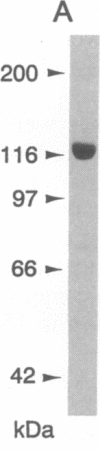
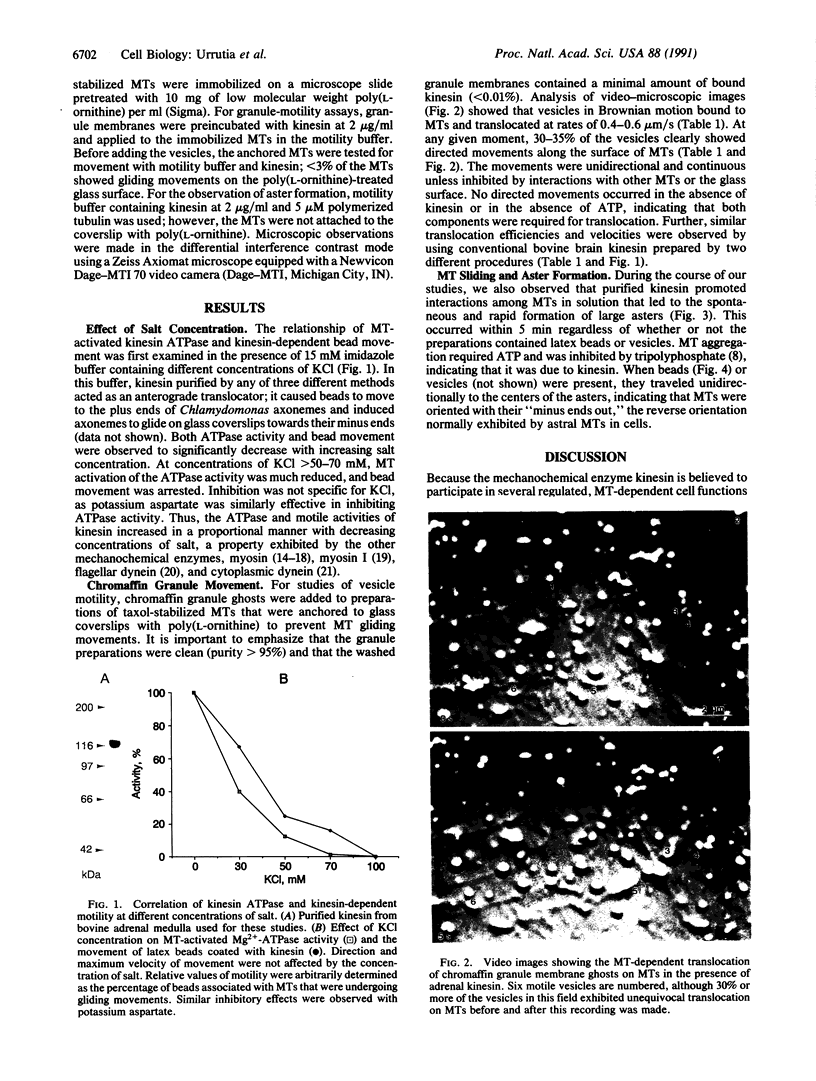
Abstract
We examined the ability of kinesin to support the movement of adrenal medullary chromaffin granules on microtubules in a defined in vitro system. We found that kinesin and ATP are all that is required to support efficient (33% vesicle motility) and rapid (0.4-0.6 micron/s) translocation of secretory granule membranes on microtubules in the presence of a low-salt motility buffer. Kinesin also induced the formation of microtubule asters in this buffer, with the plus ends of microtubules located at the center of each aster. This observation indicates that kinesin is capable of promoting active sliding between microtubules toward their respective plus ends, a movement analogous to that of anaphase b in the mitotic spindle. The fact that vesicle translocation, microtubule sliding, and microtubule-dependent kinesin ATPase activities are all enhanced in low-salt buffer establishes a functional parallel between this translocator and other motility ATPases, myosin, and dynein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albanesi J. P., Hammer J. A., 3rd, Korn E. D. The interaction of F-actin with phosphorylated and unphosphorylated myosins IA and IB from Acanthamoeba castellanii. J Biol Chem. 1983 Aug 25;258(16):10176–10181. [PubMed] [Google Scholar]
- Brady S. T., Pfister K. K., Bloom G. S. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman P. C., Kachar B., Reese T. S. The structure of cytoplasm in directly frozen cultured cells. II. Cytoplasmic domains associated with organelle movements. J Cell Biol. 1986 Apr;102(4):1510–1521. doi: 10.1083/jcb.102.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Cohn S. A., Ingold A. L., Scholey J. M. Correlation between the ATPase and microtubule translocating activities of sea urchin egg kinesin. Nature. 1987 Jul 9;328(6126):160–163. doi: 10.1038/328160a0. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Henikoff S., Soler-Niedziela L. Mediation of meiotic and early mitotic chromosome segregation in Drosophila by a protein related to kinesin. Nature. 1990 May 3;345(6270):81–83. doi: 10.1038/345081a0. [DOI] [PubMed] [Google Scholar]
- Enos A. P., Morris N. R. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell. 1990 Mar 23;60(6):1019–1027. doi: 10.1016/0092-8674(90)90350-n. [DOI] [PubMed] [Google Scholar]
- Gibbons I. R. Studies on the adenosine triphosphatase activity of 14 S and 30 S dynein from cilia of Tetrahymena. J Biol Chem. 1966 Dec 10;241(23):5590–5596. [PubMed] [Google Scholar]
- Hackney D. D. Kinesin ATPase: rate-limiting ADP release. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6314–6318. doi: 10.1073/pnas.85.17.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B., Albanesi J. P., Fujisaki H., Korn E. D. Extensive purification from Acanthamoeba castellanii of a microtubule-dependent translocator with microtubule-activated Mg2+-ATPase activity. J Biol Chem. 1987 Nov 25;262(33):16180–16185. [PubMed] [Google Scholar]
- Kuznetsov S. A., Gelfand V. I. Bovine brain kinesin is a microtubule-activated ATPase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8530–8534. doi: 10.1073/pnas.83.22.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E. M., Herrmann M., Rühl U. Tubulin domains probed by limited proteolysis and subunit-specific antibodies. J Mol Biol. 1985 Sep 20;185(2):311–327. doi: 10.1016/0022-2836(85)90406-1. [DOI] [PubMed] [Google Scholar]
- Meluh P. B., Rose M. D. KAR3, a kinesin-related gene required for yeast nuclear fusion. Cell. 1990 Mar 23;60(6):1029–1041. doi: 10.1016/0092-8674(90)90351-e. [DOI] [PubMed] [Google Scholar]
