Summary
The randomized, double‐blind, double‐dummy, phase 3b RELIEF trial evaluated polycythaemia vera (PV)‐related symptoms in patients who were well controlled with a stable dose of hydroxycarbamide (also termed hydroxyurea) but reported PV‐related symptoms. Patients were randomized 1:1 to ruxolitinib 10 mg BID (n = 54) or hydroxycarbamide (prerandomization dose/schedule; n = 56); crossover to ruxolitinib was permitted after Week 16. The primary endpoint, ≥50% improvement from baseline in myeloproliferative neoplasm ‐symptom assessment form total symptom score cytokine symptom cluster (TSS‐C; sum of tiredness, itching, muscle aches, night sweats, and sweats while awake) at Week 16, was achieved by 43·4% vs. 29·6% of ruxolitinib‐ and hydroxycarbamide‐treated patients, respectively (odds ratio, 1·82; 95% confidence interval, 0·82–4·04; P = 0·139). The primary endpoint was achieved by 34% of a subgroup who maintained their hydroxycarbamide dose from baseline to Weeks 13–16. In a post hoc analysis, the primary endpoint was achieved by more patients with stable screening‐to‐baseline TSS‐C scores (ratio ≤ 2) receiving ruxolitinib than hydroxycarbamide (47·4% vs. 25·0%; P = 0·0346). Ruxolitinib treatment after unblinding was associated with continued symptom score improvements. Adverse events were primarily grades 1/2 with no unexpected safety signals. Ruxolitinib was associated with a nonsignificant trend towards improved PV‐related symptoms versus hydroxycarbamide, although an unexpectedly large proportion of patients who maintained their hydroxycarbamide dose reported symptom improvement.
Keywords: polycythaemia vera, quality of life, signs and symptoms, Janus kinase, hydroxycarbamide
Polycythaemia vera (PV) is a Philadelphia‐negative myeloproliferative neoplasm (MPN) characterized by primary erythrocytosis and deregulated Janus kinase/signal transducer and activator of transcription signalling (Vannucchi, 2014). Most patients with PV experience a broad symptom burden that may include fatigue/tiredness, itching, muscle aches and sweating (Emanuel et al, 2012; Vannucchi et al, 2015). The biomolecular underpinnings of PV‐related symptoms have not been fully elucidated. However, elevated serum inflammatory cytokine levels have been reported in MPN patients (Barbui et al, 2011; Vaidya et al, 2012; Pourcelot et al, 2014) and may contribute to the severity of symptoms, including itching and night sweats (Tefferi et al, 2011; Squires et al, 2013). Other common aspects of the PV disease state, including blood hyperviscosity (Barbui et al, 2013) and splenomegaly (Mesa et al, 2007), may also play a role in the symptom profile of some patients. The overall PV‐related symptom burden, as measured by the MPN Symptom Assessment Form total symptom score (MPN‐SAF TSS), as well as the severity of individual symptoms, including fatigue (Emanuel et al, 2012; Abelsson et al, 2013) and itching (Siegel et al, 2013), have been associated with reduced quality of life in patients with PV.
Polycythaemia vera is adequately managed with phlebotomy and low‐dose aspirin in some patients (Marchioli et al, 2013), but many require additional therapy to achieve their treatment goals. The most common cytoreductive treatment is hydroxycarbamide (HC, also termed hydroxyurea) (Vannucchi, 2014), which is effective for controlling blood cell counts in some patients (Najean & Rain, 1997; Alvarez‐Larran et al, 2012). However, PV‐related symptoms are generally not well controlled with HC treatment (Johansson et al, 2012; Scherber et al, 2012).
In the phase 3 RESPONSE trial, ruxolitinib treatment was superior to best available therapy for the control of haematocrit without phlebotomy and management of splenomegaly in patients with PV who had an inadequate response to, or were intolerant of, HC (Vannucchi et al, 2015). Ruxolitinib treatment was also associated with improvements in PV‐related symptoms (Vannucchi et al, 2015; Mesa et al, 2016). We therefore conducted a randomized, double‐dummy, placebo‐controlled phase 3b trial (RELIEF) in patients who had been receiving a stable dose of HC and were generally well controlled but still reported disease‐associated symptoms. The primary objective of the trial was to compare the change in PV‐related symptom burden in patients continuing their HC therapy with those switching to ruxolitinib.
Methods
Patients
Eligible patients were ≥18 years of age, diagnosed with PV according to the World Health Organization criteria (Tefferi & Vardiman, 2008), treated with HC monotherapy for ≥12 weeks before enrolment, had received a stable dose of HC for ≥4 weeks before enrolment and had cytokine‐related symptoms defined as a score ≥8 (maximum, 50) on the MPN‐SAF TSS cytokine symptom cluster (TSS‐C) (Vannucchi et al, 2015). The TSS‐C is the sum of individual scores for tiredness, itching, muscle aches, night sweats and sweats while awake, each rated on a scale of 0 (absent) to 10 (worst imaginable). The requirement for TSS‐C score ≥8 (i.e, mean score ≥1·6 on each of the 5 individual components) was implemented to ensure that patients had an adequate symptom score at baseline to assess meaningful changes in the primary endpoint. The TSS clusters were identified using an empirical statistical factor analysis of baseline MPN‐SAF scores from the phase 3 RESPONSE trial (Vannucchi et al, 2015). This analysis divided the 14 individual MPN‐SAF symptoms into 3 symptom clusters independent of presumed pathophysiological mechanisms. From a clinical perspective, the 3 symptom clusters agreed well with 3 presumptive pathophysiological mechanisms associated with PV‐related symptoms (cytokines, hyperviscosity and splenomegaly). Eligible patients had ≤2 phlebotomies in the 6 months before screening or no palpable splenomegaly; therefore, the TSS‐C was chosen to define patient eligibility and the primary endpoint. Eligible patients also had haematocrit values between 35% and 48% before randomization; had recovered from all phlebotomy‐associated adverse events, with ≥1 week elapsed between the last phlebotomy and baseline; and had an Eastern Cooperative Oncology Group performance status of 0–2 at baseline. Patients with inadequate liver or renal function, platelet count <100 × 109/l, neutrophil count <1 × 109/l, or peripheral blood blast count >0% at screening were not eligible.
Study design
RELIEF (ClinicalTrials.gov identifier, NCT01632904) was a randomized, multicentre, double‐blind, double‐dummy, phase 3b clinical trial evaluating ruxolitinib versus HC in patients with PV reporting disease‐related symptoms while receiving a stable dose of HC (Fig 1 ). Eligible patients were randomized 1:1 to receive ruxolitinib (10 mg twice daily) plus HC placebo or HC (prerandomization dose and schedule) plus ruxolitinib placebo.
Figure 1.
