Background
The number of percutaneous coronary interventions (PCI) in China has increased more than 20‐fold over the last decade. Consequently, there is a need for national‐level information to characterize PCI indications and long‐term patient outcomes, including health status, to understand and improve evolving practice patterns. Objectives: This nationwide prospective study of patients receiving PCI is to: (1) measure long‐term clinical outcomes (including death, acute myocardial infarction [AMI], and/or revascularization), patient‐reported outcomes (PROs), cardiovascular risk factor control and adherence to medications for secondary prevention; (2) determine patient‐ and hospital‐level factors associated with care process and outcomes; and (3) assess the appropriateness of PCI procedures. Methods: The China Patient‐centered Evaluative Assessment of Cardiac Events (PEACE) Prospective Study of PCI has enrolled 5,000 consecutive patients during 2012–2014 from 34 diverse hospitals across China undergoing PCI for any indication. We abstracted details of patient's medical history, treatments, and in‐hospital outcomes from medical charts, and conducted baseline, 1‐, 6‐, and 12‐month interviews to characterize patient demographics, risk factors, clinical presentation, healthcare utilization, and health status using validated PRO measures. The primary outcome, a composite measure of death, AMI and/or revascularization, as well as PROs, medication adherence and cardiovascular risk factor control, was assessed throughout the 12‐month follow‐up. Blood and urine samples were collected at baseline and 12 months and stored for future analyses. To validate reports of coronary anatomy, 2,000 angiograms are randomly selected and read by two independent core laboratories. Hospital characteristics regarding their facilities, processes and organizational characteristics are assessed by site surveys. Conclusion: China PEACE Prospective Study of PCI will be the first study to generate novel, high‐quality, comprehensive national data on patients’ socio‐demographic, clinical, treatment, and metabolic/genetic factors, and importantly, their long‐term outcomes following PCI, including health status. This will build the foundation for PCI performance improvement efforts in China. © 2016 The Authors. Catheterization and Cardiovascular Interventions. Published by Wiley Periodicals, Inc.
Keywords: outcomes research, patient reported outcome measures, registries
BACKGROUND
The dramatically rising prevalence of coronary heart disease in China has occurred at the same time as expansions in access to advanced cardiovascular procedures 1, 2, 3. Over the last decade, the volume of percutaneous coronary intervention (PCI) has increased more than 20‐fold, with 454,505 procedures reported in 2013 4. Despite the magnitude of PCI volume, little information is available regarding the quality of these procedures in China. There is paucity of data on long‐term clinical outcomes such as cardiac death and revascularization, as well as patient‐reported outcomes (PROs), patients’ symptoms, and function and quality of life. In addition, there is a substantial gap in understanding the hospital‐ and patient‐level determinants of patients’ health and well‐being following PCI. Data on indications for, and appropriateness of, PCI are sparse. Furthermore, there is limited knowledge about how evidence‐based therapies are applied in the management of patients after PCI. All of these data are needed to characterize the quality of care provided to patients undergoing PCI and, more importantly, to guide the creation of strategies and tools for quality improvement in China.
Assessments of early and long‐term adverse clinical outcomes after PCI are important in understanding the risk‐benefit balance of this treatment. In the case of PCI, particularly among patients with stable coronary disease, the procedure is performed with the objective of improving patients’ health status 5, 6, 7. In China, few prior studies of PCI included longitudinal data or measured patient care or outcomes, and none focused on health status or other PROs 5, 6, 7. Current evidence regarding long‐term adverse cardiac events following PCI in China are largely based on selected patients, such as those enrolled in stent registries 8, 9 or in randomized controlled trials 10, 11, or those with complex coronary diseases 12, 13, 14. In contrast, numerous studies in western countries have described the efficacy of PCI in reducing the risk of adverse events among patients with acute coronary syndromes 15, as well as improving quality‐of‐life n both acute and stable settings 16, 17. While these studies have laid a foundation for patient and health service decision‐making to improve the quality of PCI, the absence of such data in China limits the opportunity to similarly improve the quality of PCI in China.
