Abstract
Objectives
The aim of this study is to evaluate the effect of a high‐intensity functional exercise program on depressive symptoms among older care facility residents with dementia.
Methods
Residents (n = 186) with a diagnosis of dementia, age ≥ 65 years, Mini‐Mental State Examination score ≥ 10, and dependence in activities of daily living were included. Participants were randomized to a high‐intensity functional exercise program or a non‐exercise control activity conducted 45 min every other weekday for 4 months. The 15‐item Geriatric Depression Scale (GDS) and the Montgomery–Åsberg Depression Rating Scale (MADRS) were administered by blinded assessors at baseline, 4, and 7 months.
Results
No difference between the exercise and control activity was found in GDS or MADRS score at 4 or 7 months. Among participants with GDS scores ≥ 5, reductions in GDS score were observed in the exercise and control groups at 4 months (–1.58, P = 0.001 and –1.54, P = 0.004) and 7 months (–1.25, P = 0.01 and –1.45, P = 0.007). Among participants with MADRS scores ≥ 7, a reduction in MADRS score was observed at 4 months in the control group (–2.80, P = 0.009) and at 7 months in the exercise and control groups (–3.17, P = 0.003 and –3.34, P = 0.002).
Conclusions
A 4‐month high‐intensity functional exercise program has no superior effect on depressive symptoms relative to a control activity among older people with dementia living in residential care facilities. Exercise and non‐exercise group activities may reduce high levels of depressive symptoms.
Keywords: dementia, residential facilities, depression, exercise, randomized controlled trial, frail elderly
Introduction
Depression is common among older people with dementia (Bergdahl et al., 2011) and people living in residential care facilities (Bergdahl et al., 2005). Approximately 20–30% of people with Alzheimer's disease have depressive disorders, and this proportion appears to be higher among people with vascular or Lewy body dementia (Enache et al., 2011; Ballard et al., 1996). Antidepressant drugs seem to have a limited or no effect in people with dementia (Enache et al., 2011; Nelson and Devanand, 2011; Banerjee et al., 2011). Thus, alternative ways of treating depression in this group need to be evaluated.
Physical exercise has shown effect sizes similar to those of antidepressants in reducing depressive symptoms among older people without dementia (Bridle et al., 2012) and moderate–high‐intensity exercise seems to be more effective than low‐intensity exercise (Singh et al., 2005). However, these effects among older people with dementia are unclear (Forbes et al., 2013); high‐quality studies evaluating the effects of high‐intensity physical exercise programs in this population are needed. Four randomized controlled trials have evaluated the effects of physical exercise as a single intervention on depressive symptoms among older people with dementia (Rolland et al., 2007; Williams and Tappen, 2008; Steinberg et al., 2009; Vreugdenhil et al., 2012). Only one of these studies included more than 45 participants (Rolland et al., 2007), and none reported the exercise intensity achieved or included participants with non‐Alzheimer dementias. These studies compared exercise with usual care (Rolland et al., 2007; Vreugdenhil et al., 2012) or a control activity (Williams and Tappen, 2008; Steinberg et al., 2009). However, no study provided comparable attention to the control and intervention groups, which can introduce bias affecting the observed effects of exercise per se (McCarney et al., 2007), especially among people with physical and cognitive impairment, who generally have few social contacts (Perrin, 1997; Simonsick et al., 1998).
The aims of this study were to evaluate the effect of a high‐intensity functional exercise program on depressive symptoms compared with a control activity and to determine whether the effect differed in preplanned subgroups of dementia type or depressive symptom level, among older people with dementia living in residential care facilities.
Methods
Setting and participants
The Umeå Dementia and Exercise (UMDEX) Study was a rater‐blinded, stratified, cluster‐randomized controlled trial conducted in 16 residential care facilities in Umeå, Sweden, in 2011–2012. Participating facilities comprised specialized and non‐specialized units for people with dementia. Fourteen facilities had units with private rooms and staff on hand, and five facilities had units where residents in private apartments had access to dining facilities, alarms, and on‐site nursing and care. The UMDEX study was determined to require 183 participants, based on results from the Frail Older People – Activity and Nutrition Study in Umeå (Rosendahl et al., 2006). The calculation was based on Barthel activity of daily living (ADL) Index, one of the primary outcomes in the UMDEX study, and is described in detail elsewhere (Toots et al., No date).
In August 2011, physical therapists (PTs) and physicians assessed the eligibility of 864 residents. Inclusion criteria were a diagnosis of dementia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR; American Psychiatric Association, 2000), age ≥ 65 years, dependency in personal ADLs according to the Katz Index (Katz et al., 1963), ability to stand up from an armchair with help from no more than one person, Mini‐Mental State Examination (MMSE) (Folstein et al., 1975) score ≥ 10, ability to hear and understand Swedish sufficiently well to participate in assessment, and physician approval. A team of physicians, including a geriatric medicine specialist, established dementia diagnoses according to DSM‐IV‐TR criteria using medical records, MMSE scores, assessment of temporary states of confusion, and information about visual and hearing impairment. Classification of dementia type was based on medical records, in most cases, including records of brain imaging, anamnesis of memory impairment, history of other diseases (e.g., stroke), and past MMSE scores, which could be compared with present MMSE scores.
Eligible residents gave informed oral consent to participation, which was confirmed by their next of kin. Umeå's Regional Ethical Review Board approved the study in August 2011 (2011‐205‐31 M). The study protocol (ISRCTN31767087) is available at http://www.isrctn.com.
Baseline assessment
At baseline, trained PTs and physicians conducted assessments. The MMSE was used to measure cognitive function (range 0–30; mild cognitive impairment 18–23, severe impairment <18) (Folstein et al., 1975; Tombaugh and McIntyre, 1992). Visual impairment was defined as the inability to read 5‐mm‐high capitalized text with or without glasses, and hearing impairment was defined as the inability to hear a normal‐volume conversation at 1 m distance with or without a hearing aid.
