Abstract
Cognitive deficits have been regarded as one of the most significant clinical symptoms of depressive disorder. Accumulating evidence has shown that apolipoprotein B (ApoB) levels, which are responsible for inducing neurodegeneration, may be involved in cognitive deficits. This study examines cognitive deficits, and the correlation of serum ApoB levels with cognitive deficits of depressive disorder. 90 depressive patients and 90 healthy controls with matched age and gender were recruited. Cognition was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Serum ApoB levels in depressive patients were measured by immunoturbidimetric method. Our results showed that depressive patients had lower scores of cognition including RBANS total score and subscales of language and delayed memory (all, p < 0.001) than healthy controls after controlling for the variables. The differences in cognitive functions also passed Bonferroni corrections. Serum ApoB levels were negatively correlated with delayed memory score in depressive patients (r = −0.30, p = 0.01). Furthermore, stepwise multivariate regression analysis indicated that serum ApoB levels independently contributed to delayed memory in depressive patients (t = −2.68, p = 0.01). Our findings support that serum ApoB levels may be involved in delayed memory decline in depressive patients. Depressive patients also experience greater cognitive deficits, especially in delayed memory and language than healthy controls.
Depressive disorder is one of the most common psychiatric disorders affecting millions of people worldwide1,2, and is closely associated with more frequent relapse, higher burden of illness, higher risk for suicide, and poorer quality of life3,4. Although depressive disorder mainly involved in mood disturbance, cognitive deficits have now been a well-established feature of this disorder5,6. The meta-analysis studies have indicated that cognitive deficits in depressive patients mainly appeared in the following domains, such as attention, memory, processing speed, executive functioning, and selective cognitive control7,8,9. Cognitive deficits in depressive patients were found to further lead to increased disability and mortality, and shortened life time10,11,12,13. Cognitive deficits should thus be considered a critical clinical and therapeutic target of depressive disorder. Furthermore, cognitive deficits in differing stages of depressive disorder have been reported. Recent studies have shown that cognitive functions in first-episode drug-free depressive patients were impaired in a Chinese population14,15. Current depressive patients have been found to have lower scores of all domains of cognitive functions than healthy controls, and there were significant differences in immediate memory and attention between previous depressive patients and healthy controls16. Other studies have revealed that cognitive functions significantly declined in recurrent depressive patients with each successive episode of depression17,18. Cognitive deficits frequently persisted despite the remission of depressive episodes19. These studies have provided evidence that cognitive deficits in depressive patients may be pervasive and long-lasting. However, the etiology of cognitive deficits in depressive patients remains uncertain, which should be further investigated.
Serum apolipoprotein B (ApoB) is synthesized in the liver and small intestine rather than the brain, and is used for cholesterol transport in periphery20. Several human studies have indicated serum ApoB levels may be involved in cognitive dysfunction. A recent study has showed higher serum ApoB levels in older individuals with amnestic mild cognitive impairments compared to healthy controls21. The variables of serum ApoB levels have been reported to be associated with cognitive changes prior to age 6522. Intriguingly, a post-mortem study has found that higher ApoB levels were significantly associated with higher brain Abeta43 levels that were involved in the dysfunctions of verbal learning, delayed verbal recall and response inhibition in neurodegenerative disorders23,24. Several animal studies also have shown a significant association between ApoB and cognitive deficits. For example, ApoB overexpression in hAPPs1 mice alone induced memory decline compared to those in wild-type mice25. The addition of the ApoB transport domain to the secreted neprilysin was reported to be able to improve memory deficits in amyloid protein precursor transgenic model of Alzheimer’s disease26. These findings suggest that serum ApoB levels may influence cognitive functions in human and animal. However, no studies have examined the correlation between serum ApoB levels with cognitive deficits of depressive disorder. The purpose of the present study is to examine whether depressive patients have worse cognitive functions than healthy controls, and to further investigate whether serum ApoB levels contribute to cognitive deficits of depressive disorder.
