Abstract
Aims
LY2963016 (LY IGlar) and Lantus (IGlar) are insulin glargine products manufactured by distinct processes, but with identical amino acid sequences. This study compared the duration of action of LY IGlar and IGlar in subjects with type 1 diabetes mellitus (T1DM).
Materials and methods
This was a randomized, double‐blind, single‐dose, two‐period, crossover study. Twenty subjects underwent 42‐hour euglycaemic clamps after a single subcutaneous 0.3‐U/kg dose of LY IGlar or IGlar. In this study, the duration of action was defined as the time required for blood glucose levels to rise consistently above a predefined cut‐off of 8.3 mmol/L (150 mg/dL) from a state of euglycaemia. Blood samples were collected to measure blood glucose for pharmacodynamic (PD) evaluations.
Results
End of action was reached within 42 hours in 26 of 40 clamps (13 LY IGlar and 13 IGlar). The median duration of action for all subjects was 37.1 and 40.0 hours, and the mean duration of action (calculated using only patients who reached end of action) was 23.8 and 25.5 hours for LY IGlar and IGlar, respectively. The duration of action was demonstrated to be similar between the treatments using time‐to‐event analysis (log‐rank test of equality p = .859). Following administration of LY IGlar and IGlar, the PD parameters of maximum glucose infusion rate (R max) and total glucose infusion during the clamp (G tot) were comparable.
Conclusion
LY IGlar and IGlar had similar duration of action and comparable PD parameters in subjects with T1DM.
Keywords: basal insulin, biosimilar insulin, clinical trial, pharmacodynamics, phase I–II study, type 1 diabetes
1. INTRODUCTION
LY2963016 insulin glargine (LY IGlar) is a long‐acting human insulin analog manufactured by Eli Lilly and Company with an amino acid sequence identical to that of Lantus insulin glargine (a registered trademark of Sanofi; hereafter IGlar). IGlar is indicated for the treatment of type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) in adult and paediatric patients.1 LY IGlar is currently approved for use in the European Union (EU) under the name Abasaglar and the United States (USA) under the name Basaglar for treatment of diabetes mellitus as a subcutaneous (SC) injection in adults, adolescents and children aged 2 years and above.2
LY IGlar was developed in accordance with biosimilar guidelines established by the European Medicines Agency and the US Food and Drug Administration.3, 4, 5 These guidelines recommend a pharmacokinetic (PK) and pharmacodynamic (PD) comparison of a new insulin analog with a reference insulin in glucose clamp studies. We have previously demonstrated the equivalence of PK and PD parameters between LY IGlar and IGlar in euglycaemic glucose clamp studies in healthy subjects.6 However, it is difficult to determine accurately the duration of action of exogenous insulin in healthy subjects because of the presence of endogenous insulin and the induction of hormonal responses during prolonged fasting. This study was undertaken with the primary objective of comparing the duration of action of IGlar and LY IGlar in subjects with T1DM.
2. MATERIALS AND METHODS
2.1. Study subjects
The study protocol was approved by an ethical review board (Ärztekammer Nordrhein, Düsseldorf, Germany) and was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. All subjects provided written informed consent prior to participating.
Male or female subjects ≥18 and ≤60 years of age with T1DM were eligible for the study. Subjects were required to have had diabetes for ≥1 year, a body mass index ≤29 kg/m2, haemoglobin A1c (HbA1c) ≤86 mmol/mol (≤10.0%) and fasting C‐peptide ≤ 0.3 nmol/L. A summary of subject demographics and prior basal insulin therapy is provided in Table 1.
Table 1.
Subject demographics and baseline diabetes characteristics
| Parameter | Mean ± SD (N = 20) |
|---|---|
| Age (years) | 41.5 ± 9.1 |
| Sex male (%) | 100 |
| Race white (%) | 100 |
| Weight (kg) | 84.1 ± 9.8 |
| BMI (kg/m2) | 25.6 ± 2.4 |
| HbA1c (mmol/mol) | 64 ± 6.8 |
| HbA1c (%) | 7.99 ± 0.62 |
| Duration of diabetes (years) | 18.9 ± 9.8 |
| Baseline basal insulin (U/kg/day) | 0.45 ± 0.24 |
| Baseline total insulin (U/kg/day) | 1.56 ± 0.45 1 |
| Prior basal insulin therapy (N [%]) | |
| Insulin glargine | 6 (30) |
| NPH insulin | 4 (20) |
| Insulin detemir | 4 (20) |
| Insulin pump (basal/bolus) | 6 (30) |
BMI, body mass index; HbA1c, haemoglobin A1c; N, number of subjects; NPH, neutral protamine Hagedorn; SD, standard deviation.
