Abstract
Objective
Intravesical bacillus Calmette-Guerin (BCG) is the gold standard for high-grade non-muscle-invasive bladder cancer (NMIBC); however, some patients do not respond to initial therapy while others relapse and/or progress. Therefore, combination strategies that can enhance the efficacy and sustainability of BCG are needed. Herein, we explore the efficacy of lenalidomide, a thalidomide derivative with immunomodulatory effects, in combination with BCG, both in vitro and in vivo.
Materials and methods
We explored the outcomes of lenalidomide in combination with BCG in vivo using the MBT-2 cell line implanted in C3H immunocompetent mice. Apoptosis, cell proliferation, and microvessel density were measured by immunohistochemistry. In vitro, we performed Western blotting for cell cycle and apoptosis regulatory proteins and a chromatin condensation assay to evaluate TNF-α and FasL in combination with lenalidomide.
Results
In the mouse model, combination therapy with BCG and lenalidomide resulted in a statistically significant decrease in tumor size compared with the control group. IHC demonstrated a nonsignificant increase in apoptosis in the combination condition and no effect on cellular proliferation. Microvessel density was decreased in all treated conditions. In vitro, caspase-3 activation and chromatin condensation studies demonstrated increased cell death in the combinations of lenalidomide and TNF-α.
Conclusions
The immunomodulatory molecule lenalidomide augments the response to BCG in an in vivo mouse model. This provides the rationale for studying the combination in patients with high grade NMIBC.
Keywords: BCG, Lenalidomide, Non-muscle-invasive bladder cancer, Immunotherapy
1. Introduction
Bladder cancer continues to be a significant healthcare and financial liability in the USA. It is estimated that 73,510 patients will be diagnosed in 2012 [1]. The vast majority of patients present with non-muscle-invasive bladder cancer (NMIBC), and these contribute (largely due to the financial burden of routine surveillance and repeated therapies) to the fact that bladder cancer is now the most expensive malignancy in terms of cost per patient from diagnosis to death [2]. Therefore, therapeutic regimens that demonstrate a robust immediate response, durable prevention of recurrence, and diminished risk of progression are desperately needed.
Since 1976, when Morales et al reported the first usage of bacillus Calmette-Guerin (BCG) as an intravesical therapy for bladder cancer [3], multiple studies have solidified the role of BCG as the gold standard for decreasing the rates of recurrence and/or progression [4–7]. However, approximately 50% of patients will recur by 5 years [8, 9]. This poses a serious dilemma, especially in those who refuse radical cystectomy or have too many comorbidities to undergo a major operation. While other therapies such as mitomycin C are considered in this unique setting, the only Food and Drug Administration (FDA) approved intravesical option for BCG “failure” is valrubicin, which exhibits a modest response rate of 21% at 6 months [10].
Lenalidomide, a thalidomide derivative, is an attractive adjunct therapeutic option to BCG for several reasons. In non-bladder cancers, lenalidomide has displayed direct tumoricidal activity, antiangiogenic properties and, most importantly, immunomodulatory functions [11,12]. Furthermore, lenalidomide has been shown to be effective in 2 separate phase III clinical trials for relapsing multiple myeloma demonstrating improved time to progression and overall survival [13,14].
In the present study, we examine whether lenalidomide can augment the response to BCG in an immunocompetent mouse model. We demonstrate that this combination significantly reduces tumor burden and decreases tumor microvessel density. Furthermore, in our corresponding in vitro studies, we found that MBT-2 cell death occurs with the combination of lenalidomide and TNF-α.
