Abstract
Background
E‐52862 (S1RA, 4‐[2‐[[5‐methyl‐1‐(2‐naphthalenyl)‐1H‐pyrazol‐3‐yl]oxy]ethyl]‐morpholine), a novel selective sigma 1 receptor (σ1R) antagonist, has demonstrated efficacy in nociceptive and neuropathic pain models. Our aim was to test if σ1R blockade with E‐52862 may modify the signs of neuropathy in Zucker diabetic fatty (ZDF) rats, a type 2 diabetes model.
Methods
Mechanical and thermal response thresholds were tested on 7‐, 13‐, 14‐ and 15‐week‐old ZDF rats treated with saline or with E‐52862 acutely administered on week 13, followed by sub‐chronic administration (14 days). Axonal peripheral activity (skin–saphenous nerve preparation) and isolated aorta or mesenteric bed reactivity were analysed in 15‐week‐old ZDF rats treated with saline or E‐52862 and in LEAN rats.
Results
Zucker diabetic fatty rats showed significantly decreased thermal withdrawal latency and threshold to mechanical stimulation on week 13 compared to week 7 (prediabetes) and with LEAN animals; single‐dose and sub‐chronic E‐52862 administration restored both parameters to those recorded on week 7. Regarding axonal peripheral activity, E‐52862 treatment increased the mean mechanical threshold (77.3 ± 21 mN vs. 19.6 ± 1.5 mN, saline group) and reduced the response evoked by mechanical increasing stimulation (86.4 ± 36.5 vs. 352.8 ± 41.4 spikes) or by repeated mechanical supra‐threshold steps (39.4 ± 1.4 vs. 83.5 ± 0.9). E‐52862 treatment also restored contractile response to phenylephrine in aorta and mesenteric bed.
Conclusions
E‐52862 administration reverses neuropathic (behavioural and electrophysiological) and vascular signs in the ZDF rat.
Significance
Blockade of σ1R avoids the development of diabetic neuropathy in rats, and may represent a potentially useful therapeutic approach to peripheral neuropathies in diabetic patients.
What does this study add?
This study presents evidences for the potential usefulness of sigma receptor blockade on diabetic neuropathy in rats.
The methodology includes behavioural evidences, electrophysiological data and vascular‐isolated models.
1. Introduction
Sigma receptors (σR) have been classified into two subtypes (σ1R and σ2R). The therapeutic potential of σR ligands includes several diseases (Maurice and Su, 2009; Tsai et al., 2009) and it is known that blocking σ1R induces antinociception (Zamanillo et al., 2013). Moreover, recent studies using σ1R knock‐out mice and pharmacological interventions with selective σ1R antagonists (Romero et al., 2012) showed an antinociceptive effect of σ1R modulation independent of the opioid system. σ1R knock‐out mice showed attenuated nociceptive responses in the formalin test (Cendán et al., 2005), in sciatic nerve injury (de la Puente et al., 2009), in capsaicin sensitization (Entrena et al., 2009) and in antitumoral‐induced cold and mechanical allodynia (Nieto et al., 2012).
According to World Health Organization, 366 million people worldwide will suffer from diabetes by year 2030 and diabetic polyneuropathy is a common complication of diabetes, affecting approximately 50% of both type 1 and type 2 diabetic patients (Dyck et al., 1993). Current treatments are only partially effective and, at best, provide 50% pain relief in one‐third of patients (Jensen et al., 2006).
Little is known regarding the role of the σR in diabetes. A decrease in the number of binding sites for both σ1R and σ2R receptors in the brain of streptozotocin‐induced diabetic rats has been described (Mardon et al., 1999). However, no data about the role of the σR in diabetic peripheral neuropathies, particularly in type 2 diabetes models are available.
Recently, a new drug, E‐52862 (S1RA, 4‐[2‐[[5‐methyl‐1‐(2‐naphthalenyl)‐1H‐pyrazol‐3‐yl]oxy]ethyl] morpholine), has been characterized as a novel σ1R selective antagonist that shows efficacy in nociceptive as well as in neuropathic pain models (Romero et al., 2012). As σ1R antagonists are effective in several peripheral neuropathy models, including sciatic nerve ligation (de la Puente et al., 2009; Romero et al., 2012) and paclitaxel‐induced neuropathy (Nieto et al., 2012), our aim was to test if the blockade of the σ1R with E‐52862 may modify the signs of neuropathy in Zucker diabetic fatty (ZDF) rats, a widely accepted model of type 2 diabetes.
Neuropathy was evidenced by changes in mechanical and thermal response thresholds, but there is scarce information about the involvement of Aδ‐fibres in these sensory disturbances in ZDF rats. Accordingly, the electrophysiological response of Aδ‐fibres to mechanical stimulation was evaluated using the skin–saphenous nerve preparation (Reeh, 1986; Kress et al., 1992). Finally, as reduced nerve perfusion is an important factor in the aetiology of diabetic neuropathy (Cameron and Cotter, 1997) and decreased nerve blood flow and hypoxia in neuropathic diabetes patients have been related to the neurovascular dysfunction (Cameron and Cotter, 1992, 1994), the vascular reactivity of conduit (aorta) and resistance (mesenteric) vessels have been evaluated (Tagashira et al., 2010; Amer et al., 2013) as a first approach.
2. Materials and methods
2.1. Animals
Six‐week‐old male ZDF rats or their respective control (age‐matched lean non‐diabetic Zucker rats, LEAN) were obtained from Charles River Laboratories (Research Models, Barcelona, Spain). The animals were housed in a certified animal care facility, in cages (2–3 animals) and maintained in environmentally controlled conditions (temperature 20 °C, humidity 60%) with a 12‐h light/dark cycle until they reached 15 weeks of life. Animals were maintained on Purina 5008 (16.7 kcal% fat) diet and sterile tap water, chow and water being available ad libitum through all the experimental period.
In this model for non‐insulin–dependent diabetes mellitus, hyperglycaemia and insulin resistance begin to develop at, approximately, 7 weeks of age and glucose levels typically reach a plateau (more than 300 mg/dL) by 15–16 weeks of age (Peterson et al., 1990).
Naive Wistar rats were used to test the effect of E‐52862 in acute nociception in a control group.
All experimental protocols were approved by the Ethical Committee of the Universidad Rey Juan Carlos and performed in strict accordance with the EC regulation for care and use of experimental animals (2010/63/EU).
2.2. Drugs
E‐52862, the newly synthesized sigma 1 antagonist, was supplied by ESTEVE and was dissolved in saline (0.9% w/v). The compound was administered by intraperitoneal (i.p.) route. The doses of drug employed in this study were 64 mg/kg i.p. to test the acute effect, and 25 mg/kg i.p. given twice a day (b.i.d.) during 14 days to test the effect of sub‐chronic administration. The concentration of the solutions was adjusted to inject a volume of 0.5 mL. Doses were selected from pilot experiments from our laboratory using Wistar rats (data not shown) and are in agreement with published data (Nieto et al., 2012). Phenylephrine and carbachol were obtained from Sigma (Sigma Chemical Company, Poole, UK) and were dissolved in distilled water.
