Abstract
Background
The efficacy of ixekizumab, an anti‐interleukin‐17A (anti‐IL‐17A) monoclonal IgG4 antibody, was demonstrated in moderate‐to‐severe psoriasis patients when administered via prefilled syringe (PFS).
Objective
To evaluate the effect of two drug delivery devices on the pharmacokinetics (PK) of ixekizumab as well as efficacy and safety with both devices.
Methods
In the first 12 weeks of an open‐label, phase 3 study, moderate‐to‐severe psoriasis patients were randomized to ixekizumab delivery via PFS or autoinjector device. Randomization was stratified by weight (<80 kg, 80–100 kg, >100 kg), injection assistance (yes/no) and injection site (arm, thigh or abdomen). Following a 160‐mg initial dose at week 0, patients received subcutaneous 80‐mg ixekizumab as a single injection every 2 weeks for 12 weeks. Blood samples were collected following the initial 160‐mg dose on days 2, 4, 7, 10 and 14 for PK analysis. Primary PK parameters were maximum concentration (C max) and area under the curve (AUC 0‐tlast) where t last is the time of last sample (14 days ± 24 h). Efficacy was assessed by percent improvement on the Psoriasis Area and Severity Index (PASI) at week 12. Adverse event reporting, vital signs and clinical laboratory data were used to evaluate safety.
Results
Of 204 randomized patients, 192 were included in the PK analysis (PFS: 94; autoinjector: 98). The PFS and autoinjector showed similar geometric mean C max (90% CI) [15.0 μg/mL (13.9–16.1) vs. 14.8 μg/mL (13.8–15.9)] and geometric mean AUC 0‐tlast (90% CI) [157 μg × day/mL (147–168) vs. 154 μg × day/mL (144–165)]. When comparing C max and AUC 0‐tlast of the autoinjector to PFS, the geometric LS mean ratios were 0.97. At week 12, mean percent PASI improvement (via modified baseline observation carried forward) was similar with the PFS (89.3%) and autoinjector (86.9%). Both devices had safety results that were consistent with the known safety profile of ixekizumab.
Conclusion
The PK, efficacy and safety of ixekizumab administered subcutaneously by PFS and autoinjector were similar.
Clinicaltrials.gov number: NCT01777191 https://clinicaltrials.gov/ct2/show/NCT01777191
Introduction
Ixekizumab is an anti‐interleukin‐17A (IL‐17A) monoclonal IgG4 antibody with a high binding affinity to IL‐17A, a key cytokine in psoriasis pathogenesis.1, 2, 3 Specific inhibition of IL‐17A represents a targeted approach to the management of plaque psoriasis. Ixekizumab administered as a subcutaneous (SC) injection using a prefilled syringe (PFS) has demonstrated rapid, robust and durable efficacy and was well tolerated in patients with moderate‐to‐severe psoriasis in randomized, placebo‐controlled, UNCOVER phase 3 clinical trials.4
To offer patients options for self‐administration of ixekizumab, a PFS and an autoinjector have been developed. Several studies have demonstrated that an autoinjector is a drug delivery approach that can increase patient treatment adherence and benefits patients in terms of ease of use, improved self‐esteem and greater independence in their social, domestic and professional lives.5, 6 Furthermore, several clinical studies have shown that autoinjectors improve injection tolerability and patient‐reported satisfaction/acceptability, compared with a PFS.7, 8, 9, 10 However, self‐injection with a PFS is also recognized as an efficient and reliable method for drug administration. Offering patients a choice between these two different drug delivery devices provides options for patients and can increase convenience in the management of their disease.
Upon pushing a button, the autoinjector inserts the needle into the skin, delivers the drug and retracts the needle back into the device. It is designed to encase the PFS so there is no difference in the drug contact surfaces. The glass barrel, plunger and needle are identical in the autoinjector and the PFS, as are the intended dose, volume and formulation of ixekizumab. The autoinjector has an ergonomic shape and limits visibility of the needle to help reduce potential patient anxiety of needles. Similar to the autoinjector, the PFS also supports convenient self‐injection with a single dose of the drug.
