Abstract
Follistatin‐like protein 1 (FSTL1) is a newly characterized protein that can regulate the immune response in various ways. Dendritic cells (DCs) are central to immune regulation. In this study, we explored the impact of FSTL1 on DC activity in nasopharyngeal carcinoma (NPC) patients. The surface expression of CD40, CD86, and HLA‐DR on DCs was analyzed and showed significantly elevated expression levels, indicating DC maturity. After FSTL1 was added to DCs collected from NPC patients (n = 50), controls (n = 47), and healthy donors (n = 10), interferon γ secretion and T‐cell receptor expression in cytotoxic T lymphocytes were also investigated. In the experimental groups, the expression of the critical immune protein nuclear factor (NF)‐κb was upregulated, whereas Jun N‐terminal kinase (JNK) was downregulated. Our findings demonstrate that FSTL1 plays a critical role in immune regulation, enhancing the antigen presentation ability of DCs by up‐regulating NF‐κb expression and down‐regulating JNK expression.
Keywords: dendritic cell, follistatin‐like protein 1, Jun N‐terminal kinase, nasopharyngeal carcinoma, nuclear factor κb, T lymphocyte
1. INTRODUCTION
In China, the highest incidence of nasopharyngeal carcinoma (NPC) occurs in the Guangxi, Guangdong, Hunan, and Fujian provinces. People between 40 and 50 years of age are at high risk for this type of carcinoma, and the disease is 2 to 3 times more common in men than women. Ninety‐eight percent of NPC originates in the nasopharynx and is characterized as a poorly differentiated squamous cell carcinoma.1 The etiology of NPC involves genetic factors, infection with the Epstein‐Barr virus (EBV), and environmental factors. NPC has been specifically associated with race and exhibits familial clustering such that Southern Chinese descendants living abroad still have a high incidence of NPC.2
Follistatin‐like protein 1 (FSTL1), a proinflammatory cytokine, is an extracellular matrix glycoprotein secreted by a variety of cells that participates in the immune response by regulating interleukins (ILs), interferons (IFNs), and other immune molecules. FSTL1 is expressed in some malignant tumors, such as renal clear cell cancer, endometrial cancer, and lung cancer, among others, and inhibits the proliferation and migration of tumor cells instead promoting apoptosis. Therefore, FSTL1 is a newly discovered tumor suppressor gene.3, 4, 5, 6 Exogenous FSTL1 regulates the immune response by enhancing or inhibiting specific functions. For example, in rheumatoid arthritis synovium, FSTL1 promoted the cellular immune response by enhancing the function of antigen‐presenting cells surrounding the synovium.7 FSTL1 secreted by fibroblasts can increase the expression of tumor necrosis factor α (TNF‐α) and IL‐1β secreted by macrophages, and injecting an adenoviral vector encoding FSTL1 in mice induces excessive IL‐6, IL‐1β, and TNF‐α expression in the liver.8, 9 These data suggest that FSTL1 might also regulate host immunity through a paracrine mechanism. Furthermore, when examining the relationship between FSTL1 and macrophages, it was observed that FSTL1 expression significantly increased in tissue macrophages after LPS injection into mouse footpads, demonstrating that macrophages can respond to FSTL1 at sites of inflammation. In vitro, FSTL1 activates the mitochondrial electron transport chain and increases the production of ATP and IL‐1β secretion from monocytes/macrophages.10 The specific immune function of dendritic cells (DC) in NPC patients is decreased, and DC function has not been reported to be affected by FSTL1 protein.
DCs are antigen‐presenting cells that can uptake, process, and present antigen molecules with high efficiency.11, 12 Therefore, understanding the molecular mechanisms and roles of FSTL1 in DCs might contribute to the development of novel immunotherapy approaches and supplementary treatments for NPC patients.
Nuclear factor κb (NF‐κb) plays a crucial role in the induction of inflammatory mediators and is implicated in the development and progression of many chronic diseases. IL‐6 and IL‐8 release from human CF bronchial epithelial cells (IB3‐1), which is stimulated by TNF‐α, is controlled by NF‐κb.13 Similarly, monocytes, macrophages, and DCs can activate NF‐κb translocation to the nucleus through intracellular signal transduction pathways to promote the expression of IL‐1, IL‐6, IL‐8, IL‐10, IL‐12, TNF, and IFNs, among other factors.14, 15 After their release, these cytokines attract granulocytes and macrophages, increase capillary permeability, and stimulate lymphocytic infiltration, which are important for the early immune response.16 In addition, cytokines stimulated by activated NF‐κb upregulate the expression of costimulatory molecules such as CD83, CD80, CD86, and MHC II on DCs, thereby generating mature DCs.15 NF‐κb plays significant roles in host defense, tissue damage and stress, cell differentiation, apoptosis, and the inhibition of tumor growth.
There is a close relationship between the number of immune cells and overall immune function. Usually, inner and outer stimuli can lead to apoptosis, which effectively downregulates overall immune function. Jun N‐terminal kinase (JNK), which is also referred to as stress‐activated protein kinase (SAPK), is a subclass of the mitogen‐activated protein kinase signaling pathway in mammalian cells that plays a critical role in both the physiological and pathological regulation of the cell cycle, reproduction, apoptosis, and cell stress. Overexpressed JNK protein functions in the endoplasmic reticulum.17 Generally, apoptosis is triggered when endoplasmic reticulum stress is continuous and excessive by activating caspase‐12 and up‐regulating the expression of CHOP/CADD153 and JNK.18 Activated estrogen receptor type I transmembrane protein kinase binds to TNF receptor associated factor 2, forming a complex that activates TNF‐dependent apoptosis‐signaling kinase 1, which activates JNK to induce immune cell apoptosis.19 As a result of contact with foreign antigens, DCs mature and migrate to the lymphoid organs, where they present captured antigens and undergo apoptosis.20 When DCs provide immune signals to T cells, DC apoptosis is initiated through various mechanisms.21 Although JNK is a critical apoptotic protein, it is still unknown whether exogenous FSTL1 regulates immune cell apoptosis through JNK.