N = 16; does not include four subjects using an insulin pump for whom exact prandial insulin requirements were not recorded.
2.2. Study drugs
LY IGlar and IGlar were each supplied as a 100‐U/mL solution in a cartridge and each product was from a single lot. A needle and syringe were used for the injections.
2.3. Study design
This was a single‐site, randomized, investigator‐ and subject‐blind, single‐dose, 2‐period, crossover, 42‐hour euglycaemic clamp study in subjects with T1DM (NCT01600950). When subjects were admitted to the study site, they were randomized to receive either 0.3 U/kg LY IGlar or 0.3 U/kg IGlar, followed by the other study drug in Period 2. Study personnel who performed the injections were unaware of the drug allocation. The use of standard insulin syringes ensured that there was no visual difference with regards to volume or appearance between the insulin products. The study design is illustrated in Figure 1.
Figure 1.
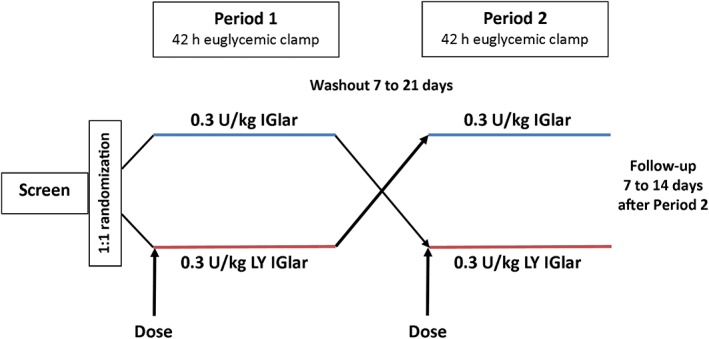
Schematic of study design. After screening to ensure that all study criteria were met, subjects were randomized to receive a single dose of 0.3 U/kg IGlar or 0.3 U/kg LY IGlar on day 1 of period 1, followed by a 42‐hour euglycaemic clamp procedure. Following a washout period of 7‐21 days, subjects received a single dose of the other insulin glargine on day 1 of period 2, followed by a 42‐hour euglycaemic clamp procedure. Subjects returned for a follow‐up visit 7‐14 days after the second clamp procedure.
For each subject, the study consisted of a screening visit, two treatment periods separated by a washout period of seven to 21 days, and a follow‐up visit 7‐14 days after the last treatment. Subjects received a single dose of LY IGlar or IGlar (0.3 U/kg) by SC injection following an overnight fast of at least 8 hours. All insulin injections were given subcutaneously by means of a conventional insulin syringe. The needle was inserted at approximately 90° into a raised skinfold. The injection site was alternated between the lower two quadrants of the abdominal wall, approximately 10 cm from the umbilicus. Doses were administered at approximately the same time each day.
Subjects continued their existing diabetes treatments until 48 hours prior to each study drug administration; subjects who were on pre‐existing insulin detemir or insulin glargine therapy were then transitioned to Neutral Protein Hagedorn (NPH) insulin. The last dose of NPH insulin was administered no later than 22 hours prior to the study drug. Short‐ or rapid‐acting insulin was not permitted to be used later than 9 hours prior to study drug administration. Subjects who were on continuous SC insulin infusion stopped their basal rate not later than 4 hours prior to insulin glargine administration.
The run‐in period started approximately 1‐6 hour(s) before administration of the study drug (LY IGlar or IGlar). A variable intravenous infusion of insulin lispro or glucose was initiated to obtain a target blood glucose level of 5.6 mmol/L (100 mg/dL). This level [±20% (4.5‐6.7 mmol/L or 80‐120 mg/dL]) was maintained continuously for at least 1 hour before study drug administration.
2.4. Euglycaemic clamp procedures
After study drug administration, insulin lispro infusion (if any) was continued at a constant rate using the immediate postdose rate. The insulin lispro infusion was completely terminated when an effect from the study drug (insulin glargine), indicated by a blood glucose drop of approximately 0.3 mmol/L (5 mg/dL) compared to the individual blood glucose level (defined as a mean of three blood glucose measurements at −10, −5 and −2 minutes), was observed.