The primary objective of this study was to evaluate the effect of drug delivery, either by PFS or autoinjector, on the pharmacokinetics (PK) of ixekizumab after the administration of the initial 160‐mg dose as a SC injection in patients with moderate‐to‐severe psoriasis. Additionally, the efficacy and safety were evaluated after 12 weeks of SC administration of ixekizumab with the PFS and the autoinjector.
Materials and methods
The clinical study was approved by the investigational review boards and all patients provided written informed consent before enrollment. The study was conducted in accordance with Good Clinical Practice guidelines, with ethical principles having their origin in the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines and applicable laws and regulations.
Study design
Here, we report the first 12 weeks of a randomized, open‐label, parallel‐arm, multicentre, outpatient, phase 3 study in patients with moderate‐to‐severe plaque psoriasis. Patients were assigned by a computer generated random sequence to an injection device (PFS or autoinjector). To achieve an equal number of patients assigned to the PFS and autoinjector within weight and injection‐site category, randomization was stratified by weight (low: <80 kg, medium: 80–100 kg, high: >100 kg), injection assistance (yes/no; a caregiver or adult other than the patient who administered all injections of ixekizumab to the patient) and injection site (arm, thigh or abdomen). Patients were randomized based on bodyweight and injection site to ensure that a minimum of 8 PK‐evaluable patients were included in each bodyweight and injection‐site category (e.g., for each injection site, 8 patients were included in the low bodyweight category, 8 patients in the medium bodyweight category, and 8 patients in the high bodyweight category). This should provide adequate PK data across the range of bodyweights for each injection site and for both drug delivery devices. Patients utilized the assigned injection device and administration site for all injections throughout the 12‐week treatment period. Patients received SC 80‐mg ixekizumab as one injection every 2 weeks, following a 160‐mg initial dose given as two SC 80‐mg injections at week 0 (baseline visit) (Fig 1).
Figure 1.
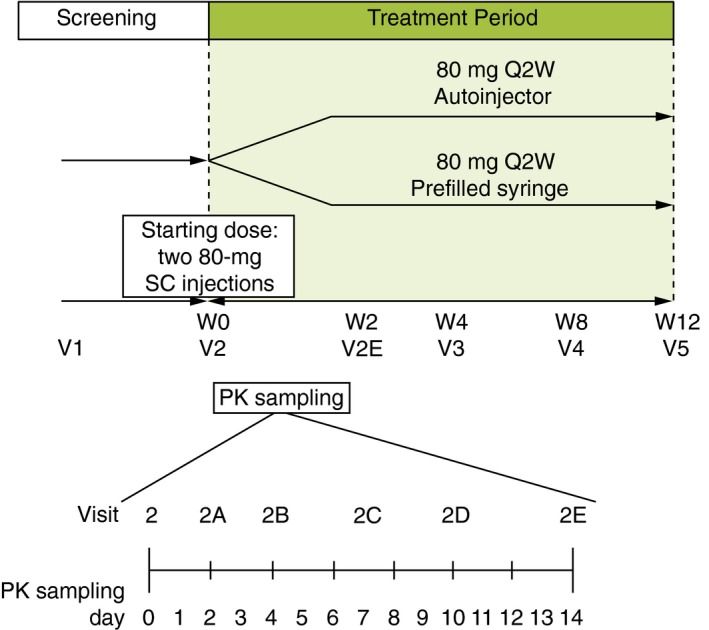
cStudy design for administration of ixekizumab via a prefilled syringe or autoinjector. 80 mg Q2W, 80‐mg ixekizumab every 2 weeks; PK, pharmacokinetic; SC, subcutaneous; W, week; V, visit.
Patients
Eligible patients from study sites in the United States and Puerto Rico were 18 years of age or older; were using a reliable method of birth control; had a diagnosis of plaque psoriasis for at least 6 months prior to baseline; had at least 10% body surface area of plaque psoriasis, a static Physician's Global Assessment score of at least three, and a Psoriasis Area and Severity Index (PASI) score of at least 12 at both screening and baseline; were candidates for phototherapy and/or systemic therapy; and were willing and able to self‐administer (or have an assistant inject) the study drug and have blood drawn for PK sampling.
