Abstract
Aim
This study assessed the feasibility and obtrusiveness of measuring salivary oxytocin in preterm infants receiving Kangaroo care, because this is a period of maximal bonding or co‐regulation. We also analysed possible influential determinants, including maternal oxytocin.
Methods
The saliva of preterm infants and their mothers was collected prior to, and during, Kangaroo care using cotton swabs and pooled into vials until sufficient volumes were obtained to measure oxytocin levels using a radioimmunoassay. The obtrusiveness of the infants’ collections was measured with a Likert scale.
Results
Saliva was collected unobtrusively prior to, and during, 30 Kangaroo care sessions in 21 preterm infants. This resulted in three vials with sufficient volumes of before‐Kangaroo care saliva and three with during‐Kangaroo care saliva. Oxytocin was detectable in all six vials. The Kangaroo care duration and the intensity of the mother–infant interaction before and during Kangaroo care seemed to be the most important determinants, and these should preferably be standardised in any future trials.
Conclusion
Oxytocin was measured unobtrusively in the pooled saliva of preterm infants both before and during Kangaroo care and could therefore be investigated as a biomarker in future studies.
Keywords: Bonding, Co‐regulation, Kangaroo care, Oxytocin, Preterm infants
Abbreviation
- NICU
Neonatal intensive care unit
Key notes.
We assessed the feasibility and obtrusiveness of measuring salivary oxytocin in preterm infants receiving Kangaroo care, which is a period of maximal bonding and co‐regulation.
Saliva collections were nonobtrusive, and when the saliva from multiple collections was pooled, sufficient volumes were obtained to measure oxytocin.
Oxytocin was detected both before Kangaroo care and during Kangaroo care, suggesting that oxytocin could be investigated as a biomarker for bonding in future studies.
Introduction
Prematurity impairs bonding, which is neurobiologically defined as the process of co‐regulation 1, 2. Regulation, or homoeostasis, is the constant adjustment of an organism's internal environment to the external environment and co‐regulation, or bonding, is when organisms influence the internal environment of other organisms. This is enabled by specific cues that organisms either consciously or unconsciously express and the sensitivities to these cues that other organisms have 2, 3 (Fig. 1). When it is adequate, co‐regulation is a stabilising and very powerful positive stimulus for organisms. However, the physical immaturity of preterm infants and the mechanical barrier caused by incubators make the cues expressed by preterm infants more difficult to recognise. Furthermore, fear and stress might make parents less receptive to those cues 1, 2. Adequate parent–infant co‐regulation is therefore challenging in the case of prematurity.
Figure 1.
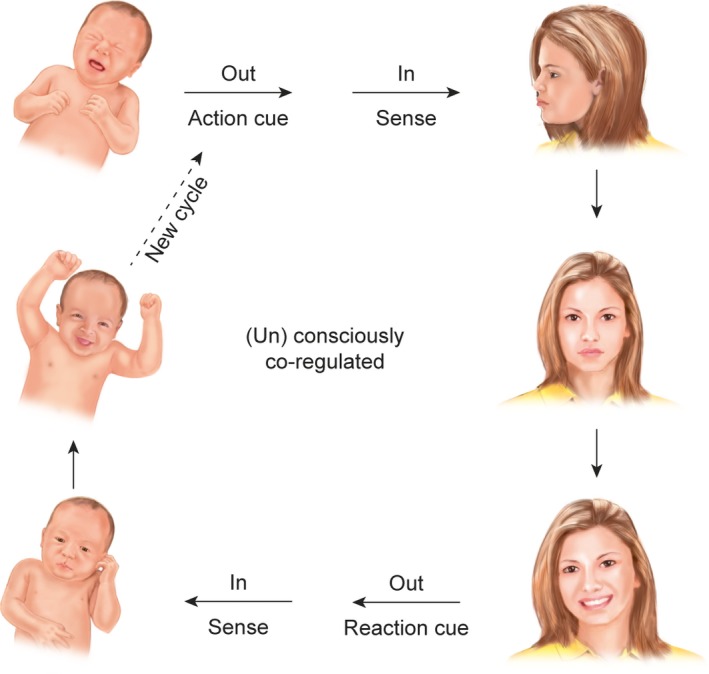
The process of bonding envisioned by a co‐regulation cycle. Bonding is driven by the expression of cues, such as scent, heartbeat, vocalisations, or changes in facial expression (top left). The cue is then perceived (top right), which leads to the co‐regulation of the internal environment of the receiving organism (middle right), resulting in reaction‐cues to be expressed (bottom right), to be perceived (bottom left) and to be (un)consciously used for co‐regulation (middle left). This cycle is virtually endless.
We reasoned that techniques evaluating the co‐regulation or bonding in parents and their preterm infants could lead to improvements in care, as evaluating bonding could lead to optimising bonding and that has been reported to enhance the physiological development of preterm infants 2. It would thus be very valuable to identify nonobtrusively available biological markers that reflect elements of the co‐regulative process of bonding. For this, candidate markers should be studied during a time of maximal parent–infant bonding. In a neonatal intensive care unit (NICU), such a time of maximal bonding is Kangaroo care, when the regulating function of nurses and the incubator is entirely replaced by the co‐regulation of the parental chest 4. The numerous studies in preterm infants that demonstrate both the regulatory and developmental benefits of Kangaroo care support the threefold theory that Kangaroo care is a period of maximal co‐regulation or parent–infant bonding, that this optimal bonding enhances the well‐being of preterm infants and that optimising parent–infant bonding should thus be prioritised in NICUs 2, 4.