Nutritional status was assessed using the Mini Nutritional Assessment (range 0–30; Guigoz and Vellas, 1999). Dependency in personal ADLs was recorded using the 10‐item Barthel ADL Index (range 0–20; Collin et al., 1988). The Berg Balance Scale (BBS; range 0–56) was used to measure functional balance capacity (Berg, 1989). Behavioral and psychological symptoms of dementia were measured using the Neuropsychiatric Inventory (range 0–144; Cummings et al., 1994). Self‐reported presence of pain during a walking test was recorded. Self‐reported health was extracted from the first item of the 36‐item short‐form questionnaire (Ware and Sherbourne, 1992). Diagnoses were based on data from medical records and prescribed medications. Delirium in the last week was assessed using the confusion subscale of the Organic Brain Syndrome scale (Jensen et al., 1993). A specialist in geriatric medicine diagnosed depressive disorders using DSM‐IV‐TR criteria, based on measures such as the Geriatric Depression Scale (GDS; Sheikh and Yesavage, 1986) and Montgomery–Åsberg Depression Rating Scale (MADRS; Montgomery and Åsberg, 1979), medical records, and indications of prescribed drugs. Participants with an ongoing antidepressant treatment (indicated for a depressive disorder) were classified as having a depressive disorder, regardless of the baseline assessments' results.
Clusters and randomization
To avoid contamination between activities, participants were divided into 36 clusters (activity groups) comprising residents of the same wing, unit, or floor of a facility. The mean ± standard deviation (SD) number of participants in a cluster was 5.2 ± 1.2, range 3–8. To reduce the impacts of facility of residence and living conditions, randomization was stratified with the intention to define at least one exercise cluster and one control cluster at each facility. Researchers not involved in the study performed concealed randomization (using sealed, opaque envelopes), starting with the order of the cluster allocation followed by the allocation to intervention or control, after participant enrollment and baseline assessment.
Procedure
In October 2011, participants in 36 clusters each began the intervention and control activities. Both activities were performed for 45 min every other weekday for 4 months (total 40 sessions). In both activities, individually supervised sessions were offered participants not attending the group sessions, if possible.
Exercise intervention
Two PTs supervised every exercise session. The intervention was based on the High‐Intensity Functional Exercise (HIFE) program, which has been described in detail elsewhere (Toots et al., No date; Littbrand et al., 2006; Littbrand et al., 2014). The program was developed by, and can be obtained from, members of the research team. Its aim is to improve lower limb strength, balance, and mobility in older people with various levels of functional capacity. It comprises 39 exercises, intended to be performed at high intensity and designed to imitate daily functional movements, such as rising from a chair or climbing stairs. The strength exercises were defined as high‐intensive when 8–12 repetition maximum (RM; DeLorme, 1945) was performed, and the balance exercises when performed near the limit of maintaining postural stability. The load in strength exercises was increased gradually by, for example, adding weight to a weighted belt worn around the waist (maximum 12 kg), increasing height of stairs or lowering height of chairs. The balance exercises progressed by, for example, narrowing base of support or by altering support surface. All participants were individually supervised, and each participant had an individually based exercise program. Throughout the intervention period, the PTs chose and adapted exercises and intensity for each participant based on his/her current physical and functional capacity, cognitive function, behavioral and psychological symptoms of dementia, and health status. PTs (and control activity leaders) could contact physicians or nurses to clarify participants' health status when necessary. Participants were encouraged to exercise with moderate intensity (i.e., 13–15 RM for strength exercises) in the first 2 weeks and with high intensity thereafter. After each session, PTs evaluated the exercise intensity achieved by each participant (high, moderate, or low), according to a predefined scale (Littbrand et al., 2006).
Control activity
The control activity was a non‐exercise activity program developed for the study by occupational therapists (OTs) and an OT assistant. One OT/assistant led each session, which comprised seated activities (e.g., conversing, singing, picture viewing, listening to readings or music). Session topics included seasons, wildlife, cooking, authors, and famous artists.
Outcomes
At baseline and 4 and 7 months, physicians blinded to group allocation and previous test results interviewed participants using the 15‐item GDS and the MADRS. GDS items are structured by yes/no responses; scores range from 0 to 15 (normal 0–4, mild depression 5–9, and moderate to severe depression 10–15; Alden et al., 1989). The scale has shown high levels of sensitivity and specificity for the detection of clinical depression among people living in residential care facilities (Smalbrugge et al., 2008) and is considered to be applicable to people with cognitive impairment (Smalbrugge et al., 2008; Conradsson et al., 2013b). The MADRS was designed to detect changes in depressive symptoms and has been used widely in clinical trials involving antidepressants (Montgomery and Åsberg, 1979). It is based on a clinical interview; 10 depressive symptoms are rated on a scale of 0 to 6, with higher scores reflecting greater severity. Total scores range from 0 to 60, with a score ≥ 7 indicating clinically relevant depressive symptoms (Snaith and Taylor, 1985).
Statistical methods
Differences in baseline characteristics between groups, stratified by activity (exercise or control) and GDS score, were examined using the independent samples t‐test or χ 2 test. GDS score stratification was based on the cutoff of 4/5 points. Scores were considered missing when less than 10/15 questions were answered. For incomplete scores with ≥10/15 answered GDS items or ≥8/10 answered MADRS items, a total score was imputed by dividing the score by the number of questions answered and multiplying by 15 for GDS and 10 for MADRS (with rounding up to an integer; Shrive et al., 2006). The number of participants with imputed GDS and MADRS total scores, respectively, were 3 and 0 at baseline, 3 and 2 at 4 months, and 17 and 4 at 7 months. The only attribute that differed significantly (P < 0.05) between activity groups was antidepressant use, which was adjusted for in subsequent analyses. Correlations between baseline variables and changes in GDS and MADRS scores were examined with the intention of adjusting for variables correlated (r ≥ 0.3) with the outcome, but none were found (data not shown).