In this study, we investigated the functional role and molecular mechanism of FSTL1 in the immune regulation of DCs and T lymphocytes from NPC patients. We examined antigen‐presenting molecule expression, IFN‐γ secretion, and T‐cell receptor (TCR) presentation on the surface of cytotoxic T lymphocytes in FSTL1‐treated and control groups. We also measured NF‐κb and JNK expression, as these proteins are involved in many critical inflammation‐related pathways. Our results demonstrate that FSTL1 contributes to the activation of DCs and T lymphocytes from NPC patients by modulating NF‐κb and JNK expression.
2. MATERIALS AND METHODS
2.1. Specimens
From September 2013 to May 2015, 50 cases of NPC were confirmed in HLA‐A2+ patients, who were diagnosed for the first time by the Pathology Department of the First Affiliated Hospital of Guangxi Medical University. Forty patients were male, and 10 patients were female, with an average age of 46 years. There were 47 HLA‐A2+ NPC patients included in the control group, with an average age of 42 years. All of the patients were recruited from the outpatient Otorhinolaryngology Head and Neck (ENT and HN) Surgery Department. Ten healthy control samples, 6 male and 4 female, from Guangxi Medical University were also used for this study, with an average age of 25 years.
2.2. Peripheral blood mononuclear cell separation and DC induction
Before collecting blood, participants were clearly informed such that they understood the study and signed their informed consent. The project was approved by our ethics committee. Twenty‐five milliliters of blood were collected from each subject in ethylenediaminetetraacetic acid–anticoagulant tubes. Peripheral blood mononuclear cells (PBMCs) were separated with Lymphoprep™ (STEMCELL™ Technologies, Vancouver, BC, Canada) according to the manufacturer's instructions. Approximately 1.1 × 106 to 3 × 106 cells/mL PBMCs were cultured in 6‐well flat bottom plates in RPMI 1640 medium (Hyclone, Thermo Fisher Scientific, Logan, UT) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin‐streptomycin. The plates were incubated at 37°C and 5% CO2 for 4 h. Four hours later, the adhesion method was used to separate the lymphocytes and the PBMCs, and the lymphocytes were stored at −80°C. Recombinant human granulocyte macrophage colony stimulating factor (rhGM‐CSF; 1,000 U/mL, American PeproTech Inc. Rocky Hill, NJ), recombinant human IL‐4 (rhIL‐4; 500 U/mL, American PeproTech Inc. Rocky Hill, NJ), and recombinant human TNF‐α (rhTNF‐α; 1,000 U/mL, American PeproTech Inc.) were used to induce PBMCs to become DCs. The cultures were refreshed every 3 days with RPMI 1640 medium containing growth factors and were split upon confluence. DCs were harvested on the eighth day of culture.
2.3. Cell surface molecule expression detected by flow cytometry
On the third and eighth days of culture, CD40, CD80, CD86, and HLA‐DR expression on the DC surface was examined for FITC fluorescence by flow cytometry to judge the maturity of the DCs. The cells were labeled with antibodies against the above‐mentioned surface markers by resuspending 1× 105 cells in 100 μL 1× PBS containing 5 μL antibody, and 5 μL isotype control was used as control (mouse IgG1 K isotype control FITC). Flow cytometry analysis was performed on a FACSaria (BD Biosciences) instrument.
2.4. Experimental groups
Mature DCs were collected and separated into the following 4 groups: (1) NPC patients (mixed cells + FSTL1), (2) control group (NPC patient mixed cells), (3) healthy control sample 1 (mixed cells + FSTL1), and (4) healthy control samples 2 (mixed cells). Mixed cells refer to mature DCs and lymphocytes. FSTL1 (R&D Systems Inc., Minneapolis, MN) was diluted to a final concentration was 40 ng/mL. After 24 h, the DCs were washed twice with PBS and then stimulated with a mixture of EBV (LMP2) (JPT Peptide Technologies, Berlin, Germany) and a peptide fragment with EBV antigenic characteristics and cocultured with corresponding lymphocytes from the same NPC patient or healthy donor.
2.5. DC and lymphocyte coculture
The ratio of 2 types of cells was approximately 1:8, and during coculture, recombinant human interleukin 7 (rhIL‐7) (American PeproTech Inc.) was added to a final concentration of 10 mg/mL. Half of the medium was changed every 3 days, and 20 mg/mL IL‐7 was added to all of the groups. On the seventh day, new medium containing IL‐7 (20 mg/mL) and IL‐2 (American PeproTech Inc.) (40 U/mL) was added to all of the groups, which were cocultured in a 5% CO2 incubator at 37°C. Finally, all of the cells were harvested on the 15th day.
2.6. Detection of IFN‐γ production and TCR expression
After 24 h, approximately 1 × 105 cells from the DC/lymphocyte mixture (mixed cells) exposed to peptides were separated, washed twice with 1 × PBS, and transferred into an ELISpot plate, which was coated with antihuman IFN‐γ antibody (Human INF‐γ ELISpot kit, Mabtech Company, Nacka Strand, Sweden). The plate was then incubated overnight at 37°C at 5% CO2. The positive control mAb CD3‐2 was diluted 1:1000 in RPMI‐1640 with 10% FBS, and 100 μL was added to the positive control wells. The negative control was ELISA/ELISPOT Coating Buffer, which was in the kit. Next, 100 μL of the chromogenic agent 5‐bromo‐4‐chloro‐3‐indolyl phosphate/nitrotetrazolium blue chloride BCIP/NBT‐plus was added to each well and incubated at room temperature protected from light. Finally, the plate was observed every 10 minutes until spots appeared in the positive control wells. All of the steps were performed in accordance with the operation manual. IFN‐γ spots per well were counted using an ELR02 ELISpot reader and analyzed using the ELISpot Reader v4.0 software (Autoimmune Diagnostic, Strassberg, Germany). Approximately 1 × 105 cells from the mixed cells of each group were counted for the analysis of TCR expression. The cells were dyed with the major histocompatibility complex (MHC)–peptide complex tetramer (R‐PE A0201/LMP2356 Tetramer, Beijing Kuangbo Biological Technology Limited), which had been marked with a PE fluorescent dye. Positive controls were stained with antihuman CD8a‐FITC antibody (American eBioscience company).