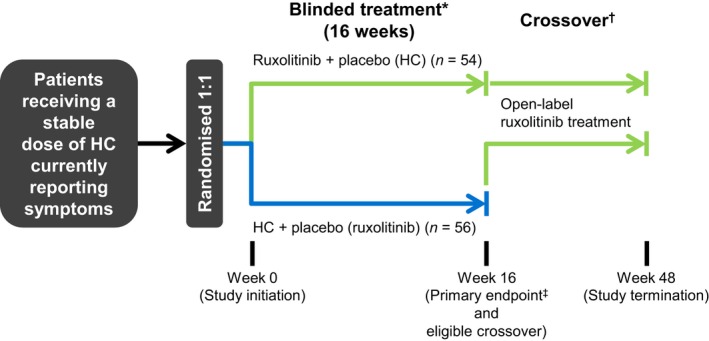
Study design. HC, hydroxycarbamide. *All patients received low‐dose aspirin unless contraindicated. †Patients randomized to HC plus placebo were eligible to cross over after Week 16 to receive open‐label ruxolitinib if safety criteria were met. ‡The primary endpoint was the proportion of patients who achieved ≥50% improvement in Myeloproliferative Neoplasm Symptom Assessment Form total symptom score cytokine symptom cluster at Week 16.
Treatment dose modifications were permitted for safety and efficacy and to optimize response for each patient. HC dose modifications were per investigator judgment; increases to a dose that was not previously tolerated were not permitted. Ruxolitinib dose increases were permitted for patients demonstrating both inadequate efficacy (defined as ≥1 of the following: haematocrit ≥45% or >40% and ≥3 percentage points above baseline; white blood cell or platelet count above the upper limit of normal; reduction from baseline in palpable spleen length of <25% at Week 4 and <50% at Week 8; or minimal improvement, no change or worsening on the Patient Global Impression of Change [PGIC]) and adequate haematological parameters (defined as platelet count ≥140 × 109/l, haemoglobin ≥120 g/l, and absolute neutrophil count ≥1·5 × 109/l). Blood counts were assessed at Day 1 and every 4 weeks during the blinded treatment phase, and every 12 weeks starting at Week 24 during the open‐label treatment phase. For patients who crossed over to ruxolitinib, blood counts were also assessed at 4 and 8 weeks after crossover. Ruxolitinib dose increase was also permitted after a prior dose reduction for safety. Ruxolitinib dose increases were permitted in the absence of select grade 1 cytopaenias; dose reductions or interruptions were required for select grade ≥2 cytopaenias. Dose modifications were made to the study drug and placebo to ensure continued blinding, where applicable.
After the 16‐week blinded treatment phase, patients in either treatment arm with adequate haematological parameters (i.e, platelet count ≥100 × 109/l, neutrophil count ≥1 × 109/l and haemoglobin ≥120 g/l) were eligible to receive open‐label ruxolitinib until study completion at Week 48.
Endpoints
The primary endpoint was the proportion of patients with ≥50% reduction (improvement) from baseline in TSS‐C at Week 16. Secondary endpoints included the proportion of patients with ≥50% reduction (improvement) from baseline in individual TSS‐C symptom severity scores at Week 16 and safety parameters, including nonhaematological and haematological adverse events. Exploratory endpoints included median changes from baseline in TSS‐C individual symptom severity scores at Week 16 and the proportion of patients reporting treatment‐related improvements in PV symptoms at Week 16 using the PGIC (Dworkin et al, 2005).
Assessments
Polycythaemia vera−related symptom severity was assessed with the MPN‐SAF questionnaire once during screening between Days −28 and −7 (using a 7‐day recall), daily during the baseline phase between Days −7 and −1, and daily starting at randomization and continuing until the end of treatment. Baseline score was defined as the average score during the 7 days before randomization (a minimum of 4 days of TSS‐C scores was required before randomization). The Week 16 TSS‐C score was defined as the average over the last 28 days before the Week 16 visit. Week 16 scores were considered missing if there were <20 days of data recorded before the Week 16 visit.
The single‐item PGIC instrument asks patients to grade changes in their PV‐related symptoms since starting study treatment using the following response options: “very much improved” to “much improved”, “minimally improved”, “no change”, “minimally worse”, “much worse” and “very much worse “(Dworkin et al, 2005). Patient responses on the PGIC were captured at Weeks 4, 8, 12, and 16.
Safety
All adverse events, regardless of causality, were assessed according to the National Cancer Institute. Common Terminology Criteria for Adverse Events, version 3·0 (2016 http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). Haematological adverse events were evaluated based on laboratory values.
Statistical analyses
A previous study of patients with primary myelofibrosis (MF), post‐PV MF or post–essential thrombocythaemia‐MF found that approximately 46% who received ruxolitinib and 5% who received placebo experienced a ≥50% reduction (improvement) in total symptom score at Week 24 (Verstovsek et al, 2012). As such, the sample size of the current study was selected based on an assumption that ≥35% of patients in the ruxolitinib arm and 7·5% of patients in the HC arm would achieve a ≥50% reduction (improvement) in TSS‐C at Week 16. Based on this assumption, a sample size of 100 patients (n = 50 per treatment arm) would provide 90% power (2‐sided alpha, 0·05).
Efficacy endpoints were evaluated in the intent‐to‐treat population, with the following exception: for analyses of individual TSS‐C symptom scores, patients were excluded if scores were either missing at baseline or 0 at baseline and Week 16; patients with a score of 0 at baseline and a score >0 at Week 16 were considered nonresponders and included in the analyses. The safety analysis set included all randomized patients who received ≥1 dose of study treatment.
The primary endpoint was estimated with 95% confidence intervals (CIs); the odds ratio (OR) and corresponding 95% CIs were also calculated for the primary endpoint using the Fisher exact test.
A post hoc subgroup analysis was performed to evaluate the association between changes in TSS‐C during the time between screening and baseline (up to 3 weeks) and corresponding changes in TSS‐C at Week 16. The proportion of patients achieving ≥50% improvement in TSS‐C at Week 16 was calculated among patient subgroups with TSS‐C screening‐to‐baseline ratios ≤2 or >2; 95% CIs were calculated using the Clopper‐Pearson exact method, and P values were calculated using the chi‐square test.
A second post hoc analysis evaluated the effect of treatment dose modifications during the blinded treatment phase on changes in TSS‐C. The proportion of patients achieving ≥50% improvement in TSS‐C at Week 16 was calculated among those who required a dose reduction, maintained a consistent dose, and required a dose increase from baseline to Weeks 13 through 16. The coefficient of determination was used to evaluate the correlation between individual changes in study treatment dose from baseline to Weeks 13 through 16 and the percentage change in TSS‐C.
Treatment adherence was assessed using pill counts recorded by study site staff members and calculated for each patient as the percentage of the intended dose (i.e, sum of doses prescribed by the investigator) that was taken.
All other analyses were summarized by descriptive statistics.