Longitudinal changes in GDS and MADRS scores over 4 and 7 months were analyzed using linear mixed models, with baseline and follow‐up values composing the outcome variables. Analyses were adjusted for cluster and test‐subject as random effects and age, sex, and antidepressant use as fixed effects. Between‐group differences were estimated using an activity × timepoint interaction term, and within‐group differences (follow‐up–baseline values) were estimated using least square means (LSMs). Adjusted intention‐to‐treat analyses included all participants with at least one (baseline or follow‐up) outcome measurement and were performed according to group allocation, irrespective of activity adherence. Intracluster correlation coefficients were calculated as the proportions of total variance attributed to cluster variance in GDS and MADRS scores in the total sample. Because previous studies included only participants with Alzheimer's disease, dementia type was dichotomized as Alzheimer and non‐Alzheimer (including mixed) dementia. Within‐group LSM analyses were performed according to dementia type, GDS score (≥5 and <5), and MADRS score (≥7 and <7) following the same procedure as in the whole sample. Between‐group analyses compared within‐group LSM changes using the independent samples t‐test with 50% fewer degrees of freedom to obtain conservative P‐values. Activity × timepoint interaction terms and subgroup divisions (dementia type, GDS score, and MADRS score) were also tested using linear mixed models in the total sample. R version 3.0.1 (R Core Team, 2014) with the LME4 package (Bates et al., 2013), and SPSS version 21.0 (IBM Corporation, Armonk, NY, USA) were used to perform statistical analyses. All statistical tests were two tailed, and P < 0.05 was considered to indicate significance.
Results
A flowchart of the inclusion process is presented in Figure 1. Age and MMSE score did not differ between the 186 study participants and the 55 (23%) residents who declined participation. A larger proportion of men than women declined to participate (34% vs 18%, P = 0.008).
Figure 1.
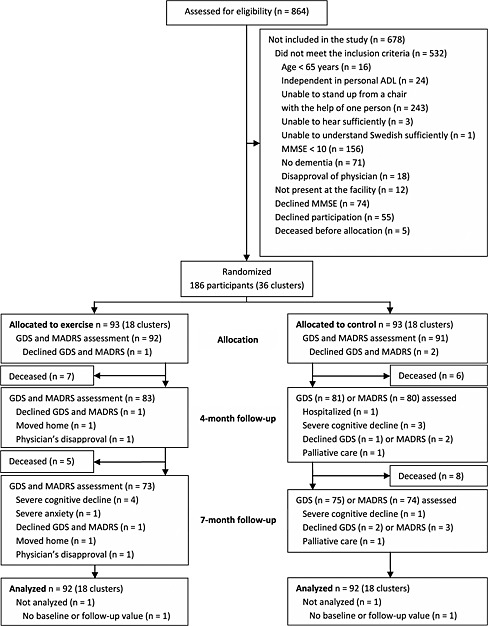
Flowchart of the study. Note: ADL, activities of daily living; MMSE, Mini‐Mental State Examination; GDS, 15‐item Geriatric Depression Scale; MADRS, Montgomery–Åsberg Depression Rating Scale.
Depressive disorders were diagnosed in 107 (58%) participants. Antidepressant use (n = 102 [55%]) was more prevalent in the exercise than in the control group (62% vs 47%, P = 0.04; Table 1). The 55 (30%) participants with GDS scores ≥ 5 were more likely to have angina pectoris (38% vs 22%, P = 0.02) and constipation (78% vs 59%, P = 0.01) and had a lower average BBS score (23.0 ± 14.8 vs 31.6 ± 13.7, P < 0.001). Dementia type ratios differed according to GDS score, with a higher ratio of vascular dementia and lower ratio of Alzheimer's disease among those with GDS scores ≥ 5 (P = 0.03; Table 1).
Table 1.
Baseline characteristics of study participants
| Characteristic | Total (n = 186) | Exercise (n = 93) | Control (n = 93) | P | GDS ≥ 5 (n = 55) | GDS < 5 (n = 128) | P |
|---|---|---|---|---|---|---|---|
| Age (years) | 85.1 ± 7.1 (65–105) | 84.4 ± 6.2 (67–97) | 85.9 ± 7.8 (65–105) | 0.15 | 84.5 ± 8.2 (65–99) | 85.5 ± 6.5 (67–105) | 0.38 |
| Female sex | 141 (76) | 70 (75) | 71 (76) | 0.86 | 41 (75) | 97 (76) | 0.86 |
| Diagnoses and medical conditions | |||||||
| Dementia type | 0.86 | 0.03 | |||||
| Vascular | 77 (41) | 36 (39) | 41 (44) | 32 (58) | 44 (34) | ||
| Alzheimer | 67 (36) | 34 (37) | 33 (35) | 14 (25) | 51 (40) | ||
| Mixed Alzheimer and vascular | 15 (8) | 8 (9) | 7 (8) | 4 (7) | 11 (9) | ||
| Other | 27 (15) | 14 (16) | 12 (13) | 5 (9) | 22 (17) | ||
| Depressive disorder | 107 (58) | 53 (57) | 54 (58) | 0.88 | 51 (93) | 54 (42) | <0.001 |
| Delirium in the last week | 102 (55) | 48 (52) | 54 (58) | 0.38 | 35 (64) | 66 (52) | 0.13 |
| Previous stroke | 57 (31) | 33 (35) | 24 (26) | 0.15 | 21 (38) | 35 (27) | 0.14 |
| Diabetes mellitus | 29 (16) | 18 (19) | 11 (12) | 0.16 | 11 (20) | 18 (14) | 0.31 |
| Heart failure | 55 (30) | 24 (26) | 31 (33) | 0.26 | 19 (35) | 36 (28) | 0.39 |
| Angina pectoris | 49 (26) | 21 (23) | 28 (30) | 0.24 | 21 (38) | 28 (22) | 0.02 |
| Malignancy, current or in the last 5 years | 20 (11) | 13 (14) | 7 (8) | 0.16 | 8 (15) | 12 (9) | 0.30 |
| Constipation | 121 (65) | 60 (65) | 61 (66) | 0.88 | 43 (78) | 75 (59) | 0.01 |