The literature on parent–infant bonding is clear about the fact that oxytocin plays an important role in this process 5, 6. The functioning of the oxytocin system has frequently been reported as crucial for the expression of, and readiness for, caregiving behaviours 7, 8, and we therefore selected oxytocin as a biological marker of interest to assess during Kangaroo care. This had been done once before by Cong et al. 9, who showed that after 30 minutes, Kangaroo care appeared to activate a release of oxytocin in the saliva of both mothers and fathers. However, the salivary oxytocin of preterm infants was not measured. The reason for this probably is that both the collection of saliva in preterm infants and the measurement of oxytocin in saliva are challenging. Average collected saliva volumes in preterm infants are very small 10, and oxytocin may be broken down in saliva and thereby avoid detection by oxytocin‐specific antibodies used in commercially available enzyme immunoassays 11. Furthermore, the specificity of those antibodies allows detection of some other oxytocin‐like substances that can be misleading 12, especially when investigating the possibly very low concentrations of oxytocin in the saliva of preterm infants. We hypothesised that some of the barriers of measuring dynamic changes in oxytocin in the saliva of preterm infants could be overcome, by having a specialist laboratory perform a more sensitive radioimmunoassay 12 on the pooled saliva of multiple saliva collections before Kangaroo care and during Kangaroo care. We conducted a pilot study to investigate the feasibility and obtrusiveness of doing this. Determinants that possibly influenced oxytocin responses were analysed as secondary outcomes, such as the duration of Kangaroo care, the intensity of the mother–infant interaction during Kangaroo care, maternal characteristics and the maternal salivary oxytocin responses.
Materials and methods
Study population and design
We included preterm infants with cardiorespiratory stability, who were born between 29 and 36 weeks of gestation and weighing over 1500 g. The exclusion criteria were congenital anomalies, current signs of infection and current contraindications for Kangaroo care. The baseline characteristics can be seen in Table 1. This study was approved by the Medical Ethical Committee of Máxima Medical Centre Veldhoven and conducted according to the principles of the seventh revision of the Declaration of Helsinki in 2013. Once written informed consent had been obtained from the parent(s) of each participant, we agreed on the day that all four saliva collections would be carried out. The first collection was from the infants before Kangaroo care, preferably performed before the mother arrived at the hospital. The second was from the mothers before Kangaroo care, which was obtained immediately after the mother's arrival at the hospital. The two collections during Kangaroo care were performed successively during a Kangaroo care session that was planned by the mother, so that the study did not interfere with the normal maternal caregiving routine. Those collections were performed after at least 30 minutes of Kangaroo care had passed. To minimise the potential effect of feeding on the oxytocin concentration, we aimed to keep the time between both infant saliva collections and the last moment of feeding before that collection equal. Furthermore, the obtrusiveness of the collection procedures was analysed during these two collections.
Table 1.
Characteristics of the 21 preterm infants participating in 30 Kangaroo care sessions
| Characteristics | Mean | Standard deviation |
|---|---|---|
| Gestational age (weeks) | 31 + 3 | 3 + 0 |
| Birthweight (g) | 1705 | 449 |
| Infant age at study participation (weeks) | 35 + 3 | 1 + 2 |
| Infant weight at study participation (g) | 2302 | 314 |
| Post‐natal age (days after birth) | 28.4 | 24.3 |
Assessment of the obtrusiveness
After each infant collection, the researcher asked the nurse about the monitor alarm frequency during the procedure. As differences in alarms were not expected to occur, an additional scale to measure the obtrusiveness was constructed, the so‐called collection intolerance. Because a validated scale for this purpose did not exist, a modified five‐point Likert‐type scale based on the facial expression section of the COMFORTneo scale was generated 13 (Table 2). A form was used to register these Likert scores (Appendix S1). Any particularities during the collections, such as the presence of siblings or guests, possible interruptions or the infant having the hiccups, were also noted on this form. These were in addition to the patient data that were gathered in order to be able to identify factors that altered the collection intolerance, such as the gestational age, postnatal age, birthweight and current weight of the infant. Another purpose of the form was to assemble the information to screen for determinants that could have possibly influenced the oxytocin release, if oxytocin appeared measurable.
Table 2.
Likert scale to score the infant's saliva collection intolerance
| Score | Definition |
|---|---|
| 0 | Continuously normal, relaxed facial expression according to COMFORTneo |
| 1 | Totally relaxed facial expression for almost all of the time (minus 2 seconds) |
| 2 | Relaxed facial expression for most of the time (minus <10 seconds) |
| 3 | A lot of times a not totally relaxed expression (>10 seconds <1 minute) |
| 4 | A continuously intolerant expression or crying (abortion of the collection) |
Assessment of the feasibility
Succeeding in measuring oxytocin was the main criterion for the analysis of the feasibility of a future trial. This depended on the collected saliva volumes and on whether the oxytocin concentration in the saliva of preterm infants exceeded the radioimmunoassay's detection limit. The other criterion for determining the feasibility was a required sample size that could be realised in a future trial. Furthermore, to optimally standardise such a future trial, determinants that possibly influenced the oxytocin release were screened for in this pilot study.