Results
Clinical and Demographic Characteristics
Table 1 showed no significant difference in gender, age, and education between depressive patients and healthy controls (all p > 0.05). However, there were significant differences in body mass index (BMI) (F = 23.72, p < 0.001), smoking status (χ2 = 11.75, df = 1, p < 0.001), and suicide status (χ2 = 81.29, df = 1, p < 0.001) between two groups. The mean and standard deviation (Mean ± SD) of age of illness onset, age of first hospitalization, self-rating depression scale (SDS) and self-rating anxiety scale (SAS) standards score, and serum ApoB levels in depressive patients were 31.30 ± 10.23 years, 33.67 ± 10.54 years, 61.77 ± 12.73, 52.00 ± 12.14, and 0.86 ± 0.21 g/L. They had duration of illness for an average 50.70 ± 90.31 months, with hospitalization number for a mean of 0.96 ± 0.92 time. The types of antidepressants included serotonergic and noradrenergic reputake inhibitor (SNRI, 22.22%), selective serotonergic reuptake inhibitor (SSRI, 58.89%), and never taking antidepressants (18.89%).
Table 1. Clinical and demographic characteristics in depressive patients and healthy controls.
| Variables | Depressive Patients (n = 90) | Healthy Controls (n = 90) | F or χ2 | P-value |
|---|---|---|---|---|
| Gender (male/female) | 30/60 | 30/60 | 0.00 | 1.00 |
| Age (years) | 34.98 ± 10.78 | 34.98 ± 10.70 | 0.00 | 1.00 |
| Education (years) | 10.12 ± 3.34 | 9.74 ± 3.55 | 0.57 | 0.45 |
| BMI (kg/m2) | 21.62 ± 3.16 | 24.13 ± 3.70 | 23.72 | <0.001 |
| Smoking (smoker/nonsmoker) | 8/82 | 26/64 | 11.75 | <0.001 |
| Suicide (attempter/no-attempter) | 56/34 | 0/90 | 81.29 | <0.001 |
| Age of Illness Onset (years) | 31.30 ± 10.23 | |||
| Age of First Hospitalization (years) | 33.67 ± 10.54 | |||
| Duration of Illness (months) | 50.70 ± 90.31 | |||
| Number of Hospitalizations | 0.96 ± 0.92 | |||
| Types of Antidepressants | ||||
| SNRI | 20 (22.22%) | |||
| SSRI | 53 (58.89%) | |||
| Never Taking Antidepressants | 17 (18.89%) | |||
| SDS Standard Score | 61.77 ± 12.73 | |||
| SAS Standard Score | 52.0 ± 12.14 | |||
| Apolipoprotein B (g/L) | 0.86 ± 0.21 | |||
Mean ± SD (standard deviation); BMI = body mass index; SNRI = serotonergic and noradrenergic reuptake inhibitor; SSRI = selective serotonergic reuptake inhibitor; SDS: self-rating depression scale; SAS: self-rating anxiety scale.
Main Findings
The Mean ± SD of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total and index scores of cognitive functions in 90 depressive patients and 90 healthy controls were shown in Table 2. There were significant differences in cognitive functions including RBANS total score (75.17 ± 15.15 vs. 84.73 ± 13.15, F = 20.46, df = 1, p < 0.001) and subscales of language (76.70 ± 15.25 vs. 96.36 ± 13.57, F = 83.42, df = 1, p < 0.001), and delayed memory (77.43 ± 19.02 vs. 95.97 ± 54.73, F = 9.21, df = 1, p = 0.003) between depressive patients and healthy controls. All these differences remained significant with low p values for RBANS total score (F = 19.56, df = 1, p < 0.001) and subscales of language (F = 58.21, df = 1, p < 0.001), and delayed memory (F = 7.72, df = 1, p = 0.006) between two groups after controlling for gender, age, education, BMI, smoking, and suicide status using MANOVA (Table 2). Furthermore, significant differences in RBANS total score and subscales of language, and delayed memory between two groups also passed Bonferroni corrections (all p < 0.05) (Table 2).