2.7. Reverse transcriptase polymerase chain reaction detection of IFN‐γ and critical proteins in the TLR4 pathway
Purified genomic DNA was isolated from DCs using the CellAmp™ Whole Transcriptome Amplification kit (Real Time) Ver2 (Code No. 3734, TaKara). Relative quantitative reverse transcriptase polymerase chain reaction (RT‐PCR) was performed to detect the expression of NF‐κb, JNK, IFN‐γ, and GAPDH with SYBR Green PCR kit (Takara, Dalian, China) using the StepOnePlus™ Real‐Time PCR System (Life Technoligies, Carlsbad, NM, USA). The specific RT‐PCR primers are shown in Table 1. The following PCR amplification protocol was used: (1) an initial denaturation at 95°C for 30 s, (2) 40 cycles of 95°C for 5 s and 60°C for 34 s, and (3) a final extension at 95°C for 15 s, 60°C for 1 min, and 95°C for 15 min. The expression changes of genes were calculated using the △Ct method with GAPDH as internal control.
Table 1.
RT‐PCR primers to amplify the desired genes
| Genes | Primer ID | Primer sequences | Product size (bp) |
|---|---|---|---|
| NF‐κb | HA078982 | R:5′‐TCGCACCCAGAATTGTCAAAGATA‐3′ | 139 |
| F:5′‐ACGAATGACAGAGGCGTGTATAAGG‐3′ | |||
| JNK | HA220173 | R:5′‐CAGAGCTGCTTGGCGGATTAG‐3′ | 114 |
| F:5′‐TGTGTGGAATCAAGCACCTTCA‐3′ | |||
| INF‐γ | HA004918 | R:5′‐TGGCCAGACCGAAGTCAAGA‐3′ | 189 |
| F:5′‐CTTTAAAGATGACCAGAGCATCCAA‐3′ | |||
| GAPDH | HA067812 | R:5′‐GGCGACAGTTCAGCCATCAC‐3′ | 138 |
| F:5′‐GCACCGTCAAGGCTGAGAAC‐3′ | |||
| R:5′‐TGGTGAAGACGCCAGTGGA‐3′ |
2.8. NF‐κb and JNK detection by Western blot
Total proteins were extracted from cells from each group. Western blot was used to detect the expression of NF‐κb (NF‐κb1 p105/p50(D7H5M) rabbit mAb, CST), JNK (SAPK/JNK antibody, CST), and GAPDH (GAPDH(14C10) rabbit mAb, CST). Primary antibodies were diluted with Western blot primary antibody diluent to 1:750, and the secondary antibody (antirabbit IgG (H + L) (DyLight 680 Conjugate), CST) was diluted in 1 × TBST (Tris‐buffered saline with Tween‐20, pH 8.0) 1:8,000.
2.9. Data analysis
The data were analyzed using SPSS17.0 and GraphPad Prism v. 5 software. Data are shown as the mean ± SEM of at least 3 independent experiments. Significant differences were determined using 2‐way analysis of variance and 2‐tailed t‐tests. A P value <.05 was considered statistically significant.
3. RESULTS
3.1. Induction and cultivation of DCs
We measured the expression of CD40, CD86, and HLA‐DR on the surface of DCs by flow cytometry. On the third day of culture, the respective expression levels were 20.3%, 32.6%, and 30.0%, which increased to 54.3%, 94.8%, and 67.8% on the eighth day of culture (Figure 1). These results suggested that the DCs matured during the induction and cultivation period.
Figure 1.
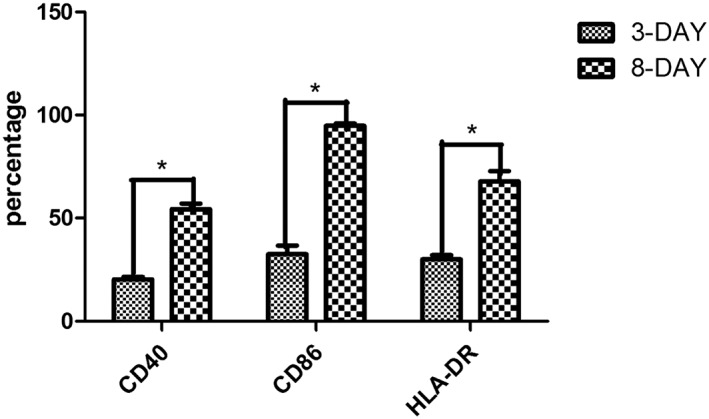
On the third day of culture, the DCs were small and exhibited anchorage‐dependent growth and slightly altered morphology. On the eighth day of culture, the DC suspension significantly increased, and the cells became large and irregular with branching morphology. CD40, CD86, and HLA‐DR expression levels on the DC surface were higher on day 8 than day 3. *P < .05
3.2. The number of T lymphocytes producing IFN‐γ increased with culture
The ELISpot assay was performed to quantify the number of T lymphocytes producing INF‐γ (termed spots). The results showed that the average number of spots for the NPC patient group was 51.00 (31.00–118.00), whereas the control group exhibited only 40.00 spots (11.00–93.00, P < .05) (Figure 2A). "At the same time, as shown in Figure 2B, the mean spot number in the mixed cells + FSTL1 healthy control sample 1 group was 72.00 (41.00–104.00), whereas the healthy control sample 2 group showed 59.50 spots (34.33–88.67, P < .05). However, the NPC patient group and healthy control sample 1 values (Figure 2C) were not significantly different (P > .05). IFN‐γ gene expression was also investigated using RT‐PCR (Figures 2D and 2E). Comparison of the NPC patient group with the control group revealed an RQ expression quantity ratio of 2.49:1 (P < .05). These results demonstrate a significant increase in IFN‐γ expression after FSTL1 addition to the culture media of mixed cells.