Determinants possibly influencing the oxytocin release
Data on the infant characteristics, the Kangaroo care duration, feeding differences during the day, the collection intolerance and the amount of eye contact, vocalisations and caressing strokes during Kangaroo care, or in other words, the mother–infant interaction intensity, were gathered. Because no validated scale existed to evaluate the mother–infant interaction intensity, a five‐point Likert scale was constructed to reflect the interactions prior to and during Kangaroo care. The Likert scores were a quantitative representation of some of the items of the Nursing Child Assessment Satellite Training Scale 14, the Arnett Caregiver Interaction Scale in the wordings recently studied by Colwell et al. 15 and the Ainsworth Maternal Sensitivity Scales 16, which all focus on the infant‐centred attention of the mother during mother–infant interactions (Table 3).
Table 3.
Likert scale to score the mother–infant interaction intensity during the collections
| Score | Definition: During the before‐Kangaroo care saliva collection, there was: |
|---|---|
| 0 | Maternal absence |
| 1 | Maternal presence but silent and without touching |
| 2 | Maternal presence, her voice was audible but speech not directed at infant |
| 3 | Maternal presence including infant‐directed speech |
| 4 | Maternal presence plus some touching of for instance the head |
| Score | Definition: During the during‐Kangaroo care saliva collection: |
|---|---|
| 0 | Mother seemed somewhat stressed or distant towards the infant |
| 1 | Mother was not stressed, but provided no extra vocalisations or caressing |
| 2 | Some infant‐directed vocalisations and extra caressing was provided |
| 3 | Frequent infant‐directed vocalisations and extra caressing was provided |
| 4 | Constant infant‐directed vocalisations and extra caressing |
Furthermore, the maternal salivary oxytocin was collected both before and during Kangaroo care, to inspect for another factor that could also possibly influence the infants’ oxytocin responses and to be used as an additional tool for optimising the standardisation of a future trial. For the latter, we examined whether there were effects of, for instance, a different Kangaroo care duration or a different mother–infant interaction intensity on the maternal oxytocin responses.
Maternal and infant saliva collection procedures
The maternal saliva was collected using Salivettes (Sarstedt, Nümbrecht, Germany). Mothers were asked to move a swab around their clean mouth and gently chew on it for at least one minute. A second minute was added when the swab did not appear saturated. When it was saturated, the swab was immediately placed into a tube, sent to the laboratory and frozen at −20°C.
The infants’ saliva was collected using tiny Sorbettes (Salimetrics, Carlsbad, CA, USA). Infants sucked on one Sorbette for four minutes and consecutively on a second Sorbette, also for four minutes. Although a 20‐minute sampling time was advocated 10, we reduced this time so that we could develop a saliva collection procedure that would fit into the caregiving routines of nurses. During the procedure, Sorbettes were gently moved around through the entire mouth, spending most of the time in both cheek pouches and underneath the tongue. After four minutes, the first Sorbette was directly put into a Salimetrics tube that was provided with a study label and that was kept on ice. After finishing the saliva collection with the second Sorbette, and adding it to the tube, the tube was immediately sent to the laboratory and stored at −20°C.
Amount of saliva collections
Based on an approximate harvest of 90 μL of saliva per infant during a 20‐minute collection procedure 10, an average of 30 μL of saliva was a reasonable expectation with the eight‐minute collection procedure applied in this study. The required saliva volume to measure oxytocin is 300 μL, according to the standard operating procedure of a specialised laboratory, RIAgnosis, Munich, Germany. In this feasibility study, therefore, we collected saliva in preterm infants both before and during 30 Kangaroo sessions to yield at least three oxytocin measurements in pooled before‐Kangaroo care saliva and three oxytocin measurements in pooled during‐Kangaroo care saliva.
Saliva handling
When all the samples had been collected, they were allowed to reach room temperature for 30 minutes and were subsequently centrifuged at 1000 × g, at 4°C for five minutes. The maternal saliva was transferred to 1.5‐mL vials (Eppendorf, Hamburg, Germany) and immediately refrozen at −20°C until shipment. The infant saliva samples were transferred into 1.5‐ml vials as well while pooling the harvests. After transferring the randomly picked first infant saliva sample to the first vial, a second randomly picked saliva sample was added to the same vial, until that vial contained at least 300 μL. Each vial contained just saliva from collections before Kangaroo care or just saliva from collections during Kangaroo care. Allocation to the vials was random, but attention was paid to ensure that a vial pair contained corresponding saliva collections. For example, when an infant's before‐Kangaroo care saliva was randomly added to the first before‐Kangaroo care vial, the same infant's during‐Kangaroo care saliva was added to the first vial that was filled with during‐Kangaroo care saliva. The same procedures were used when the infant's saliva was added to subsequent vials. Filled vials were immediately refrozen at −20°C.