Table 2. Comparisons of total and index scores of the RBANS between depressive patients and healthy controls.
| RBANS Score | Depressive Patients (n = 90) | Healthy Controls (n = 90) | F | P-valuea | P-valueb [Corrected] |
|---|---|---|---|---|---|
| Immediate Memory | 75.92 ± 42.67 | 80.57 ± 17.21 | 3.81 | 0.053 | 0.318 |
| Attention | 92.82 ± 14.65 | 92.30 ± 18.44 | 0.04 | 0.837 | 1.000 |
| Language | 76.70 ± 15.25 | 96.36 ± 13.57 | 58.21 | <0.001 | <0.001 |
| Visuospatial/Constructiona | 85.29 ± 15.74 | 80.94 ± 14.37 | 0.84 | 0.361 | 1.000 |
| Delayed Memory | 77.43 ± 19.02 | 95.97 ± 54.73 | 7.72 | 0.006 | 0.036 |
| Total Score | 75.17 ± 15.15 | 84.73 ± 13.15 | 19.56 | <0.001 | <0.001 |
The nominally significant P-values (p < 0.05) are showed in bold.
aP-values were analyzed by controlling for gender, age, education, BMI, smoking and suicide status.
bP-values were further adjusted by Bonferroni correction.
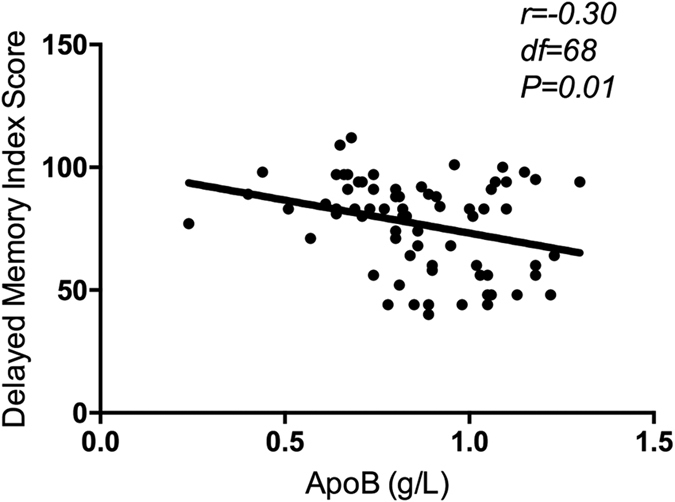
For depressive patients, correlation analysis showed a significant negative association between serum ApoB levels and delayed memory index score (r = −0.30, df = 68, p = 0.01) (Fig. 1). Further stepwise multivariate regression analysis showed that serum ApoB levels (ß = −27.42, t = −2.68, p = 0.01), education (ß = 1.83, t = 2.55, p = 0.01), and age of illness onset (ß = −1.33, t = −2.21, p = 0.03) independently contributed to delayed memory score, which together accounted for 23.3% of the variance in delayed memory score of depressive disorder. However, serum ApoB levels were not found to be associated with RBANS total score and any other cognitive index scores in depressive patients (all p > 0.05).
Figure 1. Significantly negative correlation between Apo B and delayed memory index score in depressive patients (r = −0.3, df = 68, p = 0.01).
Discussion
To the best of our knowledge, this is the first study to investigate the relationship between serum ApoB levels and cognitive functions in depressive patients. We find the effect of higher serum ApoB levels on delayed memory decline of depressive disorder. Depressive patients also have poorer cognitive functions than healthy controls, especially in delayed memory and language.
This study found cognitive functions were significantly impaired in depressive patients, which was consistent with other clinical studies that depressive patients experienced greater cognitive deficts5,27,28. Our finding was supported by several meta-analysis studies7,8,9. Another study adopting RBNAS for cognitive performance has shown that RBANS total score and five subscales of cognitive functions were significantly lower in depressive patients than healthy controls16. However, our results showed that there were significant differences in RBANS total score and subscales of delayed memory and language between depressive patients and healthy controls. The inconsistent findings between two studies could be due to demographic and clinical information differences, and ethnic background.