| Rheumatic disease | 28 (15) | 14 (15) | 14 (15) | 1.00 | 5 (9) | 23 (18) | 0.13 |
| Regular use of drugs | |||||||
| Diuretics | 88 (47) | 41 (44) | 47 (51) | 0.38 | 26 (47) | 62 (48) | 0.89 |
| Analgesics (not ASA) | 112 (60) | 55 (59) | 57 (61) | 0.76 | 36 (65) | 73 (57) | 0.29 |
| Benzodiazepines | 40 (22) | 19 (20) | 21 (23) | 0.72 | 14 (25) | 25 (20) | 0.37 |
| Antidepressants | 102 (55) | 58 (62) | 44 (47) | 0.04 | 31 (56) | 69 (54) | 0.76 |
| Neuroleptics | 31 (17) | 11 (12) | 20 (22) | 0.08 | 13 (24) | 17 (13) | 0.08 |
| Anti‐dementia drugsa | 47 (25) | 28 (30) | 19 (20) | 0.13 | 9 (16) | 36 (28) | 0.09 |
| Number of drugs used regularly | 8.3 ± 3.8 (0–22) | 8.4 ± 4.0 (0–22) | 8.2 ± 3.7 (0–20) | 0.75 | 9.1 ± 4.1 (0–21) | 8.0 ± 3.7 (0–22) | 0.07 |
| Assessments | |||||||
| Visual impairment | 26 (14) | 10 (11) | 16 (17) | 0.20 | 8 (15) | 18 (14) | 0.93 |
| Hearing impairment | 32 (17) | 12 (13) | 20 (22) | 0.12 | 13 (24) | 17 (13) | 0.08 |
| Mini‐Mental State Examination (0–30) | 14.9 ± 3.5 (10–26) | 15.4 ± 3.4 (10–23) | 14.4 ± 3.5 (10–26) | 0.06 | 14.9 ± 3.5 (10–26) | 15.0 ± 3.5 (10–25) | 0.99 |
| Mini Nutritional Assessment (0–30) | 21.0 ± 2.7 (12.5–26) | 21.2 ± 2.7 (12.5–26.0) | 20.9 ± 2.6 (14.5–26.0) | 0.35 | 20.2 ± 2.7 (14.5–25.0) | 21.5 ± 2.5 (12.5–26.0) | <0.01 |
| Barthel ADL Index (0–20) | 10.8 ± 4.4 (2–18) | 10.7 ± 4.5 (2–17) | 11.0 ± 4.4 (2–18) | 0.66 | 10.1 ± 4.7 (2–18) | 11.3 ± 4.2 (2–18) | 0.09 |
| Berg Balance Scale (0–56) | 28.9 ± 14.5 (2–54) | 28.6 ± 14.3 (2–52) | 29.3 ± 14.7 (3–54) | 0.71 | 23.0 ± 14.8 (3–49) | 31.6 ± 13.7 (2–54) | <0.001 |
| Neuropsychiatric Inventory (0–144) | 14.8 ± 14.2 (0–82) | 15.2 ± 15.8 (0–82) | 14.4 ± 12.6 (0–42) | 0.68 | 14.3 ± 16.0 (0–82) | 14.7 ± 13.5 (0–58) | 0.84 |
| Pain when walking (n = 185) | 35 (20) | 15 (16) (n = 92) | 20 (22) | 0.37 | 14 (26) (n = 54) | 20 (16) | 0.14 |
| Self‐reported health as good, very good, or excellent | 119 (64) | 60 (65) | 59 (63) | 0.88 | 28 (51) | 90 (70) | 0.02 |
| Outcomes | |||||||
| MADRS (0–60, n = 183) | 5.7 ± 6.3 (0–31) | 5.6 ± 6.5 (0–31) (n = 92) | 5.9 ± 6.1 (0–27) (n = 91) | 0.80 | 11.6 ± 7.7 (0–31) | 3.2 ± 3.3 (0–19) | <0.001 |
| GDS (0–15, n = 183) | 3.8 ± 3.2 (0–13) | 4.0 ± 3.4 (0–13) (n = 92) | 3.6 ± 2.9 (0–13) (n = 91) | 0.37 | 7.8 ± 2.5 (5–13) | 2.0 ± 1.2 (0–4) | <0.001 |
Note
GDS, 15‐item Geriatric Depression Scale; ASA, acetylsalicylic acid; ADL, activities of daily living; MADRS, Montgomery–Åsberg Depression Rating Scale.
Data are presented as mean ± standard deviation (range) or n (%). For all assessment scales except Neuropsychiatric Inventory, MADRS, and GDS, higher scores indicate higher function. Differences between activity groups and between high and low GDS scores were analyzed using the χ 2 or independent samples t‐test.
Anti‐dementia drugs include acetylcholinesterase inhibitors and memantine.
Rates of adherence to the exercise and control activities were 73% and 70%, respectively. Participants reached high intensity during strength exercises at a median of 47% of sessions attended, and moderate–high intensity at a median of 76% of sessions.
No difference in effect on GDS or MADRS score at 4 or 7 months was observed between the exercise and control activities (Table 2). In addition, no difference in effect between the exercise and control activities was found in subgroups of dementia type or depressive symptom level (data not shown), and no interaction effect was found in respective subgroup analyses (Table 3, Table 4).
Table 2.
Within‐group and between‐group differences in GDS and MADRS scores at 4 and 7 months
| Adjusted within‐groupa | Adjusted between‐groupb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise | Control | ||||||||||
| Test | n c | Month | n d | Mean (95% CI) | P | n d | Mean (95% CI) | P | Mean (95% CI) | P | ICCe |
| GDS | 183 | 4 | 83 | 0.03 (–0.53, 0.59) | 0.91 | 81 | 0.08 (–0.49, 0.64) | 0.78 | –0.05 (–0.84, 0.75) | 0.91 | 0.032 |
| 184 | 7 | 73 | –0.03 (–0.61, 0.56) | 0.92 | 75 | 0.03 (–0.55, 0.61) | 0.91 | –0.06 (–0.89, 0.76) | 0.88 | ||
| MADRS | 183 | 4 | 83 | 0.40 (–0.77, 1.57) | 0.50 | 80 | 0.33 (–0.85, 1.52) | 0.58 | 0.06 (–1.60, 1.73) | 0.94 | <0.001 |
| 184 | 7 | 73 | –0.08 (–1.30, 1.15) | 0.90 | 74 | –0.24 (–1.47, 0.99) | 0.70 | 0.16 (–1.57, 1.89) | 0.86 | ||
Note
GDS, 15‐item Geriatric Depression Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; CI, confidence interval; ICC, intracluster correlation coefficient.
