Abstract
Introduction
Despite multiple studies reporting marked benefit of botulinum toxin (BTX) for treatment of cricopharyngeal dysphagia, little is known about its safety for this indication. We examined the safety of cricopharyngeal BTX for dysphagia in oculopharyngeal muscular dystrophy (OPMD).
Methods
We reviewed records of patients with OPMD who received cricopharyngeal BTX.
Results
Twenty-four patients underwent 66 procedures. Overall adverse event frequency was 44%. The most common adverse events were dysphonia (24%) and worsened dysphagia (14%). Logistic regression demonstrated that dose was a significant predictor of worsened dysphagia (P = 0.036) and of the composite event of dysphonia or worsened dysphagia (P = 0.009). There was a nonsignificant trend for dose as a predictor of dysphonia (P = 0.073). 59% of procedures were associated with symptomatic improvement.
Conclusions
While BTX appears to be beneficial for treatment of dysphagia in OPMD, caution is warranted when injecting the cricopharyngeus muscle due to dose-related risk of dysphonia or worsened dysphagia.
Keywords: botulinum toxin, dysphagia, neuromuscular diseases, oculopharyngeal muscular dystrophy, therapeutics
Oculopharyngeal muscular dystrophy (OPMD) is a late-onset, autosomal dominant muscle disease characterized by dysphagia, ptosis, and limb weakness.1,2 Radiologic and manometric studies of OPMD have shown that the upper esophageal sphincter (UES) obstructs bolus transit.3,4 The cricopharyngeus (CP) muscle, the major component of the UES, is normally tonically active at rest and relaxes during swallow.5 In OPMD, weak pharyngeal contractions are ineffective in transporting boluses across the UES.6,7 Surgical myotomy of the UES lowers sphincter pressure and improves swallowing in OPMD6-11 but poses serious risks, including postsurgical fistula, pneumonia, and death.12
An alternative to surgery is chemical myotomy using botulinum toxin (BTX). To date, 33 observational studies have reported 300 adults, including 6 with myopathy,13–17 who received BTX for cricopharyngeal dysphagia of disparate causes. The aggregate improvement rate was 73% (see Supp. Table S1, which is available online). All 6 patients with myopathy improved, including 2 with OPMD.13,14
Given the proximity of the CP muscle to posterior arytenoid and inferior pharyngeal muscles and the risk of local diffusion of BTX, transient dysphonia and worsened dysphagia are expected adverse events (AEs).18 Remarkably, only 11% of individuals in prior studies experienced any AE, and only 3% experienced dysphonia or worsened dysphagia (Supp. Table S1). No prior study of cricopharyngeal BTX examined the relationship between dose and AEs.
We encounter many patients with OPMD at our center due to high disease prevalence in New Mexico.19 We sought to better characterize risks associated with cricopharyngeal BTX in OPMD.
MATERIALS AND METHODS
We reviewed health records of all patients with a confirmed diagnosis of OPMD20 who had at least 1 cricopharyngeal BTX injection at our center between January 1, 2000, and December 31, 2011. This study was approved by the local institutional review board. Requirement for informed consent was waived because the study was a retrospective chart review. AEs and symptomatic improvement were ascertained by reviewing notes in the 6-month period after each procedure. Two of the 66 procedures (3%) were not followed by a clinic visit. In these cases, missing data were imputed as follows: no AE and no improvement.
We used generalized linear mixed models (GLIMMIX in SAS 9.3) to identify predictors of worsened dysphagia, dysphonia, and a composite event defined as dysphonia or worsened dysphagia. These models account for correlations due to repeated procedures on the same individual. If the likelihood ratio test of the random effect (individual patient) in the generalized linear mixed model was not significant, then the model was reduced to simple logistic regression, where each procedure was considered to be independent. All initial models included the following variables: age, gender, dose, procedure type (percutaneous or endoscopic), injection site (bilateral or unilateral), and time since last injection. We included the last term to account for residual effects from any previous injection. We used backward elimination to remove nonsignificant predictors. P-values <0.05 were considered significant.
RESULTS
Sixty-six BTX injections were administered to 24 patients with OPMD (mean age, 63±68 years, mean age of dysphagia onset 52±65 years, 14 men, 100% Hispanic). Of 19 patients who had videofluoroscopic swallowing studies before the first injection, 14 had CP prominence or reduced CP opening.
The median number of procedures per patient was 3 (range, 1–13). For individuals with more than 1 procedure, the median time between treatments was 6 months (range, 2–39 months). Median onabotulinumtoxinA (Botox®, Allergan, Irvine, California) dose was 20 units (range, 10–30 units). All but 3 procedures were performed by the same otolaryngologist. Toxin was injected either percutaneously with electromyographic guidance (88%) or endoscopically with direct visualization (12%). Sixty-eight percent of procedures involved bilateral cricopharyngeal injections; the rest were unilateral.
