Abstract
Purpose
Anthracycline-induced congestive heart failure (CHF) is a rare but serious toxicity associated with this commonly employed anti-cancer therapy. The ability to predict which patients might be at increased risk prior to exposure would be valuable to optimally counsel risk to benefit ratio for each patient. Herein we present a genome wide approach for biomarker discovery with two validation cohorts to predict CHF from adult patients planning to receive an anthracycline.
Experimental Design
We performed a genome wide association study (GWAS) in 3431patients from the randomized phase III adjuvant breast cancer trial E5103 to identify SNP genotypes associated with an increased risk of anthracycline-induced CHF. We further attempted candidate validation in two independent phase III adjuvant trials, E1199 and BEATRICE.
Results
When evaluating for cardiologist adjudicated CHF, 11 SNPs had a p-value <10−5 of which nine independent chromosomal regions were associated with increased risk. Validation of the 2 top SNPs in E1199 revealed one SNP, rs28714259 that demonstrated a borderline increased CHF risk (p=0.04, OR=1.9). rs28714259 was subsequently tested in BEATRICE and was significantly associated with a decreased left ventricular ejection fraction (p=0.018, OR=4.2).
Conclusion
rs28714259 represents a validated SNP that is associated with anthracycline-induced CHF in three independent, phase III adjuvant breast cancer clinical trials.
Keywords: Genome-wide association study, Single nucleotide polymorphisms, Anthracyclines, Congestive heart failure
INTRODUCTION
Anthracyclines are a widely used chemotherapeutic class. Despite substantial anti-tumor activity, anthracyclines are not without toxicity. Cardiotoxicity, including congestive heart failure (CHF), is a dose-limiting side effect (1). Patients that received an anthracycline were five-times more likely to develop cardiac symptoms (2). The overall incidence of CHF in patients receiving a cumulative dose of 240–360mg/m2 of doxorubicin is 1.7–2.1% (3). The optimal definition of cardiac dysfunction is debatable but the most common mode of evaluation includes assessment of left ventricular ejection fraction (LVEF). Unfortunately, even with expert interpretation, there is variability in its estimation (4).
Anthracycline-induced cardiotoxicity can occur in two distinct forms: acute toxicity that develops early and presents as arrhythmias or depressed LVEF, or chronic toxicity, that occurs years later. The mechanism of cardiotoxicity is likely related to oxidative stress although not completely elucidated (5). Known risk factors for developing clinical cardiomyopathy include cumulative dose, age, history of cardiac conditions, hypertension, liver disease, and mediastinal radiotherapy (5, 6). Risk of a cardiac event is further increased when anthracyclines are combined with other therapies, including trastuzamab (7) and bevacizumab (8, 9).
The risk of serious, irreversible cardiac damage has spurred the implementation of competing non-anthracycline regimens to obviate this concern (10), although many patients still benefit from anthracyclines based on risk or biology. The severity of the side-effect necessitates a means to identify high risk patients. Germline genetic biomarkers for CHF have been studied, but their ability to reliably predict risk remains unclear as populations studied have been heterogeneous and results inconsistent(11–22).
We report a comprehensive evaluation of a large phase III, adjuvant breast cancer trial, E5103(9), to identify common single nucleotide polymorphisms (SNPs) that are associated with the risk of anthracycline-induced CHF. We performed a genome-wide association study (GWAS), which assessed SNPs throughout the genome. Furthermore, we tested our most promising SNPs in two independent, randomized phase III trials, E1199 (23) and BEATRICE (8), which used a similar anthracycline backbone.
MATERIAL and METHODS
Genome wide association study to identify common variants in ECOG-5103
E5103 Overview
E5103(9) was a phase III adjuvant breast cancer trial that randomized 4994 patients to doxorubicin (60 mg/m2) and cyclophosphamide (AC) for four cycles, followed by 12 weeks of weekly paclitaxel (Arm A) or to the same chemotherapy with either concurrent bevacizumab (Arm B) or concurrent plus sequential bevacizumab (Arm C). Patients were allowed to receive AC every two or three weeks at the discretion of the treating physician and patients from all arms were scheduled to receive the same cumulative dose of doxorubicin Table 1. Analyses were performed across all arms to identify SNPs associated with CHF. This correlative study was approved by the Indiana University IRB.
Table 1.