To address the substantial gap in understanding the selection, treatment, and outcomes of patients with PCI, we designed and implemented the China Patient‐centered Evaluative Assessment of Cardiac Events (PEACE) Prospective Study of PCI. As the first longitudinal study on clinical outcomes and PROs among patients with PCI in China, it aims to: (1) assess indications for and appropriateness of the PCI procedures performed; (2) evaluate the control of risk factors (blood pressure, hemoglobin A1c, blood lipid, obesity, smoking and alcohol consumption) and adherence to medications for secondary prevention in patients hospitalized with PCI; (3) measure long‐term clinical outcomes (i.e., death, AMI and/or revascularization) and PROs (i.e., health status, depression, and stress) among patients undergoing PCI in the short (i.e., 1 month) and long term (6 and 12 months); (4) determine patient‐level factors associated with these outcomes and generate risk prediction tools using these data; (5) assess hospital level variation in outcomes and the institutional factors associated with this variation.
METHODS
Study Overview
China PEACE is a collaboration between the China National Center for Cardiovascular Disease (NCCD), the Yale‐New Haven Hospital Center for Outcomes Research and Evaluation, the Chinese government, and more than 200 Chinese hospitals to improve cardiovascular disease outcomes in China (Fig. 1). The China PEACE Prospective Study of PCI leveraged this research network by recruiting 5,000 consecutive patients undergoing PCI for any indication from 34 tertiary hospitals located in 20 provinces across China (Fig. 2), and followed them prospectively for 12 months.
Figure 1.
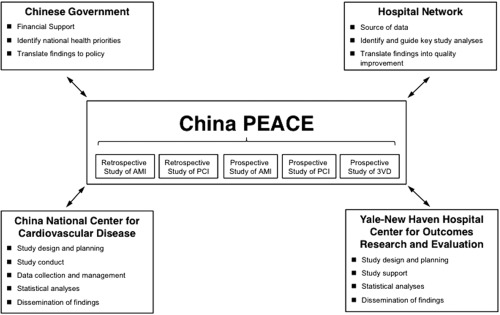
The China patient‐centered evaluative assessment of cardiac events (PEACE) Initiative. Key partners include the Chinese government, collaborating hospitals, the China National Center for Cardiovascular Disease, and the Yale‐New Haven Hospital Center for Outcomes Research and Evaluation. The China PEACE Prospective Study of PCI is one of five initial studies from the China PEACE initiative. The topic areas for these five projects concern acute myocardial infarction, coronary catheterization/percutaneous coronary intervention, and multi‐vessel coronary artery disease. Future studies will focus on cerebrovascular disease and other cardiovascular conditions. AMI, acute myocardial infarction; PCI, percutaneous coronary intervention. 3VD, Triple‐vessel Disease.
Figure 2.
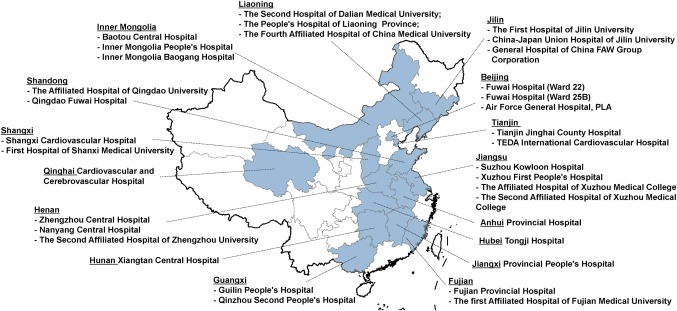
Geographic distribution of participating hospitals in the China patient‐centered evaluative assessment of cardiac events (PEACE), prospective study of percutaneous coronary intervention (PCI). The 34 participating centers are located in 20 of the 31 provinces in China.
Figure 3.
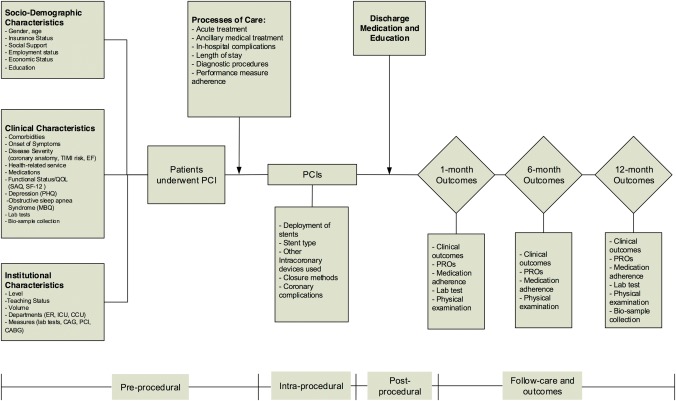
Detailed overview of potential mediators of post‐PCI outcomes, including in‐hospital and 12‐month outcomes (clinical events, patient‐reported outcomes [PROs]) and long‐term disease management). EF: ejection fraction; ER, emergency room; ICU, intensive care unit; CCU, cardiac care unit; CAG: coronary angiography; PCI, percutaneous coronary intervention; CABG: coronary artery bypass surgery; SAQ, Seattle Angina Questionnaire; EQ‐5D, EuroQol Group 5‐Dimension Self‐Report Questionnaire; PHQ‐8, Patient Health Questionnaire 8‐item depression scale.