Subjects were connected to a Biostator (MTB Medizintechnik, Amstetten, Germany) beginning 1‐6 hour(s) prior to administration of the study drug. A catheter was placed in a forearm or hand vein and connected to the glucose sensor of the Biostator. A second catheter was placed into the same arm for obtaining blood samples for analysis of insulin and blood glucose. The hand of the subject was heated with a warming device to maintain a temperature of approximately 55°C throughout the clamp procedure, resulting in an arterialization of the venous blood due to a reflexive opening of arteriovenous shunts. A catheter was placed in a vein of the contralateral forearm for infusion of insulin lispro (if needed during the run‐in period), 20% glucose and saline.
The Biostator was then programmed to maintain a blood glucose level of 5.6 mmol/L (100 mg/dL) by a variable rate of glucose infusion, and was recalibrated at least every 30 minutes using a Super GL glucose analyser (Hitado Diagnostic Systems, Möhnesee, Germany). The clamp continued until 42 hours after study drug administration or until end of action was reached. The duration of action was defined as the period from dosing until the end of action [i.e. the time at which the blood glucose level was consistently >8.3 mmol/L (150 mg/dL) without any glucose infusion for five consecutive Biostator blood glucose readings with at least one confirmatory measurement using the glucose analyser]. To assess clamp quality, both the coefficient of variation of blood glucose values and the mean difference of actual blood glucose values compared to the target level (5.6 mmol/L) were calculated as described previously7 between the time of 10% and 90% of Gtot. At the end of the clamp procedure, vital signs and electrocardiograms (ECGs) were checked and the subject received a meal. The subject's blood glucose levels were monitored and a medical assessment was conducted prior to discharge from the clinical research unit.
2.5. Pharmacokinetics and bioanalytical methods
Venous blood collection for the determination of serum concentrations of IGlar and LY IGlar was initiated prior to and continued up to 42 hours after the administration of each insulin glargine. Serum samples were analysed for immunoreactive insulin glargine using a validated, competitive radioimmunoassay method at Covance Laboratories, Inc. (Chantilly, VA, USA) as described previously.6 The lower limit of quantification was 50 pM and the upper limit was 2000 pM. The radioimmunoassay showed similar precision and accuracy in the measurement of immunoreactive insulin glargine against a standard curve prepared using Lantus® (insulin glargine) as it did in the measurement of immunoreactive insulin glargine against a standard curve prepared using LY2963016. Serum samples were also analysed for insulin lispro using a validated radioimmunoassay with a lower limit of quantification of 0.200 ng/mL and an upper limit of 15.000 ng/mL (Covance Laboratories, Inc.).
2.6. Pharmacodynamic analysis
The time profiles of glucose infusion rate (GIR) and blood glucose concentration were recorded during each clamp for each individual following administration of LY IGlar or IGlar. A locally weighted scatterplot smoothing function (the smoothing function ranged from 0.075‐0.2) was applied to all individual GIR‐time profiles in each treatment group using TIBCO Spotfire S+ software (Version 8.2, Insightful Corp., Seattle, WA, USA). The fitted GIR‐time profiles were used to calculate total glucose infusion during the clamp (Gtot), maximum GIR (Rmax), time of Rmax (TRmax), time to 50% maximal GIR before TRmax (early TRmax50%), and time to 50% maximal GIR after TRmax (late TRmax50%).
2.7. Pharmacodynamic statistical analysis
A time‐to‐event (survival) statistical analysis was conducted, allowing for censored observations. The duration of action was censored (i.e. not recorded) when a subject did not reach end of action before 42 hours. Each end of action was considered an “event”. The survival curves were compared using the log‐rank test of equality. In addition, the Cox proportional hazards regression, which included treatment and period as covariates, was fitted to the data. The Cox proportional hazards model8 was used to estimate the hazard ratio; the corresponding 95% confidence intervals (CIs) and p‐value (based on Wald test) were reported.
The PD parameters Gtot and Rmax were log‐transformed and analysed using a linear mixed‐effects model in which treatment, period and sequence were considered fixed effects and subject was a random effect. The difference in least squares (LS) mean estimates and the corresponding 90% CIs for the difference between treatments were estimated and back‐transformed from the log scale to provide estimates of the ratios of the geometric means and 90% CI for the ratio of these means. Other PD time parameters characterizing the time profile for GIR were evaluated using the same mixed‐effects model but without transformation. The differences in means between the treatments and the associated 95% CIs for the differences were reported.