Results
Patient characteristics and treatment adherence
In total, 110 patients were randomized between 2 July 2012 and 27 March 27 2014 (ruxolitinib, n = 54; HC, n = 56; Fig 2). Median age at baseline was similar between the treatment arms (Table 1 ), and most patients did not have baseline white blood cell or platelet counts higher than European LeukaemiaNet thresholds (Barosi et al, 2013) (white blood cell count, 10 × 109/l; platelet count, 400 × 109/l). The majority of patients in the ruxolitinib arm were women, whereas the majority of patients in the HC arm were men. A greater proportion of patients in the ruxolitinib arm had a history of thromboembolic events (ruxolitinib, 33·3%; HC, 21·4%). The median treatment adherence rate was 98·3% in the ruxolitinib arm and 97·7% in the HC arm. A total of 35 patients randomized to the ruxolitinib arm and 36 patients randomized to the HC arm began open‐label treatment with ruxolitinib after completing the double‐blind phase (Fig 2 ).
Figure 2.

Patient disposition. HC, hydroxycarbamide. *Arterial occlusive disease, blurred vision, diarrhoea, fatigue, increased platelet count, muscular weakness, musculoskeletal and connective tissue disorders, pyrexia, unstable angina, upper abdominal pain, and urinary tract pain; >1 adverse event could be the cause of discontinuation in a given patient. †Deep vein thrombosis. ‡Data cut‐off for this analysis occurred when the last patient completed the Week 16 visit. Patients were still in the blinded phase at data cut‐off if the unblinding date was missing or occurred after the cut‐off date.
Table 1.
Baseline characteristics
| Ruxolitinib (n = 54) | HC (n = 56) | |
|---|---|---|
| Median (range) age, years | 64 (36–87) | 66 (19–85) |
| Male, n (%) | 24 (44·4) | 34 (60·7) |
| Race, n (%) | ||
| White | 53 (98·1) | 56 (100) |
| Asian | 1 (1·9) | 0 |
| Median (range) time since PV diagnosis, months | 58·5 (7·6–395·0) | 62·4 (3·5–277·5) |
| History of thromboembolic events, n (%) | 18 (33·3) | 12 (21·4) |
| Mean (SD) JAK2V617F allele burden, % | 47·7 (29·0) | 47·9 (30·2) |
| ECOG performance status, n (%) | ||
| 0 | 25 (46·3) | 32 (57·1) |
| 1 | 27 (50·0) | 22 (39·3) |
| 2 | 2 (3·7) | 2 (3·6) |
| Median (range) palpable spleen length below the costal margin, cm | 0·0 (0·0–13·0) | 0·0 (0·0–9·0)a |
| Mean (SD) WBC count, ×109/l | 9·0 (5·5) | 10·6 (9·8) |
| ≤10, n (%) | 39 (72·2) | 39 (69·6) |
| >10–15, n (%) | 9 (16·7) | 9 (16·1) |
| >15, n (%) | 6 (11·1) | 8 (14·3) |
| Mean (SD) platelet count, ×109/l | 357·4 (145·4) | 348·2 (189·3) |
| ≤400, n (%) | 35 (64·8) | 35 (62·5) |
| >400–600, n (%) | 17 (31·5) | 16 (28·6) |
| >600, n (%) | 2 (3·7) | 5 (8·9) |
| Mean (SD) haematocrit, % | 42·1 (3·4) | 43·7 (3·4) |
| <40, n (%) | 15 (27·8) | 8 (14·3) |
| 40–45, n (%) | 27 (50·0) | 27 (48·2) |
| >45, n (%) | 12 (22·2) | 21 (37·5) |
| Mean (SD) MPN‐SAF TSS‐C scoreb | 16·7 (9·8) | 18·0 (10·0) |
| Tiredness | 4·6 (2·5) | 5·2 (2·7) |
| Itching | 3·6 (2·9) | 4·0 (2·7) |
| Muscle aches | 3·3 (2·6) | 3·7 (2·7) |
| Night sweats | 2·8 (2·6) | 2·6 (2·3) |
| Sweats while awake | 2·4 (2·3) | 2·5 (2·7) |
| PV treatment history, n (%) | ||
| HC | 54 (100) | 56 (100) |
| Interferon | 2 (3·7) | 4 (7·1) |
| Anagrelide | 4 (7·4) | 1 (1·8) |
| Pipobroman | 1 (1·9) | 0 |
| Cladribine | 0 | 1 (1·8) |
| Investigational drug | 0 | 1 (1·8) |
ECOG, Eastern Cooperative Oncology Group; HC, hydroxycarbamide; MPN‐SAF TSS‐C, Myeloproliferative Neoplasm Symptom Assessment Form total symptom score cytokine symptom cluster; PV, polycythaemia vera; SD, standard deviation; WBC, white blood cell.
n = 53.
The TSS‐C is the sum of the individual symptom scores for tiredness, itching, muscle aches, night sweats, and sweating while awake (maximum, 50). Individual symptom scores were each rated on a scale of 0 (absent) to 10 (worst imaginable).
Efficacy
The primary endpoint, ≥50% improvement from baseline in TSS‐C at Week 16, was achieved by 43·4% of patients in the ruxolitinib arm and 29·6% in the HC arm; however, the difference between arms was not statistically significant (OR, 1·82; 95% CI, 0·82–4·04; P = 0·139; Table 2). There was a trend towards a greater proportion of patients in the ruxolitinib arm achieving ≥50% improvement from baseline in the individual TSS‐C symptoms compared with the HC arm; however, only the difference for itching was statistically significant (OR, 2·51; 95% CI, 1·10–5·71; P = 0·027; Table 2). In addition, there was a trend towards greater improvement in the median percentage change from baseline in the individual TSS‐C symptoms in favour of ruxolitinib at Week 16 (Table 2 ). Treatment with ruxolitinib during the unblinded treatment phase (randomized ruxolitinib and crossover) was associated with continued benefit based on the median percentage change from baseline in TSS‐C and individual TSS‐C symptom severity scores at 24 and 48 weeks after initiation of ruxolitinib therapy (Table 3 ). The proportion of patients who achieved a ≥50% improvement in the MPN‐SAF TSS or individual hyperviscosity‐ or splenomegaly‐related symptoms was similar between the treatment arms (data not shown), as expected for this PV patient population with generally well‐controlled haematocrit and/or no splenomegaly.
Table 2.