Summary of CHF definitions and events across the clinical trials
| Trial | Definition of CHF | Cumulative dose of anthracycline | Frequency of events | Bevacizumab administered |
|---|---|---|---|---|
| E5103 | Centrally reviewed, cardiologist –adjudicated (Composite of symptoms and lowered LVEF) |
All patients received 240 mg/m2 of doxorubicin | 2.0% | Two of the three study arms |
| E1199 | Common toxicity criteria v. 2.0 | All patients received 240 mg/m2 of doxorubicin | Parent trial: 1.5% GWAS subgroup: 1.6% |
No |
| BEATRICE | Parent trial: NYHA class 3/4 CHF Current GWAS: LVEF <45% |
94.8% of patients received an anthracycline at various doses per physician`s choice | Parent trial: 1.6% GWAS subgroup: 2.9% |
One of the two study arms |
LVEF=left ventricular ejection fraction; NYHA=New York Heart Association; GWAS=genome wide association study
E5103 genotyping and imputation
Due to the availability of germline DNA, genotyping was performed at two independent time points in non-overlapping study subsets (Figure 1). DNA from an initial 2209 and an additional 1222 patients was genotyped by Illumina Genotyping Services using the HumanOmni1-Quad (>1 million SNPs) and the HumanOmniExpress (730,525 SNPs) array, respectively. Both sample sets used the Illumina BeadChip microarray platform for genotyping and the Illumina GenomeStudio software for initial genotyping calls. Of note, SNPs on the HumanOmniExpress were a subset of those on the HumanOmni1-Quad. Those SNPs not on the HumanOmniExpress were imputed in the second sample subset (Supplemental data).
FIGURE 1.
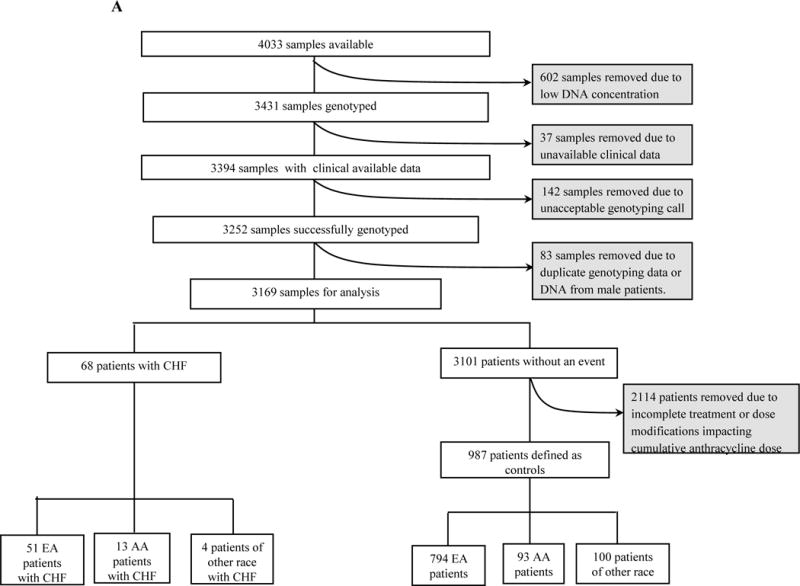

Consort Diagram. A) E5103-(the genome wide discovery cohort); B) E1199-(first candidate SNP validation cohort); C) BEATRICE-(second candidate SNP validation cohort).
E5103 case and control definitions for CHF
To be selected for inclusion in E5103, patients must have not had a history of clinically significant cardiovascular disease. Cardiovascular health was monitored at the start and during the trial by MUGA or echocardiograms. Additionally, a cardiac symptoms assessment was performed two years post-registration. Cardiac events including: CHF, decrease in LVEF, acute coronary syndrome, supraventricular tachycardia and myocardial dysfunction, were diagnosed by a cardiologist. Cases to be considered for this correlative study included individuals that had centrally reviewed, cardiologist-adjudicated CHF. Controls were strictly defined and included patients who did not experience CHF or other reported events of cardiotoxicity including: LVEF <50% or drop in LVEF ≥20% from baseline. All controls received the full intended dose of AC with no modification.
Statistical analysis
A case-control GWAS was performed in the genetically defined European American (EA) subsamples (Supplement Material) to identify SNPs associated with the presence or absence of CHF. Age, menopausal status, experimental arm, tumor grade, body surface area (BSA), hypertension during therapy and use of anti-hypertension medications at baseline or anti-hypertensive therapy added during treatment, were considered as covariates in analyses. The p-value threshold was < 0.05 for inclusion of covariates in the regression model. SNPs available on the HumanOmni1-Quad array were used as the common basis for all statistical analysis. An additive model of SNP effect was used when testing all hypotheses. The case-control analysis was conducted using SNPTEST v2.4 (https://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html). SNP and sample quality control (QC) were performed as previously (24) and outlined in Supplemental Material.