2. Materials and methods
2.1. Cell lines and reagents
Mouse MBT-2 bladder cancer cells (originally generated by Soloway et al [15–17]) were obtained from Dr. Weigo Jian and Dr. Seth Lerner (Baylor College of Medicine, Houston, TX). MBT-2 cells were cultured in RPMI supplemented with 10% FBS, penicillin, streptomycin, vitamins, L-glutamine, nonessential amino acids, pyruvate supplements, and 2 mM HEPES. Lenalidomide was obtained from Celgene Corporation (Summit, NJ). TheraCys BCG was obtained from Sanofi Pasteur Inc. (Swiftwater, PA). Antibodies to BCLXL (Sc-8392) and caspase-3 (Sc-7148) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to Ki-67 (M7240) were purchased from DAKO (Glostrup, Denmark). Carboxymethyl cellulose (21902) was purchased from Sigma-Aldrich (St. Louis, MO). Antibodies to p27 (2552) and cyclin-D1 (2922) were from Cell Signaling Technology (Danvers, MA). Mouse anti-CD-31 (53370) was purchased from BD Biosciences (Franklin Lakes, NJ).
2.2. Animals
Female C3H/HEN MTV–/– immunocompetent mice (age 3–5 weeks) were purchased from the National Cancer Institute-Frederick Research and Development Center. All animal experiments were performed as per the M. D. Anderson Institutional Animal Care and Use Committee (IACUC) approved protocol 11-09-13531.
2.3. In vivo evaluation of lenalidomide and BCG combination therapy
MBT-2 cells were trypsinized at 75%–85% confluence and washed (1,200 rpm for 3 min at room temperature) once with 10% FBS containing RPMI and once with serum free RPMI. The cells were resuspended in HBSS and counted after trypan blue staining. Single-cell suspensions of 2 × 105 viable cells in 100 μl HBSS were injected subcutaneously in the flank region. Mice were grouped into the following treatment conditions after verification of similar tumor volumes (n = 10): (a) vehicle control (1% carboxymethyl cellulose by oral gavage daily plus intratumoral normal saline injection weekly); (b) lenalidomide alone (30 mg/kg), once daily, oral gavage; (c) BCG (105 CFU/50 μl), once weekly via intratumoral injection; and (d) combination of lenalidomide (30 mg/kg), once daily, and BCG (105 CFU), once weekly. The BCG dose and timing was determined by an independent animal experiment where 1 × 105, 1 × 106, and 1 × 107 doses were tested to reduce tumor volume (data not shown). Lenalidomide treatment was initiated 24 hours before the first BCG injection (which was initiated on day 6 post-injection) to allow the drug to reach its target and to have a modulated immune tumor microenvironment. This immunocompetent model of MBT-2 cells and C3H mice is consistent with previously published literature [18–20]. The endpoint of the experiment was determined to be the time at which the mouse tumors exceeded the size of 1.5 cm as per the IACUC guidelines on tumor burden. Tumors were inspected and measured every third day using a Vernier caliper. Tumor volume calculations were performed as previously described [18].
2.4. TUNEL detection of apoptosis in tumor sections
Formalin-fixed, paraffin-embedded sections were deparaffinized, fixed in paraformaldehyde, and the apoptotic cells were stained using dead end fluorescent terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) kit (G3250) as per manufacturer's instructions (Promega, Madison, WI). Total nuclei were stained using propidium iodide. The sections were imaged using a Zeiss, Plan-Neofluar lens on an epifluorescence microscope (Olympus IX). Imaging was done using a 20× objective lens. The ratio of TUNEL positive nuclei to propidium iodide positive total nuclei from 3 random fields per treatment condition were counted and expressed in terms of percentage.
2.5. Assessment of proliferating cells and microvessel density in vivo
Formalin-fixed, paraffin-embedded sections were deparaffinized, fixed in paraformaldehyde, and stained with anti-Ki-67 antibody as described previously [21]. For CD-31 immunocytochemistry, cryosections from OCT preserved tumor samples were used. The sections were stained using diaminobenzidine (DAB). Imaging was performed using a 20× objective lens. The images were used to count Ki-67 positive nuclei and total nuclei. The percentage of positive nuclei were calculated and plotted. For microvessel density, the CD-31 positive foci were counted and the controls were considered as 100%. The reduction in CD-31 positive cells in treated conditions was represented in percentages.