2.8. Safety assessments
Safety assessments included physical examinations, clinical laboratory evaluations, and evaluations of vital signs, ECGs and adverse events (AEs).
3. RESULTS
3.1. Demographics and baseline characteristics
Twenty male subjects with T1DM, aged 23‐54 years, participated in and completed the study. Subject demographics, baseline diabetic characteristics and prior basal and total daily insulin dose are presented in Table 1.
3.2. Pharmacodynamics
Following single SC injections, the mean GIR profiles (Figure 2A) and blood glucose levels (Figure 2B) were comparable between LY IGlar and IGlar over the 42‐hour clamp period. The median duration of action of LY IGlar and IGlar was 37.1 and 40.0 hours, respectively. The mean duration of action, calculated using only subjects that reached the end of action during the 42‐hour clamp period, was 23.8 and 25.5 hours for LY IGlar and IGlar, respectively. Summary statistics for duration of action, defined as the period from dosing until end of action [i.e. the time at which the blood glucose level was consistently >8.3 mmol/L (150 mg/dL) without any glucose infusion] are presented in Table 2. The end of action was reached before 42 hours in 26 of 40 clamps (65%), equally distributed between subjects receiving IGlar or LY IGlar. A time‐to‐event analysis was conducted to compare the duration of action of the study drugs. The survival curves for LY IGlar and IGlar were similar over the 42‐hour clamp interval (log‐rank test of equality p = .859, Figure 3) and the Cox proportional hazards ratio (LY IGlar/IGlar) was 1.063 (p = .8777). For the PD parameters Gtot and Rmax, the 90% CIs for the ratios of geometric LS means (LY IGlar/IGlar) overlapped 1, being 0.46‐1.30 and 0.52‐1.61 for Gtot and Rmax, respectively. The additional PD parameters TRmax, early TRmax50% and late TRmax50% had 95% CIs for the difference in LS means that included 0 (Table S1).
Figure 2.
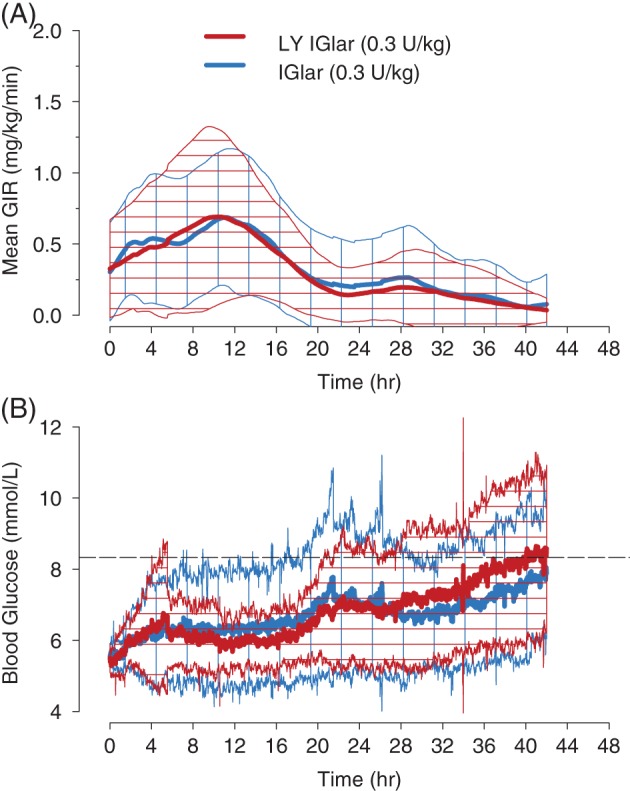
Mean glucose infusion rate and blood glucose versus time profile. Subjects underwent a euglycaemic clamp procedure with the glucose infusion rate A, and blood glucose levels B, monitored for up to a 42‐hour period. The mean (bold lines) and standard deviation (cross hatched area) following administration of 0.3 U/kg LY IGlar (red) or IGlar (blue) are shown. The end of action was defined as the time at which the subject's blood glucose was consistently >8.3 mmol/L (150 mg/dL) without glucose infusion (dashed line in bottom graph). GIR, glucose infusion rate.
Table 2.