Saliva analysis
Saliva samples were assayed by RIAgnosis according to a previously described procedure 17. Briefly, samples were extracted using LiChroprep Si60 (Merck, Hesse, Germany) which was heat‐activated at 700°C for three hours. After evaporation using a SpeedVac High Capacity Concentrator (Thermo Scientific, Waltham, MA, USA), 50 μL of assay buffer was added. The oxytocin was then measured with a highly sensitive and specific radioimmunoassay, standardised and validated in human studies. The detection limit was in the 0.5 pg/sample range, the intra‐assay variation was <8%, and the <0.7% cross‐reactivities were considered negligibly small 18.
Data analysis
First, the obtrusiveness of the collection procedure was analysed. Second, the feasibility was assessed by determining the salivary yields, the oxytocin detectability and the required sample size for the execution of a future trial investigating oxytocin as a biomarker. The Statistical Package for the Social Sciences, 22nd edition (IBM Corp, Armonk, IL, USA) was used to calculate the mean overall oxytocin concentration, the mean before‐Kangaroo care and during‐Kangaroo care concentrations, the mean differences before Kangaroo care versus during Kangaroo care and the mean standard deviations. A sample size power calculation was performed using the mean difference and mean standard deviation in a dependent means t‐test of the free software programme G*Power 3.0.10 19. Finally, to identify determinants that could possibly influence oxytocin responses, the characteristics of the different groups of infants were displayed and the characteristics and oxytocin responses of the mothers and Kangaroo care sessions were examined using t‐tests and analysis of variance tests. Two extra characteristics were calculated using the gathered data, namely the difference in collection intolerance and the difference in interaction intensity prior to and during Kangaroo care. These characteristics were termed tolerance increase and intensity increase. To compute these variables, the collection intolerance during Kangaroo care minus the collection intolerance before Kangaroo care was calculated and the interaction intensity before Kangaroo care minus the interaction intensity during Kangaroo care was calculated. A positive tolerance increase value would suggest that the collection during Kangaroo care was tolerated better than the collection before Kangaroo care. A high‐intensity increase value would suggest a better fit to the study protocol, that is an absent mother during the collection before Kangaroo care and an apparently infant‐centred mother during the collection during Kangaroo care.
Results
Baseline characteristics of the preterm infants
A total of 21 preterm infants were included: 13 contributed to one Kangaroo care collection session, seven contributed to two sessions and one contributed to three sessions on different days. Seven of the included infants were part of a multiple pregnancy: three were included without their siblings and the other four were two fully included twins.
Obtrusiveness
In total, the saliva of these 21 preterm infants was collected at 60 different times: 30 times before Kangaroo care and 30 times during Kangaroo care. Monitor alarms had not increased during any of these collections, and a critical monitor alarm was never reported. The obtrusiveness was minimal, and a Likert score of four was not reported during any of the collections. Almost all of the collections (88%) were performed during sleep. There were no awakenings during these 53 collection procedures, with Likert scores of zero in 12 instances, a score of one in 24 instances, two in 13 instances and three in four instances. The collection intolerance was not related to any of the infant characteristics.
Two of the awake collections were shortened due to crying, which started before the collection and continued during the collection, and were noted as a peculiarity. The mothers of the two infants deliberately suggested that we collected the saliva at these moments, because the crying resulted in an excessive, visible amount of saliva. During these two nonobtrusive collections, one before Kangaroo care and one during Kangaroo care, it only took 10 seconds for the Sorbettes to be saturated with saliva. The obtrusiveness during the other five awake collections was also minimal, with three Likert scores below two and two Likert scores of two.
Feasibility
The 60 collected samples provided almost sufficient saliva to fill six vials with at least the desired 300 μL. After the pooling process, the three before‐Kangaroo care vials contained 320–380 μL, whereas the first two vials with during‐Kangaroo care saliva contained 305–310 μL and the last during‐Kangaroo care vial contained 290 μL. It was possible to measure the salivary oxytocin in all six vials. The mean overall oxytocin was 4.79 pg/mL (SD = 0.86 pg/mL). Before Kangaroo care, it was 4.34 pg/mL, and during Kangaroo care, it was 5.24 pg/mL. Therefore, the mean difference in oxytocin was +0.9 pg/mL (SD 1.38 pg/mL, p = 0.38), which led to a required sample size of 21 vials for a future trial, using a standardised α‐error probability of 0.05, a standard power of 0.8 and a deduced Cohen's d of 0.65.
Determinants possibly influencing the oxytocin release
Two of the three vials that contained during‐Kangaroo care saliva showed an increase in oxytocin concentration, and one showed a decrease (Table 4). Determinants that could have possibly influenced the oxytocin release, and would be necessary to track in a future trial, were investigated by screening for differences in baseline characteristics of the groups of infants that contributed to each oxytocin measurement (Table 5). Except for the current weight, birthweight, Kangaroo care duration and interaction intensity during Kangaroo care, all of the baseline characteristics of the group that did not show an oxytocin increase (group two) were in between the baseline characteristics of the other two groups, including the average increase in the oxytocin levels of the mothers. In groups one and three, the average increases in maternal oxytocin were +0.6 pg/mL and +0.1 pg/mL, whereas in group two, it was +0.2 pg/mL, a respective 21%, 4% and 8% over the mean maternal baseline oxytocin concentration of 2.7 pg/mL.