Validation in E1199
Overview of E1199
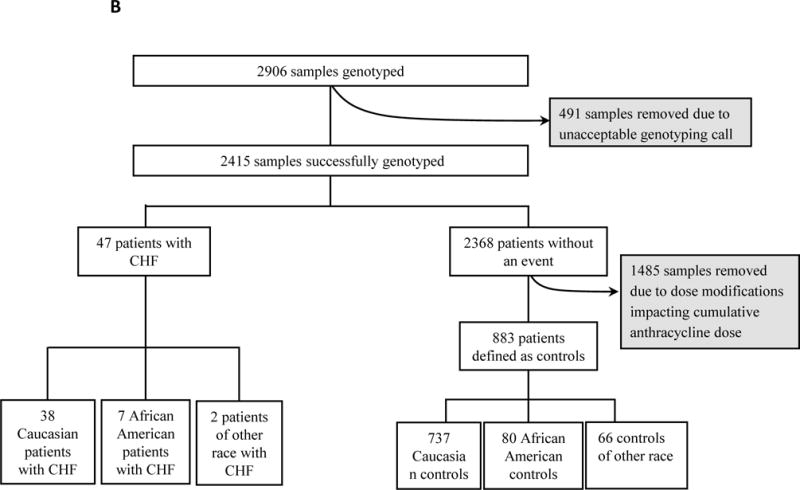
E1199(23) was a phase III adjuvant breast cancer trial that randomized 5052 patients with node-positive or high-risk, node-negative breast cancer to one of four treatment arms. First, all patients received doxorubicin (60 mg/m2) and cyclophosphamide (AC) for four cycles followed by paclitaxel every three weeks for four cycles, or paclitaxel weekly for 12 cycles, docetaxel every three weeks for four cycles, or docetaxel at weekly for 12 cycles. Tumor derived DNA (formalin fixed paraffin embedded) was available from 2906 patients; Figure 1
Candidate validation of top SNPS in E1199
All association results were reviewed and priority were given to two SNPs with further evidence of association from other top SNPs (p-value <10−5) in linkage disequilibrium (LD) to reduce the risk of false positive signals from technical genotyping errors; Figure 2. These two SNPs were genotyped using the QuantStudio™ 12K Flex OpenArray® AccuFill™ System (Thermo Fisher Scientific) platform.
FIGURE 2.
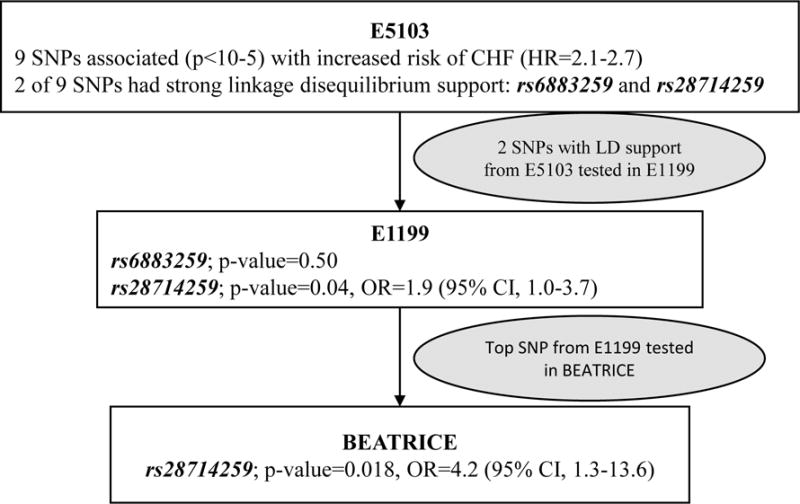
Flow diagram for candidate SNP selection.
E1199 case and control definitions for CHF
To be included in E1199, patients must have not had a history of myocardial infarction, CHF or significant ischemic or valvular heart disease. Cases included patients with reported CHF as defined by the Common Toxicity Criteria version 2.0. Raw LVEF data points were not available. Patients were considered controls if they had no reported cardiac events and received the full dose of intended AC with no dose modification.
Statistical analysis
Comparing cases and controls in the EA subpopulation, logistic regression analysis was performed to identify SNPs associated with CHF. Age, BSA and treatment arm were considered as covariates. The p-value threshold for significance was set at 0.025 after Bonferroni correction was made.
Validation in BEATRICE
Overview of BEATRICE
BEATRICE(8) was a phase III adjuvant breast cancer trial that randomized 2591 patients to a minimum of four cycles of standard adjuvant chemotherapy (including an anthracycline, taxane or both) at the investigator’s discretion with or without bevacizumab for one year. Germline DNA was available from 828 patients; Figure 1.
Candidate SNP validation in BEATRICE
The SNP with borderline association with CHF in the E1199 was further tested for association with decreased LVEF in BEATRICE; Figure 2. The SNP was genotyped using a TaqMan SNP assay.
BEATRICE case and control definitions for CHF
To be included in BEATRICE, patients had a baseline LVEF >55%. In the parent trial, CHF was defined as New York Heart Association (NYHA) class III or IV CHF accompanied by a drop in LVEF> 10% to below 50. There were only 16 cases of NYHA CHF or cardiac death across the parent trial and therefore insufficient cases in the cohort of genotyped patients. Thus we enriched statistical power by including cases based on low LVEF. Prior to genetic analysis, we selected LVEF <45% to be considered an event. Because of inconsistency and inaccuracy of LVEF measurement, we did not select <50%, a commonly used criterion for mild dysfunction, to minimize false positive events. We also chose 45% based on prior data suggesting that LVEF <45% was a strong contributor to future cardiovascular risk (25). Patients were considered controls if they had no reported cardiac events, had no hypertension and received the full dose of intended anthracycline without dose modification.
Statistical analysis
Comparing cases and controls in the EA subpopulation, logistic regression analysis was performed to identify SNPs associated with decreased LVEF. Age, BSA and treatment arm were considered as covariates. Since only one statistical comparison was performed, statistical significance was set at a p-value <0.05.