Summary statistics for duration of action of LY IGlar and IGlar
| IGlar (0.3 U/kg) | LY IGlar (0.3 U/kg) | |
|---|---|---|
| (N = 20) | (N = 20) | |
| Number (%) reaching end of action | 13 (65.0%) | 13 (65.0%) |
| Number (%) censored 1 | 7 (35.0%) | 7 (35.0%) |
| Duration of action (h) | ||
| Range 2 | 2.0‐41.5 | 2.8‐40.5 |
| 25th Percentile 3 (95% CI) | 19.50 (12.23, 39.50) | 19.75 (7.00, 37.00) |
| Median (95% CI) | 40.00 (20.00, NA 4 ) | 37.13 (20.00, NA 4 ) |
| Mean 2 (SE) | 25.54 (3.91) | 23.78 (3.75) |
CI, confidence interval; N, number of subjects; NA, not applicable; SE, standard error.
The duration of action was censored (i.e. not recorded) when a subject did not reach end of action before clamp termination at 42 hours.
The range and mean are based only on those subjects who reached end of action before 42 hours.
The Xth percentile of the duration of action is the time beyond which (100‐X)% of the subjects have not reached the end of action.
Not applicable because of censoring.
Figure 3.
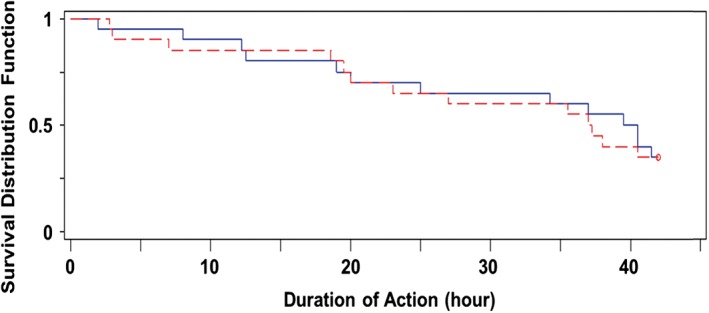
Time‐to‐event (survival) plot of duration of action. Survival curves for 0.3 U/kg LY IGlar (red) and IGlar (blue) showing the proportion of subjects expected to “survive” (i.e. the end of action has not been reached), where end of action was defined as the time at which blood glucose was consistently >8.3 mmol/L (150 mg/dL).
The mean difference in blood glucose from the target value (5.6 mmol/L) and the mean coefficient of variation of blood glucose levels were estimated to assess clamp quality. The mean difference [±standard deviation (SD)] between measured and target blood glucose values was 0.09 ± 0.13 mmol/L (1.6 ± 2.3 mg/dL) and 0.12 ± 0.17 mmol/L (2.2 ± 3.1 mg/dL) for IGlar and LY IGlar, respectively. The mean coefficient of variation (±SD) of blood glucose values was 6.3% ± 1.6% and 6.5% ± 2.0% for IGlar and LY IGlar, respectively.
3.3. Pharmacokinetics
Immunoreactive IGlar and LY IGlar were measured to determine insulin PK during the clamp procedure. Nine subjects had analysable PK data for both treatment periods, but insufficient concentration data were available for the remaining subjects, mainly because serum concentration levels were below the quantifiable lower limit (BQL) of the assay (50 pM) for either one or both treatment periods. Insulin lispro levels were detectable in only 18 of 280 serum samples across both clamp periods.
3.4. Safety and tolerability
There were no notable differences in the incidence of treatment‐emergent AEs, clinical laboratory evaluations, vital signs, or ECGs following administration of LY IGlar compared to IGlar. There was a single hypoglycaemic event [defined as a blood glucose value ≤3.5 mmol/L (63 mg/dL)] that occurred after completion of the clamp procedure, approximately 2 days after the dose of IGlar.
4. DISCUSSION
In the current study, the primary objective was to compare the duration of action of LY IGlar and IGlar using a 42‐hour euglycaemic clamp procedure following a single SC administration of 0.3 U/kg in subjects with T1DM. The duration of action was similar for LY IGlar and IGlar, with a median duration of 37.1 and 40.0 hours and a mean duration of action of 23.8 and 25.5 hours, respectively. The mean values do not include clamps where the end of action was not reached by 42 hours and thus are underestimates of the actual duration of action. Based on the time‐to‐event analysis, it was demonstrated that there was no statistically significant difference in the duration of action of LY IGlar compared to IGlar.