Patients were ineligible if they had erythrodermic, generalized pustular or guttate forms of psoriasis; were pregnant or breastfeeding; recently received any systemic, biologic or non‐biologic therapy or topical psoriasis therapy; were concurrently using biologic therapy; were positive for human immunodeficiency virus, hepatitis B or hepatitis C or had any other active or recent infection; or had an uncontrolled cerebrovascular condition, unstable medical condition and/or any significant, uncontrolled neuropsychiatric disorders.
Bioanalytical methods
Following the initial 160‐mg dose of ixekizumab at week 0 (baseline visit, day 0), blood samples for the measurement of serum ixekizumab concentrations were collected on days 2, 4, 7 and 10, and prior to ixekizumab administration on day 14 (Fig 1). Each blood draw was at approximately the same time of the day for the majority of the patients. Serum samples were analyzed for ixekizumab concentrations using an Enzyme Linked Immunosorbent Assay method at Intertek ALTA Analytical (San Diego, CA, USA). The range of quantitation was 7.5–300 ng/mL. Samples above the upper limit of quantification were further diluted and reanalyzed to yield results within the calibrated range. The intra‐assay accuracy (% relative error) during validation ranged from −25.0% to 27.5%. The inter‐assay precision (% relative SD) during validation ranged from 11.8% to 17.3%.
Pharmacokinetic analyses
All PK analyses were conducted on an evaluable PK population (i.e. patients who were compliant with the dosing regimen and the full PK sampling scheme or patients who had at least four of the five serum PK samples and were not missing the last PK sample on day 14). Serum ixekizumab concentration‐time data obtained from individual patients were analyzed using non‐compartmental PK methods (Phoenix WinNonlin Professional version 6.3; Pharsight Corporation, Mountain View, CA, USA). The PK parameters determined for ixekizumab included the maximum plasma concentration (C max), time to maximum concentration (t max) and the area under the curve (AUC0‐tlast) where t last is the time of the last sample (14 days ± 24 h). The C max and AUC0‐tlast were summarized as geometric means and 90% confidence intervals (CIs) for the PFS and autoinjector. The t max was summarized and reported as median (minimum–maximum) number of days.
Efficacy and safety assessments
Efficacy was evaluated at specific visits and safety was assessed at each visit following SC administration of ixekizumab with the PFS or the autoinjector. PASI scores were recorded at weeks 0, 4, 8 and 12 and efficacy was evaluated by the mean percent improvement on the PASI at week 12. Safety assessments included analysis of treatment‐emergent adverse events (TEAEs) and serious adverse events (SAEs), which were recorded at each visit, as well as vital signs and clinical laboratory data, which were recorded at regular intervals throughout the study.
Statistical analysis
No hypothesis was tested; the estimation approach was used to evaluate the effect of drug delivery on ixekizumab PK. The primary PK parameters for analysis were C max and AUC0‐tlast. PK parameters were loge‐transformed and analyzed by an analysis of variance (ANOVA) model with device, injection location and weight category as factors. For each loge‐transformed PK parameter, the point estimates and the associated 90% CIs were constructed for the device difference and were then exponentiated back to obtain the ratio of geometric LS mean and 90% CIs. The median difference and 90% CIs for t max were based on Hodges‐Lehmann estimation using SAS NPAR1WAY procedure.
Demographics, baseline characteristics and efficacy analyses were conducted on the intent‐to‐treat population, which included all randomized patients. Demographic variables and baseline characteristics were summarized by drug delivery device. The change from baseline and percent improvement from baseline in PASI total score were analyzed by drug delivery device for each scheduled visit. Missing data for the efficacy analysis were imputed using the modified baseline observation carried forward (mBOCF) approach. All statistical tests were performed at a two‐sided significance level of 0.05.
Safety analyses included all randomized patients who received at least one dose of study drug. Safety was assessed by summarizing and analyzing adverse events, laboratory analytes and vital signs.
Results
Patient disposition and baseline characteristics
Of the 282 enrolled patients, 204 were randomized to receive ixekizumab: 102 by PFS and 102 by autoinjector (Fig 2). The majority of patients (93%) completed the 12‐week treatment period. Baseline demographics and clinical characteristics were similar between groups (Table 1). The majority of patients were male (70%) and Caucasian (87%) with a mean age of 46.5 years.
Figure 2.