2.6. Western blotting
Cells were treated as indicated in the figures and were scrapped in the medium, pelleted at 3,500 rpm for 5 minutes, and washed once with ice cold PBS at 3,500 rpm for 5 minutes. The pellets were lysed [lysis buffer: 50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 5 mM EDTA; 25 mM NaF; 1% Triton-X 100; 1% NP-40; 0.1 mM Na3VO4; 12.5 mM β-glycerophosphate; 1 mM PMSF and complete protease inhibitors] and clarified at 13,000 rpm for 10 minutes at 4°C. The lysates were subjected to SDS-PAGE and Western blotted using nitrocellulose membranes. The blots were developed using ECL reagent (GE Healthcare, Pittsburgh, PA).
2.7. Statistical analyses
All statistical analyses for relevance were performed using Student's t-test (2-tailed distribution, 2-sample unequal variance) in Microsoft Excel 2010. P value less than 0.05 was considered significant.
3. Results
3.1. Lenalidomide enhances the anticancer activity of BCG immunotherapy in an immunocompetent mouse model
Based on the proposed mechanisms of BCG and lena-lidomide therapy as described in the introduction, we explored the ability of this drug combination to enhance the anticancer activity over BCG alone in a mouse model. An intact immune system is necessary for the proposed mechanisms of action; therefore, we chose to use MBT-2 cells implanted into C3H mice.
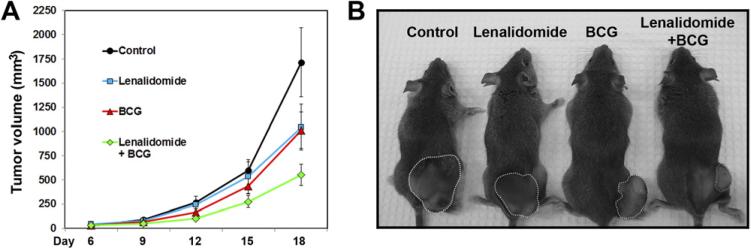
We initiated the lenalidomide oral treatment 24 hours before BCG therapy to ensure the bioavailability of lenalidomide in the tumor microenvironment at the time of BCG treatment. The average tumor sizes were reduced insignificantly by single agents of BCG (P = 0.101) and lenalidomide (P = 0.136) compared with the control group. However, the combination treatment reduced the average tumor size to a significant level compared with the control (P < 0.01) (Fig. 1A). Representative images of mouse tumors from each group are shown in Fig. 1B.
Fig. 1.
Lenalidomide reduces tumor growth in combination with BCG in immunocompetent mice. (A) C3H mice were injected with MBT-2 cells and treated as described in the Materials and Methods section. Tumor volumes are plotted. See text for P values. Error bars = standard error. (B) Photomicrograph of representative mice from each group. (Color version of figure is available online.)
3.2. The antitumor activity of lenalidomide and BCG is independent of effects on apoptosis or cell proliferation
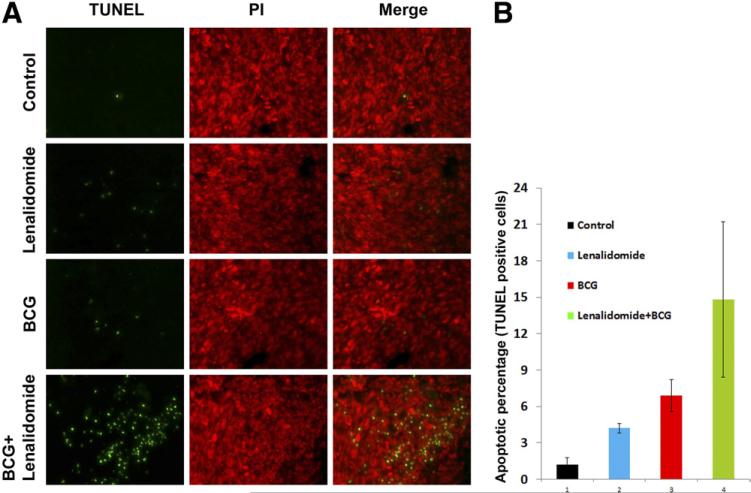
We used fluorescent TUNEL staining to determine whether the reduction in tumor size was due to increased apoptosis. We found that lenalidomide and BCG as single agents resulted in modest apoptotic activity compared with the control, while the combination treatment resulted in higher densities of apoptotic cells (Fig. 2A). However, there was no statistically significant difference between the treatment groups when apoptotic cells were quantified using the percentage of apoptotic nuclei compared with total cells (Fig. 2B).