Figure 2.
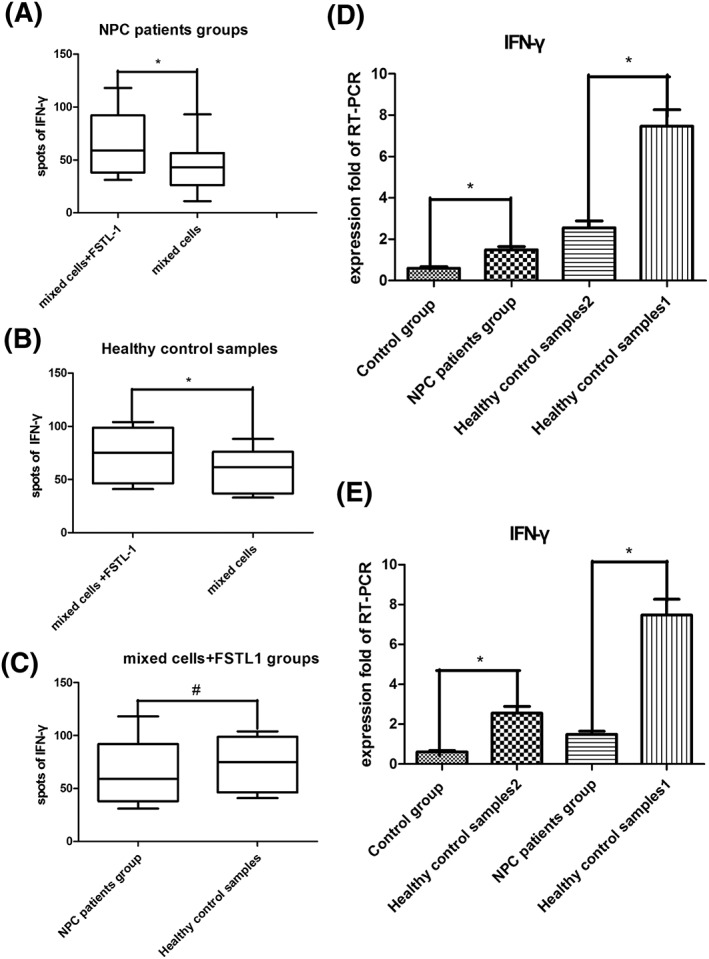
A, The IFN‐γ spots for the different groups. There were more spots in the NPC patient group than in the control group. B, IFN‐γ levels increased after FSTL1 addition to the healthy control sample 1 group. C, No significant differences were observed between the NPC patients and healthy control sample 1 after FSTL1 addition. D and E, IFN‐γ expression was measured by RT‐PCR. The fold RQ of the NPC patient group was 2.49, whereas the RQ of the control group was set to 1. *P < .05 and # P > .05
3.3. The number of T lymphocytes expressing TCRs increased
Tetramers were used to detect TCR expression on CD8 + T cells. The increase in TCR expression in the NPC patient group was larger than in the control group; the same results were observed for the control groups (Figure 3). Interestingly, these results are in accordance with the detection of INF‐γ spots.
Figure 3.
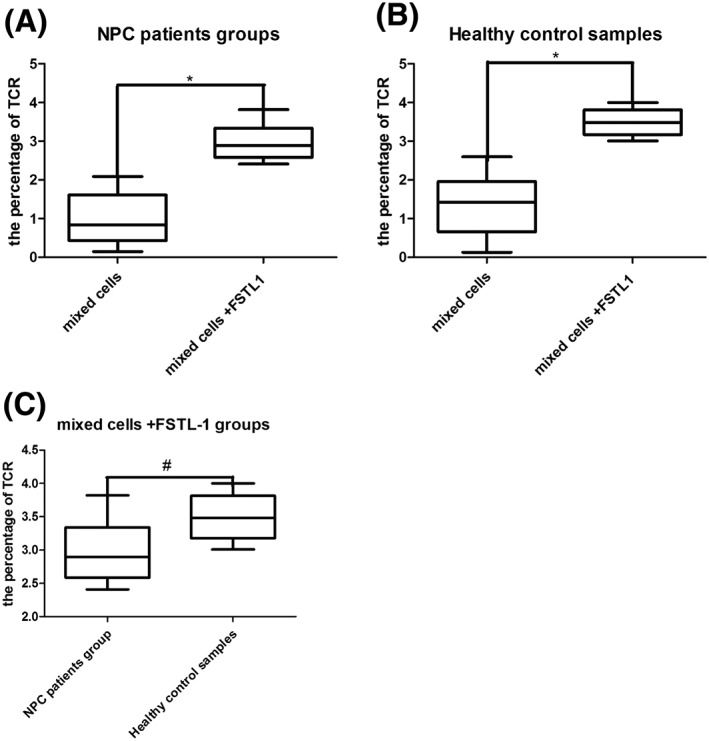
A, The median TCR expression level in the NPC patient group was 2.99% (2.05%–3.82%) compared with 0.99% in the control group (0.33%–2.09%, P < .05). B, Among healthy control samples, these percentages were 3.49% (3.01%–4.00%) and 1.36% (0.13%–1.75%, P < .05) for group 1 and group 2, respectively.3 Comparison of the NPC patient and healthy control groups showed no statistically significant differences (P > .05). *P < .05 and # P > .05
3.4. RT‐PCR and Western blot for NF‐κb and JNK
The gene and protein expression levels of NF‐κb and JNK were measured using RT‐PCR and Western blot, respectively. NF‐κb and JNK expression showed clear changes (P < .05); NF‐κb expression was upregulated, whereas JNK expression was downregulated after treatment with FSTL1. There was also an obvious increase in NF‐κb expression and a significant decrease in JNK expression in the NPC patient group (P < .05). Interestingly, the Western blot results showed the same trends as the RT‐PCR results. These data suggest that NF‐κb mRNA and protein expression were upregulated by FSTL1, whereas JNK expression was downregulated (Figure 4).