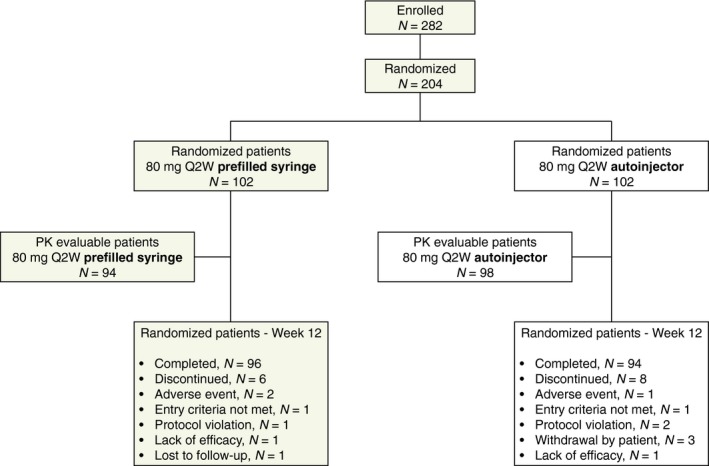
Patient disposition for all patients randomized to ixekizumab treatment via prefilled syringe or autoinjector and for PK‐evaluable patients (patients who were compliant with the dosing regimen and PK sampling scheme, and patients who had at least four serum samples and were not missing the last sample on day 14). PK, pharmacokinetic; 80 mg Q2W, 80‐mg ixekizumab every 2 weeks.
Table 1.
Baseline demographics and clinical characteristics – all randomized patients
| 80 mg Q2W PFS N = 102 | 80 mg Q2W autoinjector N = 102 | |
|---|---|---|
| Age, year | 46.3 ± 14.5 | 46.8 ± 13.1 |
| Male, n (%) | 71 (70%) | 71 (70%) |
| Race, n (%) | ||
| Caucasian | 82 (80%) | 95 (93%) |
| Black/African American | 13 (13%) | 5 (5%) |
| Asian | 4 (4%) | 2 (2%) |
| Other | 3 (3%) | 0 (0%) |
| BMI, kg/m2 | 31.0 ± 7.6 | 31.7 ± 9.1 |
| Weight, kg | 92.4 ± 25.2 | 95.6 ± 27.7 |
| Weight category, n (%) | ||
| <80 kg | 36 (35%) | 35 (34%) |
| 80–100 kg | 32 (31%) | 33 (32%) |
| >100 kg | 34 (33%) | 34 (33%) |
| Disease duration, year | 18.7 ± 13.7 | 19.1 ± 13.0 |
| Percentage of BSA | 27.7 ± 18.4 | 22.8 ± 14.7 |
| <20%, n (%) | 44 (43%) | 56 (55%) |
| ≥20%, n (%) | 58 (57%) | 46 (45%) |
| PASI score | 21.1 ± 9.4 | 17.8 ± 6.1 |
All data are mean ± SD unless otherwise indicated as n (%).
BMI, body mass index; BSA, body surface area; PASI, Psoriasis Area and Severity Index; 80 mg Q2W, 80‐mg ixekizumab every 2 weeks.
For the PK analyses, 192 of the 204 patients had PK‐evaluable data: 94 in the PFS group and 98 in the autoinjector group (Fig 2). Of the 12 patients excluded from the PK analysis, three patients had no evaluable samples, eight patients had ≤3 samples and one patient had the last sample taken at day 17 instead of day 14.
Pharmacokinetics
The mean ixekizumab serum concentrations vs. time profiles were similar with the PFS and the autoinjector (Fig 3). The PFS and autoinjector showed similar maximum concentration [geometric mean C max (90% CI): 15.0 μg/mL (13.9–16.1) vs. 14.8 μg/mL (13.8–15.9)] (Table 2). The geometric LS mean ratio when comparing the C max of the autoinjector to the PFS was 0.97 (90% CI: 0.89–1.06). The geometric mean AUC0‐tlast (90% CI) with the PFS was 157 μg × day/mL (147–168) and 154 μg × day/mL (144–165) with the autoinjector. The geometric LS mean ratio when comparing the AUC0‐tlast of the autoinjector to the PFS was 0.97 (90% CI: 0.89–1.05). Variability in these parameters was also similar for each device group, with percent coefficient of variation estimates in the 41% to 46% range for C max and AUC0‐tlast. The time to maximum concentration [median t max (minimum–maximum)] was approximately 4 days after dosing for each device [PFS: 3.97 days (90% CI: 1.88–13.96); autoinjector: 4.00 days (90% CI: 1.88–14.01)]. When the t max of the autoinjector was compared to the PFS, the median difference was 0.046 days (90% CI: 0.01–0.09).