RESULTS
Phenotypes: rate of CHF and clinical predictors
E5103
Of 4033 patients who had available DNA (Figure 1), phenotype data were available only from 3394 genotyped patients. In this subgroup, 2% experienced cardiologist-adjudicated CHF (Table 1). This included 68 cases; 51 EA, 13 African Americans (AA) and 4 of other race. There was no increased risk across the genetically determined racial groups (EA vs. AA vs. other; p-value=0.50). Due to the insufficient number of non-EA cases, genetic association analysis was limited to the EA subgroup. Non-genetic demographic predictors were compared for the entire group as well as for EAs and AAs separately (Table 2). Older age was a significant risk factor, with a 43% relative increased risk with each decade of life (p=0.009). There was an increased risk in arm C (p=0.04) but not in arm B (p=0.87) when compared with arm A. There was an increased risk for patients who were on anti-hypertensives at baseline (p=7.2×10−4) but not for those patients who experienced therapy-induced hypertension. BSA was not associated with an increased risk in the entire cohort (p=0.10) or in EA patients (p=0.79) but was associated with increased risk in AA patients (p=0.01).
Table 2.
Comprehensive comparisons of non-genetic demographic predictors of CHF
| Clinical Trial | Samples with clinical data available | |||||
|---|---|---|---|---|---|---|
| All | AA | EA | ||||
| Age | BSA | Age | BSA | Age | BSA | |
|
E5103* (genotyped) N=3394 (68 cases) AA=386 (13 cases) EA=2525 (51 cases) Others=484 (4 cases) |
0.009 | 0.10 | 0.76 | 0.01 | 0.002 | 0.79 |
|
E1199 (Parent trial) N=4735 (71 cases) AA=401 (11 cases) EA=4042 (58 cases) Others=292 (2 cases) |
0.005 | 0.04 | 0.62 | 0.10 | 0.005 | 0.48 |
|
E1199 (genotyped) N=2906 (47 cases) AA=267(38 cases) EA=2616 (7 cases) Others=23 (2 cases) |
0.044 | 0.07 | 0.50 | 0.07 | 0.08 | 0.61 |
|
BEATRICE* (genotyped) N=828 (24 cases) AA=31 (1 case) EA=705 (21 cases) Others=92 (2 cases) |
0.774 | 0.91 | 0.99 | 0.99 | 0.87 | 0.72 |
Clinical data was only available for those patients who had companion DNA samples
AA=African American; EA=European American; BSA=Body Surface Area
E1199
Phenotypic data were available from a majority of patients in the parent trial (n=4735) as well as the entire genotyped group (n=2906); Figure 1. The risk of CHF was 1.5% in the parent trial and 1.6% in the genotyped group (Table 1). In the genotyped group, there were 47 cases of CHF; 38 among self-defined EA and 7 cases in AA and 2 cases among other races. There was no significant increase in risk based on race (EA vs. AA vs. other; p-value=0.09). Again, genetic association analyses were limited to the EA subgroup due to an insufficient number of non-EA cases. Non-genetic demographic predictors were compared for the entire group as well as for EAs and AAs separately (Table 2). Older age demonstrated a 39% relative increased risk with each decade of life in the parent population (p=0.005). BSA was not associated with risk in the EA population (p=0.61) but a trend for increased risk in the AA population (p=0.07). Neither hypertension nor use of anti-hypertensive data was available in this trial.
BEATRICE
Phenotypic data were available only from the 828 genotyped patients (Figure 1). In this subgroup of patients, 2.9% experienced a decline in LVEF <45%. This value is higher than the 1.6% of population reported to experience NYHA class III or IV CHF in the parent trial (8) (Table 1). The subgroup included a total of 24 cases; 21 among self-defined EA and the remaining 3 were of other race. There was no increased risk of CHF across the self-defined racial groups (EA vs. other; p-value=0.45). Because of insufficient numbers of cases, the genetic association analyses were limited to the EA subgroup. Non-genetic demographic predictors were compared for the entire group as well as for EAs and AAs separately (Table 2). Older age (p=0.77) and increased BSA (p=0.91) were not associated with increased likelihood of decreased LVEF. There was also no increased risk for those who experienced significant therapy-induced hypertension (p=0.98).
GWAS results for CHF in E5103
After genotyping and quality control (QC), 810,907 SNPs were used for the GWAS in E5103. The strength of association between genotype and CHF (Figure 3) are demonstrated in Table 2. 112 SNPs had a p-value <1×10−4 of which 99 were associated with increased risk of CHF (OR=1.4–3.7) (Supplemental table 1). Nine independent chromosomal regions represented by eleven top SNPs were associated with the risk of CHF (p-value <10−5) and in two of the nine regions, there are multiple SNPs that are in LD with top SNPs and also provide evidence of association; therefore, the likelihood of a false positive association is substantially reduced. One SNP from each of these two regions was selected for validation in independent data sets; Figure 2. SNPs previously reported to be associated with anthracycline-induced CHF did not provide evidence of association in this study; Table 3.