Table 4.
The oxytocin concentrations per vial in pg/mL before Kangaroo care, during Kangaroo care and the difference during Kangaroo care versus before Kangaroo care
| Pooled saliva of 10 collections | Before KC pg/mL | During KC pg/mL | Difference pg/mL |
|---|---|---|---|
| Vial 1 | 4.32 | 5.86 | 1.54 |
| Vial 2 | 4.70 | 4.02 | −0.68 |
| Vial 3 | 3.99 | 5.83 | 1.84 |
KC, Kangaroo care.
Table 5.
Characteristics of the infants grouped per vial in which oxytocin was measured
| Group characteristics | Vial 1 Mean (SD) | Vial 2 Mean (SD) | Vial 3 Mean (SD) |
|---|---|---|---|
| Age (weeks) | 35.8 (1.1) | 35.3 (1.1) | 35.1 (1.5) |
| Weight (g) | 2249 (270) | 2386 (329) | 2270 (352) |
| Gestational age (weeks) | 32.1 (3.2) | 31.3 (3.4) | 31.0 (2.3) |
| Birthweight (g) | 1716 (421) | 1796 (537) | 1606 (406) |
| Number of days after birth | 25.8 (25.3) | 27.7 (26.7) | 28.9 (23.0) |
| Number of caesarean sections | 5 | 5 | 6 |
| Kangaroo care duration (min) | 44 (20) | 39 (27) | 51 (16) |
| Ml feeding before collection 1 | 39.4 (12.6) | 40.5 (15.0) | 44.5 (5.5) |
| Ml breastmilk thereof | 28.9 | 18.5 | 36.0 |
| Ml ingested through sucking | 3.5 | 0.1 | 17.4 |
| Ml actually fed per breast | 0.0 | 0.0 | 3.0 |
| Time since feeding (min) | 19.1 (4.3) | 22.8 (7.6) | 21.2 (7.0) |
| Ml feeding before collection 2 | 39.4 (12.6) | 40.5 (15.0) | 45.0 (5.8) |
| Ml breastmilk thereof | 32.4 | 24.0 | 36.5 |
| Ml ingested through sucking | 12.2 | 11.2 | 17.1 |
| Ml actually fed per breast | 7.7 | 5.6 | 3.6 |
| Time since feeding (min) | 21.0 (4.0) | 23.0 (7.8) | 21.0 (6.1) |
| Maternal OT response (pg/mL) | +0.6 (1.2) | +0.2 (0.9) | +0.1 (1.6) |
| First collection intolerance | 1.4 (0.1) | 1.5 (0.1) | 0.9 (0.1) |
| Second collection intolerance | 0.9 (0.1) | 1.2 (0.1) | 1.0 (0.1) |
| Tolerance increase (1–2) | 0.5 | 0.3 | −0.1 |
| First interaction intensity | 1.0 (0.1) | 1.4 (0.1) | 0.4 (0.1) |
| Second interaction intensity | 2.8 (0.1) | 2.3 (0.1) | 2.3 (0.1) |
| Intensity increase (2–1) | 1.8 | 0.9 | 1.9 |
SD = standard deviation; OT = oxytocin.
Bold value: If group 2 is not in between group 1 and 3, AND (only if it is a collection‐dependent variable) the value of group 2 is in between 1 and 3 before Kangaroo care but not anymore during Kangaroo care.
When using the maternal oxytocin responses as an additional tool to screen for determinants that could possibly influence oxytocin responses, we noted that weight‐related determinants and the duration of Kangaroo care did not seem to have an effect, whereas the interaction intensity during Kangaroo care and the interaction intensity increase did reveal significant differences (p < 0.001 and p = 0.005) (Table 6). Furthermore, the distinction between a singleton or a multiple pregnancy also showed a significant difference (p = 0.004). Just under half (46%) of the mothers without an oxytocin increase during Kangaroo care gave birth to a twin or triplet. In contrast, none of the mothers with an oxytocin increase during Kangaroo care gave birth to a twin or triplet. The average number of days after labour was higher for the Kangaroo care sessions where oxytocin increased, but this was not significant.
Table 6.