The NCCD/Fuwai Hospital ethics committee approved this study and, where required, individual hospitals received approval from their local ethics committee. The Chinese government, which provides financial support for the study, had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation or approval of the article or any of the articles that will be derived from the study.
Participant
Eligible patients were defined as those admitted to a participating hospital that received PCI for any indication and had at least one coronary stent implanted during the procedure. Only patients who sign informed consent are then enrolled and interviewed at baseline and follow‐up. To evaluate potential selection biases, we collected information on patient baseline characteristics, in‐hospital care, and in‐hospital outcomes for all registered patients through medical chart abstraction (Fig. 4).
Figure 4.
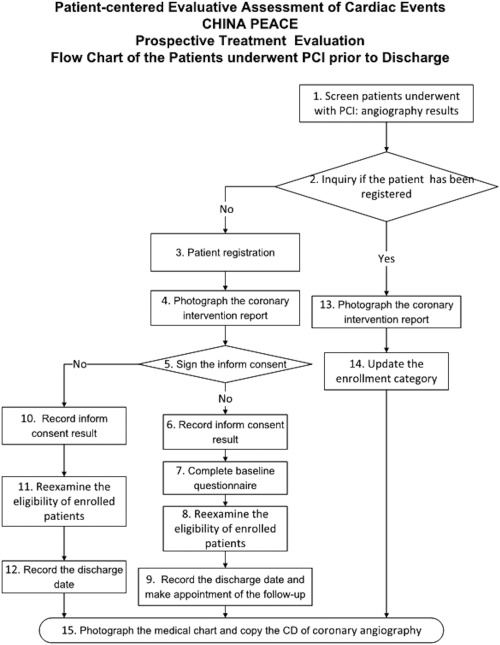
The study flowchart of China patient‐centered evaluative assessment of cardiac events (PEACE), Prospective Study of Percutaneous Coronary Intervention (PCI).
Outcomes
The primary outcome is a composite measure of major adverse cardiovascular events, including death, non‐fatal AMI and/or revascularization. It is assessed at follow‐up interviews conducted at 1, 6, and 12 months in local sites after discharge from initial hospitalization. Secondary endpoints include PROs at 12 month as well as the individual components of major adverse vascular events, re‐hospitalization, adherence to medications for secondary prevention (e.g., aspirin, clopidogrel, statin, and beta‐blockers), and control of cardiovascular risk factors (e.g., blood pressure, hemoglobin A1c, blood lipid, obesity, smoking, and alcohol consumption) at 12 months.
Data Collection Overview
Data are collected through central medical chart abstraction, interviews by site investigators, physical examinations, and central analysis of blood/urine specimens (Table 1, Figure 3). Data elements and variable definitions for this study were modeled after the National Cardiovascular Data Registry Cath PCI registry (NCDR‐Cath PCI) 18, the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery Study (PREMIER) 19, the (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status study (TRIUMPH) 20 and the Variation in Recovery: Role of Gender on Outcomes Young Acute Myocardial Infarction Patients (VIRGO) 21. The adoption of these standardized elements enables direct comparisons with US data. PROs were assessed using instruments previously validated in the Chinese population 19, 20, 21, facilitating comparisons of these outcomes as well.
Table 1.