Fig. 2.
Lenalidomide, in combination with BCG, has no significant effect on apoptosis. (A) TUNEL staining of fragmented apoptotic DNA/nuclei to detect the efficacy of lenalidomide and BCG in vivo (green) and the total nuclei stained using propidium iodide (red). Magnification 20×. (B) TUNEL positive nuclear percentage with respect to total nuclear count. (Color version of figure is available online.)
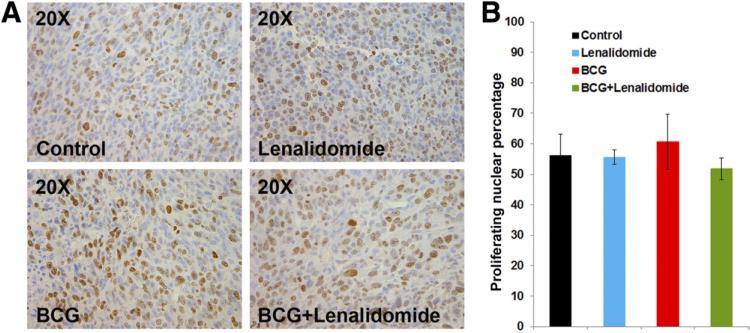
To examine whether the tumor size reduction was due to reduced cellular proliferation, we examined the tumor sections for Ki-67 positivity by immunohistochemistry. We identified no significant differences between the treatment groups, suggesting that the therapy does not exert its effect via cell cycle arrest (Fig .3).
Fig. 3.
The combination of lenalidomide and BCG does not affect the proliferation of MBT-2 cells in vivo. (A) Ki-67 immunohistochemistry of formalin fixed paraffin embedded tumor sections to study the proliferation of MBT-2 cells in vivo under various treatment conditions. (B) Quantification of proliferative nuclei was performed as described in the Materials and Methods section and plotted. Error bars SE. (Color version of figure is available online.)
3.3. Lenalidomide and BCG immunotherapy reduces tumor microvessel density in vivo
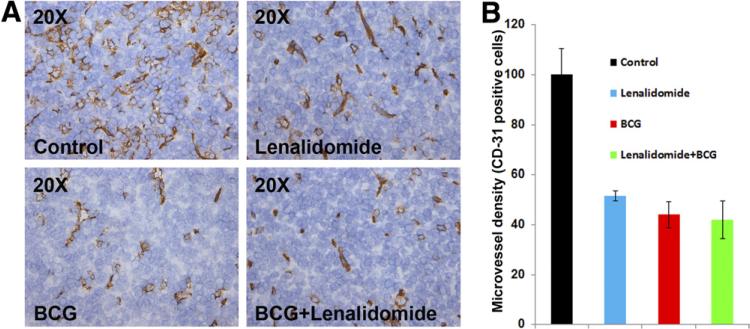
Lenalidomide has been shown to decrease microvessel density in non-bladder solid tumors [22]. Furthermore, our laboratory has previously shown that BCG, as a single agent, reduces tumor microvessel density [18]. Hence, we examined the tumor microvessel density by CD-31 immunohistochemistry and found that lenalidomide and BCG as single agents, as well as the combination treatment, all reduced the tumor microvessel density to a significant extent compared with control (P = 0.0169, 0.0065, P = 0.0050, respectively) (Fig. 4A, B). However, there was no significant reduction in tumor microvessel density when the combination was compared with single agent treatments with lenalidomide and BCG (P = 0.29 and 0.828, respectively).