Proportion of patients with ≥50% improvement in MPN‐SAF TSS‐C and individual symptoms at Week 16a
| Symptom, n/N b (%) | Ruxolitinib | HC | P value OR (95% CI) |
|---|---|---|---|
| Primary endpoint | |||
| TSS‐C | 23/53 (43·4) | 16/54 (29·6) |
0·139 1·82 (0·82–4·04) |
| Individual symptoms | |||
| Tiredness | 20/50 (40·0) | 14/53 (26·4) |
0·143 1·86 (0·81–4·27) |
| Itching | 26/48 (54·2) | 16/50 (32·0) |
0·027 2·51 (1·10–5·71) |
| Muscle aches | 18/47 (38·3) | 15/49 (30·6) |
0·428 1·41 (0·60–3·28) |
| Night sweats | 20/42 (47·6) | 20/48 (41·7) |
0·571 1·27 (0·55–2·93) |
| Sweats while awake | 23/42 (54·8) | 16/46 (34·8) |
0·059 2·27 (0·96–40·38) |
HC, hydroxycarbamide; OR, odds ratio; MPN‐SAF TSS C, Myeloproliferative Neoplasm Symptom Assessment Form total symptom score cytokine symptom cluster.
For individual symptoms within the TSS C cluster, all patients with a score >0 at baseline were included in the analysis. If the baseline score was 0 and Week 16 score was >0, the patient was considered a nonresponder. If the baseline and Week 16 scores were both 0, the patient was excluded from the responder analysis.
The denominator is the number of evaluable patients.
Table 3.
Median percentage change from baseline or crossover in MPN‐SAF TSS‐C and individual symptomsa
| Treatment duration after baseline or crossover, weeksb | Ruxolitinib | Ruxolitinib crossover group | HC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 8 | 16 | 24 | 48 | 8 | 24 | 48 | 8 | 16 | |
| Median percentage change in symptom score, % (evaluable n) | |||||||||
| TSS‐C | −41·2 (49) | −50·3 (45) | −47·8 (34) | −81·9 (10) | −45·1 (28) | −17·7 (15) | −53·1 (16) | −30·6 (52) | −30·2 (50) |
| Tiredness | −22·8 (47) | −39·5 (43) | −37·8 (32) | −87·0 (9) | −23·9 (26) | −7·2 (15) | −37·5 (15) | −20·7 (51) | −27·9 (49) |
| Itching | −68·0 (41) | −68·0 (38) | −69·2 (28) | −92·1 (8) | −61·8 (23) | −35·6 (13) | −64·7 (12) | −28·4 (47) | −31·3 (45) |
| Muscle aches | −39·5 (44) | −43·6 (41) | −30·2 (31) | −93·3 (9) | −23·3 (23) | −15·7 (13) | −42·6 (14) | −27·3 (46) | −29·4 (45) |
| Night sweats | −62·1 (37) | −59·5 (33) | −65·0 (24) | −95·8 (8) | −67·6 (18) | −13·9 (13) | −65·2 (9) | −50·8 (42) | −46·9 (41) |
| Sweats while awake | −54·9 (39) | −65·6 (36) | −62·5 (27) | −98·9 (8) | −59·3 (14) | −5·9 (10) | −85·1 (8) | −32·6 (40) | −39·9 (39) |
HC, hydroxycarbamide; MPN‐SAF TSS‐C, Myeloproliferative Neoplasm Symptom Assessment Form total symptom score cytokine symptom cluster.
All patients with a score >0 at baseline were included in the analysis.
Treatment duration: (1) after baseline in the ruxolitinib and HC groups; (2) after crossover from HC to ruxolitinib in the ruxolitinib crossover group.
Some patients reported large changes in symptom severity between screening and baseline, with TSS‐C symptom scores at least twice as severe at screening compared with baseline in 15/53 (28·3%) evaluable patients in the ruxolitinib arm and 10/54 (18·5%) evaluable patients in the HC arm. Therefore, a post hoc subgroup analysis was performed among patients with relatively stable TSS‐C scores between screening and baseline (i.e, screening‐to‐baseline TSS‐C ratio ≤2). Among these patients, a significantly greater proportion in the ruxolitinib arm compared with the HC arm achieved a ≥50% improvement from baseline in TSS‐C at Week 16 (ruxolitinib, 47·4%; HC, 25·0%; P = 0·0346; Table 4). The proportion of patients who achieved a ≥50% reduction from baseline in TSS‐C at Week 16 was not significantly different between treatment arms for patients with a screening‐to‐baseline TSS‐C ratio >2.
Table 4.
Proportion of patients achieving ≥50% improvement in MPN‐SAF TSS‐C at Week 16, by screening‐to‐baseline TSS‐C ratioa
| Ruxolitinib (n = 53b) | HC (n = 54b) | |
|---|---|---|
| Screening‐to‐baseline TSS‐C ratio ≤2, n | 38 | 44 |
| Response rate, % (95% CI) | 47·4 (31·0–64·2) | 25·0 (13·2–40·3) |
| P value | 0·0346 | |
| Screening‐to‐baseline TSS‐C ratio >2, n | 15 | 10 |
| Response rate, % (95% CI) | 33·3 (11·8–61·6) | 50·0 (18·7–81·3) |
| P value | 0·4422 | |
HC, hydroxycarbamide; MPN‐SAF TSS‐C, Myeloproliferative Neoplasm Symptom Assessment Form total symptom score cytokine symptom cluster.
The time between screening and baseline was up to 3 weeks for all patients except 1 in the HC arm (7 weeks).
One patient in the ruxolitinib arm and 2 patients in the HC arm were excluded because TSS‐C at baseline and Week 16 were 0 or missing.
Dose modifications occurred in 24 patients in the ruxolitinib arm (reduction, n = 11; increase, n = 13) and 21 patients in the HC arm (reduction, n = 9; increase, n = 12). There was no statistically significant correlation between changes in treatment dose from baseline to Weeks 13 through 16 and the percentage change in TSS‐C in either treatment arm (coefficient of determination: ruxolitinib arm, 0·018; HC arm, 0·030). Among patients who continued to receive the same HC dose between baseline and Weeks 13–16, 34·3% achieved ≥50% improvement in TSS‐C (Table 5 ).
Table 5.
Proportion of patients with ≥50% improvement in MPN‐SAF TSS‐C at Week 16, by change in dose between baseline and Weeks 13–16
| Patients, n/N (%) | Ruxolitinib (n = 54) | HC (n = 56) |
|---|---|---|
| Dose reduction | 2/11 (18·2) | 0/9 (0) |
| Consistent dose | 13/30 (43·3) | 12/35 (34·3) |
| Dose increase | 8/13 (61·5) | 4/12 (33·3) |
HC, hydroxycarbamide; MPN‐SAF TSS‐C, Myeloproliferative Neoplasm Symptom Assessment Form total symptom score cytokine symptom cluster.
Using the PGIC, a greater proportion of patients in the ruxolitinib arm reported that their PV‐related symptoms were “very much improved” or “much improved” at Week 16 as a result of treatment (ruxolitinib, 48·1%; HC, 30·4%; OR, 2·13; 95% CI, 0·98–4·65), whereas patients in the HC arm were more likely to describe their symptoms as “minimally improved” or “no change” (ruxolitinib, 33·3%; HC, 55·4%). The proportions of patients who reported each PGIC response option are presented in Fig 3.