Information Collected During the PCI Index Hospitalization and Follow‐up
| Assessment | |||||
|---|---|---|---|---|---|
| Domain | Scale | Baseline | 1 month | 6 month | 12 month |
| Medical record review | |||||
| Medical history/risk factors | √ | ||||
| Family history | √ | ||||
| Clinical characteristics | √ | ||||
| Diagnostic tests | √ | ||||
| Treatments/procedures | √ | ||||
| Discharge medications | √ | ||||
| In‐hospital outcomes | √ | ||||
| Patient interviews | |||||
| Clinical outcomes | |||||
| Death | √ | √ | √ | ||
| Myocardial infarction | √ | √ | √ | ||
| Re‐hospitalization | √ | √ | √ | ||
| PROs | |||||
| CVD functional status | SAQ(21) | √ | √ | √ | √ |
| Health‐related quality of life | EQ‐5D(19) | √ | √ | √ | √ |
| Depression | PHQ‐8(23) | √ | √ | √ | √ |
| Stress | PSS(24) | √ | √ | √ | √ |
| Social support | ESSI(44) | √ | √ | ||
| Obstructive sleep apnea | MBQ(32) | √ | |||
| Onset of symptoms | |||||
| Seeking care for symptoms | √ | ||||
| Health care service | √ | √ | |||
| TCM clinic/therapies | √ | √ | |||
| Health care insurance | √ | √ | |||
| Medical expenses | √ | √ | |||
| Socio‐economic status | √ | √ | |||
| Education | √ | ||||
| Work status | √ | √ | |||
| Marital/living status | √ | √ | |||
| Household income | √ | √ | |||
| Health knowledge | √ | √ | |||
| Risk factors | |||||
| Lifestyle factors | √ | √ | |||
| Physical activity | √ | √ | |||
| Smoking status | √ | √ | |||
| Alcohol consumption | √ | √ | |||
| Adherence to secondary preventive medications | √ | √ | √ | ||
| BMI/weight/hip circumference | √ | √ | √ | √ | |
| Local tests | |||||
| Blood cell count | √ | √ | |||
| Urine analysis | √ | √ | |||
| Alanine transaminase | √ | √ | √ | ||
| Creatinine/BUN | √ | √ | √ | ||
| Blood glucose | √ | √ | |||
| Electrocardiogram | √ | √ | √ | ||
| Bio‐samples for central analysis | |||||
| Blood lipid profile | √ | √ | |||
| HbA1c | √ | √ | |||
| Alanine transaminase | √ | √ | |||
| Creatinine/BUN | √ | √ | |||
| Blood glucose | √ | √ | |||
| CK | √ | √ | |||
| Hs‐CRP | √ | √ | |||
| Bio‐samples for long‐term storage | |||||
| Plasma/serum | √ | √ | |||
| DNA | √ | ||||
| Urine | √ | √ | |||
| Coronary angiogram | √ | ||||
PROs: patient‐reported outcomes; CVD= cardiovascular disease; SAQ: Seattle Angina Questionnaire; EQ‐5D: EuroQol Group 5‐Dimension Self‐Report Questionnaire; PHQ‐8: Patient Health Questionnaire 8‐item depression scale; PSS: Perceived Stress Scale; ESSI: Enhancing Recovery in Coronary Heart Disease (ENRICHD) Social Support Inventory; MBQ: Modified Berlin Questionnaire; BUN: blood urea nitrogen; HbA1c: hemoglobin A1c; HsCRP: high‐sensitivity c‐reactive Protein; CK: creatine kinase; RNA: riboNucleic acid.
Angiogram Review
We collected all imaging CDs of index PCI procedures (n = 5,000). A total of 2,000 angiograms of patients’ index PCIs are randomly selected, and reviewed by independent angiographic core labs in China [Core Laboratory in Company of Cardiovascular Research Foundation (Beijing)] and the US (Yale Angiographic Core Laboratory). Staff at these angiographic core labs record angiographic information into predesigned case report forms including the SYNTAX Score 22, vessel morphology, and lesion condition (which is used for assessment of the appropriateness of the index PCI). To assess the consistency of results, 100 angiograms are being reviewed by both core labs. Results of this double‐review will be compared, then shared in the two core labs, and ultimately used to guide the rectification of all reviewing results.
Participant Interviews and Laboratories
Participants were interviewed in‐person at baseline (i.e., during index hospitalization) by local site coordinators. The baseline questionnaire (Supporting Information 3) used for patient interviews include 130 questions ascertaining detailed information on patient demographics, socio‐economic status, risk factors, medical history as well as sleep apnea (Modified Berlin Questionnaire [MBQ]) 23, generic (EQ‐5D) 24, 25and disease‐specific health status (SAQ) 26, 27, depression 28, and stress 29, 30. Site investigators administered the interviews using an electronic data capture program on a tablet computer, in which the real‐time logic checks were implemented to ensure the accuracy and completeness of data.