Figure 3.
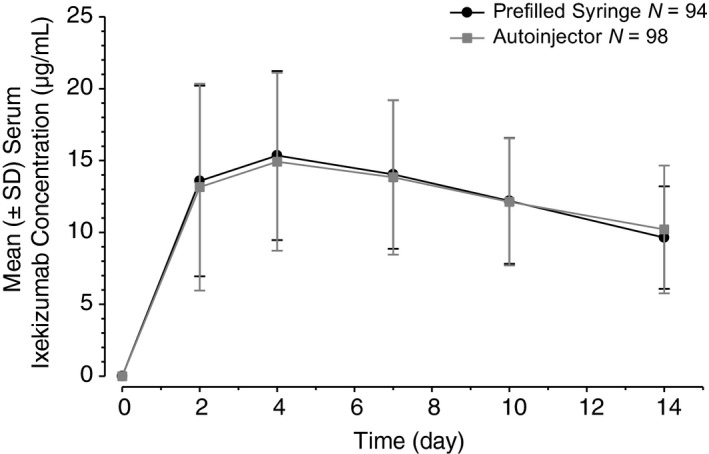
Mean (±SD) serum ixekizumab concentration vs. time profiles following a 160‐mg subcutaneous dose using either a prefilled syringe or an autoinjector in patients with moderate‐to‐severe plaque psoriasis. SD, standard deviation.
Table 2.
Summary of ixekizumab pharmacokinetic (PK) parameters in serum using either a PFS or an autoinjector – PK‐evaluable patients
| 80 mg Q2W PFS N = 94 | 80 mg Q2W autoinjector N = 98 | Autoinjector to PFS ratio/median difference | |
|---|---|---|---|
| C max, μg/mL | 15.0 (13.9–16.1) | 14.8 (13.8–15.9) | 0.97 (0.89–1.06)a |
| t max, day | 3.97 (1.88–13.96)b | 4.00 (1.88–14.01)b | 0.046 (0.01–0.09)c |
| t last, day | 13.97 (13.80–14.18) | 13.98 (13.86–14.89) | – |
| C last, μg/mL | 8.98 (8.41–9.59) | 9.22 (8.52–9.98) | – |
| AUC0‐tlast, μg × day/mLd | 157 (147–168) | 154 (144–165) | 0.97 (0.89–1.05)a |
Data are reported as mean (90% CI) unless otherwise noted.
Geometric LS mean ratio of autoinjector to PFS (90% CI for ratio).
Median (minimum–maximum).
Median difference of autoinjector to PFS (90% CI of difference).
AUC0‐tlast is equal to AUC0–14 days, where the last time point was 14 days ± 24 h.
AUC0‐last, area under the curve up to last time point; C last, observed concentration at the last time point; C max, maximum plasma concentration; CI, confidence interval; LS, least squares; PFS, prefilled syringe; 80 mg Q2W, 80‐mg ixekizumab every 2 weeks; t last, last time point; t max, time of C max.
Efficacy and safety
At week 12, mean percent improvement in PASI (mBOCF) was 89.3% (95% CI: 83.8–94.9) with the PFS and 86.9% (95% CI: 82.2–91.6) with the autoinjector (P < 0.001 vs. baseline for both groups) (Table 3).
Table 3.