The efficacy and safety of LY IGlar was previously assessed in two randomized, controlled clinical trials in patients with T1DM and T2DM.9, 10 These trials demonstrated equivalent efficacy of LY IGlar to IGlar, as measured by change in HbA1c from baseline. In addition, the insulin glargine products demonstrated similar safety profiles. The clinical safety and efficacy studies,9, 10 in addition to the extensive manufacturing comparisons,11 non‐clinical comparisons,11 and prior PK/PD clinical pharmacology studies,6 were all part of a comprehensive program that established the similarity of LY IGlar and IGlar. These studies were not conducted, however, with the intent to establish the interchangeability of LY IGlar and IGlar. Interchangeability requires a different scientific standard than biosimilarity, including a demonstration of safety for patients who are switched back and forth between the biosimilar and reference products.12 The USA is unlike the EU and most other markets in that a decision regarding interchangeability is made by the US regulatory authority and can serve as a basis for a biosimilar to be substituted for the reference biological product at the pharmacy, and without advance notice or agreement of the prescriber. It remains to be seen whether pharmacy substitution of insulin products will be accepted by patients and providers, many of whom may be justifiably reluctant to permit substitution for a well‐controlled patient or to support any such switch without medical supervision.12
Because the previous LY IGlar PK/PD studies designed to meet bioequivalence criteria were conducted in healthy subjects, it has not been possible to compare the PD duration of action of LY IGlar and that of IGlar because of the presence of endogenous insulin. A higher dose of 0.5 U/kg and a clamp duration limited to 24 hours also contributed to preventing determination of the duration of action.
Regulatory guidelines recommend clamp durations of at least 24 hours for determining the duration of action of intermediate or long‐acting insulins.3 A clamp duration of 42 hours was used in the present study in an effort to allow a higher percentage of subjects to reach the end of action compared to shorter clamp durations. To our knowledge, this is the first euglycaemic clamp study reporting the duration of action for an insulin glargine using a clamp duration as long as 42 hours; although a clamp duration of 42 hours was also used in a study comparing IGlar and insulin degludec in subjects with T1DM, only data to 24 hours was reported.13 While extending clamp duration further would allow for more subjects to reach end of action and potentially eliminate the need for right‐censoring, practical considerations and safety concerns related to the length of fasting and the relatively high blood loss make it problematic to extend glucose clamps beyond 42 hours.
The 0.3‐U/kg dose used here was similar to doses used in the previously reported Phase 3 trial in T1DM patients, in which the mean dose at 52 weeks was 0.36 U/kg/day for IGlar and 0.38 U/kg/day for LY IGlar.9 The dose of 0.3 U/kg was chosen so that the entire duration of action could be captured for the majority of subjects while maintaining a reasonable clamp duration. At the same time, the selected dose should, ideally, be high enough to allow for detection of the study drug by the bioanalytical assay. However, serum insulin levels were BQL (50 pM) following administration of the 0.3‐U/kg dose of insulin glargine in many instances. Because less than half of the subjects had evaluable insulin PK profiles during both treatment periods and because there was considerable variability in the available data, definitive conclusions regarding insulin PK could not be made. The sample preparation method used included extraction of all insulin glargine‐antibody complexes, and thus antidrug antibody interference could not have been a confounding factor in this study. Comparing the PK parameters of IGlar and LY IGlar was a secondary endpoint of the current study; however, the pivotal Phase 1 studies designed to meet bioequivalence criteria were statistically powered to demonstrate that the area under the concentration‐time curve from time 0‐24 hours [AUC (0‐24)], maximum observed drug concentration (Cmax) and time of Cmax (tmax) were similar after doses of 0.5 U/kg IGlar and LY IGlar [6].
A blood glucose cut‐off of 8.3 mmol/L (150 mg/dL) was used for determining the end of action. While there is no universally accepted definition of end of action for an insulin product, the cut‐off of 8.3 mmol/L (150 mg/dL) was chosen for this study as it has been a commonly used definition in IGlar euglycaemic clamp studies in T1DM patients14, 15, 16, 17, 18, 19 and has also been recommended in regulatory guidance.3 The idea of defining the duration of action in euglycaemic clamp studies using blood glucose rather than GIR is based on the concept that the main aim of basal insulin is to control blood glucose under fasting conditions.16 While this approach can be useful, the data generated for basal insulins to this point indicate that the duration of action determined in clamp studies using this definition results in values that exceed the clinical duration of action. In a previous study, the end of action of NPH insulin was not reached at 24 hours in 10 of 69 clamps (14%) when given at a dose of 0.4 U/kg,20 whereas it is common for patients to use NPH insulin at least twice daily as a basal insulin therapy. The prolonged fasting that occurs in euglycaemic clamp procedures contributes to an extended duration of action. Blood glucose cut‐offs as low as 105 mg/dL have been introduced recently to reach the end of action earlier in glucose clamp studies, but data on end of action was still right‐censored (i.e. did not reach the blood glucose cut‐off) at this endpoint in some clamps,14 demonstrating the difficulties in designing euglycaemic clamp studies using long‐acting insulins. Despite the relatively low dose of 0.3 U/kg and the 42‐hour clamp duration, the end of action cut‐off was not reached in 14 of 40 clamps in this study, also demonstrating limitations in studies of this type.