Fig. 4.
Lenalidomide and BCG reduced tumor microvessel density in vivo. (A) Immunohistochemistry of OCT cryopreserved tumor samples was performed using CD-31 antibody to detect tumor blood vessels. (B) Lenalidomide and BCG as single agents and the combination all reduced the tumor microvessel density to a significant extent compared to control (P = 0.0169, 0.0065, P = 0.0050, respectively) Error bars: Standard error. Magnification 20×. (Color version of figure is available online.)
3.4. In vitro, lenalidomide enhances the cell death of MBT-2 cells in combination with recombinant TNF-α but not with recombinant FasL
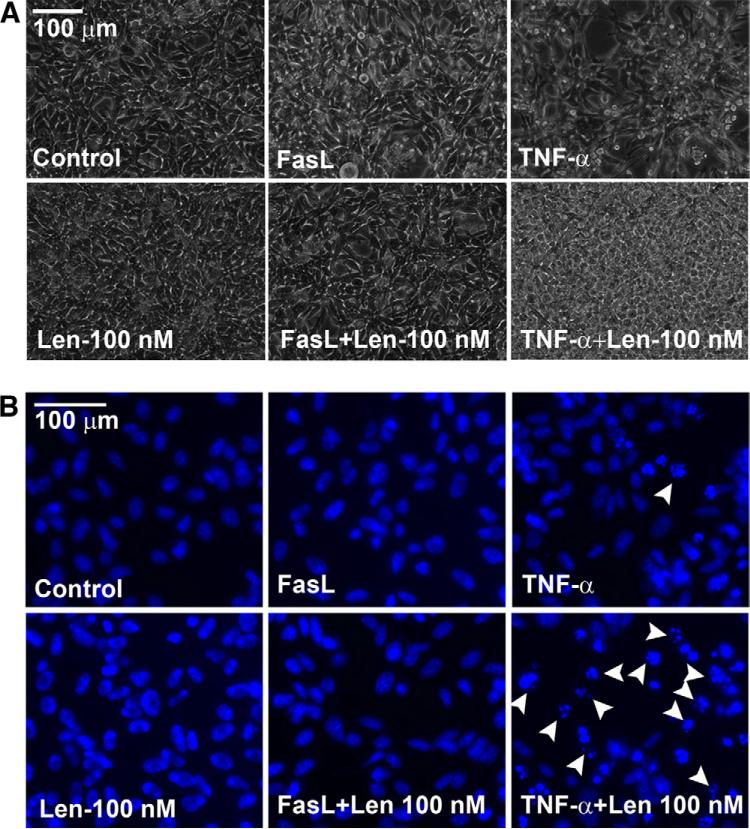
It has been demonstrated that neutrophil granulocytes are required for effective BCG immunotherapy [23] and, in vitro, we have found that BCG exerts its effects through the secretion of TNF-α from BCG stimulated neutrophils [24]. Furthermore, in a phase I metastatic melanoma trial, lena-lidomide was found to induce the secretion of TNF-α into patient sera [25]. Therefore, we examined whether cytotoxicity observed in vivo could be produced in vitro by using recombinant TNF-α. We also evaluated FasL to determine whether the effects of TNF-α were reproducible with an alternative effector ligand. Qualitatively, co-treatment of lenalidomide 100 nM with TNF-α resulted in MBT-2 cell death, however, cell death was not observed with the combination containing FasL and lenalidomide as assessed by microscopy and chromatin condensation (Fig. 5).
Fig. 5.
