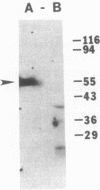
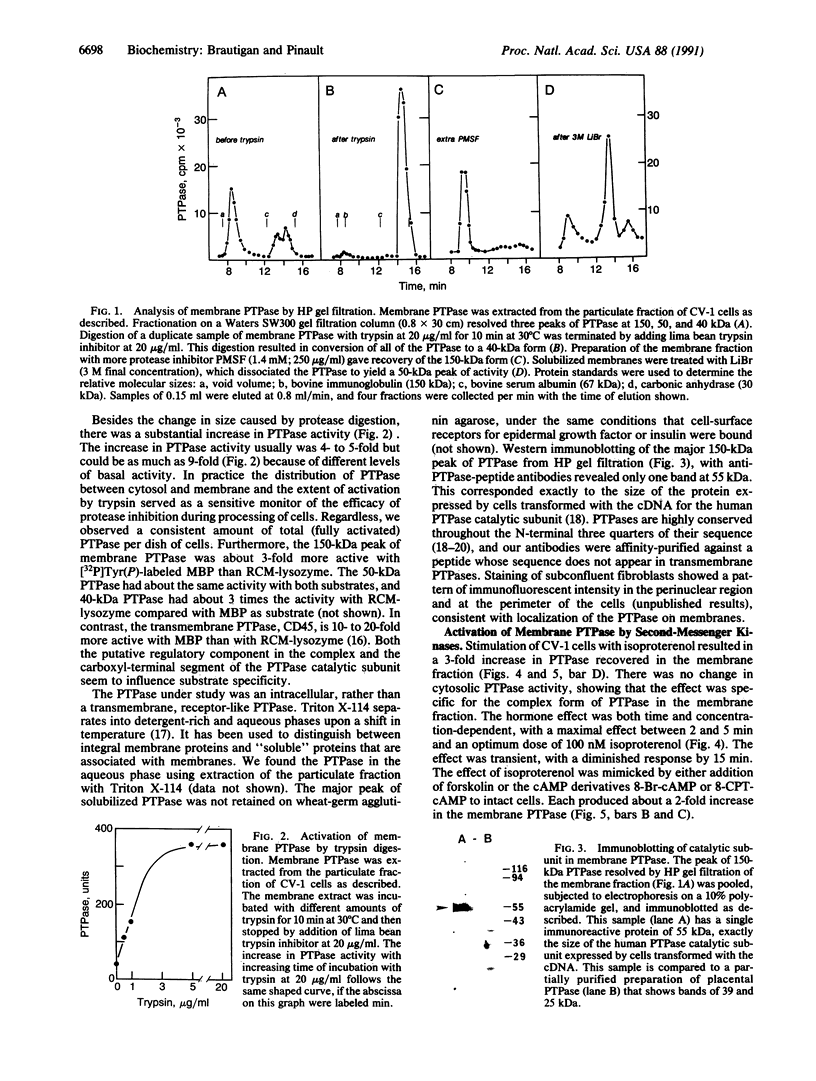
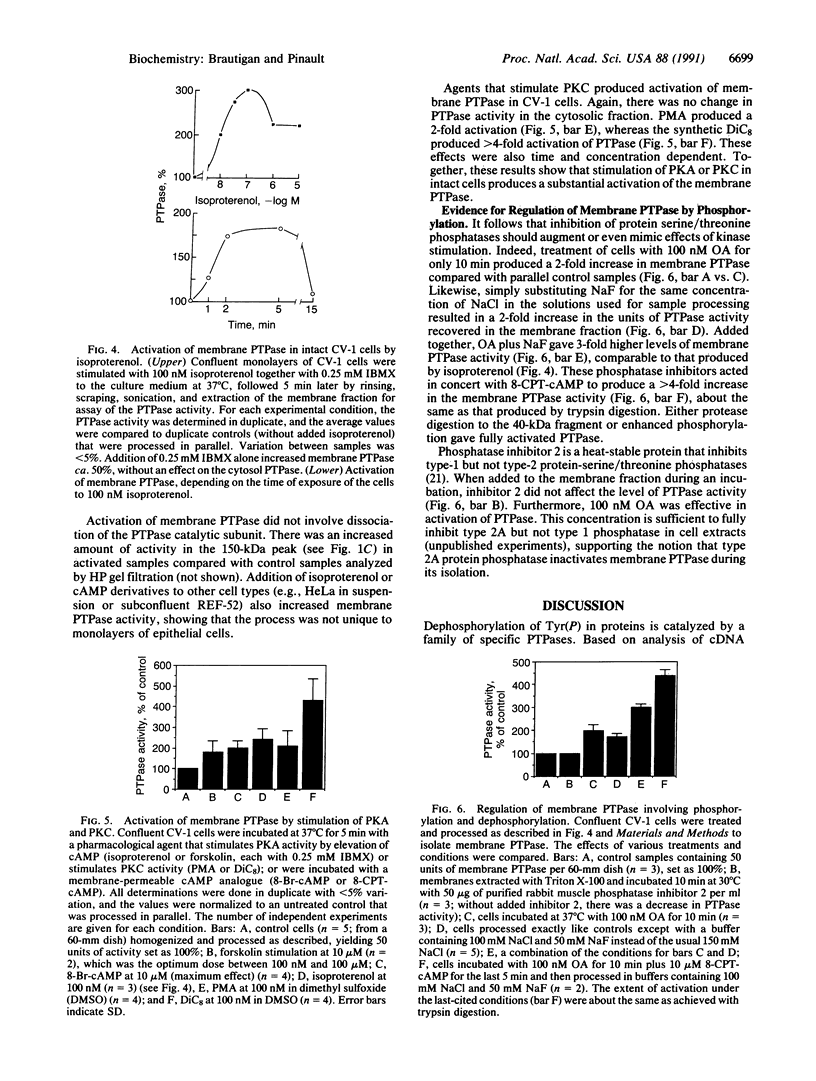
Abstract
Essential to signal transduction are mechanisms of "cross-talk" to coordinate different pathways. This study shows that stimulation of serine/threonine protein kinases activates protein-tyrosine phosphatase (PTPase; protein-tyrosine-phosphate phosphohydrolase, EC 3.1.3.48). More than 95% of intracellular PTPase was in the particulate fraction of various cell lines and was extracted with detergent as a 150-kDa complex that contained a 55-kDa catalytic subunit. The complex was activated by protease digestion, which changed its substrate specificity coincident with reduction in size. The complex was dissociated by treatment of the membrane fraction with 3 M LiBr. Treatment of intact cells with isoproterenol, forskolin, or cAMP analogues to stimulate cAMP-dependent protein kinase (PKA) or with phorbol ester or dioctanoylglycerol to stimulate Ca2+/phospholipid-dependent protein kinase (PKC) produced activation of membrane PTPase complex without a change in its size. Inhibition of protein-serine/threonine phosphatases with okadaic acid or fluoride also resulted in activation of the membrane PTPase. These results support a model for regulation of PTPase by phosphorylation and dephosphorylation of serine/threonine residues in a regulatory component complexed with the 55-kDa PTPase catalytic subunit. This mechanism may be important in regulating sensitivity to extracellular signals transduced via tyrosine phosphorylation and in the synchronization of events during the cell cycle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R. The role of phosphatases in signal transduction. New Biol. 1990 Dec;2(12):1049–1062. [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bowen W. D., Kirschner B. N., Newman A. H., Rice K. C. Sigma receptors negatively modulate agonist-stimulated phosphoinositide metabolism in rat brain. Eur J Pharmacol. 1988 May 10;149(3):399–400. doi: 10.1016/0014-2999(88)90678-4. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Bornstein P., Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J Biol Chem. 1981 Jul 10;256(13):6519–6522. [PubMed] [Google Scholar]
- Brown-Shimer S., Johnson K. A., Lawrence J. B., Johnson C., Bruskin A., Green N. R., Hill D. E. Molecular cloning and chromosome mapping of the human gene encoding protein phosphotyrosyl phosphatase 1B. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5148–5152. doi: 10.1073/pnas.87.13.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K., Kumar S., Diltz C. D., Harrylock M., Cool D. E., Krebs E. G., Fischer E. H., Walsh K. A. Human placenta protein-tyrosine-phosphatase: amino acid sequence and relationship to a family of receptor-like proteins. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5252–5256. doi: 10.1073/pnas.86.14.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff J., Schievella A. R., Jost C. A., Erikson R. L., Neel B. G. Cloning of a cDNA for a major human protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2735–2739. doi: 10.1073/pnas.87.