Figure 4.
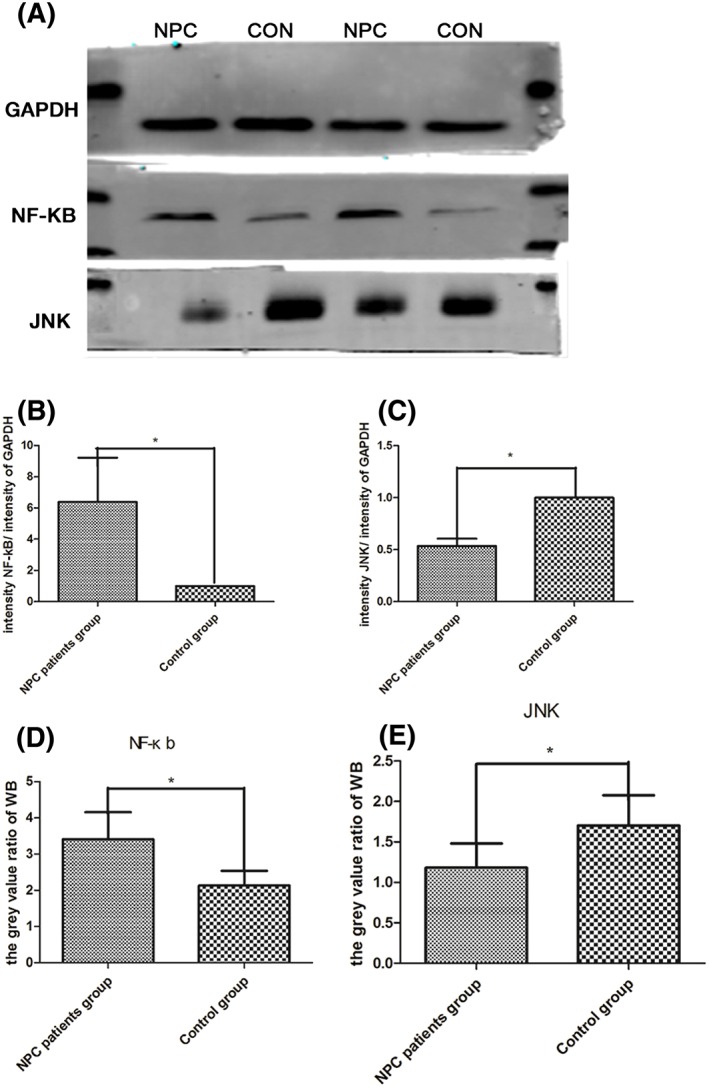
A, Western blot was performed with antibodies against GAPDH, NF‐κb, and JNK. B and C, On the basis of the RQ values from the NPC patient and the control group, NF‐κb levels increased to 6.38:1 (P < .05), and JNK mRNA expression decreased to 0.53:1 (P < .05). B and D, NF‐κb gene and protein expression levels were upregulated in the NPC patient group. The RQ relative value was 6.38, and the gray value ratio was 5.74, whereas the RQ relative value and the gray value ratio for the control group were 1 and 3.47, respectively.4 JNK expression was downregulated. C and E, The RQ expression value was 0.53:1 (P = .00), and the gray value ratio for the NPC patient group was 1.53, whereas the RQ relative value and the gray value ratio for the control group were 1 and 2.25, respectively. *P < .05
4. DISCUSSION
Here, we report that the activation of DCs and T lymphocytes is weaker in NPC patients compared with healthy individuals (Figure 2E left 2 bars) based on the lower IFN‐γ and TCR expression observed in the NPC patient groups, which means that healthy donors (non‐NPC patients) responded better to the LMP and EBV peptides. Previous work has shown that DCs from patients show decreased functionality compared with those from healthy donors, although they are not different in number or morphology.22 Angiogenic factors released by head and neck cancer (HNSCC) patients can induce tolerogenic ability in DCs.23 Upon activation in response to an inflammatory stimulus (exogenous or endogenous), migratory and lymphoid tissue‐resident cDCs display a decrease in phagocytic activity.24 Carcinoma tissue can drive iDCs to differentiate into endothelial‐like cells instead of differentiation into mature DCs, thereby preventing antigen presentation functions.25 Therefore, there are 2 hypotheses regarding the mechanism by which cancer cells avoid immune attack and establish immune evasion. First, during excrescence generation, carcinoma cells alter the microenvironment by modifying their surface antigens.26, 27 Second, the antigen presentation function of DCs is suppressed, which prevents the activation of T lymphocytes and suppresses the body's immunity. Thus, it is important to understand the antitumor immune mechanisms of DCs and T lymphocytes.28, 29 FSTL1 has been acknowledged as a novel inflammatory protein that enhances the composition of inflammatory factors in vivo and in vitro.3 However, experiments assessing the effects of FSTL1 on DCs from NPC patients are limited. Only 1 report showed that FSTL1 was associated with immunomodulation after heart allograft transplantation.30 Recent studies have demonstrated that mesenchymal cells secrete cytokines to regulate immune cell activation, which has both pro‐ and anti‐inflammatory effects.31, 32, 33 Our results demonstrated the up‐regulation of IFN‐γ secretion and TCR expression on T lymphocytes, indicating that DC immunity was enhanced.