Lenalidomide augments cell death in vitro with TNF-α but not with FasL. (A) MBT-2 cells were treated as indicated in the figure; 24 hours after treatment, the cells were imaged. Len = lenalidomide, TNF-α and FasL concentrations were 17 ng/ml. (B) The cell death was confirmed by chromatin condensation (because of caspase-3 activation) by staining the cells using live stain Hoechst-33,342. Note the chromatin condensation (arrow heads) in TNF-α treated conditions but not in FasL treated conditions. (Color version of figure is available online.)
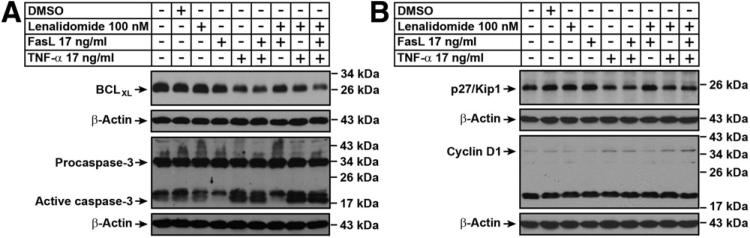
Next, we evaluated the effects of TNF-α and FasL both alone and in combination with lenalidomide on MBT-2 cells in vitro by Western blotting for apoptosis and cell cycle regulators. The results demonstrate that conditions, including TNF-α, but not FasL, induced the processing of active caspase-3. Further, expression of the cytoprotective protein BCLXL was decreased in the conditions involving TNF-α (Fig. 6A). Cellular proliferation regulatory proteins demonstrated minimal differences in the treatment conditions. There was minimal down-regulation of p27 and up-regulation in cyclin-D1 (Fig. 6B) in the TNF-α treated conditions.
Fig. 6.
TNF-α but not FasL induce apoptosis and cell proliferation events in vitro. MBT-2 cells were treated as indicated in figures and were lysed and subjected to immunoblotting as described in the Materials and Methods section. (A) Western analysis of apoptotic regulators BCLXL and Caspase-3. TNF-α, but not FasL is able to induce active caspase-3 either alone or in combination with lenalidomide. (B) Western analysis of cell cycle regulators p27 and cyclin-D1.
4. Discussion
The treatment of BCG failure in patients with non-muscle-invasive bladder cancer poses a serious dilemma for clinicians since there is no truly effective salvage intravesical therapy [26]. Therefore, in patients who fail BCG, the next step is radical cystectomy. While oncological results of early cystectomy are excellent, especially in those with localized disease [27], this comes with the trade-off of potential morbidity and complication rates in the range of 40%–50% [28]. Therefore, effective combination treatment strategies are desperately needed in patients either to use after BCG failure or in conjunction with initial BCG to augment its effect.
We feel that lenalidomide is a reasonable option to be used in combination with BCG for patients who fail initial therapy. In our report, we found that BCG and lenalidomide had significant anticancer activity in an immunocompetent mouse model. The combination was able to decrease mouse tumor burden and both drugs, even individually, were able decrease microvessel density compared with the control condition. However, the exact mechanism of anticancer activity in vivo is unclear and seems to be independent of effects on apoptosis or cell proliferation.
It has been shown that neutrophil granulocytes are necessary for the effective treatment of BCG immunotherapy in bladder cancer [23]. Furthermore, we have determined that BCG induces the secretion of functional TNF-α from neutrophils [24]. Lenalidomide, which has pro-immunomodulatory effects, apoptotic inducing capabilities, and antiangiogenic properties, has also been reported to increase TNF-α [25]. Our in vitro data demonstrate that MBT-2 cells are sensitized to cell death when TNF-α and lenalidomide are treated in combination. Moreover, our in vivo data demonstrate that the BCG and lenalidomide combination condition had increased cell death and reduced tumor volume. Potentially, this may be modulated through TNF-α induction, although this is a correlative assumption, and further studies are necessary to extract the exact mechanism of action.