Previous studies have indicated that hippocampal formation could play a critical role in the regulation of cognitive functions29,30. A study has also found that reduced hippocampal volumes were associated with memory loss of depressive disorder31. The variables of hippocampus have been reported to contribute to language beyond its traditional role in memory in several recent studies32,33,34,35. These findings suggest that abnormal hippocampal formation may be related to cognitive deficits of depressive disorder. Therefore, a further study on brain imaging-cognition in depressive patients should be performed, focusing on language and memory.
Interestingly, this study was the first to find that serum ApoB levels were negatively correlated with delayed memory decline of depressive disorder. The underlying mechanisms responsible for this could be that serum high ApoB levels are related to the dysfunctions of lipid peroxidation, malondialdehyde (MDA) and immune system that could been caused by oxidative stress and free radicals of depressive disorders that is associated with cognitive decline36,37,38,39. Elevated levels of MDA have been reported to adversely affect the efficiency of delayed memory in patients with depressive disorder40. The postmortem and blood studies have shown that oxidative stress and cytokines played an important role in patients with depressive disorder41,42,43, which influenced cognitive functions44. Several previous postmortem studies have indicated that oxidative stress was involved in the etiology of neurodegenerative disorders, which was significantly associated with cognitive decline45,46,47,48,49,50. Furthermore, serum ApoB change was significantly correlated with the decline of cognitive performance in older individuals with mild cognitive impairments36,51. Serum ApoB levels have been reported to be positively associated with brain Abeta43 levels which influenced delayed memory of neurodegenerative disorders23,24. ApoB overexpression has been found to induce memory decline in hAPPs1 mice25. The fusion protein of ApoB transport domain and neprilysin was involved in improving memory deficits in animal model of Alzheimer’s disease26. These results support that serum ApoB levels, which are influenced by oxidative stress and free radicals, may contribute to delayed memory decline of depressive disorder.
Several limitations in this study should be noted. First, the association between serum ApoB levels and delayed memory in depressive patients was investigated by correlation and stepwise multivariate regression analyses. Therefore, the explanation of causal relationships was rather caution. Second, our samples were collected from Suzhou area, thus our findings may not be completely applicable to depressive patients in other cities of China. Finally, some other clinical information including residual symptom, recurrent episodes and remission status were not collected, which should be considered in statistical analysis as they can influence cognitive functions in depressive patients. The above factors could lead to deviation of our results. Therefore, a strictly designed study may be performed in the future to confirm our results.
In summary, we found that serum ApoB levels might play a crucial role in delayed memory decline in depressive patients. Depressive patients experienced greater cognitive deficits than healthy controls, especially in delayed memory and language. However, this study should be viewed as a preliminary investigation, and further studies in larger, independent samples with case-control matching in different ethnicities are warranted to exclude the possibility of false-positive findings.
Methods
Study Population
This study was conducted between September 2014 and February 2016. Depressive patients (n = 90; male/female = 30/60) were recruited from the inpatient unit and outpatient clinic of the Affiliated Guangji Hospital of Soochow University. The catchment area of this hospital covered a population of approximately 9,060,000. Patients who met the following criteria were recruited to participate in the study: (a) age between 17 and 65 years; (b) diagnosis of unipolar depression according to the Diagnostic and Statistical Manual of Mental Disorders, Forth (DSM–IV); (c) received education for at least 4 years; and (d) provided written informed consent and were able to take part in the cognitive assessment. Diagnoses were performed for each patient by two independent experienced clinical psychologists and confirmed using the Structured Clinical Interview for DSM-IV.
Healthy controls (n = 90; male/female = 30/60) were recruited from the employees of the Affiliated Guangji Hospital of Soochow University. Current mental status and personal or family history of mental disorders were assessed using unstructured interviews. None of the healthy controls presented a personal or family history of psychiatric disorders.