Follow‐up interviews were conducted in‐person at 1, 6, and 12 months after discharge from the initial hospital stay (Figure 3). Telephone interviews were conducted when in‐person interviews were not feasible, such as elderly with mobility. At follow‐up interviews, clinical events and PROs were assessed, as well as adherence to medication for secondary prevention and control of risk factors. Blood was obtained at baseline and 12‐month follow‐up visits (Supporting Information 4: blood and urine collection). A consent form for the long‐term storage and future analysis of blood/urine sample was obtained from each enrolled patient. The bio‐samples were then transported to NCC and analyzed for lipid profiles and metabolic markers at a central laboratory. All blood/urine samples are stored in the China National Cardiovascular Bio‐Bank for future genetic and metabolic analysis.
Statistical Analyses
To address the primary aim of this study, we will calculate summary statistics for major adverse vascular events, as well as PROMs at 1, 6, and 12 months after PCI (Supporting Information 6: analytic methods and plan). We will report summary statistics for patient demographic, clinical, psychosocial, and behavioral characteristics; use of diagnostic tests; treatments received; and control of cardiovascular risk factors. To help identify factors associated with the primary outcomes, we will use standard parametric and non‐parametric tests for bivariate analyses, including t tests, chi‐square tests, Fisher's exact test, and Wilcoxon rank sum tests. In addition, appropriate multivariable regression analyses, such as linear, logistic, Cox proportional hazard, and Poisson models, will be conducted to determine independent factors of outcome measures, while adjusting for potential confounders. As patients are clustered within hospitals, the analyses will account for clustering in data when examining alternative treatment strategies (e.g., via generalized estimating equation approaches or random effects models). If longitudinal outcomes are considered, we will further account for the clustering effect within patients at different measurement points. We will carefully evaluate potential selection biases introduced by missing data and if needed, conduct inverse probability weighting (i.e., preferentially weight the experiences of patients who were most like those who did not participate in follow‐up) based upon a propensity model for participation in the follow‐up assessments. For observational comparative effectiveness studies, propensity score matching or instrumental variable methods will be used, when necessary, to better account for measured confounders. The creation of risk‐standardized adjustment models will serve as a foundation for comparing outcomes among centers for future quality improvement.
DISCUSSION
The China PEACE Prospective Study of PCI is the first longitudinal study in China to capture clinical events and PROs of patients over 12 months following a PCI procedure. This study addresses an important knowledge gap by providing information about long‐term outcomes after PCI, including health status improvement, the principal objective this procedure in stable patients 16. The collection of detailed demographic, clinical, treatment, and metabolic information will enable a comprehensive understanding of the disease trajectories and patients’ experiences, and define the prognostic importance of clinical, biological and institutional factors for a broad range of outcomes. Such information could provide insights into improving the quality and effectiveness of health care among PCI patients in China, as well as those in other low‐ and middle‐income countries.
As PCI, outside of the setting of primary PCI for reperfusion in the setting of an ST‐elevation myocardial infarction, is generally undertaken to relieve the signs and symptoms of myocardial ischemia, the effects on symptom relief and quality of life are critically important for patients and health service decision‐making 16. PROs quantify patients’ perspectives about the frequency and severity of their symptoms, how their disease impacts functioning, and the degree to which it limits their quality of life 31. Despite the increasing recognition of the importance of PROs in health care, they have seldom been evaluated in clinical research for PCI in China 32, 33. Moreover, few previous studies have assessed PROs at multiple time points 16, limiting our ability to understand changes in patients’ experience over time 34, 35. The China PEACE Prospective Study of PCI will bridge these knowledge gaps, and expand our knowledge on patients’ long‐term outcomes following PCI.
The data collected in China PEACE Prospective Study of PCI is unprecedented in scope for several reasons. First, we collected detailed data on both patient characteristics (e.g., demographic, clinical, psychosocial, behavioral factors, and hospital care received) and hospital attributes, which is useful in defining the determinants of patients’ recovery. Then, the 12‐month follow‐up period extends traditional assessments of long‐term mortality by including PROs, rate of adverse events, adherence to secondary prevention and risk control management. These data can also serve as a foundation for future comparative effectiveness studies of alternative treatment strategies to better understand their association with outcomes in China. In addition, risk‐standardized outcomes models can be created to enable inter‐institutional comparisons to inform the design of quality improvement strategies. Third, risk models and clinical prediction tools for clinical outcomes and PROs among PCI patients can be developed, which could fill in the gaps that none of existing scores used widely in routine clinical care have included Chinese patients and prediction models of health status outcomes are rare 36. Moreover, the biosamples collected in this study will satisfy the basic science, translational, or clinical need that advances the care of patient undergoing PCI.