2.3. Experimental protocol
After 1 week of adaptation (week 7 of life) non‐fasting blood glucose levels, body weight, spontaneous activity, thermal hyperalgesia and mechanical allodynia were monitored on LEAN and ZDF rats for the first time to facilitate the detection of the development of diabetes and neuropathies. Behavioural tests were repeated at the end of the week 13 of life and, then, ZDF rats were randomly assigned to two separated groups to be treated with a single dose of E‐52862 (64 mg/kg i.p, 0.5 mL, n = 8) or with the same volume of saline solution (n = 7). LEAN rats group (n = 7) was treated with saline. Thirty minutes after E‐52862 or saline administration mechanical allodynia, thermal hyperalgesia and spontaneous activity were sequentially evaluated.
Sub‐chronic treatment was started 24 h after this first administration. Rats received two daily intraperitoneal injections for 14 days (weeks 14 and 15) of compound E‐52862 (25 mg/kg) or saline solution. To disregard acute effects of the last dose, behavioural tests were carried out 12 h after the last administration (week 15) because E‐52862 maximum plasma concentration is achieved shortly after its administration to rodents (t max = 15 min after i.p. administration to rats) and it is quickly metabolized, having a short half‐life in rats (t 1/2 = 1.4 h) (Romero et al., 2012; Vidal‐Torres et al., 2014).
After behavioural tests rats were sacrificed and tissues were dissected to perform in vitro electrophysiological (skin–saphenous nerve preparations) and vascular (aorta rings and mesenteric bed) experiments, glycaemia and body weight were monitored throughout all the experimental period.
2.4. Experimental procedures
Animals were habituated to the corresponding test environments 2 days before the experiment by leaving them daily inside the recording device for 10 min.
2.4.1. Behavioural tests
Von Frey and plantar tests and locomotor activity were carried out as previously described (Supporting Information Methods S1).
2.4.2. Glycaemia
Non‐fasting blood glucose levels were daily measured in the morning (9 h) using a glucose strip tester (Glucocard sensor; Arkray, Inc., Kyoto, Japan), throughout the experimental period (weeks 7, 10, 13, 14 and 15).
2.4.3. Body weight
The body weight of each rat was daily recorded and body weight gain (g) has been included in the graphics.
2.4.4. Skin–saphenous nerve preparation
Our aim was not to analyse the electrophysiology of diabetes, but just to check if there is any alteration in Aδ‐peripheral axons studying their mechanical sensitivity and if these changes may be modified by E‐52862 treatment.
Eight ZDF rats sub‐chronically treated with E‐52862, other seven ZDF and seven LEAN rats receiving saline solution, were used to perform electrophysiological experiments. Tissues were obtained as previously described (Supporting Information Methods S2).
In the Aδ‐fibres the parameters measured were the presence of spontaneous discharge and the responsiveness to mechanical stimuli. Thus, once a single unit was identified, it was left to a control period of 1 min to record spontaneous activity, defined as a discharge rate ≥1 spike/min (Roza et al., 2003). First, a ramp‐pressure stimulation was applied [constantly increasing stimulus from 0 to 200 mN (Schlegel et al., 2004)] with a speed of 8 mN/s to determine the electromechanical threshold of the fibres, defined as the pressure that evoked the first spike that was followed by another spike within the next increment of the force [modified from Suzuki et al. (2002a)]; when ongoing discharge was present the threshold was determined as the lowest force at which the instantaneous frequency of spikes continuously exceeded the mean basal activity +1 standard deviation (Suzuki et al., 2002a). Second, eight stimuli of constant supra‐threshold pressure (~threshold + 40 mN, step‐pressure stimulation) were delivered for 5 s to test sensitization. Third, a noxious step stimulus of 300 mN was applied for 10 s (Wenk et al., 2006). Time interval between two consecutive stimuli all along the experiment was 5 min.
Using the ramp‐shaped mechanical stimulation, the threshold and supra‐threshold responses of the fibres were analysed by counting the number of spikes elicited by each pressure increment and the total response to the ramp. To analyse the response of the fibres to the step‐pressure stimulation, the total number of spikes evoked during each step stimulation was quantified, and the response pattern to the noxious step stimulus was analysed by counting in 2‐s bins and averaged (Suzuki et al., 2002b). For those ongoing discharge fibres, the basal activity (mean imp/s during the 30 s preceding the stimulus) was multiplied by the stimulus duration to calculate the ongoing discharge level throughout the stimulation period. This ongoing count was subtracted from the total count during the stimulus. All results are expressed as the mean ± standard error of the mean (SEM), and n refers to the number of fibres.
2.4.5. Isolated rat vessels
Zucker diabetic fatty rats and LEAN rats were treated with saline‐ or E‐52862 and then rat aorta and mesenteric bed were obtained as previously described (Supporting Information Methods S3).
Contraction responses of the aorta rings are expressed as mean absolute values and relaxation responses are expressed as the percentage relaxation of the tone induced by phenylephrine by the concentration–response curves of carbachol (Abboud et al., 2009).
In the experiments carried in intact mesenteric beds, the evaluation of the effect of the sub‐chronic treatment with E‐52862 on the functionality of the vascular bed was carried out following two different procedures: (1) mesenteric bed contractile function (Carvalho Leone and Coelho, 2004) was evaluated by a concentration–response curve of phenylephrine 10−8 mol/L – 8 × 10−8 mol/L; and (2) mesenteric bed vasorelaxant function was evaluated by the response to the precontracted bed (phenyleprine 50 × 10−6 mol/L) to a concentration–response curve of carbachol 0.5–5 × 103 pmol (endothelium‐dependent vasodilatation) (Makino et al., 2000).
Contraction responses of superior mesenteric artery bed are expressed as mean absolute values and relaxation responses are expressed as the percentage relaxation of the tone induced by phenylephrine by the concentration–response curves of carbachol.
2.5. Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis of drug effects was performed by unpaired two‐tailed t‐test or analysis of variance (ANOVA), followed, when appropriate, by post‐hoc Newman–Keuls test or Bonferroni test. p < 0.05 was considered as statistically significant.
3. Results
The ZDF rats showed a mean blood glucose concentration of 88.1 ± 5.1, 329.3 ± 26.0 and 413.6 ± 22.6 mg/dL, on weeks 7, 10 and 13, respectively, being the difference in weeks 10 and 13 versus week 7 values statistically significant (p < 0.001). The LEAN mean blood glucose concentration was of 64.8 ± 1.4, 60.6 ± 1.3 and 69.9 ± 2.6 mg/dL, on weeks 7, 10 and 13, respectively. Values recorded on LEAN rats were significantly different from those recorded in both saline‐ and E‐52862‐treated ZDF rats (p < 0.001).
Non‐fasting blood glucose levels in ZDF rats were slight, but significantly (p < 0.01) decreased from the first administration of E‐52862 in comparison with values recorded in saline‐treated ZDF animals (Fig. 1A).
Figure 1.