Confirmation of clamp quality is an important consideration to ensure confidence in the results obtained in euglycaemic clamp studies.7 In the current study, the mean difference between measured and target blood glucose levels was similarly low for both study insulins. The mean coefficient of variation of blood glucose levels was also similar between study insulins, with mean values of 6.3% and 6.5% for IGlar and LY IGlar, respectively.
Careful consideration was given to whether to conduct the study after a single dose or at steady state. Single‐dose studies are more sensitive than steady‐state studies for assessing PK and PD properties and can detect early differences in PD parameters that may not be apparent at steady state. Regulatory guidance3, 4 recommends single‐dose studies as the default when comparing the PKs and PDs of a biosimilar and a reference product. Furthermore, data suggest that the PK of insulin glargine is linear in terms of time; therefore, PK/PD after a single dose should predict PK/PD after multiple doses.
The ratios for the geometric LS means for Gtot and Rmax were comparable between LY IGlar and IGlar. Other PD parameters characterizing the time profile for GIR (TRmax, early TRmax50%, late TRmax50%) were also comparable between LY IGlar and IGlar. While the current study was designed to compare the duration of action of LY IGlar and IGlar, the PD results are consistent with previous studies demonstrating that LY IGlar and IGlar met bioequivalence criteria with regard to the PD parameters Gtot and Rmax at a dose of 0.5 U/kg.6
In the present study, no safety concerns were noted in subjects with T1DM following administration of either LY IGlar or IGlar.
In summary, the results presented here demonstrate a similar duration of action for LY IGlar and IGlar in subjects with T1DM and also showed comparable PD parameters. In conjunction with previous studies demonstrating that LY IGlar PK/PD parameters met bioequivalence criteria in healthy subjects and clinical trials showing similar safety and efficacy in T1DM and T2DM subjects, the current study adds to the totality of evidence supporting the similarity of LY IGlar and IGlar.
Supporting information
Table S1. Glucodynamic parameters of LY IGlar and IGlar.
ACKNOWLEDGMENTS
The authors wish to acknowledge the investigators and subjects who participated in this study, in particular Dr. Leszek Nosek (Profil, Neuss, Germany) and his team, as well as the following individuals: Helen Desmier, MSc, formerly of Eli Lilly and Company, for project management support; Aik Hoe Seah, MSc, of Eli Lilly and Company, for statistical computing and analysis support; and Jay Tichelaar, PhD for providing medical writing services on behalf of Covance, Inc.
Conflict of interest
C. K. and T. H. are employees and co‐owners of Profil, which has received research funds from Adocia, Astra Zeneca, Biocon, Boehringer Ingelheim, Dance Pharmaceuticals, Gulf Pharmaceutical Industries, Johnson & Johnson, Eli Lilly, Marvel Life Sciences Ltd, Medtronic, Medimmune, Novo Nordisk, Novartis, Roche Diagnostics, Sanofi, Senseonics and Zealand Pharma. C. K. has received advisory and speaker fees and travel grants from Sanofi. T.H. has received speaker and advisory fees or travel grants from Eli Lilly, Mylan and Novo Nordisk. All other authors are employees (H. L., E. C. Q. L., X. Z., D. C. and L. C.) or retired employee (M. E. S.) of, and serve as authors for, Eli Lilly and Company. H. L., M. E. S., D. C. and X. Z. also hold stock/shares in Eli Lilly and Company.
Author contributions
H. L., E. C. Q. L., X. Z., M. E. S., D. C., L. C., C. K. and T. H. contributed to the study design, data analysis, interpretation and discussion of the research, and reviewed and edited the manuscript. All authors approved the version to be published. H. L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Linnebjerg H, Lam ECQ, Zhang X, Seger ME, Coutant D, Chua L, Kapitza C and Heise T. Duration of action of two insulin glargine products, LY2963016 insulin glargine and Lantus insulin glargine, in subjects with type 1 diabetes mellitus, Diabetes Obes Metab, 2017;19(1):33–39.