Another unique feature of this study is the imaging and/or angiogram review of index PCI by specialized “core” labs in China and the US. The lack of contemporary information about the quality of clinical interpretations of coronary angiograms may undermine the benefit of PCI, as the presence and severity of coronary stenosis play a key role in the selection of patients 37. Recent data revealed that physicians tended to assess coronary lesions treated with PCI as more severe than measurements by angiographic core lab 38, 39. Importantly, with the rapid growth of PCI procedures in China, there is rising concern about the selection of patients and the appropriateness of PCI performance 40. Thus, angiogram review in our study, to a certain degree, might provide valued opportunities to inform clinical interpretation of coronary angiography and, together with the assessment of appropriateness of PCI using Appropriate Use Criteria, will optimize the selection and care of patients undergoing PCI in contemporary practice.
CONCLUSION
The China PEACE Prospective Study of PCI is designed to explicitly examine short‐ and long‐term survival, hospitalization, and PROs up to 12 months after PCI, to characterize trajectories of recovery, and to identify determinants of recovery. This study is uniquely positioned to inform strategies for improving care performance and patients’ outcomes by generating novel, high‐quality, and comprehensive data on patients’ experience following PCI. Findings are intended to improve the efficient and appropriate use of PCI in a manner that optimizes patient outcomes.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors appreciate the multiple contributions made by study teams at the China Oxford Centre for International Health Research and the Yale‐New Haven Hospital Center for Outcomes Research and Evaluation in the realms of study design and operations, particularly the data collection by Jiapeng Lu, Xuekun Wu, Hao Yang, and Jiamin Liu. They appreciate the advice by Yongfei Wang, Nihar R. Desai, Joseph S. Ross, Khurram Nasir, Haiqun Lin, and Brian Wayda. They are grateful for the support provided by the Chinese government.
Conflict of interest: Nothing to report
REFERENCES
- 1. He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, Klag MJ, Whelton PK. Major causes of death among men and women in China. N Engl J Med 2005;353:1124–1134. [DOI] [PubMed] [Google Scholar]
- 2. Moran A, Gu D, Zhao D, Coxson P, Wang YC, Chen CS, Liu J, Cheng J, Bibbins‐Domingo K, Shen YM, He J, Goldman L. Future cardiovascular disease in china: Markov model and risk factor scenario projections from the coronary heart disease policy model‐china. Circ Cardiovasc Qual Outcomes 2010;3:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang G, Kong L, Zhao W, Wan X, Zhai Y, Chen LC, Koplan JP. Emergence of chronic non‐communicable diseases in China. Lancet 2008;372:1697–1705. [DOI] [PubMed] [Google Scholar]
- 4. Gao R. Evolution and future perspectives for cardiovascular interventions in China. EuroIntervention 2012;8:773–778. [DOI] [PubMed] [Google Scholar]
- 5. Fei Y, Xiantao S, Hong L, Rui T, Xin C, Shuzheng L and Registry of PCIICSI. Percutaneous coronary interventions in Chinese mainland 2008. Int J Cardiol 2010;145:314–315. [DOI] [PubMed] [Google Scholar]
- 6. Gao R. Current status of percutaneous coronary intervention in China. Heart 2010;96:415–418. [DOI] [PubMed] [Google Scholar]
- 7. Gao R, Patel A, Gao W, Hu D, Huang D, Kong L, Qi W, Wu Y, Yang Y, Harris P, Algert C, Groenestein P, Turnbull F, and Investigators C. Prospective observational study of acute coronary syndromes in China: Practice patterns and outcomes. Heart 2008;94:554–560. [DOI] [PubMed] [Google Scholar]
- 8. Gao RL, Xu B, Lu SZ, Chen JL, Han YL, Chen JZ, Gai LY, Ge JB, Wang WM, Du ZM, Huo Y, Wang LF, Gao W, Chen JY, He B, Jia GL, Yang ZJ, Cao KJ, Li WM, Shen WF, Wan Z, Huang DJ, Zhu GY, and Investigators C. Safety and efficacy of the CYPHER select sirolimus‐eluting stent in the “Real World”—Clinical and angiographic results from the China CYPHER Select registry. Int J Cardiol 2008;125:339–346. [DOI] [PubMed] [Google Scholar]