Figure 3.
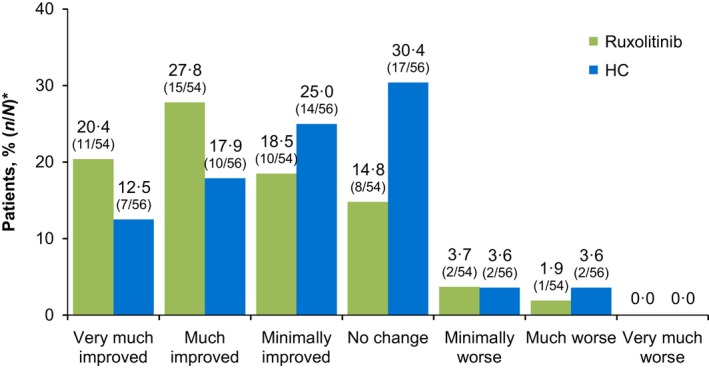
Patient Global Impression of Change at Week 16. *HC, hydroxycarbamide. Percentage of patients with missing data: ruxolitinib, 13·0%; HC, 7·1%. *N is the number of patients in each treatment arm; n is the number of patients who selected each response option.
Safety
Adverse events during blinded treatment are summarized in Table 6. Events were primarily grades 1 or 2 in both treatment arms. The most common nonhaematological adverse events in the ruxolitinib arm included fatigue, headache, and dizziness. Diarrhoea, constipation, fatigue, and pruritus were the most common nonhaematological adverse events in the HC arm. Anaemia and thrombocytopaenia were mainly grades 1 or 2 in each treatment arm. Five patients in the ruxolitinib arm and 4 patients in the HC arm experienced serious adverse events during blinded treatment: thromboembolic events occurred in 2 patients in the ruxolitinib arm and 2 patients in the HC arm.
Table 6.
Adverse events during the blinded phase
| Adverse event | Ruxolitinib (n = 54) | HC (n = 56) | ||
|---|---|---|---|---|
| All Gradesa | Grade 3/4a | All Gradesa | Grade 3/4a | |
| Nonhaematological,b n (%) | ||||
| Fatigue | 11 (20·4) | 1 (1·9) | 6 (10·7) | 1 (1·8) |
| Headache | 9 (16·7) | 0 | 3 (5·4) | 0 |
| Dizziness | 7 (13·0) | 0 | 5 (8·9) | 0 |
| Nausea | 6 (11·1) | 0 | 3 (5·4) | 0 |
| Pruritus | 6 (11·1) | 0 | 6 (10·7) | 0 |
| Rash | 6 (11·1) | 0 | 0 | 0 |
| Diarrhoea | 5 (9·3) | 0 | 11 (19·6) | 0 |
| Constipation | 4 (7·4) | 0 | 7 (12·5) | 0 |
| Haematological,c n/N (%) | ||||
| Anaemia | 20/54 (37·0) | 0 | 13/56 (23·2) | 1/55 (1·8) |
| Thrombocytopaenia | 5/54 (9·3) | 0 | 15/56 (26·8) | 1/55 (1·8) |
| Leucopaenia | 6/54 (11·1) | 1/54 (1·9) | 13/56 (23·2) | 1/55 (1·8) |
| Lymphopaenia | 10/54 (18·5) | 3/50 (6·0) | 20/56 (35·7) | 2/49 (4·1) |
| Neutropaenia | 2/54 (3·7) | 2/54 (3·7) | 7/56 (12·5) | 1/54 (1·9) |
HC, hydroxycarbamide.
Per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3·0.
Adverse events reported by >10% of patients in either arm.
New or worsening haematology laboratory values; n indicates the number of patients with abnormal laboratory values, N indicates the number of evaluable patients (patients were evaluable for new or worsening grade 3/4 haematology laboratory values if post‐baseline data were available and data were missing at baseline or the severity at baseline was grade ≤2).
Herpes zoster infection was observed in 1 patient in the ruxolitinib arm during blinded treatment; no patients received prophylaxis treatment for herpes zoster infection before or during the trial. During blinded treatment, squamous cell carcinoma was observed in 1 patient in the ruxolitinib arm, and no patients had disease transformation to MF or acute myeloid leukaemia (AML). After completion of the blinded treatment phase, 1 additional patient in the ruxolitinib arm developed squamous cell carcinoma and 1 patient in the ruxolitinib arm was diagnosed with disease transformation to MF (Day 211, 24 days after the final dose of ruxolitinib) and AML (Day 216, 29 days after the final dose of ruxolitinib); no additional cases of herpes zoster infection or nonmelanoma skin cancer were observed in either treatment arm.
Four patients in the ruxolitinib arm and 1 patient in the HC arm discontinued because of adverse events (Fig 2 ). There were 2 deaths during the trial, both of which occurred after completion of the blinded treatment phase; 1 patient died from pneumonia after discontinuing HC but before crossing over to ruxolitinib, and 1 patient randomized to ruxolitinib died because of transformation to AML.
Discussion
This report of the RELIEF trial primary results indicates that for patients with generally well‐controlled PV receiving a stable dose of HC, a change in treatment to ruxolitinib monotherapy was associated with a positive trend in symptom improvement compared with those continuing on HC, although this trend was not statistically significant. At Week 16, median changes from baseline in all individual TSS‐C symptom scores favoured ruxolitinib and were still improving at Week 48. Itching was the symptom with the most pronounced improvements observed with ruxolitinib at Week 16. Patients who crossed over to ruxolitinib after randomization to HC experienced improvements in all individual TSS‐C symptom scores within 8 weeks of ruxolitinib treatment; symptom scores stabilised or continued to improve with 48 weeks of treatment. However, data on long‐term and crossover treatment with ruxolitinib were limited by small patient populations. Patient‐reported improvements in symptom severity were better with ruxolitinib compared with HC; a greater proportion of patients receiving ruxolitinib reported that their symptoms were “very much improved” or “much improved”.