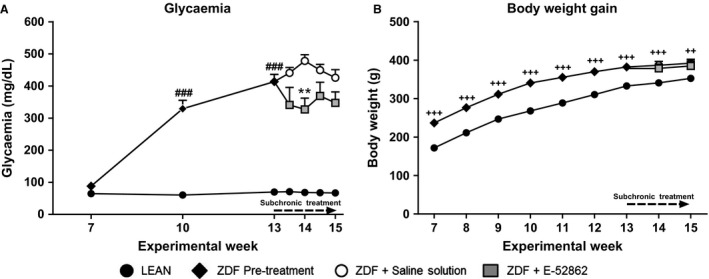
Evolution of the glycaemia and body weight gain in Zucker diabetic fatty (ZDF) treated with saline or E‐52862 and LEAN rats. (A) Lines represent the mean ± SEM of the glucose levels on weeks 7, 10, 13, 14 and 15. (B) Weekly increase in body weight. A one‐way ANOVA followed by Bonferroni post hoc test was used for statistical analysis of glycaemia differences between week 7 values (###p < 0.001) and between saline‐ and E‐52862‐treated ZDF‐rats (**p < 0.01). A two‐way ANOVA followed by Bonferroni post‐hoc test was used when comparing body weight among the three groups (++p < 0.01; +++p < 0.001 vs. LEAN) (n = 7–8).
As previously described in Adegoke et al. (2015). Body weight was significantly higher in ZDF than in LEAN rats and E‐52862 administration did not significantly affect body weight gain (Fig. 1B).
No significant differences were found between responses to thermal or mechanical stimulation between ZDF rats (thermal: 12 ± 0.7 s; mechanical: 26.0 ± 0.0 g) and LEAN rats (thermal: 10.2 ± 0.6 s; mechanical: 25.3 ± 0.7 g) in week 7 (Fig. 2A and B). However, when the responses to thermal and mechanical stimulation were tested in ZDF rats in week 13 and compared to the values obtained in week 7, the thermal withdrawal latency (Fig. 2A) was significantly (p < 0.001) decreased (7.6 ± 0.5 s) and the response threshold to mechanical stimulation (Fig. 2B) was also significantly (p < 0.001) lower in week 13 (16.3 ± 1.2 g). Values recorded in LEAN rats did not show modifications along the experimental procedure either thermal (10.0 ± 0.4 s) or mechanical responses (26.0 ± 0.0 g). These values were not significantly different from those recorded on week 7. As expected, values recorded in ZDF rats before treatment were significantly lower than those obtained in LEAN rats (thermal: p < 0.05; mechanical: p < 0.001).
Figure 2.
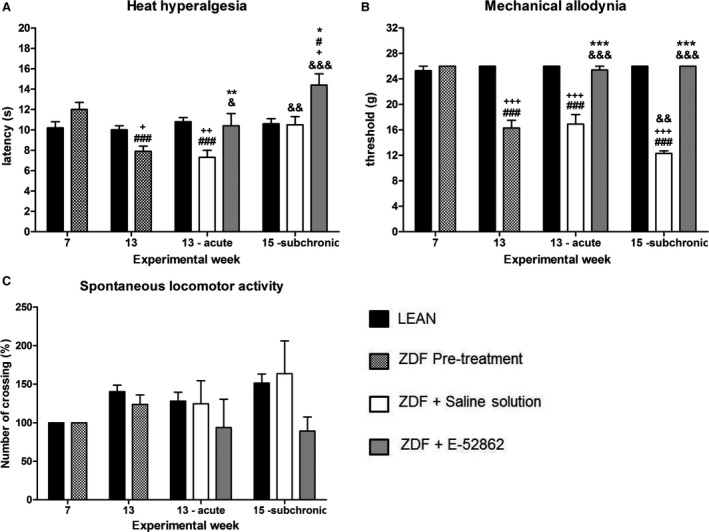
Evolution of the sensorial signs of peripheral neuropathy in ZDF and LEAN rats. Bars show the thermal latency (A), the threshold in response to mechanical stimulation (B) and the percentage of modification of the spontaneous motility (number of crosses) (C), in rats aged 7, 13 (before and after acute treatment) and 15 (sub‐chronic treatment) weeks. Values are expressed as the mean ± SEM. A one‐way ANOVA followed by Bonferroni post‐hoc test was used for statistical analysis. # versus week 7, & versus week 13, + versus LEAN, * versus saline (#, &, +, * p < 0.05; &&, ** p < 0.01; ###, &&&, +++, *** p < 0.001) (n = 7–8).
3.1. Effect of the treatment with E‐52862 on nociception and locomotor activity
In naive rats E‐52862 did not modify the response in von Frey test (19.45 ± 0.28 g before administration and 19.67 ± 0.37 after treatment) and increased the thermal threshold in the plantar test (9.31 ± 0.40 s before administration and 13.23 ± 0.45 after treatment).
Saline solution administration to 13‐week‐old ZDF and LEAN rats did not modify thermal withdrawal latency (ZDF: 7.3 ± 0.7 s; LEAN: 10.8 ± 0.4 s) or mechanical threshold responses (ZDF: 16.9 ± 1.5 g; LEAN: 26.0 ± 0.0 g), these values being not statistically different from those recorded in each group in week 13 before saline solution administration (Fig. 2A and B). Thresholds obtained in ZDF rats were significantly lower than those recorded in LEAN rats (thermal: p < 0.01; mechanical: p < 0.001).
When a single dose of E‐52862 (64 mg/kg i.p.) was administered to 13‐week‐old ZDF rats 30 min before behavioural tests, both the thermal withdrawal latency (10.4 ± 1.2 s) (Fig. 2A) and the mechanical threshold (25.4 ± 0.6 g) (Fig. 2B) increased, reaching the difference significant values (thermal: p < 0.01; mechanical: p < 0.001) with respect to those recorded in the group treated with saline solution and being no statistically different from those recorded on week 7 in ZDF rats (baseline values). Values recorded in ZDF rats treated with E‐52862 were similar to those recorded in saline‐treated LEAN rats.
Twenty‐four hours after acute treatments, E‐52862 (25 mg/kg i.p.) in ZDF rats or saline in ZDF and LEAN rats were administered b.i.d. for 14 days, and the effect of this treatment on nociception and on spontaneous motility was evaluated (week 15).
For the group of ZDF animals treated 14 days with saline (week 15), the thermal withdrawal latency was increased (10.5 ± 0.8 s) being this increase non‐statistically significant with respect to week 7, but significant (p < 0.01) with respect to week 13 in ZDF saline‐treated rats. The mechanical threshold was significantly (p < 0.01) decreased (12.3 ± 0.4 g) versus values recorded in the ZDF saline‐treated rats in week 13. In LEAN rats thermal response values remain unchanged and were not statistically different from those recorded in ZDF group treated with saline. On the contrary, the response to mechanical stimulation was statistically (p < 0.001) higher than that obtained in ZDF treated with saline.
After 14 days of E‐52862 administration, values of thermal withdrawal latency and mechanical threshold were 14.4 ± 1.1 s and 26.0 ± 0.0 g, respectively (Fig. 2A and B). Thermal withdrawal latency was increased after E‐52862 sub‐chronic administration, this increase being statistically significant (p < 0.05) versus ZDF control rats (week 7), versus saline‐treated ZDF rats (week 15) (p < 0.05) and versus LEAN rats (week 15) (p < 0.05), suggesting that E‐52862 induced antinociceptive effect. With respect to mechanical response, the sigma antagonist significantly (p < 0.001) increased the threshold when compared with ZDF treated with saline solution (weeks 13 and 15) to restore it to a normal baseline value, not statistically different from ZDF control values (week 7) or from LEAN animals, thus suggesting an antiallodynic effect.