Performed using linear mixed models with least square means (follow‐up – baseline values), adjusted for age, sex, antidepressant use, cluster, and test‐subject.
Three‐way interactions of activity, time, and subgroup were analyzed in the total sample using linear mixed models adjusting for cluster and test‐subject as random effects and age, sex, and antidepressant use as fixed effects.
Number of participants in analyses with baseline or follow‐up outcome measurement.
Number of participants in subgroup with complete before‐and‐after measurement.
Calculated as the proportion of the total variance attributed to cluster variance in GDS and MADRS scores in the total sample.
Table 3.
Within‐group differences and interaction effects of GDS and MADRS scores in subgroups of participants with high and low levels of depressive symptoms
| Adjusted within‐groupa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | n b | Month | Group | Exercise | Control | Interactionc | |||||
| n d | Mean (95% CI) | P | n d | Mean (95% CI) | P | Mean (95% CI) | P | ||||
| GDS | 183 | 4 | GDS ≥ 5 | 26 | –1.58 (–2.53, –0.63) | 0.001 | 21 | –1.54 (–2.56, –0.50) | 0.004 | –0.09 (–1.76, 1.57) | 0.91 |
| GDS < 5 | 57 | 0.78 (0.14, 1.42) | 0.02 | 60 | 0.72 (0.10, 1.35) | 0.02 | |||||
| 184 | 7 | GDS ≥ 5 | 23 | –1.25 (–2.23, –0.26) | 0.01 | 21 | –1.45 (–2.49, –0.41) | 0.007 | 0.26 (–1.45, 1.98) | 0.76 | |
| GDS < 5 | 50 | 0.53 (–0.14, 1.21) | 0.12 | 53 | 0.59 (–0.06, 1.25) | 0.08 | |||||
| MADRS | 183 | 4 | MADRS ≥ 7 | 27 | –1.69 (–3.66, 0.27) | 0.09 | 23 | –2.80 (–4.90, –0.71) | 0.009 | 1.54 (–1.90, 4.99) | 0.38 |
| MADRS < 7 | 56 | 1.31 (–0.05, 2.67) | 0.06 | 57 | 1.74 (0.40, 3.09) | 0.01 | |||||
| 184 | 7 | MADRS ≥ 7 | 22 | –3.17 (–5.28, –1.06) | 0.003 | 22 | –3.34 (–5.47, –1.21) | 0.002 | –0.08 (–3.67, 3.51) | 0.97 | |
| MADRS < 7 | 51 | 1.32 (–0.08, 2.72) | 0.07 | 51 | 1.07 (–0.33, 2.47) | 0.13 | |||||
Note
GDS, 15‐item Geriatric Depression Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; CI, confidence interval.
Performed using linear mixed models with least square means (follow‐up – baseline values), adjusted for age, sex, antidepressant use, cluster and test‐subject.
Number of participants in analyses with baseline or follow‐up outcome measurement.
Three‐way interactions of activity, time, and subgroup were analyzed in the total sample using linear mixed models adjusted for cluster and test‐subject as random effects and age, sex, and antidepressant use as fixed effects.
Number of participants in subgroup with complete before‐and‐after measurement.
Table 4.
Within‐group differences and interaction effects of GDS and MADRS scores in subgroups of dementia
| Adjusted within‐groupa | Interactionc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise | Control | ||||||||||
| Group | n b | Month | Subgroup | n d | Mean (95% CI) | P | n d | Mean (95% CI) | P | Mean (95% CI) | P |
| GDS | 183 | 4 | AD | 29 | –0.09 (–1.04, 0.86) | 0.85 | 31 | 0.47 (–0.46, 1.39) | 0.32 | 0.80 (–0.86, 2.46) | 0.34 |
| Non‐AD | 54 | 0.10 (–0.60, 0.79) | 0.79 | 50 | –0.15 (–0.87, 0.57) | 0.68 | |||||
| 184 | 7 | AD | 25 | –0.10 (–1.10, 0.90) | 0.85 | 27 | 0.47 (–0.50, 1.44) | 0.34 | 0.79 (–0.94, 2.53) | 0.37 | |
| Non‐AD | 48 | <0.01 (–0.73, 0.73) | 1.00 | 47 | –0.22 (–0.95, 0.51) | 0.56 | |||||
| MADRS | 183 | 4 | AD | 29 | –0.12 (–2.09, 1.85) | 0.90 | 31 | 1.10 (–0.82, 3.02) | 0.26 | 2.03 (–1.43, 5.49) | 0.25 |
| Non‐AD | 54 | 0.67 (–0.78, 2.12) | 0.37 | 49 | –0.14 (–1.65, 1.37) | 0.85 | |||||
| 184 | 7 | AD | 25 | 1.02 (–1.06, 3.11) | 0.34 | 26 | 0.30 (–1.75, 2.35) | 0.78 | –0.84 (–4.47, 2.78) | 0.65 | |
| Non‐AD | 48 | –0.67 (–2.18, 0.85) | 0.39 | 47 | –0.55 (–2.08, 0.98) | 0.48 | |||||
Note
GDS, 15‐item Geriatric Depression Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; CI, confidence interval; AD, Alzheimer's disease; Non‐AD, non‐Alzheimer dementia.
Performed using linear mixed models with least square means (follow‐up–baseline values), adjusted for age, sex, antidepressant use, cluster and test‐subject.
Number of participants in analyses with baseline or follow‐up outcome measurement.