- 9. Han Y, Jing Q, Xu B, Yang L, Liu H, Shang X, Jiang T, Li Z, Zhang H, Li H, Qiu J, Liu Y, Li Y, Chen X, Gao R, Investigators C. Safety and efficacy of biodegradable polymer‐coated sirolimus‐eluting stents in “real‐world” practice: 18‐month clinical and 9‐month angiographic outcomes. JACC Cardiovasc Intervent 2009;2:303–309. [DOI] [PubMed] [Google Scholar]
- 10. Ge J, Han Y, Jiang H, Sun B, Chen J, Zhang S, Du Z, and Investigators RT. RACTS: A prospective randomized antiplatelet trial of cilostazol versus ticlopidine in patients undergoing coronary stenting: Long‐term clinical and angiographic outcome. J Cardiovasc Pharmacol 2005;46:162–166. [DOI] [PubMed] [Google Scholar]
- 11. Han YL, Wang B, Li Y, Xu K, Wang SL, Jing QM, Wang ZL, Wang DM, Ma YY, Wang G. A high maintenance dose of clopidogrel improves short‐term clinical outcomes in patients with acute coronary syndrome undergoing drug‐eluting stent implantation. Chin Med J 2009;122:793–797. [PubMed] [Google Scholar]
- 12. Chen SL, Zhang JJ, Ye F, Chen YD, Patel T, Kawajiri K, Lee M, Kwan TW, Mintz G, Tan HC. Study comparing the double kissing (DK) crush with classical crush for the treatment of coronary bifurcation lesions: The DKCRUSH‐1 bifurcation study with drug‐eluting stents. Eur J Clin Investig 2008;38:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao RL, Xu B, Chen JL, Han YL, Li ZQ, Lu SZ, Qi XY, Huo Y, Wang LF, Chen JZ, Shen WF, Fang WY, Jia SQ. Chinese Registry of Unprotected Left Main Coronary Artery Stenting I. Prognosis of unprotected left main coronary artery stenting and the factors affecting the outcomes in Chinese. Chin Med J 2006;119:14–20. [PubMed] [Google Scholar]
- 14. Gao RL, Xu B, Chen JL, Yang YJ, Qiao SB, Li JJ, Qin XW, Yao M, Liu HB, Wu YJ, Yuan JQ, Chen J. Immediate and long‐term outcomes of drug‐eluting stent implantation for unprotected left main coronary artery disease: Comparison with bare‐metal stent implantation. Am Heart J 2008;155:553–561. [DOI] [PubMed] [Google Scholar]
- 15. Wallentin L, Lagerqvist B, Husted S, Kontny F, Stahle E, Swahn E. Outcome at 1 year after an invasive compared with a non‐invasive strategy in unstable coronary‐artery disease: The FRISC II invasive randomised trial. FRISC II Investigators. Fast revascularisation during instability in coronary artery disease. Lancet 2000;356:9–16. [DOI] [PubMed] [Google Scholar]
- 16. Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O'Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008;359:677–687. [DOI] [PubMed] [Google Scholar]
- 17. Kim J, Henderson RA, Pocock SJ, Clayton T, Sculpher MJ, Fox KA, and Investigators R‐T. Health‐related quality of life after interventional or conservative strategy in patients with unstable angina or non‐ST‐segment elevation myocardial infarction: One‐year results of the third randomized intervention trial of unstable angina (RITA‐3). J Am Coll Cardiol 2005;45:221–228. [DOI] [PubMed] [Google Scholar]
- 18. Moussa I, Hermann A, Messenger JC, Dehmer GJ, Weaver WD, Rumsfeld JS, Masoudi FA. The NCDR CathPCI Registry: A US national perspective on care and outcomes for percutaneous coronary intervention. Heart 2013;99:297–303. [DOI] [PubMed] [Google Scholar]
- 19. Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The prospective registry evaluating myocardial infarction: Events and recovery (PREMIER)—Evaluating the impact of myocardial infarction on patient outcomes. Am Heart J 2006;151:589–597. [DOI] [PubMed] [Google Scholar]
- 20. Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA. Translational research investigating underlying disparities in acute myocardial infarction patients' health status (TRIUMPH): Design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes 2011;4:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lichtman JH, Lorenze NP, D'Onofrio G, Spertus JA, Lindau ST, Morgan TM, Herrin J, Bueno H, Mattera JA, Ridker PM, Krumholz HM. Variation in recovery: Role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes 2010;3:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW. The SYNTAX score: An angiographic tool grading the complexity of coronary artery disease. EuroIntervention J EuroPCR Collaborat Work Group Intervent Cardiol Eur Soc Cardiol 2005;1:219–227. [PubMed] [Google Scholar]