Efficacy and safety of ixekizumab delivered by either PFS or autoinjector over the 12‐week treatment period
| 80 mg Q2W PFS N = 102 | 80 mg Q2W autoinjector N = 102 | |
|---|---|---|
| Percent of patients – ITT population | ||
| Mean PASI improvement, mBOCF (95% CI) | 89.3% (83.8–94.9)a | 86.9% (82.2–91.6)a |
| Number of patients (%) – safety analysis population | ||
| Discontinued due to an AE | 2 (2.0%)b | 1 (1.0%)c |
| TEAEs | 51 (50.0%) | 46 (45.1%) |
| Mild | 23 (22.5%) | 25 (24.5%) |
| Moderate | 25 (24.5%) | 17 (16.7%) |
| Severe | 3 (2.9%) | 4 (3.9%) |
| TEAEs in ≥5% of patients Injection site reactiond | 8 (7.8%) | 5 (4.9%) |
| SAEs | 3 (2.9%) | 4 (3.9%) |
| Deaths | 0 | 0 |
Table includes all events, regardless of investigator‐reported relatedness to the drug.
P < 0.001 vs. baseline.
Drug hypersensitivity, rash erythematous.
Anaphylactic reaction.
MedDRA preferred term.
AE, adverse event; ITT, intent‐to‐treat; mBOCF, modified baseline observation carried forward; PASI, Psoriasis Area and Severity Index; PFS, prefilled syringe; 80 mg Q2W, 80‐mg ixekizumab every 2 weeks; SAE, serious adverse events; TEAE, treatment‐emergent adverse events.
Both devices had safety results that were consistent with the known safety profile of ixekizumab. Of the 48% of patients who experienced a TEAE, the majority of adverse events were mild (n = 48, 24%) or moderate (n = 42, 21%) in severity (Table 3). Injection‐site reaction was the most frequently reported TEAE (≥5% of patients in either device group). Most TEAEs were judged by the investigators as possibly related to the study drug (n = 40, 20%) and few were possibly related to the device (n = 11, 5%). Each of the 11 possibly device‐related TEAEs involved administration‐site conditions, 10 of which occurred in the autoinjector group and one of which occurred in the PFS group. Three patients (1.5%) experienced an adverse event that led to study drug discontinuation (Table 3). One was an SAE reported as anaphylactic reaction 13 days after the initial injection of the 160‐mg initial dose using the autoinjector and 1 day after starting an antibiotic for sinusitis. The lack of temporal relationship of the event to the study drug suggested that this was not a case of true anaphylaxis. This patient experienced shortness of breath and hives on the legs and arms, and was treated with an antihistamine and prednisone as an outpatient. A total of seven SAEs were reported during the 12‐week treatment period, three in the PFS and four in the autoinjector group (Table 3). Each SAE was different and was not reported in more than one patient. There were no deaths reported during the 12‐week treatment period (Table 3).
Treatment‐emergent abnormal laboratory values occurring in ≥5% of patients included low bicarbonate (41.9%), high very‐low‐density lipoprotein cholesterol (20.7%) and high creatinine clearance (14.0%). The majority of patients had leukocyte (94.6%), lymphocyte (96.5%), neutrophil (94.1%) and platelet count (98.5%) results that remained in the same Common Terminology Criteria for Adverse Events grade category from the baseline to post‐baseline observation. No patient had post‐baseline Grade 3 or 4 leukocytes, lymphocytes or neutrophils. No patient had post‐baseline Grades 2 to 4 platelets. The proportion of patients with treatment‐emergent high systolic blood pressure was 2.8% and for diastolic pressure was 4.7% overall. No patient experienced a treatment‐emergent low systolic or diastolic blood pressure value or low or high pulse rate.
Discussion
This study evaluated the PK of ixekizumab for two different devices in addition to evaluating the efficacy and safety of each device with approximately 100 patients per treatment arm. This design allowed for a robust assessment of ixekizumab PK and its variability by the administration of ixekizumab via PFS and autoinjector. In this study, the PK of ixekizumab was similar when administered SC by either the PFS or autoinjector; therefore, both devices appear to be suitable options for the administration of ixekizumab in patients with moderate‐to‐severe psoriasis.
Patients receiving ixekizumab with either device demonstrated at least 85% improvement in PASI at week 12. Additionally, similar percentages of patients reported TEAEs and SAEs. These findings indicate that SC ixekizumab administered either with an autoinjector or PFS is consistent with randomized, placebo‐controlled, phase 3 clinical trials that demonstrated the efficacy and safety of ixekizumab.