Three‐way interactions of activity, time, and subgroup were analyzed in the total sample using linear mixed models adjusted for cluster and test‐subject as random effects and age, sex, and antidepressant use as fixed effects.
Number of participants in subgroup with complete before‐and‐after measurement.
Among participants with GDS scores ≥ 5, adjusted within‐group analyses showed similar significant reductions in GDS score at 4 months in the exercise and control groups (–1.58, P = 0.001 and –1.54, P = 0.004, respectively; Table 3, Figure 2). At 7 months, the GDS‐scores in both groups were still improved compared with baseline (exercise: –1.25, P = 0.01; control: –1.45, P = 0.007; Table 3, Figure 2). Among participants with MADRS scores ≥ 7, significant reductions in MADRS score were observed at 4 months in the control group (–2.80, P = 0.009) and at 7 months in the exercise and control groups, compared with baseline (–3.17, P = 0.003 and –3.34, P = 0.002; Table 3, Figure 2).
Figure 2.
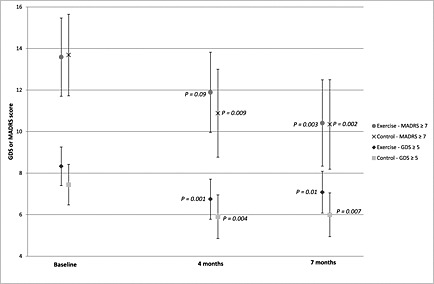
Adjusted GDS and MADRS scores of participants with high levels of depressive symptoms by activity and follow‐up timepoint. Note: GDS, 15‐item Geriatric Depression Scale; MADRS, Montgomery–Åsberg Depression Rating Scale. P‐values represent comparisons of follow‐up values at 4 and 7 months with baseline values using least square means, adjusted for age, sex, antidepressant use, cluster, and test‐subject. Lower scores represents lower levels of depressive symptoms.
Discussion
This study demonstrated no difference in the effects between a 4‐month high‐intensity functional exercise program and a control activity on depressive symptoms in older people with dementia living in residential care facilities, in the total sample or in subgroups defined by dementia type or depressive symptom level. Participants with higher levels of depressive symptoms at baseline showed significant reductions in depressive symptoms at 4‐month and 7‐month follow‐ups, which were of comparable magnitude in the exercise and control groups.
The finding that physical exercise had no superior effect on depressive symptoms compared with a non‐exercise control activity is in line with previous research in older people with dementia (Williams and Tappen, 2008; Steinberg et al., 2009). It contrasts with the finding that 3–4 months of moderate‐intensity and high‐intensity physical exercises reduce depressive symptoms in older people without dementia (Bridle et al., 2012). These contrasting results may be explained by differences in the etiology of depression, which may be related to organic brain disorders in people with dementia (Enache et al., 2011; Taylor et al., 2013). Physical exercise has been suggested to prevent or reduce depressive symptoms through different pathways, such as changes in endorphin and monoamine levels (Rimer et al., 2012), reduced ADL dependency, or improved functional capacity (Netz et al., 2005; Lenze et al., 2001). The latter two may be mediated by factors such as improved self‐efficacy, self‐esteem, sense of control, and increased daily physical activity (Netz et al., 2005; Lenze et al., 2001; Lee and Park, 2008; Yang, 2006). As presented by Toots et al. (No date), the HIFE program reduced ADL decline in the present trial, among participants with non‐Alzheimer dementia and improved functional balance capacity in the total sample compared with the control activity. The lack of a superior effect of exercise on depressive symptoms despite these positive results may be due to insufficient improvement in functional capacity (Boström et al., 2014) or may be because the suggested pathway does not apply to older people living in residential care facilities (Conradsson et al., 2013a), including those with dementia.
A similar study also demonstrated that exercise and non‐exercise activities reduced high levels of depressive symptoms in older people with dementia in residential care facilities (Williams and Tappen, 2008). These results are of particular interest, as antidepressants seem to have little or no effect in this population (Nelson and Devanand, 2011; Banerjee et al., 2011). As the exercise per se did not seem to reduce depressive symptoms in the present study, it suggests that maybe the social contacts mediated in both group activities could be a part of the explanations why positive effects were found in participants with high levels of depressive symptoms (Lenze et al., 2001; Yang, 2006; Cohen‐Mansfield et al., 2011). However, as the exercise and control interventions were not compared with usual care in the present study, we cannot disregard the possibility that the observed effects reflect the natural course of depressive symptoms in this group. The results may also have been influenced by regression toward the mean, considering that participants with low baseline levels of depressive symptoms tended to have more depressive symptoms at 4 and 7 months.
Strengths of the present study are that it involved the inclusion of participants with non‐Alzheimer dementia, assessment of exercise intensity at each session, and assessor blinding. In addition, the effect may be applicable to many older people with dementia in residential care facilities as the inclusion criteria were wide, the proportion of people who declined to participate in the study (23%) was comparably low (Rolland et al., 2007; Vreugdenhil et al., 2012), and intention‐to‐treat analyses were used. The assessment of intensity enables comparison of the HIFE intervention with exercise trials that have shown effects on depressive symptoms in people without dementia. Furthermore, the study design comprised an attention control activity with adherence comparable with the exercise activity, enabling evaluation of exercise effects per se. The study also included participants with low levels of depressive symptoms. Because of floor effects, the ability to reduce depressive symptoms in this group is limited. However, it is of high interest to evaluate whether physical exercise can prevent, as well as reduce, depressive symptoms among people with dementia, where reduced functional capacity and dependency in ADL may be risk factors for an increase in depressive symptoms (Lenze et al., 2001, Boström et al., 2014). A limitation in the present study is that the power calculation was not based on the outcome measures used in this study. However, the sample size in the present study was relatively large, and further, the between‐group changes found were small, indicating no clinically relevant effects (Duru and Fantino, 2008). Another limitation was that two participants were unintentionally not assessed at 4 and 7 months because of relocation and a physician's recommendation for discontinuation, respectively.