Characteristics of the Kangaroo care sessions with an oxytocin increase versus the Kangaroo care sessions without an oxytocin increase
| Characteristics | Increase in OT n = 15 (SD or %) | No increase in OT n = 13 (SD or %) | t‐test p‐value |
|---|---|---|---|
| Maternal age (years) | 31.1 (3.7) | 32.5 (4.5) | 0.4 |
| Maternal weight (kg) | 66.7 (9.7) | 70.6 (7.7) | 0.248 |
| Maternal length (cm) | 165 (6.1) | 165 (7.2) | 0.966 |
| Infant was delivered vaginally | 8 (53.3%) | 6 (46.2%) | 0.272 |
| Current pregnancy was multiple | 0 (0%) | 6 (46.2%) | 0.004a |
| Average # previous pregnancies | 1.20 (0.4) | 1.54 (0.5) | 0.066 |
| Average # of days after labour | 35.7 (27.0) | 20.7 (20.6) | 0.114 |
| Mean Kangaroo care duration | 44.8 (23.5) | 42.7 (22/9) | 0.813 |
| Collection intolerance (collection 2) | 1.20 (0.9) | 0.92 (0.5) | 0.317 |
| Ml breastmilk prior to collection 1 | 0 | 0 | – |
| Ml breastmilk prior to collection 2 | 25.3 (27.4) | 13.3 (14.8) | 0.379 |
| Rating of the milk production | 1.6 (0.7) | 1.9 (1.0) | 0.458 |
| Interaction intensity collection 1 | 1.0 (1.1) | 0.9 (1.0) | 0.697 |
| Interaction intensity collection 2 | 3.3 (0.6) | 1.6 (0.7) | <0.001a |
| Interaction intensity increase (2–1) | 2.3 (1.2) | 0.8 (1.5) | 0.005a |
OT = oxytocin; SD = standard deviation; # = number.
Indicates significance as defined by a p‐value <0.05.
Discussion
This pilot study focused on 21 infants, and saliva was collected unobtrusively prior to, and during, 30 Kangaroo care sessions. Pooling of this saliva resulted in three sufficiently filled vials with before‐Kangaroo care saliva and three with during‐Kangaroo care saliva. Oxytocin was detectable in all six samples. The Sorbettes cotton swabs provided a minimally obtrusive way for oxytocin detection. The majority of the infants did not appear to notice the Sorbettes at all or got used to them in less than two‐seconds after initial signs of disgust. Moreover, infants were not woken up by the procedure, even though nearly all of the collections were performed while the infant was asleep. This means that a presumably important biological factor for social interactions, salivary oxytocin, could be investigated during Kangaroo care in a trial with a determined required sample size of 21 vials. However, there has been a controversy in the literature about the existence of a direct relationship between such peripheral oxytocin concentrations and central oxytocin concentrations 20, 21.
The majority of human oxytocin is produced in the hypothalamus, where it is either transported to the pituitary gland to enter the peripheral bloodstream or transported in axons to be released in target brain areas to function as a neurotransmitter there or in nearby areas via diffusion 20, 22. Once released into the brain, the large oxytocin molecules do not cross the blood–brain barrier easily 20. Indeed, several studies demonstrated no correlation between central and peripheral oxytocin 17, 23. However, accumulating evidence shows that certain stimuli can cause a coordinated release of central and peripheral oxytocin 21, 24. Kangaroo care could therefore be such a stimulus, evoking both a central and peripheral oxytocin surge. A peripheral surge, however, does not necessarily mean an increase in the salivary oxytocin. In fact, Horvat‐Gordon et al. 11 were unable to detect any oxytocin in saliva using three commercially available immunoassays. They stated that due to its size and short half‐life, oxytocin might be unable to enter the oral mucosa from the blood. On the other hand, other studies did detect oxytocin in the saliva of humans, including changes in that oxytocin after exposure to different behavioural paradigms 8, 9, 25. As stated in the introduction, these enzyme immunoassay‐based findings could be due to the cross‐reactivity of molecules other than oxytocin 12, but it is also possible that the detected molecules were indeed oxytocin from a central or even a local origin. The hypothalamus is not the only site that produces oxytocin molecules, as endothelial and epithelial cells at various other sites throughout the body have been reported to produce it 26. Perhaps Kangaroo care and maternal scent are stimuli causing a local oxytocin release. So far, many uncertainties remain about salivary oxytocin and its potential as a biomarker. These uncertainties were not addressed in this pilot study, and, therefore, it would be very interesting to perform a larger trial assessing salivary oxytocin both before and during Kangaroo care.
For this larger trial, a study design with 10 saliva collections from the same infant was discussed, because that would reduce the number of varying determinants. The burden would be for mothers to have 10 Kangaroo care sessions disturbed by the procedure, and it was felt that this outweighed the advantage. Nonetheless, in any future trial, the number of participations per infant should preferably be the same, as it is unknown whether the unequal participations affected the study results of this pilot. Responses to a second or third participation could differ from the first response, just like many other determinants could influence oxytocin responses. This study was not powered to draw any conclusions regarding that issue, as determinants were merely screened to identify those that should be standardised in a future trial to optimise its protocol to the best of our knowledge. One such determinant, the collection intolerance, did not differ between the infant groups where the oxytocin had increased or decreased. Overall, the collection intolerance was seven points lower during Kangaroo care, which could indicate some comforting effect. It would therefore appear interesting to retain the registering of the collection intolerance in a future trial. Four other determinants were of interest in relation to the oxytocin concentrations in the infant vials, which were the Kangaroo care duration, the mother–infant interaction intensity at baseline and the average birthweight and current weight of the groups of infants.
