane twin‐pore translocase. J Biol Chem 280, 6215–6221. [DOI] [PubMed] [Google Scholar]
- 13. Sokol AM, Sztolsztener ME, Wasilewski M, Heinz E and Chacinska A (2014) Mitochondrial protein translocases for survival and wellbeing. FEBS Lett 588, 2484–2495. [DOI] [PubMed] [Google Scholar]
- 14. Sirrenberg C, Bauer M, Guiard B, Neupert W and Brunner M (1996) Import of carrier proteins into the mitochondrial inner membrane mediated by Tim22. Nature 384, 582–585. [DOI] [PubMed] [Google Scholar]
- 15. Kerscher O, Holder J, Srinivasan M, Leung R and Jensen R (1997) The Tim54p‐Tim22p complex mediates insertion of proteins into the mitochondrial inner membrane. J Cell Biol 139, 1663–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kovermann P, Truscott KN, Guiard B, Rehling P, Sepuri NB, Müller H, Jensen RE, Wagner R and Pfanner N (2002) Tim22, the essential core of the mitochondrial protein insertion complex, forms a voltage‐activated and signal‐gated channel. Mol Cell 9, 363–373. [DOI] [PubMed] [Google Scholar]
- 17. Kerscher O, Sepuri NB and Jensen RE (2000) Tim18p is a new component of the Tim54p‐Tim22p translocon in the mitochondrial inner membrane. Mol Biol Cell 11, 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gebert N, Gebert M, Oeljeklaus S, Von Der Malsburg K, Stroud DA, Kulawiak B, Wirth C, Zahedi RP, Dolezal P, Wiese S et al (2011) Dual function of Sdh3 in the respiratory chain and TIM22 protein translocase of the mitochondrial inner membrane. Mol Cell 44, 811–818. [DOI] [PubMed] [Google Scholar]
- 19. Rehling P, Model K, Brandner K, Kovermann P, Sickmann A, Meyer HE, Kühlbrandt W, Wagner R, Truscott KN and Pfanner N (2003) Protein insertion into the mitochondrial inner membrane by a twin‐pore translocase. Science 299, 1747–1751. [DOI] [PubMed] [Google Scholar]
- 20. Wrobel L, Sokol AM, Chojnacka M and Chacinska A (2016) The presence of disulfide bonds reveals an evolutionarily conserved mechanism involved in mitochondrial protein translocase assembly. Sci Rep 6, 27484–27498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bauer MF, Rothbauer U, Mühlenbein N, Smith RJ, Gerbitz K, Neupert W, Brunner M and Hofmann S (1999) The mitochondrial TIM22 preprotein translocase is highly conserved throughout the eukaryotic kingdom. FEBS Lett 464, 41–47. [DOI] [PubMed] [Google Scholar]
- 22. Wrobel L, Trojanowska A, Sztolsztener ME and Chacinska A (2013) Mitochondrial protein import: Mia40 facilitates Tim22 translocation into the inner membrane of mitochondria. Mol Biol Cell 24, 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okamoto H, Miyagawa A, Shiota T, Tamura Y and Endo T (2014) Intramolecular disulfide bond of Tim22 protein maintains integrity of the TIM22 complex in the mitochondrial inner membrane. J Biol Chem 289, 4827–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koehler C, Jarosch E, Tokatlids K, Schmid K, Schweyen R and Schatz G (1998) Import of mitochondrial carriers mediated by essential proteins of the intermembrane space. Science 279, 369–373. [DOI] [PubMed] [Google Scholar]
- 25. Endres M, Neupert W and Brunner B (1999) Transport of the ADP/ATP carrier of mitochondria from the TOM complex to the TIM22‐54 complex. EMBO J 18, 3214–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wiedemann N, Pfanner N and Ryan MT (2001) The three modules of ADP/ATP carrier cooperate in receptor recruitment and translocation into mitochondria. EMBO J 20, 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Curran S, Leuenberger D, Oppliger W and Koehler C (2002) The Tim9p‐Tim10p complex binds to the transmembrane domains of the ADP/ATP carrier. EMBO J 21, 942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Webb CT, Gorman MA, Lazarou M, Ryan MT and Gulbis JM (2006) Crystal structure of the mitochondrial chaperone TIM9.10 reveals a six‐bladed alpha‐propeller. Mol Cell 21, 123–133. [DOI] [PubMed] [Google Scholar]