AEs occurred after 44% of procedures, and 19 of 24 individuals had at least 1 AE. The most common AEs were transient dysphonia and worsened dysphagia, which occurred after 24% and 14% of procedures, respectively. Other AEs included dizziness or syncope (8%), reflux (5%), injection site pain (5%), rash (2%), and laryngospasm (2%). In 10 cases, the duration of dysphonia after treatment was recorded and ranged from 1 week to 4 months. There were 2 serious AEs requiring brief hospital visits (1 patient with 3 episodes of syncope immediately after the procedure and a second patient with laryngospasm and respiratory distress within 1 day of the procedure).
Likelihood ratio tests of the random effect (individual patient) in the generalized linear mixed models were not significant for any of the 3 dependent variables (worsened dysphagia: P = 0.15, dysphonia: P = 0.34, composite event of dysphonia or worsened dysphagia: P = 0.23). Logistic regression demonstrated that dose is a significant predictor of worsened dysphagia (P = 0.036) and of the composite event (P = 0.009). There was a nonsignificant trend for dose as a predictor of dysphonia alone (P = 0.073, see Figure 1). Age, gender, procedure type, injection site, and time since last injection were not significant predictors in any model. For a 10-unit increase in dose, the odds ratio (OR) for worsened dysphagia was 3.92 (95 % CI, 1.09–14.05), the OR for dysphonia was 2.40 (95% CI, 0.92-6.26), and the OR for the composite event was 3.49 (95% CI, 1.36-8.95).
FIGURE 1.
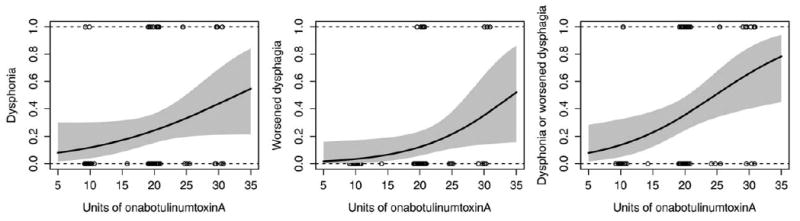
Logistic dose-response models were fit to the data. Estimated probabilities of dysphonia, worsened dysphagia, and the composite event of dysphonia or worsened dysphagia increase as a function of dose. The y-axis represents estimated probabilities. Solid black curves represent the results of the models for each adverse event. Gray regions represent 95% confidence intervals. Open circles indicate actual doses for each procedure and are displayed with jitter to make identical values visible.
Physicians documented symptom improvement following 59% of procedures.
DISCUSSION
This study examined the safety of BTX for treatment of cricopharyngeal dysphagia in a large number of cases with a single myopathy. Our observed 44% AE rate contrasts with the 11% AE rate reported in prior studies (Supp. Table S1).
Doses in prior studies varied from 5 to 120 Botox® -equivalent units. While some have predicted that injection of 100 units in the CP muscle would likely cause dysphonia and worsened dysphagia through local toxin diffusion,18 ≥100 units in prior studies were reported to experience an AE (Supp. Table S1). Furthermore, only 3% of 300 individuals in prior studies were reported to experience dysphonia or worsened dysphagia, and no study demonstrated a link between dose and AEs (Supp. Table S1). In contrast, our study shows that dysphonia and worsened dysphagia are dose-related AEs, and provides estimates for the magnitude of AE risk at different doses.
AE risk may depend on the etiology of dysphagia. Given the peripheral mechanism of action of BTX, individuals with muscle or lower motor neuron disorders may be more prone to AEs. Yet, none of 6 individuals with myopathy who received cricopharyngeal BTX in prior studies experienced dysphonia or worsened dysphagia.13–17 Moreover, a recent study that evaluated the benefit of cricopharyngeal BTX in 20 patients with amyotrophic lateral sclerosis reported no complications in the sub-group with lower motor neuron bulbar weakness.21 Our study quantifies the risks of cricopharyngeal BTX specifically in muscle disease.
Although this study did not assess efficacy, since there was no untreated comparison group and validated dysphagia outcome measures were not used, subjective improvement followed 59% of procedures. Given potential for benefit, a controlled trial is warranted. Until then, we emphasize the need for caution when administering BTX to the CP muscle.