- 23. Zeng J, Gu Y, Ke J, Li L, Yao J, Ma F. Evaluation of the diagnostic accuracy of modified Berlin questionnaire on predicting obstructive sleep apnea‐hypopnea syndrome in adults. (Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi) J Clin Otorhinolaryngol Head Neck Surg 2014;28:1658–1662. [PubMed] [Google Scholar]
- 24. Li M, Luo N. Application of EQ‐5D Chinese version. China J Pharma Econ 2009;1:49–57. [Google Scholar]
- 25. EuroQol Group . EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 26. Li J, Chang G. Assessement of SAQ for measuring quality of life among patients with coronary heart disease. Chin J Public Helath 2004;20:594. [Google Scholar]
- 27. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: A new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 28. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ‐8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–173. [DOI] [PubMed] [Google Scholar]
- 29. Yang Y, Huang H. An epidemiological study on stress among urban residentsin social transition period. Chin J Epidemiol 2003;24:760–764. [PubMed] [Google Scholar]
- 30. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–396. [PubMed] [Google Scholar]
- 31. Spertus J. Barriers to the use of patient‐reported outcomes in clinical care. Circ Cardiovasc Qual Outcomes 2014;7:2–4. [DOI] [PubMed] [Google Scholar]
- 32. Rahimi K, Malhotra A, Banning AP, Jenkinson C. Outcome selection and role of patient reported outcomes in contemporary cardiovascular trials: Systematic review. BMJ 2010;341:c5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mommersteeg PM, Denollet J, Spertus JA, Pedersen SS. Health status as a risk factor in cardiovascular disease: A systematic review of current evidence. Am Heart J 2009;157:208–218. [DOI] [PubMed] [Google Scholar]
- 34. Lenzen MJ, Scholte OP, Reimer WJ, Pedersen SS, Boersma E, Maier W, Widimsky P, Simoons ML, Mercado NF, Wijns W, and Euro Heart Survey on Coronary R. The additional value of patient‐reported health status in predicting 1‐year mortality after invasive coronary procedures: A report from the Euro Heart Survey on Coronary Revascularisation. Heart 2007;93:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biering K, Botker HE, Niemann T, Hjollund NH. Patient‐reported health as a prognostic factor for adverse events following percutaneous coronary intervention. Clin Epidemiol 2014;6:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arnold SV, Masoudi FA, Rumsfeld JS, Li Y, Jones PG, Spertus JA. Derivation and validation of a risk standardization model for benchmarking hospital performance for health‐related quality of life outcomes after acute myocardial infarction. Circulation 2014;129:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH, 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44–e122. [DOI] [PubMed] [Google Scholar]
- 38. Nallamothu BK, Spertus JA, Lansky AJ, Cohen DJ, Jones PG, Kureshi F, Dehmer GJ, Drozda JP Jr., Walsh MN, Brush JE Jr., Koenig GC, Waites TF, Gantt DS, Kichura G, Chazal RA, O'Brien PK, Valentine CM, Rumsfeld JS, Reiber JH, Elmore JG, Krumholz RA, Weaver WD, Krumholz HM. Comparison of clinical interpretation with visual assessment and quantitative coronary angiography in patients undergoing percutaneous coronary intervention in contemporary practice: The assessing angiography (A2) project. Circulation 2013;127:1793–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toth GG, Toth B, Johnson NP, De Vroey F, Di Serafino L, Pyxaras S, Rusinaru D, Di Gioia G, Pellicano M, Barbato E, Van Mieghem C, Heyndrickx GR, De Bruyne B, Wijns W. Revascularization decisions in patients with stable angina and intermediate lesions: Results of the international survey on interventional strategy. Circ Cardiovasc Intervent 2014;7:751–759. [DOI] [PubMed] [Google Scholar]
- 40. Huo Y. Current status and development of percutaneous coronary intervention in China. J Zhejiang Univ Sci B 2010;11:631–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