- Murofushi H., Ikai A., Okuhara K., Kotani S., Aizawa H., Kumakura K., Sakai H. Purification and characterization of kinesin from bovine adrenal medulla. J Biol Chem. 1988 Sep 5;263(25):12744–12750. [PubMed] [Google Scholar]
- Neighbors B. W., Williams R. C., Jr, McIntosh J. R. Localization of kinesin in cultured cells. J Cell Biol. 1988 Apr;106(4):1193–1204. doi: 10.1083/jcb.106.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K. K., Wagner M. C., Stenoien D. L., Brady S. T., Bloom G. S. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989 Apr;108(4):1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebhun L. I. Polarized intracellular particle transport: saltatory movements and cytoplasmic streaming. Int Rev Cytol. 1972;32:93–137. doi: 10.1016/s0074-7696(08)60339-3. [DOI] [PubMed] [Google Scholar]
- Saxton W. M., Porter M. E., Cohn S. A., Scholey J. M., Raff E. C., McIntosh J. R. Drosophila kinesin: characterization of microtubule motility and ATPase. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1109–1113. doi: 10.1073/pnas.85.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S. Cytoplasmic structure in rapid-frozen axons. J Cell Biol. 1982 Sep;94(3):667–669. doi: 10.1083/jcb.94.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey J. M., Porter M. E., Grissom P. M., McIntosh J. R. Identification of kinesin in sea urchin eggs, and evidence for its localization in the mitotic spindle. Nature. 1985 Dec 5;318(6045):483–486. doi: 10.1038/318483a0. [DOI] [PubMed] [Google Scholar]
- Schroer T. A., Schnapp B. J., Reese T. S., Sheetz M. P. The role of kinesin and other soluble factors in organelle movement along microtubules. J Cell Biol. 1988 Nov;107(5):1785–1792. doi: 10.1083/jcb.107.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpetner H. S., Paschal B. M., Vallee R. B. Characterization of the microtubule-activated ATPase of brain cytoplasmic dynein (MAP 1C). J Cell Biol. 1988 Sep;107(3):1001–1009. doi: 10.1083/jcb.107.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. A., Chock P. B., Eisenberg E. Mechanism of the actomyosin ATPase: effect of actin on the ATP hydrolysis step. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1346–1350. doi: 10.1073/pnas.78.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D. Intracellular transport using microtubule-based motors. Annu Rev Cell Biol. 1987;3:347–378. doi: 10.1146/annurev.cb.03.110187.002023. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Reese T. S., Sheetz M. P. Movement of organelles along filaments dissociated from the axoplasm of the squid giant axon. Cell. 1985 Feb;40(2):449–454. doi: 10.1016/0092-8674(85)90159-x. [DOI] [PubMed] [Google Scholar]
- Wagner M. C., Pfister K. K., Bloom G. S., Brady S. T. Copurification of kinesin polypeptides with microtubule-stimulated Mg-ATPase activity and kinetic analysis of enzymatic properties. Cell Motil Cytoskeleton. 1989;12(4):195–215. doi: 10.1002/cm.970120403. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Allen R. D., Inoue S. ATP-dependent formation and motility of aster-like structures with isolated calf brain microtubule proteins. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1728–1732. doi: 10.1073/pnas.83.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Jr, Greene L. E., Eisenberg E. Comparison of effects of smooth and skeletal muscle tropomyosins on interactions of actin and myosin subfragment 1. Biochemistry. 1984 Aug 28;23(18):4150–4155. doi: 10.1021/bi00313a022. [DOI] [PubMed] [Google Scholar]