Conclusion
A 4‐month high‐intensity functional exercise program has no superior effect compared with a control activity on depressive symptoms among older people with dementia living in residential care facilities, irrespective of dementia type or depressive symptom level. Both exercise and non‐exercise group activities may reduce high levels of depressive symptoms. However, this finding must be confirmed in three‐armed randomized controlled trials including control groups receiving usual care.
Conflict of interest
None declared.
Key points.
A high‐intensity functional exercise program consisting of 40 sessions over 4 months had no superior effect on depressive symptoms compared with a control activity among older care facility residents with dementia.
The exercise effect did not differ in subgroups of dementia type or depressive symptom level in interaction analyses.
In participants with high levels of depressive symptoms, both exercise and non‐exercise activities reduced depressive symptoms. However, these results should be confirmed in three‐armed randomized controlled trials, including a control group that receives usual care, to control for the natural course of depressive symptoms.
Supporting information
Supporting info item
Acknowledgements
We would like to thank Robert Wiklund, PT, Annika Toots, PT, and Beatrice Pettersson, PT, for their efforts in data collection and implementation of the exercise program; Gunbritt Lindahl, OT assistant, Elisabet Nilsson, OT, and Caroline Ollman, OT, for their efforts in development and implementation of the control activity program; Lena Molander, MD, and Ellinor Nordin, PT, for their efforts in data collection; and Lillemor Lundin‐Olsson, PT, for her efforts in planning the UMDEX Study, data collection, and implementation of the exercise program. We would like also to express our sincere gratitude to the Social Authorities of the Municipality of Umeå, care staff, and participants for their cooperation.
This study was supported by the Swedish Research Council (K2009‐69P‐21298‐01‐4, K2009‐69X‐21299‐01‐1, K2009‐69P‐21298‐04‐4, K2014‐99X‐22610‐01‐6), the Swedish Research Council for Health, Working Life and Welfare [2012‐0775, 2013‐1512] (formerly FAS – Swedish Council for Working Life and Social Research), the Vårdal Foundation, the King Gustav V and Queen Viktoria's Foundation, the Swedish Dementia Association, the Promobilia Foundation, the Swedish Society of Medicine, the Detlof Research Foundation, the Swedish Alzheimer Foundation, the County Council of Västerbotten (ALF), the Umeå University Foundation for Medical Research, and the Ragnhild and Einar Lundström's Memorial Foundation. Funding was also received from the European Union and the Regional Development Fund: the Interreg IIIA Mitt‐Scandia and the Bothnia‐Atlantica Program.
Boström, G. , Conradsson, M. , Hörnsten, C. , Rosendahl, E. , Lindelöf, N. , Holmberg, H. , Nordström, P. , Gustafson, Y. , and Littbrand, H. (2016) Effects of a high‐intensity functional exercise program on depressive symptoms among people with dementia in residential care: a randomized controlled trial. Int J Geriatr Psychiatry, 31: 868–878. doi: 10.1002/gps.4401.
References
- Alden D, Austin C, Sturgeon R. 1989. A correlation between the Geriatric Depression Scale long and short forms. J Gerontol 44: 124–125. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 2000. Diagnostic and statistical manual of mental disorders: DSM‐IV‐TR. American Psychiatric Association: Washington, DC. [Google Scholar]
- Ballard CG, Bannister C, Oyebode F. 1996. Depression in dementia sufferers. Int J Geriatr Psychiatry 11: 507–515. [Google Scholar]
- Banerjee S, Hellier J, Dewey M, et al. 2011. Sertraline or mirtazapine for depression in dementia (HTA‐SADD): a randomised, multicentre, double‐blind, placebo‐controlled trial. Lancet 378: 403–11. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, et al. 2013. lme4: Linear mixed‐effects models using Eigen and S4 . R package version 1.1‐7. From: http://CRAN.R‐project.org/package=lme4.
- Berg K. 1989. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can 41: 304–311. [Google Scholar]
- Bergdahl E, Allard P, Gustafson Y. 2011. Depression among the very old with dementia. Int Psychogeriatr 23: 756–63. [DOI] [PubMed] [Google Scholar]
- Bergdahl E, Gustavsson JM, Kallin K, et al. 2005. Depression among the oldest old: the Umeå 85+ study. Int Psychogeriatr 17: 557–75. [DOI] [PubMed] [Google Scholar]
- Boström G, Conradsson M, Rosendahl E, et al. 2014. Functional capacity and dependency in transfer and dressing are associated with depressive symptoms in older people. Clin Interv Aging 9: 249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle C, Spanjers K, Patel S, Atherton NM, Lamb SE. 2012. Effect of exercise on depression severity in older people: systematic review and meta‐analysis of randomised controlled trials. Br J Psychiatry 201: 180–5. [DOI] [PubMed] [Google Scholar]
- Cohen‐Mansfield J, Marx MS, Thein K, Dakheel‐Ali M. 2011. The impact of stimuli on affect in persons with dementia. J Clin Psychiatry 72: 480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C, Wade DT, Davies S, Horne V. 1988. The Barthel ADL Index: a reliability study. Int Disabil Stud 10: 61–3. [DOI] [PubMed] [Google Scholar]
- Conradsson M, Littbrand H, Boström G, et al. 2013a. Is a change in functional capacity or dependency in activities of daily living associated with a change in mental health among older people living in residential care facilities? Clin Interv Aging 8: 1561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradsson M, Rosendahl E, Littbrand H, et al 2013b. Usefulness of the Geriatric Depression Scale 15‐item version among very old people with and without cognitive impairment. Aging Ment Health 17: 638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, et al. 1994. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44: 2308–14. [DOI] [PubMed] [Google Scholar]
- DeLorme TL. 1945. Restoration of muscle power by heavy‐resistance exercises. J Bone Joint Surg 27: 645–667. [Google Scholar]
- Duru G, Fantino B. 2008. The clinical relevance of changes in the Montgomery–Asberg Depression Rating Scale using the minimum clinically important difference approach. Curr Med Res Opin 24: 1329–35. [DOI] [PubMed] [Google Scholar]