Supplementary Material
Acknowledgments
This project was supported in part by the Muscular Dystrophy Association (Clinical Research Training Grant) and the NIH/NCRR/NCATS (University of New Mexico Clinical and Translational Science Center, 8UL1TR000041). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- AE
adverse event
- BTX
botulinum toxin
- CI
confidence interval
- CP
cricopharyngeus
- OR
odds ratio
- OPMD
oculopharyngeal muscular dystrophy
- UES
upper esophageal sphincter
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Taylor EW. Progressive vagus-glossopharyngeal paralysis with ptosis: a contribution to the group of family diseases. J Nerve Ment Dis. 1915;42:129–139. [Google Scholar]
- 2.Victor M, Hayes R, Adams RD. Oculopharyngeal muscular dystrophy. A familial disease of late life characterized by dysphagia and progressive ptosis of the evelids. N Engl J Med. 1962;267:1267–1272. doi: 10.1056/NEJM196212202672501. [DOI] [PubMed] [Google Scholar]
- 3.Bender MD. Esophageal manometry in oculopharyngeal dystrophy. Am J Gastroenterol. 1976;65:215–221. [PubMed] [Google Scholar]
- 4.O’Laughlin JC, Bredfeldt JE, Gray JE. Hypertonic upper esophageal sphincter in the oculopharyngeal syndrome. J Clin Gastroenterol. 1980;2:93–98. doi: 10.1097/00004836-198003000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Ertekin C, Aydogdu I. Electromyography of human cricopharyngeal muscle of the upper esophageal sphincter. Muscle Nerve. 2002;26:729–739. doi: 10.1002/mus.10267. [DOI] [PubMed] [Google Scholar]
- 6.Duranceau A, Forand MD, Fauteux JP. Surgery in oculopharyngeal muscular dystrophy. Am J Surg. 1980;139:33–39. doi: 10.1016/0002-9610(80)90226-3. [DOI] [PubMed] [Google Scholar]
- 7.Fradet G, Pouliot D, Lavoie S, St-Pierre S. Inferior constrictor myotomy in oculopharyngeal muscular dystrophy: clinical and manometric evaluation. J Otolaryngol. 1988;17:68–73. [PubMed] [Google Scholar]
- 8.Peterman AF, Lillington GA, Jamplis RW. Progressive muscular dystrophy with ptosis and dysphagia. Arch Neurol. 1964;10:38–41. doi: 10.1001/archneur.1964.00460130042005. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery WW, Lynch JP. Oculopharyngeal muscular dystrophy treated by inferior constrictor myotomy. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:986–993. [PubMed] [Google Scholar]
- 10.Taillefer R, Duranceau AC. Manometric and radionuclide assessment of pharyngeal emptying before and after cricopharyngeal myotomy in patients with oculopharyngeal muscular dystrophy. J Thorac Cardiovasc Surg. 1988;95:868–875. [PubMed] [Google Scholar]
- 11.Gomez-Torres A, Abrante Jimenez A, Rivas Infante E, Menoyo Bueno A, Tirado Zamora I, Esteban Ortega F. Cricopharyngeal myotomy in the treatment of oculopharyngeal muscular dystrophy. Acta Otorrinolaringol Esp. 2012;63:465–469. doi: 10.1016/j.otorri.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Brigand C, Ferraro P, Martin J, Duranceau A. Risk factors in patients undergoing cricopharyngeal myotomy. Br J Surg. 2007;94:978–983. doi: 10.1002/bjs.5760. [DOI] [PubMed] [Google Scholar]
- 13.Restivo DA, Marchese Ragona R, Staffieri A, de Grandis D. Successful botulinum toxin treatment of dysphagia in oculopharyngeal muscular dystrophy. Gastroenterology. 2000;119:1416. doi: 10.1053/gast.2000.20113. [DOI] [PubMed] [Google Scholar]
- 14.Shaw GY, Searl JP. Botulinum toxin treatment for cricopharyngeal dysfunction. Dysphagia. 2001;16:161–167. doi: 10.1007/s00455-001-0074-8. [DOI] [PubMed] [Google Scholar]
- 15.Haapaniemi JJ, Laurikainen EA, Pulkkinen J, Marttila RJ. Botulinum toxin in the treatment of cricopharyngeal dysphagia. Dysphagia. 2001;16:171–175. doi: 10.1007/s00455-001-0059-7. [DOI] [PubMed] [Google Scholar]
- 16.Liu LW, Tarnopolsky M, Armstrong D. Injection of botulinum toxin A to the upper esophageal sphincter for oropharyngeal dysphagia in two patients with inclusion body myositis. Can J Gastroenterol. 2004;18:397–399. doi: 10.1155/2004/360537. [DOI] [PubMed] [Google Scholar]
- 17.Alberty J, Oelerich M, Ludwig K, Hartmann S, Stoll W. Efficacy of botulinum toxin A for treatment of upper esophageal sphincter dysfunction. Laryngoscope. 2000;110:1151–1156. doi: 10.1097/00005537-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Marchese-Ragona R, Marioni G, Restivo DA, Staffieri A. Solving dysphagia due to cricopharyngeal muscle dysfunction with botulinum toxin. Eur Arch Otorhinolaryngol. 2005;262:250–251. doi: 10.1007/s00405-004-0776-2. [DOI] [PubMed] [Google Scholar]
- 19.Becher MW, Morrison L, Davis LE, Maki WC, King MK, Bicknell JM, et al. Oculopharyngeal muscular dystrophy in Hispanic New Mexicans. JAMA. 2001;286:2437–2440. doi: 10.1001/jama.286.19.2437. [DOI] [PubMed] [Google Scholar]
- 20.Brais B. Oculopharyngeal muscular dystrophy. In: Griggs RC, Amato AA, editors. Handbook of clinical neurology. New York: Elsevier; 2011. pp. 181–192. [DOI] [PubMed] [Google Scholar]
- 21.Restivo DA, Casabona A, Nicotra A, Zappia M, Elia M, Romano MC, et al. ALS dysphagia pathophysiology: differential botulinum toxin response. Neurology. 2013;80:616–620. doi: 10.1212/WNL.0b013e318281cc1b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