When using the small set of maternal oxytocin responses as an additional tool to screen for determinants that should be standardised, the duration of Kangaroo care did not appear to be crucial and neither did the maternal weight. The distinction between a multiple or singleton pregnancy did seem interesting, as the oxytocin was not increased in any of the Kangaroo care sessions performed by a mother of twins or triplets, which could be clinically irrelevant, but which is an interesting finding to address in a different study design nonetheless. However, the most interesting finding was the mother–infant interaction intensity, the only determinant that seemed relevant when regarding both the infants’ results and the maternal results. Depending on the interaction intensity, or in other words depending on the amount of touching and infant‐centred vocalisations expressed by mothers during Kangaroo care, half of the mothers who participated in this pilot study more than once did demonstrate an oxytocin increase in a Kangaroo care session with high interaction intensity but not in a Kangaroo care session with lower interaction intensity. Obviously conclusions cannot be drawn based on this either, but nevertheless, in a future trial, providing extra positive caressing stimuli and speech during Kangaroo care sessions should be part of the protocol to maximise the chances of finding a consistent oxytocin increase. Indeed, it has been demonstrated before that maternal vocalisations positively affected their infants’ physiological state and oxytocin 27, 28. Furthermore, mothers should preferably not even be in the infants’ room during the saliva collections before Kangaroo care according to the impression that was given by the results from the interaction intensity at baseline and the intensity increase parameters. We argue that, like the sheer presence of rat dams appeared to influence the electro‐encephalogram of their pups in the study by Sarro et al. 29, the sheer presence of a mother might influence the infant's baseline oxytocin measurement. However, the average oxytocin response of the mothers did not seem related to the pooled oxytocin response of their preterm infants in this pilot study. To the best of our knowledge, the correlation between the oxytocin of preterm infants and their mothers has never been evaluated before. Nonetheless, as only three pooled infant samples were obtained and these samples obviously lacked individual values, a different study design is needed to evaluate the effect of maternal oxytocin responses on preterm infant oxytocin responses.
Finally, a different study evaluating the possible effect of crying on the oxytocin responses of preterm infants would be interesting. In this pilot study, crying prior to a saliva collection was noted as a particularity twice, taking place in one infant before Kangaroo care and in one infant during Kangaroo care. However, those collections were not obtained from the same infant on the same day, and therefore, they were not necessarily allocated to the same vial pair. The saliva of the before‐Kangaroo care collection was randomly allocated to the second pair of vials, the pair in which the before‐Kangaroo care oxytocin concentration exceeded the during‐Kangaroo care concentration. The crying could be an explanation for this, as crying is a very prosocial cue where the infant intensely asks for co‐regulation 30. If the during‐Kangaroo care saliva had been coincidentally allocated to the same vial pair, this could have cancelled out a possible crying effect, but that saliva was randomly allocated to the third vial pair. This implies that the increase in oxytocin in that vial pair could be due to Kangaroo care, but it could also be due to crying behaviour or to anything else. The relation between salivary oxytocin and Kangaroo care was not assessed in this pilot study, as just the feasibility and obtrusiveness of measuring salivary oxytocin prior to and during Kangaroo care were assessed. Therefore, the only conclusion that can be drawn is that oxytocin can be measured in preterm infants in a nonobtrusive way. A larger trial is needed to investigate whether Kangaroo care leads to a noncoincidental increase in salivary oxytocin. Such a trial would not reveal the oxytocin levels of individual preterm infants, and it would not address the questions how much of an oxytocin increase is clinically relevant and whether altered oxytocin levels reflect causes or consequences of behavioural alterations 21. However, the answers to those questions are not essential for our purpose, which was to identify a biological marker that could be used to evaluate future interventions that aim to improve co‐regulation in NICUs. In the current study, we demonstrated that it is possible to assess salivary oxytocin as such a biomarker in a future trial in a nonobtrusive way.
Conclusion
The oxytocin concentration of preterm infants was successfully measured in a nonobtrusive way both before Kangaroo care as well as during Kangaroo care by performing a radioimmunoassay on the pooled saliva of multiple saliva collections. This warrants a future trial to investigate whether or not the increase in parent–infant bonding that the literature states occurs during Kangaroo care is accompanied by an increase in salivary oxytocin.
Conflict of interest
We have no conflict of interest to declare.
Financial resources
This research was funded internally with a Máxima Medical Centre research budget.
Supporting information
Appendix S1 Form to register patient characteristics and collection data.
Acknowledgements
The authors would like to thank the medium care unit and NICU nurses of the Máxima Medical Center for their cooperation, especially Astrid Osagiator. Furthermore, we would like to thank Professor Dr Landgraf for his critical review of the manuscript.
