Abstract
Glaucoma is the second leading cause of blindness with ∼70 million people worldwide who are blind from this disease. The currently practiced trabeculectomy surgery, the gold standard treatment used to stop the progression of vision loss, is rather draconian, traumatic to the patient and requires much surgical skill to perform. This article summarizes the more than 10‐year development path of a novel device called the InnFocus MicroShunt®, which is a minimally invasive glaucoma drainage micro‐tube used to shunt aqueous humor from the anterior chamber of the eye to a flap formed under the conjunctiva and Tenon's Capsule. The safety and clinical performance of this device approaches that of trabeculectomy. The impetus to develop this device stemmed from the invention of a new biomaterial called poly(styrene‐block‐isobutylene‐block‐styrene), or “SIBS.” SIBS is ultra‐stable with virtually no foreign body reaction in the body, which manifests in the eye as clinically insignificant inflammation and capsule formation. The quest for an easier, safer, and more effective method of treating glaucoma led to the marriage of SIBS with this glaucoma drainage micro‐tube. This article summarizes the development of SIBS and the subsequent three iterations of design and four clinical trials that drove the one‐year qualified success rate of the device from 43% to 100%. © 2015 Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 105B: 211–221, 2017.
Keywords: glaucoma, trabeculectomy, SIBS, biodegradation, entrepreneurship
INTRODUCTION
The development of SIBS
The development of a micro‐shunt, from the polyolefin poly (styrene‐block‐isobutylene‐block‐styrene) (“SIBS”) (Figure 1) to treat glaucoma is one of several products that originated from a ten‐year quest to develop a novel synthetic thermoplastic elastomeric biomaterial that would resist biodegradation in the body. In the 1990s there were only two elastomeric polymers that were used for long‐term implant applications: polyurethane, predominantly polyether urethane and silicone rubber, predominantly polydimethylsiloxane (PDMS).
Figure 1.
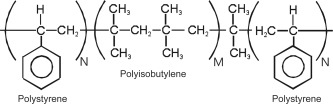
A simplified structure of poly(styrene‐block‐isobutylene‐block‐styrene) (SIBS) showing the central PIB block with polystyrene end segments (M ≫ N).
Polyether urethane demonstrates excellent abrasion resistance and fatigue life with tensile strengths in the 20–35 MPa range and elongations that can exceed 600%. However, polyether urethane is well‐known to slowly degrade in the body.1, 2
PDMS has enjoyed relative success as a long‐term implant material3; however, it suffers from several drawbacks, that is, it has low tensile strength (< 10 MPa), poor abrasion resistance, and it is relatively sticky. Besides the mechanical deficiencies, conventional PDMS contains impurities such as silica, oligomer, and unreacted starting material; most notably the cyclic monomer octamethylcyclotetrasiloxane (D4).4 These impurities may be responsible for the foreign body reaction often observed when devices made from PDMS are implanted in the eye.5
Work on investigating new polymers by the co‐authors (LP, YPK, JBM, BW) began in earnest in the early mid‐1990s when Corvita Corporation ((Miami, FL)—later acquired by Schneider/Pfizer (Zurich, Switzerland) and then Boston Scientific Corporation, (Natick, MA)) scientists observed severe biodegradation of a spun micro‐fibrous polyether urethane vascular graft that it was developing. In an attempt to fix the problem, the Corvita team developed and introduced the polycarbonate urethanes to the medical implant industry6 and began to implant spun polycarbonate urethane vascular grafts.7 Although much more biostable than the polyether urethane materials,8 the polycarbonate urethane microfilaments comprising the graft began to show signs of biodegradation in the form of fiber cracking and severing. Further, histology of the surrounding tissue showed a large influx of granulocytes which presumably was a consequence of the slow biodegradation of these polymers. Macrophages, polymorphonuclear leukocytes, and foreign body giant cells migrated toward the device to either wall‐off the degrading material by forming thick capsules around it or to disperse or remove degraded fragments by phagocytosis.2 These events led to the quest for new thermoplastic fiber‐forming elastomeric polymers. Thermoset polymers such as PDMS could not be used for this application as they are not fiber‐forming.