FIGURE 3.
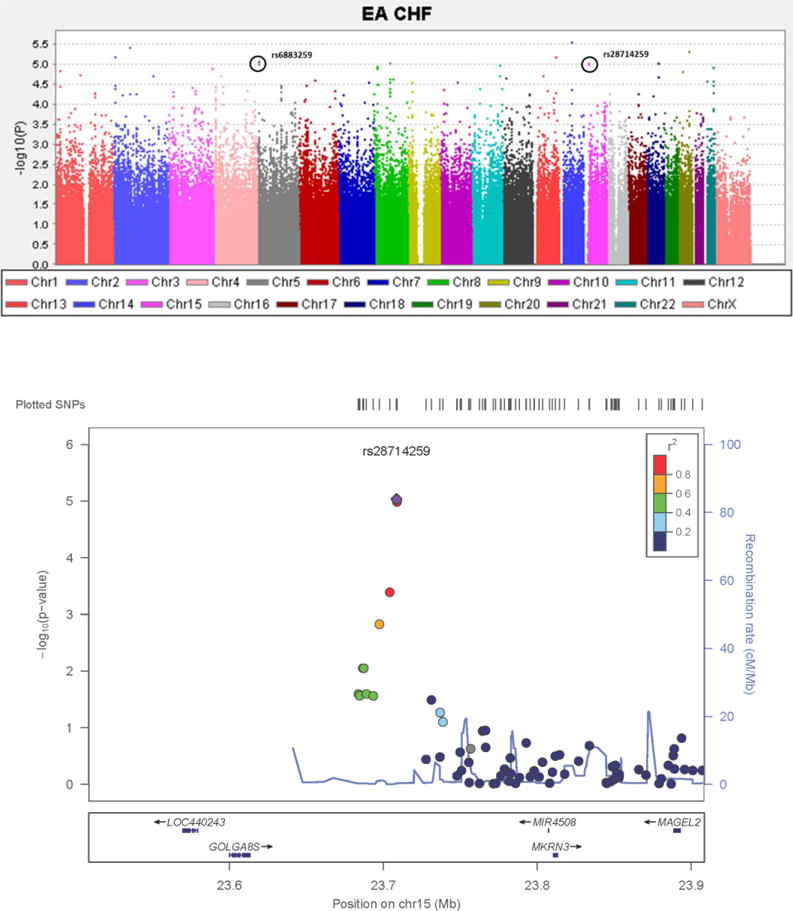
CHF GWAS in EA cohort in E5103. A) Manhattan plot for centrally reviewed, cardiologist-adjudicated congestive heart failure from EA. X-axis indicates the chromosomal position of each SNP analyzed; Y-axis denotes magnitude of the evidence for association, shown as –log10(p-value). SNPs genotyped in the two independent samples are circled; B) p-values (−log10) of SNPs near rs28714259 from SNP association analysis with CHF. SNPs are colored to reflect their LD with rs28714259.
Table 3.
Selected SNPs previously reported to be associated with congestive heart failure
| Study | Number of Patients (sample size) | Study Set | Dose and Schedule of Anthracyclines | Phenotype | Genotype | Genes of top association | Validation with number of independent cohort |
|---|---|---|---|---|---|---|---|
| Wojnowski et al.1 | 450 | DSHNHL-B1/B2 | Doxorubicin/uniform dose & schedule | WHO grade 3 and 4 cardiotoxicity | Candidate SNPs in 82 genes in anthracycline pharmacodynamics | NADPH, BCC1, ABCC2 | No |
| Blanco et al.2 | 145 | Retrospective pediatric cohort | Any anthracycline/Variable dose & schedule | Self-reported symptoms | Candidate genes NQO1, CBR3 | CBR3 | No |
| Rajić V et al.3 | 76 | Retrospective pediatric cohort | Any anthracycline/variable dose & schedule | Cardiologist defined cardiac damage | Candidate SNPs in SOD2, CAT, GSTT1, GSTM1 | CAT | No |
| Blanco et al.4 | 487 | COG-ALTE03N1 | Any anthracycline/variable dose & schedule | AHA guidelines | Candidate SNPs in CBR3 and CBR1 | CBR3 | No |
| Semsei et al.5 | 235 | Retrospective pediatric cohort | Doxorubicin & Daunorubicin/uniform dose & schedule | Cardiac function measured by LVFS | Candidate SNPs in ABCC1 | ABCC1 | No |
| Visscher et al.6 | 156 | Retrospective pediatric cohort | Doxorubicin & Daunorubicin/Variable dose & schedule | CTCAE v3 | Candidate SNPs in 220 genes in anthracycline pharmacodynamics | CBR3, CB4, HMNT,ABCC1, LC28A3 | Yes-Two cohorts |
| Volkan-Salanci et al.7 | 70 | Prospective adult cohort | Doxorubicin/Variable dose & schedule | Various measures of cardiac function | Candidate SNPs in CBR3 and GSTP1 | CBR3, STP1 | Yes |
| Armenia et al.8 | 255 | Retrospective adult cohort | Any anthracycline/Variable dose & schedule | ACC/AHA guidelines | Candidate genes in anthracycline metabolism and pharmacodynamics | NADPH, HFE, ABCC2 | No |
| Lubieniecka et al.9 | 91 | Retrospective adult cohort | Daunorubicin/Variable dose & schedule | Acute cardiotoxicity, | 60 Candidate genes in anthracycline metabolism and efflux | POR | No |
| Sachidan andam et al.10 | 2 | Two adult sisters with CHF | Doxorubicin/Variable dose & schedule | Sister 1 - cardiac transplant. Sister 2 - LVEF dropped to 22% |
Candidate gene ABCC1, NADPH, CBR1, CBR3, HMNT and ABCB4 | HNMT | No |
| Wang et al.11 | 287 | COG-ALTE03N1 | Any anthracycline/Variable dose & schedule | AHA guidelines | 2100 Candidate genes on ITMAT/Broad CARe cardiovascular SNP array | HAS3 | Yes |
| Visscher et al.12 | 344 | Retrospective pediatric cohort | Doxorubicin & Daunorubicin/variable dose & schedule | CTCAE v3 | Candidate 4578 SNPs in drug pharmacokinetic and toxicity genes | SLC22A7, SLC22A17 | Yes |
| Aminkeng et al.13 | 280 | Retrospective pediatric cohort | Doxorubicin & Daunorubicin/Variable dose & schedule | CTCAE v3 | GWAS | RARG | Yes –Two cohorts |
| Schneider et al. (current study) | 102 | ECOG-5103 | Doxorubicin/Uniform dose & schedule | cardiologist adjudicated | GWAS | rs28714259 in intergenic region of chr 15 | Yes-Two cohorts |