- Enache D, Winblad B, Aarsland D. 2011. Depression in dementia: epidemiology, mechanisms, and treatment. Curr Opin Psychiatry 24: 461–72. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–98. [DOI] [PubMed] [Google Scholar]
- Forbes D, Thiessen EJ, Blake CM, Forbes SC, Forbes S. 2013. Exercise programs for people with dementia. Cochrane Database Syst Rev 12: CD006489. [DOI] [PubMed] [Google Scholar]
- Guigoz Y, Vellas B. 1999. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Programme 3–11. [DOI] [PubMed] [Google Scholar]
- Jensen E, Dehlin O, Gustafson L. 1993. A comparison between three psychogeriatric rating scales. Int J Geriatr Psychiatry 8: 215–229. [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. 1963. Studies of illness in the aged. The Index of ADL: A standardized measure of biological and psychosocial function. JAMA 185: 914–9. [DOI] [PubMed] [Google Scholar]
- Lee Y, Park K. 2008. Does physical activity moderate the association between depressive symptoms and disability in older adults? Int J Geriatr Psychiatry 23: 249–56. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Rogers JC, Martire LM, et al. 2001. The association of late‐life depression and anxiety with physical disability: a review of the literature and prospectus for future research. Am J Geriatr Psychiatry 9: 113–35. [PubMed] [Google Scholar]
- Littbrand H, Lindelöf N, Rosendahl E. 2014. The HIFE Program: The High‐Intensity Functional Exercise Program. 2nd edn. Umeå University, Department of Community Medicine and Rehabilitation, Geriatric Medicine: Umeå. [Google Scholar]
- Littbrand H, Rosendahl E, Lindelöf N, et al. 2006. A high‐intensity functional weight‐bearing exercise program for older people dependent in activities of daily living and living in residential care facilities: evaluation of the applicability with focus on cognitive function. Phys Ther 86: 489–98. [PubMed] [Google Scholar]
- McCarney R, Warner J, Iliffe S, et al. 2007. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. 1979. A new depression scale designed to be sensitive to change. Br J Psychiatry 134: 382–389. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Devanand DP. 2011. A systematic review and meta‐analysis of placebo‐controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc 59: 577–85. [DOI] [PubMed] [Google Scholar]
- Netz Y, Wu MJ, Becker BJ, Tenenbaum G. 2005. Physical activity and psychological well‐being in advanced age: a meta‐analysis of intervention studies. Psychol Aging 20: 272–84. [DOI] [PubMed] [Google Scholar]
- Perrin T. 1997. Occupational need in severe dementia: a descriptive study. J Adv Nurs 25: 934–41. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2014. R: A language and environment for statistical computing. R foundation for Statistical Computing; Vienna, Austria: From: http://www.R‐project.org/. [Google Scholar]
- Rimer J, Dwan K, Lawlor DA, et al. 2012. Exercise for depression. Cochrane Database Syst Rev 7: CD004366. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Pillard F, Klapouszczak A, et al. 2007. Exercise program for nursing home residents with Alzheimer's disease: A 1‐year randomized, controlled trial. J Am Geriatr Soc 55: 158–165. [DOI] [PubMed] [Google Scholar]
- Rosendahl E, Lindelöf N, Littbrand H, et al. 2006. High‐intensity functional exercise program and protein‐enriched energy supplement for older persons dependent in activities of daily living: a randomised controlled trial. Aust J Physiother 52: 105–13. [DOI] [PubMed] [Google Scholar]
- Sheikh J, Yesavage J. 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol 5: 165–173. [Google Scholar]
- Shrive FM, Stuart H, Quan H, Ghali WA. 2006. Dealing with missing data in a multi‐question depression scale: a comparison of imputation methods. BMC Med Res Methodol 6: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Kasper JD, Phillips CL. 1998. Physical disability and social interaction: factors associated with low social contact and home confinement in disabled older women (The Women's Health and Aging Study). J Gerontol B Psychol Sci Soc Sci 53: S209–17. [DOI] [PubMed] [Google Scholar]
- Singh NA, Stavrinos TM, Scarbek Y, et al. 2005. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci 60: 768–76. [DOI] [PubMed] [Google Scholar]
- Smalbrugge M, Jongenelis L, Pot AM, Beekman AT, Eefsting JA. 2008. Screening for depression and assessing change in severity of depression. Is the Geriatric Depression Scale (30‐, 15‐ and 8‐item versions) useful for both purposes in nursing home patients? Aging Ment Health 12: 244–8. [DOI] [PubMed] [Google Scholar]
- Snaith R, Taylor C. 1985. Rating scales for depression and anxiety: a current perspective. Br J Clin Pharmacol 19: 17S–20S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M, Leoutsakos JMS, Podewils LJ, Lyketsos CG. 2009. Evaluation of a home‐based exercise program in the treatment of Alzheimer's disease: The Maximizing Independence in Dementia (MIND) study. Int J Geriatr Psychiatry 24: 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W, Aizenstein H, Alexopoulos G. 2013. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry 18: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. 1992. The mini‐mental state examination: a comprehensive review. J Am Geriatr Soc 40: 922–35. [DOI] [PubMed] [Google Scholar]
- Toots A, Littbrand H, Lindelöf N, et al. (No date). The effects of a high‐intensity exercise program on activities of daily living in older people with dementia living in residential care facilities: a cluster randomised controlled trial. J Am Geriatr Soc (Accepted). [Google Scholar]
- Ware JE Jr, Sherbourne CD. 1992. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 30: 473–83. [PubMed] [Google Scholar]
- Williams CL, Tappen RM. 2008. Exercise training for depressed older adults with Alzheimer's disease. Aging Ment Health 12: 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil A, Cannell J, Davies A, Razay G. 2012. A community‐based exercise programme to improve functional ability in people with Alzheimer's disease: a randomized controlled trial. Scand J Caring Sci 26: 12–9. [DOI] [PubMed] [Google Scholar]
- Yang Y. 2006. How does functional disability affect depressive symptoms in late life? The role of perceived social support and psychological resources. J Health Soc Behav 47: 355–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