An analysis of the degradation mechanism of polyurethanes9, 10 combined with an understanding of organic chemistry principles led to the hypothesis that the long‐term stability of a polymeric material in living tissue can be achieved when both the polymeric backbone and pendant groups are devoid of unprotected ester, amide, ether, carbamate, urea, or any other groups that are prone to oxidation, hydrolysis, or enzymatic cleavage. Further, the degradation of polyethylene acetabular joint liners that produce in‐chain unsaturation and crosslinking11, 12, 13, 14 that dominated the literature for the last two decades suggests that secondary carbon‐containing polymers such as polyethylene, and secondary‐and‐tertiary carbon‐containing polymers such as polypropylene15, 16, 17 are also to be avoided as double bond formation leads to embrittlement and stress cracking.
Consequently, it was hypothesized that an ideal polymer for implant application should only be comprised of oxidatively–hydrolytically–enzymatically stable alternating secondary‐and‐quaternary carbons in the backbone, and equally stable primary carbons as pendant groups. The basic structure of this nature are those comprised of polyisobutylene (PIB) shown as the center block in Figure 1. The absence of cleavable side groups in PIB, in contrast to potentially hydrolyzable ester groups in acrylic or acrylate polymers such as methacrylate, for example, the methoxy group in poly(methylmethacrylate) which contains a similar alternating secondary–quaternary carbon backbone, should provide a polymer with less biodegradation. If this hypothesis is correct, the less biodegradation, the less chronic inflammation.
Polyisobutylene, an inert non‐vulcanizable rubber used in many industrial applications (i.e., tackifiers, adhesives, sealants, thickening agents, viscosity enhancers, various additives, chewing gum, etc.), can be obtained easily and inexpensively by the cationic polymerization of isobutylene (IB). However, PIB cannot be used in applications where shape retention is essential because it is not crosslinked—it is a gum. A very close relative to PIB is butyl rubber, a commercially available copolymer of ∼98% isobutylene and ∼2% isoprene, in which the few but critically important isoprene units —CH2—C(CH3)=CH—CH2— provide vulcanizability and shape retention. However, butyl rubber is also unsuitable for implantation in living tissue as (1) it contains oxidatively vulnerable double bonds, and (2) it can be converted into a shape‐retaining rubber only by vulcanization under harsh conditions with crosslinkers and additives that are generally not tolerated in the body.
The search for PIB‐based thermoplastic elastomers, that is, for elastomers that contain PIB rubbery segments covalently linked to readily thermally‐ and/or solution‐processable glassy segments, led the lead author to Kennedy's laboratory at The University of Akron where such polymers were already synthesized.18 Kennedy's patents protecting the triblock copolymer SIBS were subsequently licensed by Corvita Corporation and strengthened by additional patents covering applications in the medical implant arena.19, 20, 21
The triblock SIBS was used for the initial studies of biocompatibility and biostability. Figure 1 shows a simplified molecular structure of SIBS in which soft PIB rubbery chains are held together by hard glassy polystyrene domains. Dangling chains are absent and all the PIB segments contribute to the load bearing capacity of the network. SIBS is a self‐assembled physically crosslinked PIB, and thus thermo‐ and solution‐formable. Furthermore, because it is soluble in various non‐polar solvents it can be spray‐coated or solvent cast to deliver soft strong coherent films.
The synthesis and properties of SIBS
SIBS is synthesized by the living cationic polymerization technique developed by Kennedy's team at The University of Akron. Living cationic polymerization, a seminal discovery in polymer science, led not only to SIBS but also to many novel compositions useful for a variety of industrial and medical applications.18
The synthesis of SIBS (Figure 2) begins with a bifunctional initiator, which becomes part of the polymer. The preferred initiator is 5‐tert‐butyl‐1,3‐bis(1‐methoxy‐l‐methylethyl)‐benzene (for brevity's sake “hindered dicumyl ether,” abbreviated HDCE). HDCE is not commercially available and is custom synthesized by either Innovia LLC (Miami, FL) or InnFocus, Inc. (Miami, FL) according to methods developed by Kennedy and coworkers.22, 23
Figure 2.
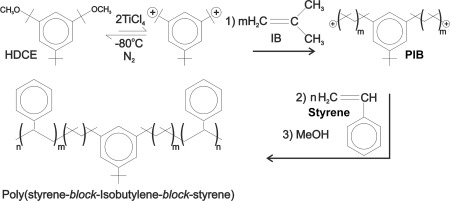
The synthesis of SIBS beginning with the di‐functional initiator—hindered dicumyl ether (HDCE), forming the di‐cation, then reacting with isobutylene (IB), then styrene to form the triblock polymer and finally quenching with methanol.