ACC = American College of Cardiology; AHA =American Heart Association ; ALL= acute lymphoblastic leukemia; AML = Acute myeloid leukemia; CHF = Congestive Heart Failure; COG = Children’s Oncology Group; CTCAE = Common Terminology Criteria for Adverse Events; DSHNHL = German High-Grade Non-Hodgkin’s Lymphoma Study Group; ECG = Electrocardiogram; ECHO = Echocardiogram; ECOG = Eastern Cooperative Oncology Group; HCT = Hematopoietic cell transplantation; LVES = Left Ventricular Ejection Fraction; LVFS = Left Ventricular Fractional Shortening; WHO = World Health Organization
Evaluation of candidate SNPs in E1199 and BEATRICE
One of the two SNPs genotyped in the E1199, rs28714259, provided evidence of borderline association with CHF (p = 0.041, OR = 1.9), whereas the other, rs6883259, was not (p=0.50). rs28714259 was subsequently genotyped in BEATRICE and was significantly associated with decreased LVEF <45% (p=0.018, OR=4.2) (Figure 2).
DICUSSION
In the present study, we aimed to identify a population at risk for developing CHF from anthracyclines using the dose and schedule commonly employed for adult solid tumors. We performed a GWAS in a large randomized phase III adjuvant trial. As a follow-up, we performed limited genotyping in two large independent phase III adjuvant trials. In total, the three large trials enrolled over 12,500 breast cancer patients. All patients in E5103 and E1199 received a uniformed dose of doxorubicin (60 mg/m2) and over 95% patients in BEATRICE received an anthyracycline. We provide evidence that the SNP, rs28714259, is associated with cardiac dysfunction across trials using slightly different phenotype definitions. This SNP was among the top SNPs associated with increased risk of CHF (OR>1) and also had LD support from another top SNP in our discovery sample set of E5103 trial. In all three trials, the A allele was associated with an increased risk of cardiac damage: E5103 (OR=2.1; p=9.25×10−6), E1199 (OR=1.9; p=0.04) and BEATRICE (OR=4.2; p=0.02).
Age was the most consistent demographic predictor for increased risk as it was associated in two of the three trials evaluated here. There was also a correlation between use of anti-hypertensive agents at baseline in E5103 but an increased risk was not seen for those who only had treatment induced hypertension. This would support the notion that underlying and potentially long term, cardiovascular disease increased the risk of this toxicity. Unfortunately details of comorbidities were not available for further evaluation. There was also a signal that BSA may be an important contributor but was only detected in the AA patients.
Previous studies have evaluated germline predictors. This included evaluation of candidate SNPs or gene panels in the metabolism and transport pathway or in the proposed mechanistic pathways of anthracycline-induced cardiomyopathy (12–22). Several of these studies have demonstrated associations, but none of these candidates were identified in the recent GWAS by Aminkeng et al.(11) or in our study (Table 3). Aminkeng et al. focused on a pediatric population of EAs with 34 cases of decreased LVEF and 248 controls (11). The study identified a SNP in RARG (OR=4.7) that was confirmed in two other independent cohorts. Our current study found the opposite effect (decreased rather than increased risk) for the RARG SNP (p=4.1×10−6; OR=0.11). There are multiple explanations for the incongruence of findings across studies. One likely reason is due to the general heterogeneity in studies, including: drug type, drug exposure, phenotype definition and population. With regard to the latter, many of the prior studies to date have focused on the pediatric patient population.