The spontaneous locomotor activity was evaluated only as an animal welfare parameter. To facilitate comparisons, Fig. 2C shows percentage changes along the experimental procedures. No significant fluctuations were found during the development of diabetic neuropathy signs in saline‐treated or E‐52862 treated ZDF rats or in LEAN rats.
3.2. Electrophysiological assays
A total of 30 Aδ‐fibres were studied: twelve fibres isolated from seven LEAN rats, nine fibres obtained from eight ZDF rats that were treated with compound E‐52862 (E‐52862 group) and nine from seven ZDF rats given saline. All fibres had a single cutaneous RF and provided stable recordings throughout the entire stimulation protocol. The CVs of the studied fibres ranged from 3.1 to 12.5 m/s, which permits to classify them as Aδ‐fibres; in the ZDF group treated with saline they ranged from 8.4 to 12.5 m/s (mean 9.5 ± 0.5 m/s) and from 5.9 to 11.1 m/s (mean 9.5 ± 0.6 m/s) in the ZDF group treated with E‐52862. In LEAN preparations, CVs ranged between 3.1 and 11.9 m/s (mean 6.7 ± 0.9 m/s). Therefore, in average, Aδ‐fibres in diabetic rats had a significantly faster CV than in control LEAN rats (p < 0.05).
With regard to spontaneous discharges prior to stimulation, they were recorded in 2 of 9 fibres in each ZDF group (22.2%) and in 3 of 12 in the LEAN group (25%); from these data differences could not be concluded.
Regarding responsiveness to mechanical stimulation, the analysis of the mechanical thresholds of the fibres revealed significant differences (p < 0.05) between the ZDF group treated with saline in which the mean threshold value was 19.6 ± 1.5 mN (range: 16–24) and, the other two groups, in which the mean values were 77.3 ± 21 mN (range: 24–176) for the ZDF group treated with E‐52862 and 54.7 ± 12.5 mN (range: 32–160) for the LEAN group.
Concerning the total number of spikes evoked by the ramp stimulation paradigm, fibres belonging to the E‐52862‐treated group showed no significant differences in the frequency of responses (86.4 ± 36.5 spikes/ramp) compared to fibres in the LEAN group (163.4 ± 33.9 spikes/ramp), whereas significantly small frequencies were found in comparison to fibres of the ZDF saline solution‐treated group (352.8 ± 41.4 spikes/ramp; p < 0.001 vs. E‐52862 group and p < 0.001 vs. LEAN). Fig. 3A shows the histogram of the averaged responses of the fibres corresponding to the three experimental groups to the ramp stimulation.
Figure 3.
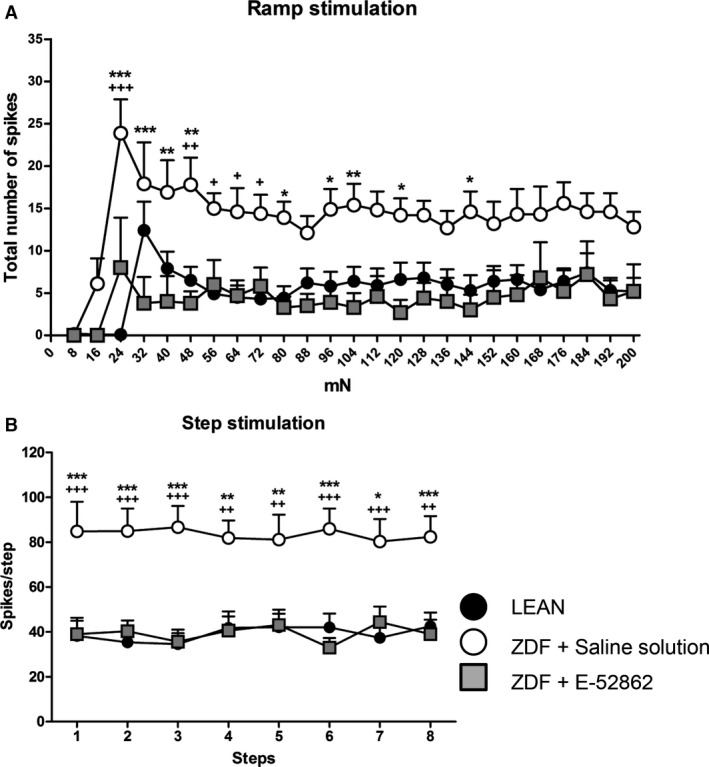
Response of Aδ‐fibres to the mechanical stimulation. (A) Averaged histogram of the responses to the ramp stimulation. Each point of the lines shows the mean number of spikes ± SEM elicited by the successive increments of pressure. (B) Response to eight supra‐threshold step stimuli; each point of the lines shows the mean number of spikes ± SEM elicited by every step. A two‐way ANOVA followed by Bonferroni/Dunn post‐hoc test was used for statistical analysis: * versus E‐52862, + versus LEAN (*, + p < 0.05; **, ++ p < 0.01; ***, +++ p < 0.001) (n = 9–12 Aδ‐fibres).
When eight stimuli of constant supra‐threshold pressure (~threshold + 40 mN) were delivered for 5 s to test sensitization, data showed that inside each group of fibres, responses to those eight step stimuli were not significantly different, thus indicating that these fibres were not or just faintly sensitized by the repeated stimulation (Fig. 3B). Nevertheless, the mean number of spikes discharged by the fibres belonging to the LEAN or E‐52862 groups was similar and significantly lower than that of the saline solution‐treated group (Fig. 3B).
The response to one‐step stimulus of 300 mN maintained during 10 s (noxious stimulation) (Fig. 4A) was similar in fibres obtained from LEAN and ZDF E‐52862‐treated rats and lower than values recorded in ZDF treated with saline solution. When the response pattern of the three groups was analysed by counting for every 2 s and normalized with the count during the first 2 s (100%), the response of the ZDF treated with saline group was different from that of the other two groups since there was no adaptation, reaching statistical significance in comparison with E‐52862 group (p < 0.05) (Fig. 4B).
Figure 4.
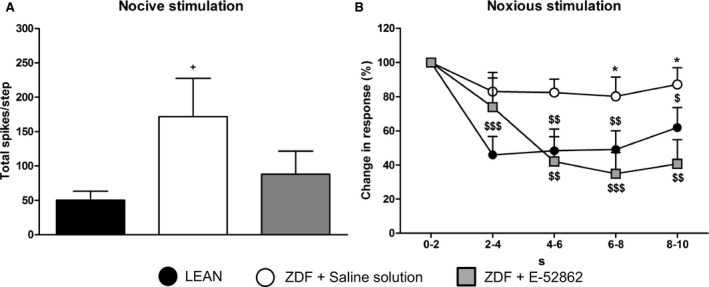
Response to one noxious step stimulus of 300 mN. (A) Bars show the mean total number of spikes ± SEM elicited by the step. (B) Figure shows the difference in the response pattern of the fibres to the step. The evoked responses are presented as the percentage of the initial response (100%). Each bin is 2 s. Unpaired t‐test and one‐way ANOVA followed by Bonferroni/Dunn post‐hoc test were used for statistical analysis: + versus LEAN, * versus E‐52862, $ versus initial response (+,*, $ p < 0.05; $$ p < 0.01; $$$ p < 0.001) (n = 9–12 Aδ‐fibres).