Indeed, DCs communicate with T lymphocytes through the presentation of antigens, the expression of surface molecules, and the secretion of immune‐enhancing cytokines. Our results, on the basis of ELISpot assays, RT‐PCR and flow cytometry (Figures 2 and 3), show the effects of FSTL1 on antigen‐antibody recognition and IFN‐γ production. INF‐γ inhibits proliferation in malignant cells, regulates the body's immune response and activates CD8+ T lymphocytes to destroy circulating tumor cells.34, 35, 36 The tetramer detection method uses a single MHC‐peptide tetramer molecule to reveal TCR expression on the surface of cytotoxic T lymphocytes, resulting in their rapid detection by flow cytometry.37 Our tetramer results demonstrated that FSTL1 enhanced the antigen‐presenting ability of DCs, which agreed with the ELISpot test results. These results support the concept that FSTL1 enhances DC immunity. TCRs are specific receptors expressed on the T‐cell surface that bind protein antigens. The homeostasis of naive T cells is maintained by TCR signals from endogenous self‐peptides/MHC complexes and cytokines.38 The suppression of immunological functions in NPC patients might occur because of downregulated TCR expression.39 When tumor antigens stimulate the immune system, the TCR structure changes, and the effectiveness of this strategy is hampered by the generation of mixed TCR heterodimers containing both exogenous and endogenous TCR chains.40 Introduced TCRα and β chains can potentially assemble with endogenous TCR chains, which not only reduces expression of the desired TCR pair but can also create a new TCR with unknown specificity that can potentially lead to autoimmunity and off‐target toxicity.41, 42 These mechanisms most likely occur in NPC patients, leading to a low recognition rate of functional TCRs, but this still requires further study.
We found that NF‐κb expression was increased after FSTL1 addition (Figures 4B and 4D). In T lymphocytes, NF‐κb not only stimulated gene transcription but also promoted lymphocyte development and differentiation. The activation of NF‐κb signaling pathways inhibits the development of carcinomas, especially via the p65 subunit, which plays an important role in p53‐mediated apoptosis. Moreover, NF‐κb up‐regulation enhances T‐cell immunity.43 MFE can increase NK cell and cytotoxic T‐lymphocyte activity by activating NF‐κb to generate IFN‐γ.44 Medicines can also suppress NF‐κb to inhibit the immune response, which leads to carcinoma cell escape.45 Our results indicated that the addition of FSTL1 downregulated JNK expression, thereby enhancing immune activity (Figures 4C and 4E). JNK also plays a critical role in immunity. For example, the absence of JNK was shown to increased T‐lymphocyte cell death.46 Simultaneously, ΔPK could activate JNK protein activity to suppress the expression of IL‐10, which is an immunosuppressive factor.47 On the basis of these results, we believe that FSTL1 plays a critical role in immune regulation by increasing the antigen presentation ability of DCs and T lymphocytes through altering NF‐κb and JNK expression.
Recently, antitumor immune therapies have shown significant advantages because of their reduced toxicity to mammalian cells.48, 49, 50 With the increased development of molecular and cellular technologies, new approaches to treat malignancy will be identified. FSTL1 can inhibit the migration and proliferation of cancer cells and promote apoptosis, which is beneficial for suppressing carcinoma development.4, 5, 51 FSTL1 can simultaneously regulate immune responses and act as an anti‐inflammatory and antineoplastic factor. As a secreted protein, FSTL1 also has the potential to be exploited as a novel antitumor therapeutic. However, some studies have suggested that FSTL1 might stimulate the development of radial gliomas52 and promote arthritis by regulating IFN‐γ1.3 By contrast, other studies have shown that FSTL1 is a pro‐inflammatory molecule that can accelerate the production of other proinflammatory molecules such as IL‐6, IL‐1β, and TNF‐α.10, 53 This investigation found that DC immunity in NPC patients was augmented by FSTL1. Similarly, TNF‐α has been shown to promote the development of immature DCs into mature DCs.54, 55 Therefore, the mechanism by which FSTL1 expedites the immune ability of DCs should be elucidated from this perspective. Above all, it is important to investigate the mechanism by which FSTL1 affects DCs to provide a basis for new antitumor immunotherapies.
ACKNOWLEDGEMENTS
This work was funded by the Nature Science Foundation of Guangxi (grant no. [2015]139). The authors thank Anzhou Tang for help with the manuscript, the First Affiliated Hospital of Guangxi Medical University (FAHGMU) Flow Cytometry Core for flow cytometry analysis, the staff at the ENT Outpatient Clinic of FAHGMU for their assistance with specimen collection, and the teachers and students in the experimental center of Guangxi Medical University for their comments.
Wang, H. , Wu, S. , Huang, S. , Yin, S. , Zou, G. , Huang, K. , Zhang, Z. , Tang, A. , and Wen, W. (2016) Follistatin‐like protein 1 contributes to dendritic cell and T‐lymphocyte activation in nasopharyngeal carcinoma patients by altering nuclear factor κb and Jun N‐terminal kinase expression, Cell Biochem Funct, 34, 554–562. doi: 10.1002/cbf.3227.
Contributor Information
Anzhou Tang, Email: anzhoutang@126.com.
Wensheng Wen, Email: wenwensheng2000@126.com.