Depressive patients and healthy controls with matched age and gender were Han Chinese from the Suzhou area. All subjects were in good physical health, and any subjects with abnormalities were excluded, such as schizoaffective disorders, dementia, neurodegenerative and neurological disorder, cardiovascular disease, cerebrovascular disease, infections, cancer, diabetes, hypertension, hyperlipidemia, and pregnant. Neither depressive patients nor healthy controls were experiencing drug or alcohol abuse/dependence, which was determined by the laboratory urine tests.
Informed consent was obtained before subjects were recruited. This protocol employed was approved by the Clinical Research Ethics Committee of the Affiliated Guangji Hospital of Soochow University, and all experiments were carried out in accordance with the approved guidelines and regulations.
Clinical Variables
A detailed questionnaire including a complete medical history, physical examination, and medical and psychological conditions was obtained from all subjects. Additional information was collected from available medical records.
Cognitive functions were measured using the RBANS (Form A)52. The RBANS was composed of 12 subtests that were used to calculate 5 age-adjusted index scores and a total score. The test indices were immediate memory (composed of listing learning and story memory tasks), attention (composed of digit span and coding tasks), language (composed of picture naming and semantic fluency tasks), visuospatial/constructional (composed of figure copy and line orientation tasks), and delayed memory (composed of list recall, story recall, figure recall, and list recognition tasks). The RBANS was previously translated into Chinese, and its clinical validity and test-retest reliability were established in healthy controls and psychiatric disorder in our previous study53. To ensure consistency and reliability of rating, the two clinical psychologists simultaneously attended a training session for standardizing their use of the RBANS prior to the start of the study. Thereafter, they maintained an intraclass correlation coefficient of 0.93 on the RBANS at the repeated assessments.
Measurement of Serum ApoB Levels
Serum samples from 70 depressive patients were collected between 7 and 9 AM following an overnight fast. HITACHI 7180 automatic biochemistry analyzer (Hitachi High-Technologies Corporation, Japan) was used for measuring serum ApoB levels by immunoturbidimetric method using a commercially available kit (Medical System Biotechnology, Ningbo, China). A full description of this method has been reported in a previous study54. Serum samples were assayed by a technician blind to the clinical situation. Each assay was run in duplicate. Inter- and intra-assay variation coefficients were 4% and 1.5%, respectively.
Statistical Analysis
Group comparisons on the clinical and demographic data used Student’s t-tests or analysis of variance (ANOVA) for continuous variables and chi-square for categorical variables. The RBANS total and index scores of cognitive functions were compared between depressive patients and healthy controls using ANOVA. When the significant differences in cognitive functions were found between two groups using ANOVA, multivariate ANOVA (MANOVA) will further analyze these differences in cognitive functions, with gender, age, education, BMI, smoking, and suicide status as the covariates. Bonferroni corrections were applied to each test to adjust for multiple testing. Relationships between serum ApoB levels and the scores of cognitive deficits of depressive disorder were measured with Pearson’s product moment correction coefficients. Stepwise multivariate analysis using the scores of cognitive deficits of depressive disorder as dependent variables was used to investigate the impact of serum ApoB levels and other variables including gender, age, education, BMI, smoking and suicide status, age of illness onset, age of first hospitalization, duration of illness, number of hospitalization, the types of antidepressants, SDS and SAS standard score. SPSS version 17.0 was used to do all statistical analysis. Continuous data were presented as Mean ± SD and all p values were 2 tailed at the significance level of <0.05.
Additional Information
How to cite this article: Hui, L. et al. Serum ApoB levels in depressive patients: associated with cognitive deficits. Sci. Rep. 7, 39992; doi: 10.1038/srep39992 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This study was funded by the grants from the Wenzhou Municipal Sci-Tech Bureau Program (H20150008), the Sci-Res Project of Medicine and Health of Wenzhou Municipal (2015B21), the Suzhou Key Diagnosis and Treatment Program (LCZX201316), the Suzhou Sci-Tech Program (SYS201372), the Jiangsu Province Natural Science Fund Project (BK20151197), the Jiangsu Province Scientific and Technological Program (BL2013018), Suzhou Key Medical Center for Psychiatric Diseases (Szzx201509), and National Natural Science Foundation of China (81501160). These sources had no further role in this study design, data collection and analysis, writing of the report, and decision to submit the paper for publication.