3.3. Cardiovascular parameters
Zucker diabetic fatty rats exhibit a hypo‐responsiveness in contractile aorta function in comparison with their control, LEAN rats. Compound E‐52862 (25 mg/kg i.p., b.i.d. during 14 days) was able to significantly increase (p < 0.001) the aorta responsiveness to phenylephrine (E max: 1.5 ± 0.08 g) (Fig. 5A) in comparison with the response obtained in ZDF rats treated with saline (E max: 1.03 ± 0.11 g) without modification of the vasorelaxation induced by carbachol (Fig. 5B). Moreover, contractile dysfunction of the aorta in ZDF rats was completely restored by E‐52862, reaching similar aorta phenylephrine responses (E max: 1.56 ± 0.06 g) than LEAN rats (Fig. 5A).
Figure 5.
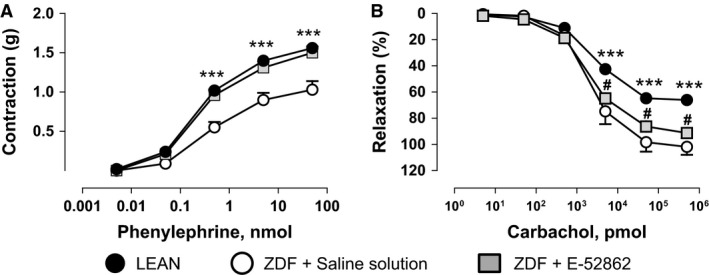
Aortic response to phenylephrine (A) and carbachol (B) in saline‐ and E‐52862‐treated ZDF and LEAN rats. Values are expressed as mean ± SEM (n = 5–8 tissues). A two‐way ANOVA followed by Bonferroni/Dunn post‐hoc test was used for statistical analysis (*p < 0.05, ***p < 0.001 vs. saline).
On the other hand, ZDF rats treated with saline solution showed significantly increased contractile responses (E max: 118.63 ± 8.36 mmHg) and significantly decreased responses (E max: 64.4 ± 4.6%) in endothelium‐dependent relaxation in mesenteric bed (resistance vessels) in comparison with LEAN (Fig. 6A and B). E‐52862 (25 mg/kg i.p., b.i.d. during 14 days) was able to significantly reduce the response to phenylephrine (Fig. 6A), and to significantly increase the response to carbachol (Fig. 6B) reaching similar values to that recorded in LEAN rats and normalizing the vasoconstrictor and the vasorelaxant functions in this vascular territory.
Figure 6.
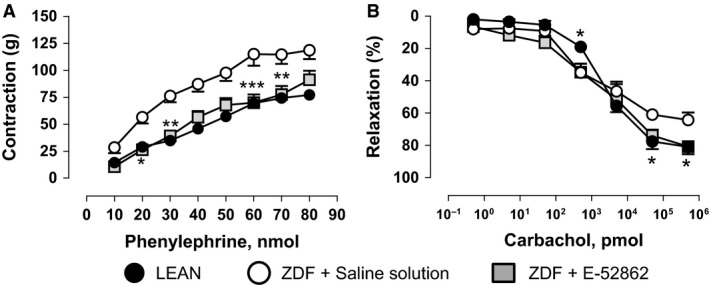
Mesenteric response to phenylephrine (A) and carbachol (B) in saline‐ and E‐52862‐treated Zucker diabetic fatty and LEAN rats. Values are expressed as mean ± SEM (n = 5–8 tissues). A two‐way ANOVA followed by Bonferroni/Dunn post‐hoc test was used for statistical analysis (*p < 0.05, **p < 0.01, ***p < 0.001 vs. saline).
4. Discussion
Treatment of pain due to diabetic neuropathy is a challenging issue as the response to drugs is often unsatisfactory. In this context, we have tested the effect of the blockade of σ1Rs in diabetic neuropathy in ZDF rats, analysing behavioural, axonal and vascular dysfunctions. The ZDF rats are genetically predisposed to obesity, hyperlipidaemia and insulin resistance (Griffen et al., 2001) and LEAN Zucker rats were used as a non‐diabetic, genetically related control group.
Glycaemia, thermal response and tactile thresholds were evaluated in LEAN and ZDF rats aged 7 weeks, before the onset of neuropathic signs in ZDF rats. This age was selected because it has been described previously that the hyperglycaemia is not detectable until 8 weeks of age and since then, glucose remains elevated throughout their lifetime (Peterson et al., 1990; Farrar et al., 2001). Signs of diabetic neuropathy, such as tactile allodynia and thermal hyperalgesia, nerve conduction deficits and cardiovascular alterations, are accepted to be present in diabetic ZDF rats at 10 weeks of age (Li et al., 2006).
A slight, but significant, reduction in the glycaemia in ZDF rats was observed from the first administration of E‐52862, when values were compared with those of the saline solution ZDF‐treated group. The presence of σ1R in rat pancreas by using the σ1R radiotracer 18F‐FTC‐146 has been described (James et al., 2014). Although some non‐selective σR ligands are known to promote insulin release from isolated rat islets (Chan and Morgan, 1998), the pathophysiological relevance of σ1R in the pancreatic function or in the control of glycaemia has not yet been established. Nevertheless, the magnitude of the effect is small and cannot explain the differences found in behavioural and electrophysiological parameters after E‐52862 treatment.
Whereas in LEAN rats values recorded for glycaemia and thermal and mechanical sensitivity remained unchanged throughout the experimental procedure, a progressive impairment of behavioural data recorded in 7‐week‐old ZDF rats was observed. This is consistent with previously reported data (Vera et al., 2012). As expected, rats developed hyperglycaemia from week 10 and behavioural signs of neuropathy were evident on week 13. At week 15, mechanical allodynia was maintained, or even increased in saline solution‐treated ZDF rats, whereas thermal hyperalgesia was reduced. This inconsistence in the response to heat compared to mechanical stimulation has been previously described in other neuropathies, including diabetic (Fuchs et al., 2010) and vincristine‐induced neuropathies (Weng et al., 2003).
The repeated administration of E‐52862 strongly reduced thermal hyperalgesia and mechanical allodynia in diabetic rats and restored initial thresholds. These antinociceptive effects are in good agreement with previous data related to the effect of σ1R antagonists in different animal models such as the sciatic nerve ligation (de la Puente et al., 2009; Romero et al., 2012) or the paclitaxel‐induced neuropathy (Nieto et al., 2012).