Our results indicate a potential role for lenalidomide in combination with BCG in patients who fail initial treatment. This concept has been considered by collaborative efforts in the past and, in fact, One multi-institutional clinical trial is currently recruiting patients (http://clinicaltrials.gov/ct2/show/NCT01373294?term=lenalidomide&cond=bladder+cancer&rank=1). Subjects are treated daily with oral lena-lidomide for 7 months, including the peri-BCG treatment period. Investigators are studying both progression-free survival and potential side effects of lenalidomide, described in a previous phase I study to include neutropenia, thrombocytopenia, fatigue, rash, and leg cramps [29].
We acknowledge there are several animal models to study treatment effects in NMIBC, especially BCG. While orthotopic models have been well established using C3H mice and MBT-2 cells [30], we chose to use the flank model with direct tumor injection of BCG based on traditional literature [19,20] to facilitate serial measurements without the usage of complicated imaging techniques. Further, the intratumoral injection of BCG, as opposed to intravesical therapy, allowed a decreased risk of anesthesia-related complications during therapy.
5. Conclusions
The immunomodulatory molecule lenalidomide augments the response to BCG by reducing tumor burden and decreasing microvessel density. This provides the rationale for studying the combination in high-grade NMIBC.
Acknowledgments
The authors thank Ms. I-Ling-Lee for technical support and Ms. Donna Reynolds for helping with cryo-immunohistochemistry.
Footnotes
This study was supported by Celgene Corporation.
The authors declare no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;2012:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Botteman MF, Pashos CL, Redaelli A, et al. The health economics of bladder cancer: A comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–30. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 3.Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–3. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 4.Herr HW, Laudone VP, Badalament RA, et al. Bacillus Calmette-Guerin therapy alters the progression of superficial bladder cancer. J Clin Oncol. 1988;6:1450–5. doi: 10.1200/JCO.1988.6.9.1450. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester RJ, van der Meijden AP, Witjes JA, et al. Bacillus Calmette-Guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: A meta-analysis of the published results of randomized clinical trials. J Urol. 2005;174:86–91. doi: 10.1097/01.ju.0000162059.64886.1c. Discussion -2. [DOI] [PubMed] [Google Scholar]
- 6.Herr HW, Schwalb DM, Zhang ZF, et al. Intravesical bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: Ten-year follow-up of a prospective randomized trial. J Clin Oncol Off J American Society Of Clinical Oncology. 1995;13:1404–8. doi: 10.1200/JCO.1995.13.6.1404. [DOI] [PubMed] [Google Scholar]
- 7.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–70. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 8.Herr HW, Dalbagni G, Donat SM. Bacillus Calmette-Guerin without maintenance therapy for high-risk non-muscle-invasive bladder cancer. Eur Urol. 2011;60:32–6. doi: 10.1016/j.eururo.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 9.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized southwest oncology group study. J Urol. 2000;163:1124–9. [PubMed] [Google Scholar]
- 10.Steinberg G, Bahnson R, Brosman S, et al. Efficacy and safety of valrubicin for the treatment of bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol. 2000;163:761–7. [PubMed] [Google Scholar]
- 11.Davies F, Baz R. Lenalidomide mode of action: Linking bench and clinical findings. Blood Rev. 2010;24(Suppl 1):S13–9. doi: 10.1016/S0268-960X(10)70004-7. [DOI] [PubMed] [Google Scholar]