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool D. E., Tonks N. K., Charbonneau H., Fischer E. H., Krebs E. G. Expression of a human T-cell protein-tyrosine-phosphatase in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7280–7284. doi: 10.1073/pnas.87.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli E., Kiss Z., Kuo J. F. Cooperative interactions of protein kinase C and cAMP-dependent protein kinase systems in human promyelocytic leukemia HL60 cells. FEBS Lett. 1988 Apr 25;231(2):407–412. doi: 10.1016/0014-5793(88)80860-3. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Newport J. W. Fission yeast p13 blocks mitotic activation and tyrosine dephosphorylation of the Xenopus cdc2 protein kinase. Cell. 1989 Jul 14;58(1):181–191. doi: 10.1016/0092-8674(89)90414-5. [DOI] [PubMed] [Google Scholar]
- Frank D. A., Sartorelli A. C. Regulation of protein phosphotyrosine content by changes in tyrosine kinase and protein phosphotyrosine phosphatase activities during induced granulocytic and monocytic differentiation of HL-60 leukemia cells. Biochem Biophys Res Commun. 1986 Oct 15;140(1):440–447. doi: 10.1016/0006-291x(86)91110-1. [DOI] [PubMed] [Google Scholar]
- Gautier J., Matsukawa T., Nurse P., Maller J. Dephosphorylation and activation of Xenopus p34cdc2 protein kinase during the cell cycle. Nature. 1989 Jun 22;339(6226):626–629. doi: 10.1038/339626a0. [DOI] [PubMed] [Google Scholar]
- Gruppuso P. A., Boylan J. M., Smiley B. L., Fallon R. J., Brautigan D. L. Hepatic protein tyrosine phosphatases in the rat. Biochem J. 1991 Mar 1;274(Pt 2):361–367. doi: 10.1042/bj2740361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruppuso P. A., Johnson G. L., Constantinides M., Brautigan D. L. Phosphorylase phosphatase regulatory subunit. "Western" blotting with immunoglobulins against inhibitor-2 reveals a protein of Mr = 60,000. J Biol Chem. 1985 Apr 10;260(7):4288–4294. [PubMed] [Google Scholar]
- Guan K. L., Haun R. S., Watson S. J., Geahlen R. L., Dixon J. E. Cloning and expression of a protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1501–1505. doi: 10.1073/pnas.87.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard M. J., Cohen P. Regulation of protein phosphatase-1G from rabbit skeletal muscle. 1. Phosphorylation by cAMP-dependent protein kinase at site 2 releases catalytic subunit from the glycogen-bound holoenzyme. Eur J Biochem. 1989 Dec 22;186(3):701–709. doi: 10.1111/j.1432-1033.1989.tb15263.x. [DOI] [PubMed] [Google Scholar]
- Hubbard M. J., Cohen P. Regulation of protein phosphatase-1G from rabbit skeletal muscle. 2. Catalytic subunit translocation is a mechanism for reversible inhibition of activity toward glycogen-bound substrates. Eur J Biochem. 1989 Dec 22;186(3):711–716. doi: 10.1111/j.1432-1033.1989.tb15264.x. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. The phosphorylation of proteins: a major mechanism for biological regulation. Fourteenth Sir Frederick Gowland Hopkins memorial lecture. Biochem Soc Trans. 1985 Oct;13(5):813–820. doi: 10.1042/bst0130813. [DOI] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Draetta G., Beach D., Wang J. Y. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell. 1989 Jul 14;58(1):193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- Pumiglia K. M., Huang C. K., Feinstein M. B. Elevation of cAMP, but not cGMP, inhibits thrombin-stimulated tyrosine phosphorylation in human platelets. Biochem Biophys Res Commun. 1990 Sep 14;171(2):738–745. doi: 10.1016/0006-291x(90)91208-a. [DOI] [PubMed] [Google Scholar]
- Roome J., O'Hare T., Pilch P. F., Brautigan D. L. Protein phosphotyrosine phosphatase purified from the particulate fraction of human placenta dephosphorylates insulin and growth-factor receptors. Biochem J. 1988 Dec 1;256(2):493–500. doi: 10.1042/bj2560493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg S. A., Brautigan D. L. Membrane protein phosphotyrosine phosphatase in rabbit kidney. Proteolysis activates the enzyme and generates soluble catalytic fragments. Biochem J. 1987 May 1;243(3):747–754. doi: 10.1042/bj2430747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. The epidermal growth factor receptor as a multifunctional allosteric protein. Biochemistry. 1988 May 3;27(9):3119–3123. doi: 10.1021/bi00409a002. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Nairn A. C. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- Shriner C. L., Brautigan D. L. Cytosolic protein phosphotyrosine phosphatases from rabbit kidney. Purification of two distinct enzymes that bind to Zn2+-iminodiacetate agarose. J Biol Chem. 1984 Sep 25;259(18):11383–11390. [PubMed] [Google Scholar]
- Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. Cyclin activation of p34cdc2. Cell. 1990 Nov 30;63(5):1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Thai T., Tang M., Saito H. Distinct functional roles of the two intracellular phosphatase like domains of the receptor-linked protein tyrosine phosphatases LCA and LAR. EMBO J. 1990 Aug;9(8):2399–2407. doi: 10.1002/j.1460-2075.1990.tb07415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Tsai A. Y., Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. CD45, an integral membrane protein tyrosine phosphatase. Characterization of enzyme activity. J Biol Chem. 1990 Jun 25;265(18):10674–10680. [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Purification of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6722–6730. [PubMed] [Google Scholar]
- Tortora G., Clair T., Katsaros D., Ally S., Colamonici O., Neckers L. M., Tagliaferri P., Jahnsen T., Robins R. K., Cho-Chung Y. S. Induction of megakaryocytic differentiation and modulation of protein kinase gene expression by site-selective cAMP analogs in K-562 human leukemic cells. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2849–2852. doi: 10.1073/pnas.86.8.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. D., Vandenheede J. R., Merlevede W. A simplified procedure for the purification of the protein phosphatase modulator (inhibitor-2) from rabbit skeletal muscle. FEBS Lett. 1981 Sep 28;132(2):293–295. doi: 10.1016/0014-5793(81)81182-9. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]