Decreased motor and sensory nerve conduction velocity have been described in ZDF rats (Shimoshige et al., 2000; Oltman et al., 2005; Li et al., 2006) and electrophysiological properties of C‐fibres have been previously reported in the streptozotocin model (Chen and Levine, 2001; Suzuki et al., 2002b; Fuchs et al., 2010); however, no data were found on the mechanical sensitivity of primary afferent nociceptive fibres in a model of type 2 diabetes. In our study the Aδ‐fibres were selected because main behavioural changes are related to tactile allodynia and hyperalgesia and they respond to mechanical stimulation (Basbaum et al., 2009) even better than C‐fibres. Our aim was to electrophysiologically study the changes in Aδ‐fibre activity and to determine if these changes were sensitive to the treatment with the σ1R antagonist.
Electrophysiological records carried out in week 15 at the end of the experimental period showed that most of the fibres from diabetic rats had no ongoing activity and this was not different in LEAN rats; these data agree with previously reported results in C‐fibres in rats rendered diabetic by administration of STZ (Chen and Levine, 2001). Regarding the slight increase in the conduction velocity found in the fibres of the ZDF groups, in ZDF rats, there are no specific data from Aδ‐fibres in the literature to compare with; there are only records from C‐fibres in STZ‐treated rats, but no differences with controls were reported (Suzuki et al., 2002b; Fuchs et al., 2010) or appeared to be enhanced in some of the studied fibres (Chen and Levine, 2001).
Diabetic preparations showed hyper‐responsiveness to mechanical stimulation (decreased threshold responses and increased discharge rate) when compared with values obtained in LEAN rats. Moreover, LEAN group showed adaptive responses to supra‐threshold stimulation, whereas ZDF group did not, which is in accordance with previous reports (Suzuki et al., 2002b). Interestingly, the responses recorded after the treatment with the sigma 1 receptor antagonist were statistically different from those recorded in ZDF treated with saline and similar to those recorded in LEAN rats.
The modifications in the responsiveness of the peripheral nerve observed in ZDF rats may be one of the causes of the decreased thresholds in the response to thermal and mechanical stimuli in awake animals, and the normalization of the electrophysiological parameters induced by blockade of σ1R may underlie the antinociceptive effect of E‐52862. Nevertheless, other factors may be also contributing to E‐52862 antinociceptive effects. Recently, Mazo et al. (2015) showed that E‐52862 inhibited spinal wind‐up responses induced by repetitive activation of C‐fibres, with no effect on either responses to single C‐fibre stimuli or on the amplitude of the non‐nociceptive monosynaptic reflex. Another contributing factor could be that E‐52862 has been described to enhance the descending noradrenaline pain inhibitory pathway, as evidenced in the formalin‐induced pain model (Vidal‐Torres et al., 2014).
Finally, it is generally accepted that the neural dysfunction in ZDF diabetic rats, as well as in diabetic humans, may be caused by vascular alterations (Cameron and Cotter, 1997; Oltman et al., 2005). In fact, experimental studies have shown that microvascular alterations occur previously to the neuronal damage in diabetes. This vascular damage occurs at 8–10 weeks of life of the animals, approximately 4 weeks before nerve conduction changes.
In this study, alterations in vascular function in either conduit (aorta) or resistance (mesenteric) vessels were found in ZDF rats of 15 weeks of life compared with their age‐matched controls, LEAN rats. Data obtained in conduit vessels are in agreement with those obtained by other authors who demonstrated that aortic phenylephrine‐induced contractile responses were attenuated in ZDF rats at 16 weeks of life (Oltman et al., 2006). The decrease in contractile function in conduit vessels could be related with alterations in sympathovagal balance and related complications (Cosson et al., 2009). In resistance vessels, an endothelium‐dependent vasorelaxant dysfunction parallel to an increase in contractile mesenteric functionality was observed in ZDF animals compared to LEAN controls. Although experiments were performed on medium‐size resistance vessels in vitro, the mechanisms are probably relevant for nerve blood flow changes. In fact, this endothelial dysfunction has been also observed by other authors in epineurial arterioles of the sciatic nerve which has been related with modification in nerve conduction in ZDF rats (Oltman et al., 2005). Besides, a deficiency in the nitric oxide system caused a marked increase in vasa nervorum epi/perineural vessel reactivity to norepinephrine in vivo (Cameron and Cotter, 1994). Our data in resistance vessels also have shown an increase in contractile vascular reactivity, as described by other authors (Okon et al., 2003), in experimental models of diabetes or metabolic syndrome.
The repeated administration of E‐52862 was able to correct both, the hypo‐responsiveness in the aorta and the endothelium‐dependent vasorelaxant dysfunction in the mesenteric artery. Some authors have described the expression of σ1R in vascular tissue (Bhuiyan et al., 2010), however, the role of this receptor in the cardiovascular system remains unclear. Thus, some studies have shown that the stimulation of σ1R was able to correct the endothelial dysfunction in ovariectomized rats (Tagashira et al., 2013), whereas others have suggested that σ1R inhibitors could have therapeutic value for the suppression of unwanted increases in vascular permeability (Amer et al., 2013). However, this data show, for the first time, that σ1R blockade could have beneficial effects in the prevention and treatment of both vascular and neural complications in patients with diabetes and/or metabolic syndrome. In fact, it has been suggested the importance of the endoneurial microenvironment and the maintenance of adequate perfusion in the selection of agents and design of clinical trials in diabetic neuropathy (Cameron and Cotter, 1994). Other authors have also proposed that the blockade of peripheral σ1R induced analgesic effect in ischemic pain (Kwon et al., 2016). The dual effect of σ1R antagonists could be interesting and more research in relation to that is needed to identify new potential levels of intervention for different neuropathies.
As there is an urgent need of new therapies for the treatment of both cardiovascular and neural complications in patients with type 2 diabetes, the σ1R could be an interesting target to be investigated in depth for this purpose.
Supporting information
Methods S1. Behavioural tests.
Methods S2. Skin‐saphenous nerve preparation.
Methods S3. Rat vessels isolation.
Acknowledgement
To Ivan Álvarez Rodríguez for his technical support.
Authors' contributions
All authors listed above have contributed sufficiently to the project to be included as authors: N. Paniagua has directly participated in the execution of the experimental work and has contributed to the critical discussion of the results. R. Girón has been responsible for the electrophysiological experiments. C. Goicoechea has been responsible for the behavioural assays. V. López‐Miranda has been responsible for the in vitro cardiovascular experiments. J.M. Vela participated in the initial planning of this study. M. Merlos participated in the planning of this study and has contributed to the elaboration of the manuscript. M.I. Martín Fontelles makes substantial contributions to the conception and design of this study, and to the analysis and interpretation of data. All the authors have revised the manuscript critically.
Funding sources
Esteve, Barcelona, Spain.
Conflicts of interest
J.M. Vela, M. Merlos work for Esteve, Barcelona, Spain. Other conflicts of interest do not exist.