Limitations
The PK of ixekizumab was evaluated for only 14 days after administration despite having an approximate 13‐day half‐life. This was considered sufficient to characterize the PK of each device in the most variable part of the exposure time profile immediately after dosing. It should be considered that in this population of patients with plaque psoriasis, the study duration was only 12 weeks, which may limit longer‐term generalizability of device use.
Conclusions
The PK of ixekizumab is similar when given by SC administration by two different delivery methods – PFS and autoinjector. Both devices provided similar drug exposure as well as significant improvements in psoriasis symptoms, as measured by percent change in PASI, and both had safety results consistent with the known safety profile of ixekizumab. Given the similar exposure delivered by both drug delivery devices, patients can be offered a choice between the two devices for the management of their disease.
Acknowledgements
The authors thank Kelly Guerrettaz, inVentiv Health Clinical (Princeton, NJ) for assistance with manuscript development.
Conflicts of interest
K. Callis Duffin has received grants and/or research support from Amgen, Eli Lilly and Company, Janssen, Stiefel, AbbVie, Bristol‐Myers Squibb, Celgene, Novartis and XenoPort and has been a consultant and/or participated on an advisory board for Amgen, Eli Lilly and Company, Janssen, Stiefel, AbbVie, Bristol‐Myers Squibb, Novartis, Celgene, Pfizer, Novartis and XenoPort. J. Bagel has received grants from Eli Lilly and Company, Amgen, AbbVie and Janssen. M. Bukhalo has been a consultant and/or speaker for Novartis, Leo Pharma, Boehringer Ingelheim and has received grants/research support from Novartis, Boehringer Ingelheim, Leo Pharma, Merck, DUSA Pharmaceuticals, Centocor, Allergan, Celgene, Galderma and Coherus; I.J. Mercado Clement is a former employee of Lilly‐NUS Centre for Clinical Pharmacology; S.L. Choi is an employee of Lilly‐NUS Centre for Clinical Pharmacology; F. Zhao, A. Gill, B. Pangallo, C. Shuler, L. Mallbris and K. Jackson are employees and stockholders of Eli Lilly and Company.
Funding sources
Eli Lilly and Company.
References
- 1. Zenobia C, Hajishengallis G. Basic biology and role of interleukin‐17 in immunity and inflammation. Periodontol 2000 2015; 69: 142–159. doi:10.1111/prd.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharma J, Balakrishnan L, Datta KK et al A knowledgebase resource for interleukin‐17 family mediated signaling. J Cell Commun Signal 2015; 9: 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raychaudhuri SP. Role of IL‐17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol 2013; 44: 183–193. [DOI] [PubMed] [Google Scholar]
- 4. Griffiths CE, Reich K, Lebwohl M et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomized trials. Lancet 2015; 386: 541–551. doi:10.1016/S0140‐6736(15)60125‐8. [DOI] [PubMed] [Google Scholar]
- 5. Berteau C, Scwarzenbach F, Donazzolo Y et al Evaluation of performance, safety, subject acceptance, and compliance of a disposable autoinjector for subcutaneous injections in healthy volunteers. Patient Prefer Adherence 2010; 4: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwarzenbach F, Dao Trong M, Grange L et al Results of a human factors experiment of the usability and patient acceptance of a new autoinjector in patients with rheumatoid arthritis. Patient Prefer Adherence 2014; 8: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kivitz A, Cohen S, Dowd JE et al Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self‐administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther 2006; 28: 1619–1629. [DOI] [PubMed] [Google Scholar]
- 8. Paul C, Stalder JF, Thaҫi D et al Patient satisfaction with injection devices: a randomized controlled study comparing two different etanercept delivery systems in moderate to severe psoriasis. J Eur Acad Dermatol Venereol 2012; 26: 448–455. [DOI] [PubMed] [Google Scholar]
- 9. Bayas A. Improving adherence to injectable disease‐modifying drugs in multiple sclerosis. Expert Opin Drug Deliv 2013; 10: 285–287. [DOI] [PubMed] [Google Scholar]
- 10. Lugaresi A. Addressing the need for increased adherence to multiple sclerosis therapy: can delivery technology enhance patient motivation? Expert Opin Drug Deliv 2009; 6: 995–1002. [DOI] [PubMed] [Google Scholar]