This study adheres to stringent criteria for biomarker discovery and the correlative study has several strengths as outlined (26). First, the discovery cohort was conducted in the context of a randomized, phase III adjuvant trial, using a standard number of doses and cycles of doxorubicin. The phenotype collection mandated close follow-up of cardiac function with serial echocardiography and events defined by cardiologist adjudication based on both imaging and clinical picture. The genomic evaluation implemented a comprehensive genome wide approach, which allowed for unbiased discovery. The top finding was further supported in two independent trials that had the same (E1199) and similar (BEATRICE) doses and schedules of anthracycline.
There were also several weaknesses. First, two of the three trials included an experimental arm that delivered bevacizumab. Thus, an association with cardiac toxicity due to the combination of an anthracycline plus bevacizumab cannot be excluded. This concern is substantially minimized as we adjusted for arm of study for both bevacizumab-containing trials, E5103 and BEATRICE, and the third trial, E1199, only included anthracycline; with the SNP being associated with an increased risk and similar effect size across all three trials. Second, the DNA from E1199 was derived from tumor DNA and thus point mutations at the SNP site of interest and loss of heterozygosity cannot be excluded. This limitation was minimized by assuring meticulous QC of genotyping and that all analyzed SNPs did not violate HWE. Finally, we used slightly different definitions of cardiac dysfunction across the three trials. E5103 utilized a clinically useful definition of CHF as defined by a centrally reviewed, cardiologist adjudication and considered a composite of both symptoms and depressed LVEF. E1199 utilized the CTC criteria for CHF and BEATRICE employed the NYHA criteria to define cardiac damage. BEATRICE also collected raw LVEF values to further refine cases and controls; whereas E1199 did not. Despite the heterogeneity of phenotype, the similar results across the three trials strengthen the clinical generalizability of this genetic predictor.
Our top SNP, rs28714259, lies in an intergenic region in chromosome 15 and had LD support from nearby SNPs (Figure 3B). There is increasing evidence that intergenic regions play an important role in gene expression and regulation through long-range interactions (27). Chromatin immunoprecipitation data from the ENCODE project revealed that rs28714259 is within the binding site for glucocorticoid receptor (GR) protein (28). Recent studies have demonstrated glucocorticoid signaling plays important roles in the structural and functional maturation of the fetal heart and in the maintenance of proper cardiac function in various animal models (29).
Future confirmatory studies might focus on even less severe phenotypes of cardiac damage, as prior data demonstrates that asymptomatic CHF has marked increase 10-year mortality (30). Rare toxicities, however, might be best explained by rare variants with large effect size and these are not evaluated on current GWAS platforms. Thus additional work evaluating the role of rare variants and mechanistic work is warranted and ongoing. The ability to better understand which patients might be at higher risk for this serious toxicity is an important step toward personalizing therapy and providing better care for our patients.
Supplementary Material
STATEMENT OF CLINICAL RELEVANCE.
Anthracyclines are a widely used chemotherapeutic class, but can cause life threatening congestive heart failure (CHF). The risk of serious cardiac damage has spurred the implementation of competing non-anthracycline regimens; although many patients still benefit from anthracyclines based on risk or underlying biology of the tumor. Germline genetic biomarkers for CHF have been studied, but their ability to reliably predict risk remains unclear as populations studied have been underpowered and heterogeneous, with inconsistent results to date. We have identified a SNP, rs28714259 that is associated with CHF risk. This SNP was identified through a GWAS from the randomized phase III adjuvant breast cancer trial E5103, and was subsequently validated in two independent phase III adjuvant breast cancer trials; E1199, and BEATRICE. rs28714259 is associated with increased risk of anthracycline induced CHF and might be useful to guide appropriate usage of anthracyclines for patients with breast cancer in the curative setting.
Acknowledgments
FINANCIAL SUPPORT
This study was conducted by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180820, CA180794, CA180795, CA180867, CA189859, CA180844, CA180816, Susan G. Komen for the Cure (BP Schneider), Conquer Cancer Foundation (BP Schneider), Breast Cancer Research Foundation (KD Miller) and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services (BP Schneider). Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
CONFLICT OF INTEREST STATEMENT
KDM has received research funds from Genentech. JAS has received personal fees from Genentech. BPS, FS, LG, MR, LL, GJ, DL, AO, ND, DC, IG, RAM, TMS, TF and GWS have declared no conflicts of interest.