References
- Abboud, K. , Bassila, J.C. , Ghali‐Ghoul, R. , Sabra, R. (2009). Temporal changes in vascular reactivity in early diabetes mellitus in rats: Role of changes in endothelial factors and in phosphodiesterase activity. Am J Physiol Heart Circ Physiol 297, H836–H845. [DOI] [PubMed] [Google Scholar]
- Adegoke, O.A. , Bates, H.E. , Kiraly, M.A. , Vranic, M. , Riddell, M.C. , Marliss, E.B. (2015). Exercise in ZDF rats does not attenuate weight gain, but prevents hyperglycemia concurrent with modulation of amino acid metabolism and AKT/mTOR activation in skeletal muscle. Eur J Nutr 54, 751–759. [DOI] [PubMed] [Google Scholar]
- Amer, M.S. , McKeown, L. , Tumova, S. , Liu, R. , Seymour, V.A. , Wilson, L.A. , Naylor, J. , Greenhalgh, K. , Hou, B. , Majeed, Y. (2013). Inhibition of endothelial cell Ca2 entry and transient receptor potential channels by Sigma‐1 receptor ligands. Br J Pharmacol 168, 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum, A.I. , Bautista, D.M. , Scherrer, G. , Julius, D. (2009). Cellular and molecular mechanisms of pain. Cell 139, 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan, M.S. , Tagashira, H. , Shioda, N. , Fukunaga, K. (2010). Targeting sigma‐1 receptor with fluvoxamine ameliorates pressure‐overload‐induced hypertrophy and dysfunctions. Expert Opin Ther Targets 14, 1009–1022. [DOI] [PubMed] [Google Scholar]
- Cameron, N. , Cotter, M. (1992). Impaired contraction and relaxation in aorta from streptozotocin‐diabetic rats: Role of polyol pathway. Diabetologia 35, 1011–1019. [DOI] [PubMed] [Google Scholar]
- Cameron, N.E. , Cotter, M.A. (1994). The relationship of vascular changes to metabolic factors in diabetes mellitus and their role in the development of peripheral nerve complications. Diabetes Metab Rev 10, 189–224. [DOI] [PubMed] [Google Scholar]
- Cameron, N.E. , Cotter, M.A. (1997). Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes 46, S31–S37. [DOI] [PubMed] [Google Scholar]
- Carvalho Leone, A.F. , Coelho, E.B. (2004). Effects of prostanoids on phenylephrine‐induced contractions in the mesenteric vascular bed of rats with streptozotocin‐induced diabetes mellitus. Life Sci 76, 239–247. [DOI] [PubMed] [Google Scholar]
- Cendán, C.M. , Pujalte, J.M. , Portillo‐Salido, E. , Montoliu, L. , Baeyens, J.M. (2005). Formalin‐induced pain is reduced in σ1 receptor knockout mice. Eur J Pharmacol 511, 73–74. [DOI] [PubMed] [Google Scholar]
- Chan, S.L. , Morgan, N.G. (1998). σ Receptor ligands and imidazoline secretagogues mediate their insulin secretory effects by activating distinct receptor systems in isolated islets. Eur J Pharmacol 350, 267–272. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Levine, J. (2001). Hyper‐responsivity in a subset of C‐fiber nociceptors in a model of painful diabetic neuropathy in the rat. Neuroscience 102, 185–192. [DOI] [PubMed] [Google Scholar]
- Cosson, E. , Valensi, P. , Laude, D. , Mesangeau, D. , Dabire, H. (2009). Arterial stiffness and the autonomic nervous system during the development of Zucker diabetic fatty rats. Diabetes Metab 35, 364–370. [DOI] [PubMed] [Google Scholar]
- Dyck, P.J. , Kratz, K.M. , Karnes, J.L. , Litchy, W.J. , Klein, R. , Pach, J.M. , Wilson, D.M. , O'Brien, P.C. , Melton, L.J. , Service, F.J. (1993). The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population‐based cohort: The Rochester Diabetic Neuropathy Study. Neurology 43, 817–824. [DOI] [PubMed] [Google Scholar]
- Entrena, J.M. , Cobos, E.J. , Nieto, F.R. , Cendán, C.M. , Baeyens, J.M. , Del Pozo, E. (2009). Antagonism by haloperidol and its metabolites of mechanical hypersensitivity induced by intraplantar capsaicin in mice: Role of sigma‐1 receptors. Psychopharmacology 205, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar, N.S. , Chambers, N.J. , Carlsson, A.R. , Denyer, G. , Johnston, G.A. (2001). Effect of a series of novel sulphonylthioureas on glucose tolerance in the obese fa/fa Zucker rat. Clin Exp Pharmacol Physiol 28, 386–391. [DOI] [PubMed] [Google Scholar]
- Fuchs, D. , Birklein, F. , Reeh, P. , Sauer, S. (2010). Sensitized peripheral nociception in experimental diabetes of the rat. Pain 151, 496–505. [DOI] [PubMed] [Google Scholar]
- Griffen, S.C. , Wang, J. , German, M.S. (2001). A genetic defect in beta‐cell gene expression segregates independently from the fa locus in the ZDF rat. Diabetes 50, 63–68. [DOI] [PubMed] [Google Scholar]
- James, M.L. , Shen, B. , Nielsen, C.H. , Behera, D. , Buckmaster, C.L. , Mesangeau, C. , Zavaleta, C. , Vuppala, P.K. , Jamalapuram, S. , Avery, B.A. , Lyons, D.M. , McCurdy, C.R. , Biswal, S. , Gambhir, S.S. , Chin, F.T. (2014). Evaluation of sigma‐1 receptor radioligand 18F‐FTC‐146 in rats and squirrel monkeys using PET. J Nucl Med 55, 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, T.S. , Backonja, M.M. , Hernandez Jimenez, S. , Tesfaye, S. , Valensi, P. , Ziegler, D. (2006). New perspectives on the management of diabetic peripheral neuropathic pain. Diab Vasc Dis Res 3, 108–119. [DOI] [PubMed] [Google Scholar]
- Kress, M. , Koltzenburg, M. , Reeh, P.W. , Handwerker, H.O. (1992). Responsiveness and functional attributes of electrically localized terminals of cutaneous C‐fibers in vivo and in vitro. J Neurophysiol 68, 581–595. [DOI] [PubMed] [Google Scholar]
- Kwon, S. , Roh, D. , Yoon, S. , Choi, S. , Choi, H. , Moon, J. , Kang, S. , Kim, H. , Han, H. , Beitz, A. (2016). Role of peripheral sigma‐1 receptors in ischaemic pain: Potential interactions with ASIC and P2X receptors. Eur J Pain 20, 594–606. [DOI] [PubMed] [Google Scholar]
- Li, F. , Abatan, O.I. , Kim, H. , Burnett, D. , Larkin, D. , Obrosova, I.G. , Stevens, M.J. (2006). Taurine reverses neurological and neurovascular deficits in Zucker diabetic fatty rats. Neurobiol Dis 22, 669–676. [DOI] [PubMed] [Google Scholar]
- Makino, A. , Ohuchi, K. , Kamata, K. (2000). Mechanisms underlying the attenuation of endothelium‐dependent vasodilatation in the mesenteric arterial bed of the streptozotocin‐induced diabetic rat. Br J Pharmacol 130, 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardon, K. , Kassiou, M. , Donald, A. (1999). Effects of streptozotocin‐induced diabetes on neuronal sigma receptors in the rat brain. Life Sci 65, PL281–PL286. [DOI] [PubMed] [Google Scholar]