Footnotes
Author Contributions Li Hui, Guang Zhong Yin, and Shu Chang He were responsible for study design, statistical analysis, and manuscript preparation. Tian Nan Shao and Xiang Dong Du were responsible for recruiting the patients, performing the clinical rating and collecting the samples. Mei Han and Bao Hua Zhang were involved in evolving the ideas and editing the manuscript. Li Hui and Guang Zhong Yin were involved in writing the protocol, cowrote the paper, and were responsible for providing the funding for the study. All authors have contributed to and have approved the final manuscript.
References
- Ustun T. B., Ayuso-Mateos J. L., Chatterji S., Mathers C. & Murray C. J. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 184, 386–392 (2004). [DOI] [PubMed] [Google Scholar]
- Andrews G., Poulton R. & Skoog I. Lifetime risk of depression: restricted to a minority or waiting for most? Br J Psychiatry. 187, 495–496 (2005). [DOI] [PubMed] [Google Scholar]
- Conley R. R. et al. The burden of depressive symptoms in the long-term treatment of patients with schizophrenia. Schizophr Res. 90, 186–197 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. J., Muller K. M. & Fellgiebel A. Detection of depression in acute schizophrenia: sensitivity and specificity of 2 standard observer rating scales. Can J Psychiatry. 51, 387–392 (2006). [DOI] [PubMed] [Google Scholar]
- Den Hartog H., Derix M., Van Bemmel A., Kremer B. & Jolles J. Cognitive functioning in young and middle-aged unmedicated out-patients with major depression: testing the effort and cognitive speed hypothesis. Psychol Med. 33, 1443–1451 (2003). [DOI] [PubMed] [Google Scholar]
- Porter R. J., Bourke C. & Gallagher P. Neuropsychological impairment in major depression: its nature, origin and clinical significance. Aust N Z J Psychiatry. 41, 115–128 (2007). [DOI] [PubMed] [Google Scholar]
- McDermott L. M. & Ebmeier K. P. A meta-analysis of depression severity and cognitive function. J Affect Disord. 119, 1–8 (2009). [DOI] [PubMed] [Google Scholar]
- Rock P. L., Roiser J. P., Riedel W. J. & Blackwell A. D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol Med. 44, 2029–2040 (2014). [DOI] [PubMed] [Google Scholar]
- Lee R. S., Hermens D. F., Porter M. A. & Redoblado-Hodge M. A. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord. 140, 113–124 (2012). [DOI] [PubMed] [Google Scholar]
- Schultz S. K. et al. The influence of cognitive impairment and psychiatric symptoms on daily functioning in nursing facilities: a longitudinal study. Ann Clin Psychiatry. 14, 209–213 (2002). [DOI] [PubMed] [Google Scholar]
- Hinton L., Tomaszewski Farias S. & Wegelin J. Neuropsychiatric symptoms are associated with disability in cognitively impaired Latino elderly with and without dementia: results from the Sacramento Area Latino study on Aging. Int J Geriatr Psychiatry. 23, 102–108 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H. et al. Association of depressed mood and mortality in older adults with and without cognitive impairment in a prospective naturalistic study. Am J Psychiatry. 167, 589–597 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura T. et al. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the aging, demographics, and memory study. J Am Geriatr Soc. 58, 330–337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. et al. Emotion, working memory, and cognitive control in patients with first-onset and previously untreated minor depressive disorders. J Int Med Res. 44, 529–541 (2016a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. et al. Cognitive Behavioral Performance of Untreated Depressed Patients With Mild Depressive Symptoms. Plos One. 11, e146356 (2016b). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune B. et al. The role of cognitive impairment in general functioning in major depression. Psychiatry Res. 176, 183–189 (2010). [DOI] [PubMed] [Google Scholar]