This randomized clinical trial is the first in patients with MPNs to evaluate symptoms as the primary endpoint and provides important insights into how best to design future trials in this setting with regard to statistical power and the impact of perceived eligibility criteria. An important limitation of this study was that it did not anticipate, and therefore underestimated, the relatively high proportion of patients who achieved the primary endpoint in the HC arm, including those who received a consistent HC dose from baseline to Weeks 13 to 16 (34%). The reason for this finding is unclear. Although compliance in a clinical trial may be better than in clinical practice, this is an unlikely explanation because previous findings in patients with PV suggest that HC treatment is not associated with clinically relevant improvements in symptoms (Johansson et al, 2012; Scherber et al, 2012; Mesa et al, 2015; Geyer et al, 2016). However, randomized controlled trial data addressing this question are lacking. It is also possible that patients in RELIEF experienced closer medical follow‐up and better availability of supportive measures that may not be typical of standard care settings, which would suggest a potential for better patient outcomes with improved supportive care. Some patients may have entered the study with an expectation of symptomatic relief, which may have contributed to a placebo effect. The substantial difference between symptom scores at screening (reported using a 1‐week recall) and baseline (average of daily reporting) suggests a possible over‐reporting bias for some patients at screening, which may have been affected by patient awareness of the eligibility requirement for pronounced symptoms. In support of this concept, a post hoc subgroup analysis demonstrated that patients with relatively stable TSS‐C between screening and baseline were significantly more likely to achieve ≥50% improvement in TSS‐C at Week 16 with ruxolitinib versus HC. These data suggest that the lower than expected baseline scores for some patients may have compromised the ability to observe clinically relevant changes from baseline during study treatment. Gender differences in treatment response and symptom assessment may also have affected study results; a smaller proportion of patients in the ruxolitinib arm were male compared with the HC arm. Finally, the study was not designed or powered to evaluate measures other than symptoms, precluding evaluation of other clinical outcomes including changes in blood counts and spleen volume.
Previous phase 3 studies suggest that ruxolitinib improves symptoms compared with best available therapy (including HC) in patients with MF or PV. In patients with MF, there is evidence of meaningful improvement in symptoms with ruxolitinib versus placebo, as evaluated by the MPN‐SAF (Mesa et al, 2013), and versus best available therapy (47% receiving HC) using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire−Core 30 (EORTC QLQ‐C30) (Harrison et al, 2012). Similar findings were observed in the randomized, open‐label, multicentre, phase 3 RESPONSE trial, which evaluated ruxolitinib versus best available therapy (59% receiving HC) in patients with PV who were intolerant of or resistant to HC (Vannucchi et al, 2015). Exploratory analyses of changes in symptom severity from baseline to Week 32 demonstrated that ruxolitinib was associated with greater improvements in items on the MPN‐SAF, EORTC QLQ‐C30, and Pruritus Symptom Impact Scale (Vannucchi et al, 2015) compared with lesser improvements or worsening symptoms/quality of life with best available therapy. There were several important differences between the patient populations included in RESPONSE and RELIEF that may explain why the ruxolitinib symptom results were not in agreement between these studies. RESPONSE included a larger patient population (N = 222) with a more severe disease state compared with RELIEF (N = 110). In RESPONSE, patients were required to have splenomegaly at baseline and to be intolerant of or resistant to HC. In contrast, RELIEF patients were generally well controlled with HC but experienced persistent PV‐related symptoms. At baseline, patients randomized to ruxolitinib in RESPONSE versus RELIEF had a longer median duration of PV (98·4 months vs. 58·5 months), longer median palpable spleen length (7·0 cm vs. 0·0 cm), higher mean blood counts (white blood cell, 17·6 × 109/l vs. 9·0 × 109/l; platelet, 484·5 × 109/l vs. 357·4 × 109/l), and a higher mean JAK2V617F allele burden (76·2% vs. 29·0%) (Vannucchi et al, 2015).
The safety and tolerability profile of ruxolitinib observed in RELIEF was consistent with that reported in previous phase 2 and 3 studies of patients intolerant of or resistant to HC (Verstovsek et al, 2014; Vannucchi et al, 2015). Adverse events were generally grades 1 or 2, with few patients (7·4%) discontinuing ruxolitinib treatment because of an adverse event.
In conclusion, treatment with ruxolitinib was associated with a nonsignificant trend towards improvements in TSS‐C compared with HC in patients who had generally well‐controlled PV with a stable dose of HC but still reported disease‐associated symptoms. A statistically significant improvement in itching was observed with ruxolitinib compared with HC, trends towards improvements with ruxolitinib were observed in all other symptoms, and patients with stable screening‐to‐baseline TSS‐C were less likely to report responses to treatment with HC. The large proportion of patients achieving the primary endpoint while continuing to receive a stable HC dose was unexpected and may have implications for the design of future clinical trials.
Conflict of interest statement
RM has received research support from Incyte Corporation, CTI BioPharma Corporation, Gilead, Genentech, Eli Lilly and Company, Promedior Inc., NS Pharma Inc., Sanofi Aventis and Celgene Corporation. AMV has received honoraria and research support from and served on advisory committees for Novartis Pharmaceuticals. AY has served on advisory committees for Incyte Corporation, Alexion Pharmaceutical, Seattle Genetics and Sanofi Aventis. RL has received research funding from Incyte Corporation, Amgen, Genentech, Celgene and Novartis Pharmaceuticals. SK has received honoraria, research support and travel/accommodation expenses and has served as a consultant and provided expert testimony for Novartis Pharmaceuticals and Bristol‐Myers Squibb. JB has received honoraria from Novartis Pharmaceuticals. SV has received funding from and participated in advisory boards for Incyte Corporation. D Hunter was an employee of Incyte Corporation at the time of this analysis. MMJ and HZ are employees of and stockholders in Incyte Corporation. D Habr is an employee and stockholder of Novartis Pharmaceuticals. PZ, MG, CR, YH, FP and BM have no conflicts of interest to disclose.
Author contributions
RM, AMV, AY, PZ, MG, RL, SK, CR, JB, YH, FP, SV and BM participated in the collection, interpretation and analysis of data and the development of the manuscript draft. D Hunter participated in study design and conduct, the interpretation and analysis of data, and the development of the manuscript draft. MMJ participated in study conduct, the analysis and interpretation of data, and the development of the manuscript draft. HZ participated in data analysis and development of the manuscript draft. D Habr participated in the interpretation and analysis of data and the development of the manuscript draft. All authors approved the final manuscript for submission.
Acknowledgements
This study was funded by Incyte Corporation. Writing assistance was provided by Cory Pfeiffenberger, PhD (Complete Healthcare Communications, LLC, an ICON plc company), whose work was funded by Incyte Corporation.
Previous presentation: American Society of Hematology Annual Meeting; December 6–9, 2014; San Francisco, CA, USA (abstract 3168).