- Maurice, T. , Su, T. (2009). The pharmacology of sigma‐1 receptors. Pharmacol Ther 124, 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo, I. , Roza, C. , Zamanillo, D. , Merlos, M. , Vela, J. , Lopez‐Garcia, J. (2015). Effects of centrally acting analgesics on spinal segmental reflexes and wind‐up. Eur J Pain 19, 1012–1020. [DOI] [PubMed] [Google Scholar]
- Nieto, F.R. , Cendán, C.M. , Sánchez‐Fernández, C. , Cobos, E.J. , Entrena, J.M. , Tejada, M.A. , Zamanillo, D. , Vela, J.M. , Baeyens, J.M. (2012). Role of sigma‐1 receptors in paclitaxel‐induced neuropathic pain in mice. J Pain 13, 1107–1121. [DOI] [PubMed] [Google Scholar]
- Okon, E.B. , Szado, T. , Laher, I. , McManus, B. , van Breemen, C. (2003). Augmented contractile response of vascular smooth muscle in a diabetic mouse model. J Vasc Res 40, 520–530. [DOI] [PubMed] [Google Scholar]
- Oltman, C.L. , Coppey, L.J. , Gellett, J.S. , Davidson, E.P. , Lund, D.D. , Yorek, M.A. (2005). Progression of vascular and neural dysfunction in sciatic nerves of Zucker diabetic fatty and Zucker rats. Am J Physiol Endocrinol Metab 289, E113–E122. [DOI] [PubMed] [Google Scholar]
- Oltman, C.L. , Richou, L.L. , Davidson, E.P. , Coppey, L.J. , Lund, D.D. , Yorek, M.A. (2006). Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 291, H1780–H1787. [DOI] [PubMed] [Google Scholar]
- Peterson, R.G. , Shaw, W.N. , Neel, M. , Little, L.A. , Eichberg, J. (1990). Zucker diabetic fatty rat as a model for non‐insulin‐dependent diabetes mellitus. ILAR J 32, 16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Puente, B. , Nadal, X. , Portillo‐Salido, E. , Sánchez‐Arroyos, R. , Ovalle, S. , Palacios, G. , Muro, A. , Romero, L. , Entrena, J.M. , Baeyens, J.M. (2009). Sigma‐1 receptors regulate activity‐induced spinal sensitization and neuropathic pain after peripheral nerve injury. Pain 145, 294–303. [DOI] [PubMed] [Google Scholar]
- Reeh, P.W. (1986). Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett 66, 141–146. [DOI] [PubMed] [Google Scholar]
- Romero, L. , Zamanillo, D. , Nadal, X. , Sánchez‐Arroyos, R. , Rivera‐Arconada, I. , Dordal, A. , Montero, A. , Muro, A. , Bura, A. , Segalés, C. (2012). Pharmacological properties of S1RA, a new sigma‐1 receptor antagonist that inhibits neuropathic pain and activity‐induced spinal sensitization. Br J Pharmacol 166, 2289–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza, C. , Laird, J.M. , Souslova, V. , Wood, J.N. , Cervero, F. (2003). The tetrodotoxin‐resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J Physiol 550, 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel, T. , Sauer, S. , Handwerker, H. , Reeh, P. (2004). Responsiveness of C‐fiber nociceptors to punctate force‐controlled stimuli in isolated rat skin: Lack of modulation by inflammatory mediators and flurbiprofen. Neurosci Lett 361, 163–167. [DOI] [PubMed] [Google Scholar]
- Shimoshige, Y. , Ikuma, K. , Yamamoto, T. , Takakura, S. , Kawamura, I. , Seki, J. , Mutoh, S. , Goto, T. (2000). The effects of zenarestat, an aldose reductase inhibitor, on peripheral neuropathy in Zucker diabetic fatty rats. Metabolism 49, 1395–1399. [DOI] [PubMed] [Google Scholar]
- Suzuki, Y. , Sato, J. , Kawanishi, M. , Mizumura, K. (2002a). Lowered response threshold and increased responsiveness to mechanical stimulation of cutaneous nociceptive fibers in streptozotocin‐diabetic rat skin in vitro – correlates of mechanical allodynia and hyperalgesia observed in the early stage of diabetes. Neurosci Res 43, 171–178. [DOI] [PubMed] [Google Scholar]
- Suzuki, Y. , Sato, J. , Kawanishi, M. , Mizumura, K. (2002b). Tissue glucose level modulates the mechanical responses of cutaneous nociceptors in streptozotocin‐diabetic rats but not normal rats in vitro. Pain 99, 475–484. [DOI] [PubMed] [Google Scholar]
- Tagashira, H. , Bhuiyan, S. , Shioda, N. , Hasegawa, H. , Kanai, H. , Fukunaga, K. (2010). Sigma1‐receptor stimulation with fluvoxamine ameliorates transverse aortic constriction‐induced myocardial hypertrophy and dysfunction in mice. Am J Physiol Heart Circ Physiol 299, H1535–H1545. [DOI] [PubMed] [Google Scholar]
- Tagashira, H. , Matsumoto, T. , Taguchi, K. , Zhang, C. , Han, F. , Ishida, K. , Nemoto, S. , Kobayashi, T. , Fukunaga, K. (2013). Vascular endothelial σ1‐receptor stimulation with SA4503 rescues aortic relaxation via Akt/eNOS signaling in ovariectomized rats with aortic banding. Circ J 77, 2831–2840. [DOI] [PubMed] [Google Scholar]
- Tsai, S.Y. , Hayashi, T. , Mori, T. , Su, T.P. (2009). Sigma‐1 receptor chaperones and diseases. Cent Nerv Syst Agents Med Chem 9, 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera, G. , López‐Miranda, V. , Herradón, E. , Martín, M.I. , Abalo, R. (2012). Characterization of cannabinoid‐induced relief of neuropathic pain in rat models of type 1 and type 2 diabetes. Pharmacol Biochem Behav 102, 335–343. [DOI] [PubMed] [Google Scholar]
- Vidal‐Torres, A. , Fernández‐Pastor, B. , Carceller, A. , Vela, J.M. , Merlos, M. , Zamanillo, D. (2014). Effects of the selective sigma‐1 receptor antagonist S1RA on formalin‐induced pain behavior and neurotransmitter release in the spinal cord in rats. J Neurochem 129, 484–494. [DOI] [PubMed] [Google Scholar]
- Weng, H. , Cordella, J. , Dougherty, P. (2003). Changes in sensory processing in the spinal dorsal horn accompany vincristine‐induced hyperalgesia and allodynia. Pain 103, 131–138. [DOI] [PubMed] [Google Scholar]
- Wenk, H.N. , Brederson, J. , Honda, C.N. (2006). Morphine directly inhibits nociceptors in inflamed skin. J Neurophysiol 95, 2083–2097. [DOI] [PubMed] [Google Scholar]
- Zamanillo, D. , Romero, L. , Merlos, M. , Vela, J.M. (2013). Sigma 1 receptor: A new therapeutic target for pain. Eur J Pharmacol 716, 78–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1. Behavioural tests.
Methods S2. Skin‐saphenous nerve preparation.
Methods S3. Rat vessels isolation.