- Basso M. & Bornstein R. Relative memory deficits in recurrent verses first-episode major depression on a word-list learning task. Neuropsychology. 13, 557–563 (1999). [DOI] [PubMed] [Google Scholar]
- Stordal K. et al. Impairment across executive functions in recurrent major depression. Nord J Psychiatry. 58, 41–47 (2004). [DOI] [PubMed] [Google Scholar]
- Airaksinen E., Larsson M., Lundberg I. & Forsell Y. Cognitive function in depression and anxiety depression. Psychol Med. 34, 83–91 (2004). [DOI] [PubMed] [Google Scholar]
- Pitas R. E., Boyles J. K., Lee S. H., Hui D. & Weisgraber K. H. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 262, 14352–14360 (1987). [PubMed] [Google Scholar]
- Gonzalez-Escamilla G., Atienza M., Garcia-Solis D. & Cantero J. L. Cerebral and blood correlates of reduced functional connectivity in mild cognitive impairment. Brain Struct Funct. 221, 631–645 (2016). [DOI] [PubMed] [Google Scholar]
- Reynolds C. A., Gatz M., Prince J. A., Berg S. & Pedersen N. L. Serum lipid levels and cognitive change in late life. J Am Geriatr Soc. 58, 501–509 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y. M. et al. Elevated low-density lipoprotein in Alzheimer’s disease correlates with brain abeta 1–42 levels. Biochem Biophys Res Commun. 252, 711–715 (1998). [DOI] [PubMed] [Google Scholar]
- Stav A. L. et al. Amyloid-β and α-synuclein cerebrospinal fluid biomarkers and cognition in early Parkinson’s disease. Parkinsonism Relat Disord. 21, 758–764 (2015). [DOI] [PubMed] [Google Scholar]
- Loffler T. et al. Impact of ApoB-100 expression on cognition and brain pathology in wild-type and hAPPsl mice. Neurobiol Aging. 34, 2379–2388 (2013). [DOI] [PubMed] [Google Scholar]
- Spencer B. et al. Peripheral delivery of a CNS targeted, metalo-protease reduces Aβ toxicity in a mouse model of Alzheimer’s disease. Plos one. 6, e16575 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin M. et al. Cognitive function in major depression. J Affect Disord. 25, 21–29 (1992). [DOI] [PubMed] [Google Scholar]
- Ravnkilde B. et al. Cognitive deficits in major depression. Scand J Psychol. 43, 239–251 (2002). [DOI] [PubMed] [Google Scholar]
- Frodl T. et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci. 31, 316–323 (2006). [PMC free article] [PubMed] [Google Scholar]
- Gray J. A. & McNaughton N. Comparison between the behavioural effects of septal and hippocampale leisons: a review. Neurosci Biobehav Rev. 7, 119–188 (1983). [DOI] [PubMed] [Google Scholar]
- Hickie I. et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 186, 197–202 (2005). [DOI] [PubMed] [Google Scholar]
- Duff M. C. & Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Front Hum Neurosci. 6, e1–11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurczek J., Brown-Schmidt S. & Duff M. Hippocampal contributions to language: evidence of referential processing deficits in amnesia. J Exp Psychol Gen. 142, 1346–1354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini G. et al. Language disorders in children with morphologic abnormalities of the hippocampus. Arch Pediatr. 17, 1008–1016 (2010). [DOI] [PubMed] [Google Scholar]
- Lee J. K. et al. Assessing hippocampal development and language in early childhood: Evidence from a new application of the Automatic Segmentation Adapter Tool. Hum Brain Mapp. 36, 4483–4496 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. et al. A randomized, controlled, double-blind trial of Huannao Yicong capsule in senile patients with mild cognitive impairment. Zhong Xi Yi Jie He Xue Bao. 6, 25–31 (2008). [DOI] [PubMed] [Google Scholar]