- 29. Adam A, Endres M, Sirrenberg C, Lottspeich F, Neupert W and Brunner M (1999) Tim9, a new component of the TIM22‐54 translocase. EMBO J 18, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wagner K, Gebert N, Guiard B, Brandner K, Truscott KN, Wiedemann N, Pfanner N and Rehling P (2008) The assembly pathway of the mitochondrial carrier translocase involves four preprotein translocases. Mol Cell Biol 28, 4251–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koehler C, Leuenberger D, Merchant S, Renold A and Junne T (1999) Human deafness dystonia syndrome is a mitochondrial disease. Proc Natl Acad Sci USA 96, 2141–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leuenberger D, Bally N, Schatz G and Koehler C (1999) Different import pathways through the mitochondrial intermembrane space for inner membrane proteins. EMBO J 18, 4816–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davis AJ, Sepuri NB, Holder J, Johnson AE and Jensen RE (2000) Two intermembrane space TIM complexes interact with different domains of Tim23p during its import into mitochondria. J Cell Biol 150, 1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paschen S, Rothbauer U, Kaldi K, Bauer M, Neupert W and Brunnner M (2000) The role of the TIM8‐13 complex in the import of Tim23 into mitochondria. EMBO J 19, 6392–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin H, Kendall E, Freeman TC, Roberts RG and Vetrie DLP (1999) The human family of deafness/dystonia peptide (DDP) related mitochondrial import proteins. Genomics 61, 259–267. [DOI] [PubMed] [Google Scholar]
- 36. Mühlenbein N, Hofmann S, Rothbauer U and Bauer MF (2004) Organization and function of the small Tim complexes acting along the import pathway of metabolite carriers into mammalian mitochondria. J Biol Chem 279, 13540–13546. [DOI] [PubMed] [Google Scholar]
- 37. Jin H, May M, Tranebjærg L, Kendall E, Fontán G, Jackson J, Subramony SH, Arena F, Lubs H, Smith S et al (1996) A novel X‐linked gene, DDP, shows mutations in families with deafness (DFN‐1), dystonia, mental deficiency and blindness. Nat Genet 14, 177–180. [DOI] [PubMed] [Google Scholar]
- 38. Ong S‐E, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A and Mann M (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 1, 376–386. [DOI] [PubMed] [Google Scholar]
- 39. Mick DU, Dennerlein S, Wiese H, Reinhold R, Pacheu‐Grau D, Lorenzi I, Sasarman F, Weraarpachai W, Shoubridge EA, Warscheid B et al (2012) MITRAC links mitochondrial protein translocation to respiratory‐chain assembly and translational regulation. Cell 151, 1528–1541. [DOI] [PubMed] [Google Scholar]
- 40. Wittig I, Braun H‐P and Schägger H (2006) Blue native PAGE. Nat Protoc 1, 418–428. [DOI] [PubMed] [Google Scholar]
- 41. Bareth B, Dennerlein S, Mick DU, Nikolov M, Urlaub H and Rehling P (2013) The heme a synthase Cox15 associates with cytochrome c oxidase assembly intermediates during Cox1 maturation. Mol Cell Biol 33, 4128–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Claros MG and Vincens P (1996) Computational method to predict mitochondrially imported proteins and their targeting sequences. FEBS J 241, 779–786. [DOI] [PubMed] [Google Scholar]
- 43. Curran S, Leuenberger D, Schmidt E and Koehler C (2002) The role of the Tim8p‐Tim13p complex in a conserved import pathway for mitochondrial polytopic inner membrane proteins. J Cell Biol 158, 1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kang Y, Baker MJ, Liem M, Louber J, Mckenzie M, Atukorala I, Ang C‐S, Keerthikumar S, Mathivanan S and Stojanovski D (2016) Tim29 is a novel subunit of the human TIM22 translocase and is involved in complex assembly and stability. Elife 5, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pfanner N, Tropschug M and Neupert W (1987) Mitochondrial protein import: nucleoside triphosphates are involved in conferring import‐competence to precursors. Cell 49, 815–823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. TIM29 is conserved among mammals and amphibians. Alignment of TIM29 homolog proteins from different species, using ClustalW with Blossum62 score matrix. Black shading, 100% similarity; dark gray, 80–100% similarity; gray, 60–80% similarity; light gray, 50–60% similarity. Shown in red are the predicted transmembrane segment and the predicted mitochondrial targeting sequence.
Table S1. Proteins enriched in SILAC‐based quantitative mass spectroscopy analysis of affinity‐purified TIM22.