REFERENCES
- 1. Li L, Tian W, Wang W, et al. NKG2C copy number variations in five distinct populations in mainland China and susceptibility to nasopharyngeal carcinoma (NPC). Hum Immunol. 2015;76(2‐3):90–94. [DOI] [PubMed] [Google Scholar]
- 2. Shen Y, Zhang S, Sun R, et al. Understanding the interplay between host immunity and Epstein‐Barr virus in NPC patients. Emerg Microbes Infect. 2015;4(3):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaly Y, Hostager B, Smith S, et al. Follistatin‐like protein 1 and its role in inflammation and inflammatory diseases. Immunol Res. 2014;59(1–3):266–272. [DOI] [PubMed] [Google Scholar]
- 4. Chan QK, Ngan HY, Ip PP, et al. Tumor suppressor effect of follistatin‐like 1 in ovarian and endometrial carcinogenesis: a differential expression and functional analysis. Carcinogenesis. 2009;30(1):114–121. [DOI] [PubMed] [Google Scholar]
- 5. Zhao W, Han HB, Zhang ZQ. Suppression of lung cancer cell invasion and metastasis by connexin43 involves the secretion of follistatin‐like 1 mediated via histone acetylation. Int J Biochem Cell Biol. 2011;43(10):1459–1468. [DOI] [PubMed] [Google Scholar]
- 6. Liu Y, Han X, Yu Y, et al. A genetic polymorphism affects the risk and prognosis of renal cell carcinoma: association with follistatin‐like protein 1 expression. Sci Rep. 2016;6:26689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaly Y, Marinov AD, Oxburgh L, et al. FSTL1 promotes arthritis in mice by enhancing inflammatory cytokine/chemokine expression. Arthritis Rheum. 2012;64(4):1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyamae T, Marinov AD, Sowders D, et al. Follistatin‐like protein‐1 is a novel proinflammatory molecule. J Immunol. 2006;177(7):4758–4762. [DOI] [PubMed] [Google Scholar]
- 9. Ni S, Miao K, Zhou X, et al. The involvement of follistatin‐like protein 1 in osteoarthritis by elevating NF‐kappaB‐mediated inflammatory cytokines and enhancing fibroblast like synoviocyte proliferation. Arthritis Res Ther. 2015;17:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaly Y, Fu Y, Marinov A, et al. Follistatin‐like protein 1 enhances NLRP3 inflammasome‐mediated IL‐1beta secretion from monocytes and macrophages. Eur J Immunol. 2014;44(5):1467–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ren Y, Yang Y, Yang J, et al. Tolerogenic dendritic cells modified by tacrolimus suppress CD4(+) T‐cell proliferation and inhibit collagen‐induced arthritis in mice. Int Immunopharmacol. 2014;21(1):247–254. [DOI] [PubMed] [Google Scholar]
- 12. Neefjes J, Sadaka C. Into the intracellular logistics of cross‐presentation. Front Immunol. 2012;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Milani R, Marcellini A, Montagner G, et al. Phloridzin derivatives inhibiting pro‐inflammatory cytokine expression in human cystic fibrosis IB3‐1 cells. Eur J Pharm Sci. 2015;78:225–233. [DOI] [PubMed] [Google Scholar]
- 14. Wang Z, Ma B, Li H, et al. Protein 4.1N acts as a potential tumor suppressor linking PP1 to JNK‐c‐Jun pathway regulation in NSCLC. Oncotarget. 2016;7(1):509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang HW, Yang W, Lu JY, et al. N‐acetylcysteine administration is associated with reduced activation of NF‐kB and preserves lung dendritic cells function in a zymosan‐induced generalized inflammation model. J Clin Immunol. 2013;33(3):649–660. [DOI] [PubMed] [Google Scholar]
- 16. Clutter SD, Wilson DC, Marinov AD, et al. Follistatin‐like protein 1 promotes arthritis by up‐regulating IFN‐gamma. J Immunol. 2009;182(1):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo KK, Tang QH, Zhang YM, et al. Identification of two internal signal peptide sequences: critical for classical swine fever virus non‐structural protein 2 to trans‐localize to the endoplasmic reticulum. Virol J , 2011. 8:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis. 2009;14(8):996–1007. [DOI] [PubMed] [Google Scholar]
- 19. Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54(4):795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kubicka‐Sierszen A, Grzegorczyk JL. The influence of infectious factors on dendritic cell apoptosis. Arch Med Sci. 2015;11(5):1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Granucci F, Zanoni I. The dendritic cell life cycle. Cell Cycle. 2009;8(23):3816–3821. [DOI] [PubMed] [Google Scholar]
- 22. Li R, Chen H, Fei R, et al. Functions of cultured dendritic cells from patients with chronic hepatitis Beta decreased. Zhonghua Yi Xue Za Zhi. 2002;82(13):887–890. [PubMed] [Google Scholar]
- 23. Strauss L, Volland D, Kunkel M, et al. Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): possible link between angiogenesis and immune tolerance. Med Sci Monit. 2005;11(8):BR280–BR292. [PubMed] [Google Scholar]
- 24. Henri S, Vremec D, Kamath A, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167(2):741–748. [DOI] [PubMed] [Google Scholar]
- 25. Lu J, Bai R, Qin Z, et al. Differentiation of immature DCs into endothelial‐like cells in human esophageal carcinoma tissue homogenates. Oncol Rep. 2013;30(2):739–744. [DOI] [PubMed] [Google Scholar]
- 26. Liu H, Wu X, Gang N, et al. Macrophage functional phenotype can be consecutively and reversibly shifted to adapt to microenvironmental changes. Int J Clin Exp Med. 2015;8(2):3044–3053. [PMC free article] [PubMed] [Google Scholar]
- 27. Amin A, Mokhdomi TA, Bukhari S, et al. Tectorigenin ablates the inflammation‐induced epithelial‐mesenchymal transition in a co‐culture model of human lung carcinoma. Pharmacol Rep. 2015;67(2):382–387. [DOI] [PubMed] [Google Scholar]