Funding information This study was funded by Eli Lilly and Company
REFERENCES
- 1.Lantus® (insulin glargine [rDNA origin] injection) solution for subcutaneous injection. Prescribing Information. https://products.sanofi.us/lantus/lantus.html. Accessed October 23, 2015.
- 2. Eli Lilly and Company . European Commission grants Lilly and Boehringer Ingelheim's insulin glargine product marketing authorisation in Europe. 2014. http://investor.lilly.com/releasedetail.cfm?ReleaseID=870088. Accessed October 23, 2015.
- 3. European Medicines Agency . Guideline on non‐clinical and clinical development of similar biological medicinal products containing recombinant human insulin and insulin analogues. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184161.pdf. Accessed October 23, 2015.
- 4. US Food and Drug Administration . Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry. 2015. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed October 23, 2015.
- 5. US Food and Drug Administration . Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference protein product. Guidance for industry. 2015. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291134.pdf. Accessed October 23, 2015.
- 6. Linnebjerg H, Lam ECQ, Seger ME, et al. Comparison of the pharmacokinetics and pharmacodynamics of LY2963016 insulin glargine and EU‐ and US‐approved versions of Lantus insulin glargine in healthy subjects: three randomized euglycemic clamp studies. Diabetes Care. 2015;38:2226–2233. [DOI] [PubMed] [Google Scholar]
- 7. Benesch C, Heise T, Klein O, Heinemann L, Arnolds S. How to assess the quality of glucose clamps? Evaluation of clamps performed with ClampArt, a novel automated clamp device. J Diabetes Sci Technol. 2015;9:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cox DR. Regression models and life‐tables. J R Stat Soc Ser B. 1972;20:187–220. [Google Scholar]
- 9. Blevins TC, Dahl D, Rosenstock J, et al. Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus®) in patients with type 1 diabetes in a randomized controlled trial: the ELEMENT 1 study. Diabetes Obes Metab. 2015;17:726–733. [DOI] [PubMed] [Google Scholar]
- 10. Rosenstock J, Hollander P, Bhargava A, et al. Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin‐naïve or previously treated with insulin glargine: a randomized, double‐blind controlled trial (the ELEMENT 2 study). Diabetes Obes Metab. 2015;17:734–741. [DOI] [PubMed] [Google Scholar]
- 11. European Medicines Agency . Assessment report: Abasria. 2014. www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/002835/WC500175383.pdf. Accessed October 23, 2015.
- 12. Dowlat HA, Kuhlmann MK, Khatami H, Ampudia-Blasco FJ. Interchangeability among reference insulin analogues and their biosimilars: regulatory framework, study design and clinical implications. Diabetes Obes Metab. 18:737–746. [DOI] [PubMed] [Google Scholar]
- 13. Heise T, Hövelmann U, Nosek L, Hermanski L, Bøttcher SG, Haahr H. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol. 2015;11:1193–1201. [DOI] [PubMed] [Google Scholar]
- 14. Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units∙mL‐1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units∙mL‐1 . Diabetes Care. 2015;38:637–643. [DOI] [PubMed] [Google Scholar]
- 15. Porcellati F, Rossetti P, Ricci NB, et al. Pharmacokinetics and pharmacodynamics of the long‐acting insulin analog glargine after 1 week of use compared with its first administration in subjects with type 1 diabetes. Diabetes Care. 2007;30:1261–1263. [DOI] [PubMed] [Google Scholar]
- 16. Heise T, Piebar TR. Towards peakless, reproducible and long‐acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;9:648–659. [DOI] [PubMed] [Google Scholar]
- 17. Koehler G, Treiber G, Wutte A, et al. Pharmacodynamics of the long‐acting insulin analogues detemir and glargine following single‐doses and under steady‐state conditions in patients with type 1 diabetes. Diabetes Obes Metab. 2014;16:57–62. [DOI] [PubMed] [Google Scholar]
- 18. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long‐acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142–2148. [DOI] [PubMed] [Google Scholar]
- 19. Lucidi P, Porcellati F, Rossetti P, et al. Pharmacokinetics and pharmacodynamics of therapeutic doses of basal insulins NPH, glargine, and detemir after 1 week of daily administration at bedtime in type 2 diabetic subjects: a randomized cross‐over study. Diabetes Care. 2011;34:1312–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heise T, Nosek L, Rønn BB, et al. Lower within‐subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614–1620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Glucodynamic parameters of LY IGlar and IGlar.