References
- 1.Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol. 2008;26:3777–84. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trudeau M, Charbonneau F, Gelmon K, Laing K, Latreille J, Mackey J, et al. Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol. 2005;6:886–98. doi: 10.1016/S1470-2045(05)70424-1. [DOI] [PubMed] [Google Scholar]
- 4.Hare JL, Brown JK, Marwick TH. Performance of conventional echocardiographic parameters and myocardial measurements in the sequential evaluation of left ventricular function. Am J Cardiol. 2008;101:706–11. doi: 10.1016/j.amjcard.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Volkova M, Russell R. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7:214–20. doi: 10.2174/157340311799960645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–5. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 7.Ades F, Zardavas D, Pinto AC, Criscitiello C, Aftimos P, de Azambuja E. Cardiotoxicity of systemic agents used in breast cancer. Breast. 2014;23:317–28. doi: 10.1016/j.breast.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Cameron D, Brown J, Dent R, Jackisch C, Mackey J, Pivot X, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14:933–42. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 9.Miller K, O’Neill AM, Dang CT, Northfelt DW, Gradishar WJ, Goldstein LJ, et al. Bevacizumab (Bv) in the adjuvant treatment of HER2-negative breast cancer: Final results from Eastern Cooperative Oncology Group E5103. Journal of Clinical Oncology. 2014;2014:32. ASCO Annual Meeting Abstracts. [Google Scholar]
- 10.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aminkeng F, Bhavsar AP, Visscher H, Rassekh SR, Li Y, Lee JW, et al. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet. 2015;47:1079–84. doi: 10.1038/ng.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armenian SH, Ding Y, Mills G, Sun C, Venkataraman K, Wong FL, et al. Genetic susceptibility to anthracycline-related congestive heart failure in survivors of haematopoietic cell transplantation. Br J Haematol. 2013;163:205–13. doi: 10.1111/bjh.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, Kawashima TI, Davies SM, Relling MV, et al. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112:2789–95. doi: 10.1002/cncr.23534. [DOI] [PubMed] [Google Scholar]
- 14.Blanco JG, Sun CL, Landier W, Chen L, Esparza-Duran D, Leisenring W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes–a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:1415–21. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubieniecka JM, Graham J, Heffner D, Mottus R, Reid R, Hogge D, et al. A discovery study of daunorubicin induced cardiotoxicity in a sample of acute myeloid leukemia patients prioritizes P450 oxidoreductase polymorphisms as a potential risk factor. Front Genet. 2013;4:231. doi: 10.3389/fgene.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajić V, Aplenc R, Debeljak M, Prestor VV, Karas-Kuzelicki N, Mlinaric-Rascan I, et al. Influence of the polymorphism in candidate genes on late cardiac damage in patients treated due to acute leukemia in childhood. Leuk Lymphoma. 2009;50:1693–8. doi: 10.1080/10428190903177212. [DOI] [PubMed] [Google Scholar]
- 17.Sachidanandam K, Gayle AA, Robins HI, Kolesar JM. Unexpected doxorubicin-mediated cardiotoxicity in sisters: possible role of polymorphisms in histamine n-methyl transferase. J Oncol Pharm Pract. 2013;19:269–72. doi: 10.1177/1078155212461022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semsei AF, Erdelyi DJ, Ungvari I, Csagoly E, Hegyi MZ, Kiszel PS, et al. ABCC1 polymorphisms in anthracycline-induced cardiotoxicity in childhood acute lymphoblastic leukaemia. Cell Biol Int. 2012;36:79–86. doi: 10.1042/CBI20110264. [DOI] [PubMed] [Google Scholar]
- 19.Visscher H, Rassekh SR, Sandor GS, Caron HN, van Dalen EC, Kremer LC, et al. Genetic variants in SLC22A17 and SLC22A7 are associated with anthracycline-induced cardiotoxicity in children. Pharmacogenomics. 2015;16:1065–76. doi: 10.2217/pgs.15.61. [DOI] [PubMed] [Google Scholar]
- 20.Visscher H, Ross CJ, Rassekh SR, Barhdadi A, Dubé MP, Al-Saloos H, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30:1422–8. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 21.Volkan-Salanci B, Aksoy H, Kiratli P, Tülümen E, Güler N, Öksüzoglu B, et al. The relationship between changes in functional cardiac parameters following anthracycline therapy and carbonyl reductase 3 and glutathione S transferase Pi polymorphisms. J Chemother. 2012;24:285–91. doi: 10.1179/1973947812Y.0000000037. [DOI] [PubMed] [Google Scholar]
- 22.Wojnowski L, Kulle B, Schirmer M, Schlüter G, Schmidt A, Rosenberger A, et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–62. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 23.Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–71. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider BP, Li L, Radovich M, Shen F, Miller KD, Flockhart DA, et al. Genome-Wide Association Studies for Taxane-Induced Peripheral Neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–44. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 26.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. REporting recommendations for tumor MARKer prognostic studies (REMARK) Nat Clin Pract Urol. 2005;2:416–22. [PubMed] [Google Scholar]
- 27.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489:109–13. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenbloom KR, Sloan CA, Malladi VS, Dreszer TR, Learned K, Kirkup VM, et al. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Res. 2013;41:D56–63. doi: 10.1093/nar/gks1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rog-Zielinska EA, Thomson A, Kenyon CJ, Brownstein DG, Moran CM, Szumska D, et al. Glucocorticoid receptor is required for foetal heart maturation. Hum Mol Genet. 2013;22:3269–82. doi: 10.1093/hmg/ddt182. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113:2851–60. doi: 10.1161/CIRCULATIONAHA.105.600437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