In brief, SIBS is prepared in two steps in one pot: First, isobutylene is polymerized by a HDCE/TiCl4 initiating system in a methyl chloride/hexanes solvent system in the presence of a proton trap under a blanket of dry nitrogen at −80°C. When the central PIB block reaches the desired molecular weight, styrene is added and the polymerization is continued until the outer polystyrene blocks also reach a predetermined length. The process is terminated by the addition of methanol.
Table 1 presents typical properties of SIBS. The molecular weight of the triblock is controlled by reaction conditions, mainly by the ratio of monomers/initiator. The hardness of SIBS can be varied by the amount of styrene employed.
Table 1.
Physical Properties of SIBS
| Shore hardness | 30A–60D |
| Mole percent styrene | 5–50 |
| Ultimate tensile strength (psi) | 2000–5000 |
| Ultimate tensile strength (MPa) | 10–20 |
| Ultimate elongation (%) | 300–1100 |
| Index of refraction | 1.525–1.535 |
| Water absorption (g/m2 at 24 h) | 0.2–0.3 |
| Weight average molecular weight | 60,000–150,000 |
| Polydispersity (M w/M n) | 1.2–2.1 |
The excellent oxidative stability of SIBS can be demonstrated by submerging a swatch of SIBS in boiling concentrated (65%) nitric acid for 30 min. Whereas, other elastomers used for implant applications, such as silicone rubber and polycarbonate urethane, severely embrittle or are completely destroyed within a few minutes, SIBS remains relatively unscathed and stable under these harsh conditions. Silicone rubber is well‐known to degrade by strong acids and strong bases and does not survive these tests.24 Although this test is unreasonably harsh, materials that withstand this test have an excellent chance of being biostable in the body.
The first medical use of SIBS was for Boston Scientific Corporation's (Natick, MA) TAXUS® stent.25, 26 TAXUS is a small balloon‐expandable metallic stent (2–3 mm in diameter and 10–20 mm long), with a SIBS coating that slowly releases the antiproliferative drug, paclitaxel, into the wall of the coronary artery to prevent restenosis. TAXUS became the largest product launch in the history of medical devices with sales of approximately $3 billion in the first year. Data collected from studies of TAXUS confirmed no biodegradation and minimal tissue reaction.27 Boston Scientific was pleased with the SIBS polymer and the work performed by Dr. Pinchuk's team; this interaction led to Boston Scientific providing the seed money for Innovia LLC which was formed in 2002. Innovia spun off InnFocus LLC in 2004 with the goal of developing novel products made from SIBS for use in the eye. A more comprehensive description of SIBS and various applications were published by Pinchuk et al.25
The development of a glaucoma tube
In 2003, Dr. Pinchuk introduced SIBS to Dr. Jean‐Marie Parel at the University of Miami's Miller School of Medicine, Bascom Palmer Eye Institute, Ophthalmic Biophysics Center (OBC). Dr. Parel's team implanted 3 mm diameter and 1 mm thick SIBS disks into the corneal stroma (see Figure 3) as well as under the conjunctiva and Tenon's Capsule in the eyes of New Zealand White Rabbits. Similar disks made from silicone rubber (PDMS) were implanted in these eyes alongside the SIBS disks as controls. The results of the two‐month implants were published by Parel et al.28 and Acosta et al.29 and, in brief, they found that there were no myofibroblasts or angiogenesis in the vicinity of the SIBS disks, nor were there integral capsules surrounding the disks. In contrast, the PDMS controls showed angiogenesis, myofibroblasts, and significant capsules attached to the disks. In summary, SIBS was found to be remarkably innocuous in the eye.
Figure 3.
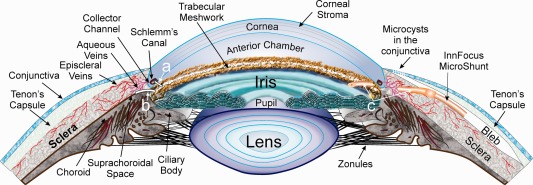
A section of the anterior segment of the eye where “a” points to a shunt across the trabecular meshwork to Schlemm's Canal, “b” points to a shunt from the anterior chamber to the suprachoroidal space and “c” points to a shunt from the anterior chamber to a space formed under the conjunctiva and Tenon's Capsule which, when filled with aqueous humor forms a bleb.