References
- Abelsson, J. , Andreasson, B. , Samuelsson, J. , Hultcrantz, M. , Ejerblad, E. , Johansson, B. , Emanuel, R. , Mesa, R. & Johansson, P. (2013) Patients with polycythemia vera have the worst impairment of quality of life among patients with newly diagnosed myeloproliferative neoplasms. Leukemia & Lymphoma, 54, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Alvarez‐Larran, A. , Pereira, A. , Cervantes, F. , Arellano‐Rodrigo, E. , Hernandez‐Boluda, J.C. , Ferrer‐Marin, F. , Angona, A. , Gomez, M. , Muina, B. , Guillen, H. , Teruel, A. , Bellosillo, B. , Burgaleta, C. , Vicente, V. & Besses, C. (2012) Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood, 119, 1363–1369. [DOI] [PubMed] [Google Scholar]
- Barbui, T. , Carobbio, A. , Finazzi, G. , Vannucchi, A.M. , Barosi, G. , Antonioli, E. , Guglielmelli, P. , Pancrazzi, A. , Salmoiraghi, S. , Zilio, P. , Ottomano, C. , Marchioli, R. , Cuccovillo, I. , Bottazzi, B. , Mantovani, A. & Rambaldi, A. ; Agimm & IIC Investigators . (2011) Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C‐reactive protein and pentraxin 3. Haematologica, 96, 315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbui, T. , Finazzi, G. & Falanga, A. (2013) Myeloproliferative neoplasms and thrombosis. Blood, 122, 2176–2184. [DOI] [PubMed] [Google Scholar]
- Barosi, G. , Mesa, R. , Finazzi, G. , Harrison, C. , Kiladjian, J.J. , Lengfelder, E. , McMullin, M.F. , Passamonti, F. , Vannucchi, A.M. , Besses, C. , Gisslinger, H. , Samuelsson, J. , Verstovsek, S. , Hoffman, R. , Pardanani, A. , Cervantes, F. , Tefferi, A. & Barbui, T. (2013) Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG‐MRT consensus project. Blood, 121, 4778–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin, R.H. , Turk, D.C. , Farrar, J.T. , Haythornthwaite, J.A. , Jensen, M.P. , Katz, N.P. , Kerns, R.D. , Stucki, G. , Allen, R.R. , Bellamy, N. , Carr, D.B. , Chandler, J. , Cowan, P. , Dionne, R. , Galer, B.S. , Hertz, S. , Jadad, A.R. , Kramer, L.D. , Manning, D.C. , Martin, S. , McCormick, C.G. , McDermott, M.P. , McGrath, P. , Quessy, S. , Rappaport, B.A. , Robbins, W. , Robinson, J.P. , Rothman, M. , Royal, M.A. , Simon, L. , Stauffer, J.W. , Stein, W. , Tollett, J. , Wernicke, J. & Witter, J. ; Immpact . (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain, 113, 9–19. [DOI] [PubMed] [Google Scholar]
- Emanuel, R.M. , Dueck, A.C. , Geyer, H.L. , Kiladjian, J.J. , Slot, S. , Zweegman, S. , te Boekhorst, P.A. , Commandeur, S. , Schouten, H.C. , Sackmann, F. , Kerguelen Fuentes, A. , Hernandez‐Maraver, D. , Pahl, H.L. , Griesshammer, M. , Stegelmann, F. , Doehner, K. , Lehmann, T. , Bonatz, K. , Reiter, A. , Boyer, F. , Etienne, G. , Ianotto, J.C. , Ranta, D. , Roy, L. , Cahn, J.Y. , Harrison, C.N. , Radia, D. , Muxi, P. , Maldonado, N. , Besses, C. , Cervantes, F. , Johansson, P.L. , Barbui, T. , Barosi, G. , Vannucchi, A.M. , Passamonti, F. , Andreasson, B. , Ferarri, M.L. , Rambaldi, A. , Samuelsson, J. , Birgegard, G. , Tefferi, A. & Mesa, R.A. (2012) Myeloproliferative Neoplasm (MPN) Symptom Assessment Form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. Journal of Clinical Oncology, 30, 4098–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer, H. , Scherber, R. , Kosiorek, H. , Dueck, A.C. , Kiladjian, J.J. , Xiao, Z. , Slot, S. , Zweegman, S. , Sackmann, F. , Fuentes, A.K. , Hernandez‐Maraver, D. , Dohner, K. , Harrison, C.N. , Radia, D. , Muxi, P. , Besses, C. , Cervantes, F. , Johansson, P.L. , Andreasson, B. , Rambaldi, A. , Barbui, T. , Bonatz, K. , Reiter, A. , Boyer, F. , Etienne, G. , Ianotto, J.C. , Ranta, D. , Roy, L. , Cahn, J.Y. , Maldonado, N. , Barosi, G. , Ferrari, M.L. , Gale, R.P. , Birgegard, G. , Xu, Z. , Zhang, Y. , Sun, X. , Xu, J. , Zhang, P. , Te Boekhorst, P.A. , Commandeur, S. , Schouten, H. , Pahl, H.L. , Griesshammer, M. , Stegelmann, F. , Lehmann, T. , Senyak, Z. , Vannucchi, A.M. , Passamonti, F. , Samuelsson, J. & Mesa, R.A. (2016) Symptomatic profiles of patients with polycythemia vera: implications of inadequately controlled disease. Journal of Clinical Oncology, 34, 151–159. [DOI] [PubMed] [Google Scholar]
- Harrison, C. , Kiladjian, J.J. , Al‐Ali, H.K. , Gisslinger, H. , Waltzman, R. , Stalbovskaya, V. , McQuitty, M. , Hunter, D.S. , Levy, R. , Knoops, L. , Cervantes, F. , Vannucchi, A.M. , Barbui, T. & Barosi, G. (2012) JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. New England Journal of Medicine, 366, 787–798. [DOI] [PubMed] [Google Scholar]
- Johansson, P. , Mesa, R. , Scherber, R. , Abelsson, J. , Samuelsson, J. , Birgegard, G. & Andreasson, B. (2012) Association between quality of life and clinical parameters in patients with myeloproliferative neoplasms. Leukemia & Lymphoma, 53, 441–444. [DOI] [PubMed] [Google Scholar]
- Marchioli, R. , Finazzi, G. , Specchia, G. , Cacciola, R. , Cavazzina, R. , Cilloni, D. , De Stefano, V. , Elli, E. , Iurlo, A. , Latagliata, R. , Lunghi, F. , Lunghi, M. , Marfisi, R.M. , Musto, P. , Masciulli, A. , Musolino, C. , Cascavilla, N. , Quarta, G. , Randi, M.L. , Rapezzi, D. , Ruggeri, M. , Rumi, E. , Scortechini, A.R. , Santini, S. , Scarano, M. , Siragusa, S. , Spadea, A. , Tieghi, A. , Angelucci, E. , Visani, G. , Vannucchi, A.M. & Barbui, T. ; CYTO‐PV Collaborative Group . (2013) Cardiovascular events and intensity of treatment in polycythemia vera. New England Journal of Medicine, 368, 22–33. [DOI] [PubMed] [Google Scholar]
- Mesa, R.A. , Niblack, J. , Wadleigh, M. , Verstovsek, S. , Camoriano, J. , Barnes, S. , Tan, A.D. , Atherton, P.J. , Sloan, J.A. & Tefferi, A. (2007) The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international internet‐based survey of 1179 MPD patients. Cancer, 109, 68–76. [DOI] [PubMed] [Google Scholar]