References
- 1. Feldman R. Parent‐infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry 2007; 48: 329–54. [DOI] [PubMed] [Google Scholar]
- 2. Kommers D, Oei G, Chen W, Feijs L, Bambang Oetomo S. Suboptimal bonding impairs hormonal, epigenetic and neuronal development in preterm infants, but these impairments can be reversed. Acta Paediatr 2016; 105: 738–51. [DOI] [PubMed] [Google Scholar]
- 3. Fleming AS, O'Day DH, Kraemer GW. Neurobiology of mother‐infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev 1999; 23: 673–85. [DOI] [PubMed] [Google Scholar]
- 4. Conde‐Agudelo A, Díaz‐Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants (Review). Cochrane Database Syst Rev 2014; 4: 1–65. [DOI] [PubMed] [Google Scholar]
- 5. Galbally M, Lewis AJ, van Ijzendoorn M, Permezel M. The role of oxytocin in mother‐infant relations: a systematic review of human studies. Harv Rev Psychiatry 2011; 19: 1–14. [DOI] [PubMed] [Google Scholar]
- 6. Uvnäs‐Moberg K. The oxytocin factor: tapping the hormone of calm, love and healing [De oxytocine factor]. 1st ed Amsterdam: Thoeris, 2007. [Google Scholar]
- 7. Porges SW. Love: an emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology 1998; 23: 837–61. [DOI] [PubMed] [Google Scholar]
- 8. Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry 2010; 18: 1–21. [DOI] [PubMed] [Google Scholar]
- 9. Cong X, Ludington‐Hoe SM, Hussain N, Cusson RM, Walsh S, Vazquez V, et al. Parental oxytocin responses during skin‐to‐skin contact in pre‐term infants. Early Hum Dev 2015; 91: 401–6. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell AJ, Chang J, Yates C, Hall RW. Challenges, guidelines, and systematic review of salivary cortisol research in preterm infants. EJ Neonatol Res 2012; 2: 44–51. [Google Scholar]
- 11. Horvat‐Gordon M, Granger DA, Schwartz EB, Nelson VJ, Kivlighan KT. Oxytocin is not a valid biomarker when measured in saliva by immunoassay. Physiol Behav 2005; 84: 445–8. [DOI] [PubMed] [Google Scholar]
- 12. McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev 2013; 37: 1485–92. [DOI] [PubMed] [Google Scholar]
- 13. van Dijk M, Roofthooft DWE, Anand KJS, Guldemond F, de Graaf J, Simons S, et al. Taking up the challenge of measuring prolonged pain in (premature) neonates: the COMFORTneo scale seems promising. Clin J Pain 2009; 25: 607–16. [DOI] [PubMed] [Google Scholar]
- 14. Sumner G, Spietz A. NCAST caregiver/parent‐child interaction teaching manual. Seattle, WA: NCAST Publications, University of Washington, School of Nursing, 1994. [Google Scholar]
- 15. Colwell N, Gordon RA, Fujimoto K, Kaestner R, Korenman S. New evidence on the validity of the Arnett Caregiver Interaction Scale: results from the Early Childhood Longitudinal Study‐Birth Cohort. Early Child Res Q 2013; 28: 218–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ainsworth MD. Maternal sensitivity scales. Power 1969; 6: 1379–88. [Google Scholar]
- 17. Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J Neuroendocrinol 2013; 25: 668–73. [DOI] [PubMed] [Google Scholar]
- 18. Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 2013; 38: 1985–93. [DOI] [PubMed] [Google Scholar]
- 19. Faul F, Erdfelder E, Lang A‐G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–91. [DOI] [PubMed] [Google Scholar]
- 20. Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol 2004; 25: 150–76. [DOI] [PubMed] [Google Scholar]
- 21. Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 2012; 35: 649–59. [DOI] [PubMed] [Google Scholar]
- 22. Carson DS, Berquist SW, Trujillo TH, Garner JP, Hannah SL, Hyde SA, et al. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol Psychiatry 2015; 20: 1085–90. [DOI] [PubMed] [Google Scholar]
- 23. Amico JA, Challinor SM, Cameron JL. Pattern of oxytocin concentrations in the plasma and cerebrospinal fluid of lactating rhesus monkeys (Macaca mulatta): evidence for functionally independent oxytocinergic pathways in primates. J Clin Endocrinol Metab 1990; 71: 1531–5. [DOI] [PubMed] [Google Scholar]
- 24. Carter CS, Pournajafi‐Nazarloo H, Kramer KM, Ziegler TE, White‐Traut R, Bello D, et al. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci 2007; 1098: 312–22. [DOI] [PubMed] [Google Scholar]
- 25. Feldman R, Gordon I, Zagoory‐Sharon O. The cross‐generation transmission of oxytocin in humans. Horm Behav 2010; 58: 669–76. [DOI] [PubMed] [Google Scholar]
- 26. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 2001; 81: 629–83. [DOI] [PubMed] [Google Scholar]
- 27. Filippa M, Devouche E, Arioni C, Imberty M, Gratier M. Live maternal speech and singing have beneficial effects on hospitalized preterm infants. Acta Paediatr 2013; 102: 1017–20. [DOI] [PubMed] [Google Scholar]
- 28. Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proc Biol Sci 2010; 277: 2661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sarro EC, Wilson DA, Sullivan RM. Maternal regulation of infant brain state. Curr Biol 2014; 24: 1664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ludington‐Hoe SM, Cong X, Hashemi F. Infant crying: nature, physiologic consequences, and select interventions. Neonatal Netw 2002; 21: 29–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Form to register patient characteristics and collection data.