- Fredrikson G. N. et al. Autoimmune responses against the apo B-100 LDL receptor-binding site protect against arterial accumulation of lipids in LDL receptor deficient mice. Autoimmunity. 40, 122–130 (2007). [DOI] [PubMed] [Google Scholar]
- Michel T. M., Pülschen D. & Thome J. The role of oxidative stress in depressive disorders. Curr Pharm Des. 18, 5890–5899 (2012). [DOI] [PubMed] [Google Scholar]
- Xiu M. H. et al. Contribution of IL-10 and its −592 A/C polymorphism to cognitive functions in first-episode drug-naive schizophrenia. Brain Behav Immun. 57, 116–124 (2016). [DOI] [PubMed] [Google Scholar]
- Talarowska M. et al. Malondialdehyde plasma concentration correlates with declarative and working memory in patients with recurrent depressive disorder. Mol Biol Rep. 39, 5359–5366 (2012). [DOI] [PubMed] [Google Scholar]
- Michel T. M. et al. Increased xanthine oxidase in the thalamus and putamen in depression. World J Biol Psychiatry. 11, 314–320 (2010). [DOI] [PubMed] [Google Scholar]
- Michel T. M. et al. Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder–a postmortem study. Psychiatry Res. 151, 145–150 (2007). [DOI] [PubMed] [Google Scholar]
- Himmeriich H. et al. Regulatory T cells increased while IL-1β decreased during antidepressant therapy. J Psychiatr Res. 44, 1052–1057 (2010). [DOI] [PubMed] [Google Scholar]
- Talarowska M. et al. Impact of oxidative/nitrosative stress and inflammation on cognitive functions in patients with recurrent depressive disorders. Med Sci Monit. 20, 110–115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durany N., Münch G. Michel T. & Riederer P. Investigations on oxidative stress and therapeutical implications in dementia. Eur Arch Psychiatry Clin Neurosci. 3, 68–73 (1999). [DOI] [PubMed] [Google Scholar]
- Michel T. M. et al. Can enzyme kinetics of prooxidants teach us a lesson about the treatment of Alzheimer’s disease: a pilot post-mortem study. World J Biol Psychiatry. 11, 677–681 (2010). [DOI] [PubMed] [Google Scholar]
- Michel T. M. et al. Increased activity of mitochondrial aldehyde dehydrogenase (ALDH) in the putamen of individuals with Alzheimer’s disease: a human postmortem study. J Alzheimers Dis. 19, 1295–12301 (2010). [DOI] [PubMed] [Google Scholar]
- Michel T. M. et al. Aldehyde dehydrogenase 2 in sporadic Parkinson’s disease. Parkinsonism Relat Disord. Suppl 1, S68–72 (2014). [DOI] [PubMed] [Google Scholar]
- Revel F. et al. Influence of oxidative stress biomarkers on cognitive decline. J Alzheimers Dis. 45, 553–560 (2015). [DOI] [PubMed] [Google Scholar]
- Zhou H., Yuan Y., Qi Z., Tong Q. & Zhang K. Study on changes of plasma levels of oxidative stress biomarkers and its relation with cognition function in patients with parkinson’s disease. Zhonghua Yi Xue Za Zhi. 95, 3357–3360 (2015). [PubMed] [Google Scholar]
- Gao P. et al. Study on the correlation between cognitive functions and regional blood flow in Alzheimer disease and mild cognitive impairment. Chinese Journal of Neuroimmunology and Neurology. 13, 205–208 (2006). [Google Scholar]
- Randolph C., Tierney M. C., Mohr E. & Chase T. N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 20, 310–319 (1998). [DOI] [PubMed] [Google Scholar]
- Zhang B. H. et al. Repeatable battery for the assessment of neuropsychological status (RBANS) as a screening test in Chinese reliability and validity. Chin Ment Heath J. 28, 865–869 (2009). [Google Scholar]
- Li B. et al. Quantitation of human apolipoprotein B100 by immunoturbidimetric assay. Hua Xi Yi Ke Da Xue Xue Bao. 28, 109–112 (1997). [PubMed] [Google Scholar]