- Mesa, R.A. , Gotlib, J. , Gupta, V. , Catalano, J.V. , Deininger, M.W. , Shields, A.L. , Miller, C.B. , Silver, R.T. , Talpaz, M. , Winton, E.F. , Harvey, J.H. , Hare, T. , Erickson‐Viitanen, S. , Sun, W. , Sandor, V. , Levy, R.S. , Kantarjian, H.M. & Verstovsek, S. (2013) Effect of ruxolitinib therapy on myelofibrosis‐related symptoms and other patient‐reported outcomes in COMFORT‐I: a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Oncology, 31, 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa, R. , Miller, C.B. , Mascarenhas, J.O. , Thyne, M. , Goldberger, S. , Paranagama, D.C. , Parasuraman, S.V. , Fazal, S. , Naim, A. & Mangan, J. (2015) Hydroxyurea treatment history and quality of life in patients with polycythemia vera: results from the MPN Landmark survey in the United States. Blood (ASH Annual Meeting Abstracts), 126, abstract 4077. [Google Scholar]
- Mesa, R. , Verstovsek, S. , Kiladjian, J.‐J. , Griesshammer, M. , Masszi, T. , Durrant, S. , Passamonti, F. , Harrison, C.N. , Pane, F. , Zachee, P. , Zhen, H. , Jones, M.M. , Parasuraman, S. , Li, J. , Côté, I. , Habr, D. & Vannucchi, A.M. (2016) Changes in quality of life and disease‐related symptoms in patients with polycythemia vera receiving ruxolitinib or standard therapy. European Journal of Haematology, 97, 192–200. [DOI] [PubMed] [Google Scholar]
- Najean, Y. & Rain, J.D. (1997) Treatment of polycythemia vera: the use of hydroxyurea and pipobroman in 292 patients under the age of 65 years. Blood, 90, 3370–3377. [PubMed] [Google Scholar]
- National Cancer Institute . Common Terminology Criteria for Adverse Events v3.0. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed February 26, 2016. [DOI] [PMC free article] [PubMed]
- Pourcelot, E. , Trocme, C. , Mondet, J. , Bailly, S. , Toussaint, B. & Mossuz, P. (2014) Cytokine profiles in polycythemia vera and essential thrombocythemia patients: clinical implications. Experimental Hematology, 42, 360–368. [DOI] [PubMed] [Google Scholar]
- Scherber, R. , Dueck, A.C. , Kiladjian, J.J. , Slot, S.S. , Zweegman, S. , Boekhorst, P. , Commandeur, S. , Schouten, H. , Sackmann, F. , Fuentes, A.K. , Hernández‐Maraver, D. , Pahl, H.L. , Greiesshammer, M. , Stegelmann, F. , Doehner, K. , Lehmann, T. , Bonatz, K. , Reiter, A. , Boyer, F. , Etienne, G. , Ianotto, J.C. , Roy, L. , Cahn, J.Y. , Harrison, C. , Radia, D. , Muxi, P. , Maldonado, N. , Besses, C. , Cervantes, F. , Johansson, P.L. , Barbui, T. , Barosi, G. , Vannucchi, A. , Passamonti, F. , Andreasson, B. , Ferarri, M. , Rambaldi, A. , Samuelsson, J. , Birgegard, G. , Tefferi, A. & Mesa, R.A. (2012) Conventional therapeutic options have limited impact on MPN symptoms: insights from a prospective analysis of the MPN‐SAF. Haematologica (EHA Annual Meeting Abstracts), 97 (s1), 147 (abstract 366). [Google Scholar]
- Siegel, F.P. , Tauscher, J. & Petrides, P.E. (2013) Aquagenic pruritus in polycythemia vera: characteristics and influence on quality of life in 441 patients. American Journal of Hematology, 88, 665–669. [DOI] [PubMed] [Google Scholar]
- Squires, M. , Harrison, C.N. , Barosi, G. , Vannucchi, A.M. , Barbui, T. , Gisslinger, H. , Passamonti, F. , Al‐Ali, H.K. , Kiladjian, J.‐J. , Marker, M.T. , Mendelson, E.T. , Stalbovskaya, V. , Cervantes, F. & Knoops, L. (2013) The relationship between cytokine levels and symptoms in patients (pts) with myelofibrosis (MF) from COMFORT‐II, a phase 3 study of ruxolitinib (RUX) vs best available therapy (BAT) [abstract]. Blood, 122, 4070. [Google Scholar]
- Tefferi, A. & Vardiman, J.W. (2008) Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point‐of‐care diagnostic algorithms. Leukemia, 22, 14–22. [DOI] [PubMed] [Google Scholar]
- Tefferi, A. , Vaidya, R. , Caramazza, D. , Finke, C. , Lasho, T. & Pardanani, A. (2011) Circulating interleukin (IL)‐8, IL‐2R, IL‐12, and IL‐15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. Journal of Clinical Oncology, 29, 1356–1363. [DOI] [PubMed] [Google Scholar]
- Vaidya, R. , Gangat, N. , Jimma, T. , Finke, C.M. , Lasho, T.L. , Pardanani, A. & Tefferi, A. (2012) Plasma cytokines in polycythemia vera: phenotypic correlates, prognostic relevance, and comparison with myelofibrosis. American Journal of Hematology, 87, 1003–1005. [DOI] [PubMed] [Google Scholar]
- Vannucchi, A.M. (2014) How I treat polycythemia vera. Blood, 124, 3212–3220. [DOI] [PubMed] [Google Scholar]
- Vannucchi, A.M. , Kiladjian, J.J. , Griesshammer, M. , Masszi, T. , Durrant, S. , Passamonti, F. , Harrison, C.N. , Pane, F. , Zachee, P. , Mesa, R. , He, S. , Jones, M.M. , Garrett, W. , Li, J. , Pirron, U. , Habr, D. & Verstovsek, S. (2015) Ruxolitinib versus standard therapy for the treatment of polycythemia vera. New England Journal of Medicine, 372, 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek, S. , Mesa, R.A. , Gotlib, J. , Levy, R.S. , Gupta, V. , DiPersio, J.F. , Catalano, J.V. , Deininger, M. , Miller, C. , Silver, R.T. , Talpaz, M. , Winton, E.F. , Harvey, J.H. Jr , Arcasoy, M.O. , Hexner, E. , Lyons, R.M. , Paquette, R. , Raza, A. , Vaddi, K. , Erickson‐Viitanen, S. , Koumenis, I.L. , Sun, W. , Sandor, V. & Kantarjian, H.M. (2012) A double‐blind, placebo‐controlled trial of ruxolitinib for myelofibrosis. New England Journal of Medicine, 366, 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek, S. , Passamonti, F. , Rambaldi, A. , Barosi, G. , Rosen, P.J. , Rumi, E. , Gattoni, E. , Pieri, L. , Guglielmelli, P. , Elena, C. , He, S. , Contel, N. , Mookerjee, B. , Sandor, V. , Cazzola, M. , Kantarjian, H.M. , Barbui, T. & Vannucchi, A.M. (2014) A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer, 120, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]