- 28. Lu Y, Gao T, Chen Y, et al. Characteristics change of the human directional highly lymphatic metastasis ovarian carcinoma cell and venous endothelial cell after establishment of their condition cultrue and co‐culture cell system. Zhonghua Fu Chan Ke Za Zhi. 2014;49(7):510–516. [PubMed] [Google Scholar]
- 29. Choi DY, You S, Jung JH, et al. Extracellular vesicles shed from gefitinib‐resistant nonsmall cell lung cancer regulate the tumor microenvironment. Proteomics. 2014;14(16):1845–1856. [DOI] [PubMed] [Google Scholar]
- 30. Le Luduec JB, Condamine T, Louvet C, et al. An immunomodulatory role for follistatin‐like 1 in heart allograft transplantation. Am J Transplant. 2008;8(11):2297–2306. [DOI] [PubMed] [Google Scholar]
- 31. Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–716. [DOI] [PubMed] [Google Scholar]
- 32. Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402. [DOI] [PubMed] [Google Scholar]
- 34. Matsueda S, Shichijo S, Nagata S, et al. Identification of novel Lck‐derived T helper epitope long peptides applicable for HLA‐A2 cancer patients as cancer vaccine. Cancer Sci. 2015;106(11):1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang JJ, Liu YH, Li GC. Induction of protective and therapeutic anti‐cancer immunity by using bispecific anti‐idiotype antibody G22‐I50 for nasopharyngeal carcinoma. Int Immunopharmacol. 2015;28(2):1026–1033. [DOI] [PubMed] [Google Scholar]
- 36. Uruena C, Gomez A, Sandoval T, et al. Multifunctional T lymphocytes generated after therapy with an antitumor gallotanin‐rich normalized fraction are related to primary tumor size reduction in a breast cancer model. Integr Cancer Ther. 2015;14(5):468–483. [DOI] [PubMed] [Google Scholar]
- 37. Tanaka Y, Kanda Y. Development of Tax‐redirected T‐cell immunotherapy using TCR gene transduction in patients with ATL. Rinsho Ketsueki. 2015;56(7):815–824. [DOI] [PubMed] [Google Scholar]
- 38. Kamimura D, Arima Y, Tsuruoka M, et al. Strong TCR‐mediated signals suppress integrated stress responses induced by KDELR1 deficiency in naive T cells. Int Immunol. 2016;28(3):117–126. [DOI] [PubMed] [Google Scholar]
- 39. Laytragoon‐Lewin N, Porwit‐MacDonald A, Mellstedt H, et al. Alteration of cellular mediated cytotoxicity, T cell receptor zeta (TcR zeta) and apoptosis related gene expression in nasopharyngeal carcinoma (NPC) patients: possible clinical relevance. Anticancer Res. 2000;20(2B):1093–1100. [PubMed] [Google Scholar]
- 40. Tao C, Shao H, Yuan Y, et al. Imaging of T‐cell receptor fused to CD3zeta reveals enhanced expression and improved pairing in living cells. Int J Mol Med. 2014;34(3):849–855. [DOI] [PubMed] [Google Scholar]
- 41. Bendle GM, Linnemann C, Hooijkaas AI, et al. Lethal graft‐versus‐host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16(5):565–570. 1p following 570. [DOI] [PubMed] [Google Scholar]
- 42. van Loenen MM, de Boer R, Amir AL, et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc Natl Acad Sci U S A. 2010;107(24):10972–10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Samivel R, Kim DW, Son HR, et al. The role of TRPV1 in the CD4+ T cell‐mediated inflammatory response of allergic rhinitis. Oncotarget. 2016;7(1):148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang BY, Kim SB, Lee MK, et al. Improved chemotherapeutic activity by Morus alba fruits through immune response of toll‐like receptor 4. Int J Mol Sci. 2015;16(10):24139–24158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wei H, Gao HQ, Li HB, et al. Correlation among RKIP expression, NF‐kappaB p65 levels, and T‐lymphocyte subsets in gastric cardia adenocarcinoma. Genet Mol Res. 2015;14(4):16491–16496. [DOI] [PubMed] [Google Scholar]
- 46. Wang YQ, Ma X, Lu L, et al. Defective antiviral CD8 T‐cell response and viral clearance in the absence of c‐Jun N‐terminal kinases. Immunology. 2014;142(4):603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bollino D, Colunga A, Li B, et al. PK oncolytic activity includes modulation of tumor cell milieu. J Gen Virol. 2016;97(2):496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith C, Khanna R. Adoptive therapy for EBV‐induced cancers: driving success with post‐transplant lymphoproliferative disorder to other EBV‐derived tumors. Immunotherapy. 2015;7(5):563–572. [DOI] [PubMed] [Google Scholar]
- 49. Li J, Qian CN, Zeng YX. Regulatory T cells and EBV associated malignancies. Int Immunopharmacol. 2009;9(5):590–592. [DOI] [PubMed] [Google Scholar]
- 50. Perez CA, Santos ES, Raez LE. Active immunotherapy for non‐small‐cell lung cancer: moving toward a reality. Expert Rev Anticancer Ther. 2011;11(10):1599–1605. [DOI] [PubMed] [Google Scholar]
- 51. Chen SX, Xu XE, Wang XQ, et al. Identification of colonic fibroblast secretomes reveals secretory factors regulating colon cancer cell proliferation. J Proteomics. 2014;110:155–171. [DOI] [PubMed] [Google Scholar]
- 52. Liang X, Hu Q, Li B, et al. Follistatin‐like 1 attenuates apoptosis via disco‐interacting protein 2 homolog A/Akt pathway after middle cerebral artery occlusion in rats. Stroke. 2014;45(10):3048–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fan N, Sun H, Wang Y, et al. Follistatin‐like 1: a potential mediator of inflammation in obesity. Mediators Inflamm. 2013;2013:752519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mobergslien A, Vasovic V, Mathiesen G, et al. Recombinant Lactobacillus plantarum induces immune responses to cancer testis antigen NY‐ESO‐1 and maturation of dendritic cells. Hum Vaccin Immunother. 2015;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Daneshmandi S, Dibazar SP, Fateh S. Effects of 3‐dimensional culture conditions (collagen‐chitosan nano‐scaffolds) on maturation of dendritic cells and their capacity to interact with T‐lymphocytes. J Immunotoxicol. 2015;1–8. [DOI] [PubMed] [Google Scholar]