- 12.Heise C, Carter T, Schafer P, et al. Pleiotropic mechanisms of action of lenalidomide efficacy in del(5q) myelodysplastic syndromes. Expert Rev Anticancer Ther. 2010;10:1663–72. doi: 10.1586/era.10.135. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–32. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 14.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–42. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 15.Soloway MS. Single and combination chemotherapy for primary murine bladder cancer. Cancer. 1975;36:333–40. doi: 10.1002/1097-0142(197508)36:2<333::aid-cncr2820360207>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.DeKernion JB, Soloway MS, Persky L. Chemotherapy of experimental transitional-cell carcinoma. Urology. 1974;4:63–8. doi: 10.1016/0090-4295(74)90110-1. [DOI] [PubMed] [Google Scholar]
- 17.Weldon TE, Soloway MS. Susceptibility of urothelium to neoplastic cellular implantation. Urology. 1975;5:824–7. doi: 10.1016/0090-4295(75)90367-2. [DOI] [PubMed] [Google Scholar]
- 18.Kamat AM, Tharakan ST, Sung B, et al. Curcumin potentiates the antitumor effects of bacillus Calmette-Guerin against bladder cancer through the down-regulation of NF-κB and up-regulation of TRAIL receptors. Cancer Res. 2009;69:8958–66. doi: 10.1158/0008-5472.CAN-09-2045. [DOI] [PubMed] [Google Scholar]
- 19.Sosnowski JT, De Haven JI, Abraham FM, et al. Sequential immunocytological evaluation of murine transitional cell carcinoma during intralesional bacillus Calmette-Guerin and interleukin-2 immunotherapy. J Urol. 1992;147:1439–43. doi: 10.1016/s0022-5347(17)37589-4. [DOI] [PubMed] [Google Scholar]
- 20.Lamm DL, Riggs DR, DeHaven JI, et al. Immunotherapy of murine bladder cancer by irradiated tumor vaccine. J Urol. 1991;145:195–8. doi: 10.1016/s0022-5347(17)38290-3. [DOI] [PubMed] [Google Scholar]
- 21.Scott IS, Morris LS, Bird K, et al. A novel immunohistochemical method to estimate cell-cycle phase distribution in archival tissue: Implications for the prediction of outcome in colorectal cancer. J Pathol. 2003;201:187–97. doi: 10.1002/path.1444. [DOI] [PubMed] [Google Scholar]
- 22.Crane E, List A. Lenalidomide: An immunomodulatory drug. Futures Oncol. 2005;1:575–83. doi: 10.2217/14796694.1.5.575. [DOI] [PubMed] [Google Scholar]
- 23.Suttmann H, Riemensberger J, Bentien G, et al. Neutrophil granulocytes are required for effective bacillus Calmette-Guerin immuno-therapy of bladder cancer and orchestrate local immune responses. Cancer Res. 2006;66:8250–7. doi: 10.1158/0008-5472.CAN-06-1416. [DOI] [PubMed] [Google Scholar]
- 24.Jinesh GG, Chunduru S, Kamat AM. Smac mimetic enables the anticancer action of BCG-stimulated neutrophils through TNF-α but not through TRAIL and FasL. J Leukoc Biol. 2012 Apr 19; doi: 10.1189/jlb.1211623. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marriott JB, Clarke IA, Dredge K, et al. Thalidomide and its analogues have distinct and opposing effects on TNF-alpha and TNFR2 during co-stimulation of both CD4(+]) and CD8(+]) T cells. Clin Exp Immunol. 2002;130:75–84. doi: 10.1046/j.1365-2249.2002.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bui TT, Schellhammer PF. Additional bacillus Calmette-Guerin therapy for recurrent transitional cell carcinoma after an initial complete response. Urology. 1997;49:687–90. doi: 10.1016/S0090-4295(97)00067-8. Discussion 90–1. [DOI] [PubMed] [Google Scholar]
- 27.Cheng L, Weaver AL, Leibovich BC, et al. Predicting the survival of bladder carcinoma patients treated with radical cystectomy. Cancer. 2000;88:2326–32. doi: 10.1002/(sici)1097-0142(20000515)88:10<2326::aid-cncr17>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Stein JP, Skinner DG. Results with radical cystectomy for treating bladder cancer: A ‘reference standard’ for high-grade, invasive bladder cancer. BJU Int. 2003;92:12–7. [PubMed] [Google Scholar]
- 29.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–7. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 30.Chan ES, Patel AR, Smith AK, et al. Optimizing orthotopic bladder tumor implantation in a syngeneic mouse model. J Urol. 2009;182:2926–31. doi: 10.1016/j.juro.2009.08.020. [DOI] [PubMed] [Google Scholar]