Shortly thereafter, Dr. Pinchuk and Dr. Parel met with Dr. Francisco Fantes, a glaucoma specialist, to determine how best to exploit this non‐encapsulating SIBS material. It was decided there was an unmet need for a simple glaucoma drainage device that could be implanted using minimally invasive surgery. The premise was that if such a device was available, with similar efficacy to trabeculectomy and with a better safety profile, it would be used earlier in the treatment paradigm for glaucoma, before the cornea and conjunctiva were irreversibly damaged by the preservatives in glaucoma medications.30 Trabeculectomy is a surgical procedure where the conjunctiva is incised to expose the sclera. A flap is made in the sclera, a section of sclera is removed (sclerectomy), a hole is then punched from within this flap into the anterior chamber, a piece of iris is often removed (iridectomy) and the flap sutured closed with sufficient tension to prevent the eye from deflating (hypotony). Aqueous humor flows from the anterior chamber through the hole into the scleral flap and then into a reservoir formed under the conjunctiva and Tenons (called a “bleb”), thereby lowering intraocular pressure (IOP).
The first question was where to shunt aqueous humor. Three outflow paths originating from the anterior chamber were considered: (1) to Schlemm's Canal; (2) to the suprachoroidal space; and (3) to a flap formed under the conjunctiva and Tenon's Capsule (bleb‐based).
Shunting from the anterior chamber and across the trabecular meshwork to Schlemm's Canal, as shown by “a” in Figure 3, implies that the trabecular meshwork was the source of high resistance; however, data gleaned from early results of the Trabectome31 procedure in which most of the trabecular meshwork is removed by an electrocautery‐type action, still showed high IOP relative to what is achievable by trabeculectomy. This observation suggested that the major source of resistance was further downstream, perhaps in the collector channels, intrascleral venous plexus or episcleral veins. Shunting to Schlemm's Canal was therefore eliminated as an option.
It was also known that IOP reduction by implanting into the suprachoroidal space, shown by “b” in Figure 3, was unpredictable. It could at times be under vacuum which can induce hypotony; however, once the vacuum pressure was relieved by forming a cyclodialysis cleft, it often remained proportional and only slightly lower (1–3 mm) than the IOP in the anterior chamber, implying that if the IOP in the anterior chamber was 24 mm Hg, it would not drop below perhaps 21 mm Hg by draining to the suprachoroidal space.32 This incremental reduction in IOP from the suprachoroidal space was not acceptable to the glaucoma specialist as it was believed to be too minute to stop the progression of glaucoma. Data from the AGIS study suggested that IOP must be reduced to the mid to low teens to stop the progression of vision loss.33 In addition, the literature suggested that flow from the suprachoroidal space invariably ended up in the same episcleral and intrascleral veins as conventional flow via Schlemm's Canal and if these downstream scleral veins were the source of high resistance, then they needed to be bypassed.34 The option of draining to the suprachoroidal space was therefore eliminated.
It was decided that draining to a flap under the conjunctiva and Tenon's Capsule, shown by “c” in Figure 3, much like trabeculectomy, made the most sense as this path bypasses the trabecular meshwork, Schlemm's Canal, the suprachoroidal space, and possibly the intrascleral venous plexus. Draining to a bleb, as shown on the right side of Figure 3, also enables filtration through microcysts35 (small pore‐like structures) in the conjunctiva and into the tear film, thereby bypassing the episcleral veins; that is, if the resistance in the episcleral venous system is higher than that through the microcysts (as fluid dynamics teaches that fluid will flow through the path of least resistance).
Once the outflow location was established, it was decided that a glaucoma drainage device without a plate might be achievable if the tube did not encapsulate nor occlude. This design would require that the lumen of the tube be larger than the diameter of a sloughed endothelial cell, which is about 40–50 µm, while at the same time sufficiently small to prevent hypotony. The lumen size was approximated from the Hagen–Poiseuille equation36 and a series of rabbit eye implants by Arrieta et al.37 and Fantes et al.38 confirmed that a lumen diameter of ∼70 µm would satisfy these safety requirements. (It is noteworthy that the Hagen–Poiseuille equation breaks down at small diameter in extremely hydrophobic materials, as is the case with SIBS; however, the empirical data generated by Arrieta helped fine‐tune the lumen diameter.) Implantation of prototypes into more than forty rabbit eyes, with some being implanted for periods over one year, confirmed the biocompatibility of SIBS in the eye as well as the safety of the device.
The team believed a priori that advantages of the MicroShunt would include the avoidance of cutting the sclera and suturing the scleral flap with sutures placed under the proper tension to control outflow, a process that requires significant surgical skill. There would also be no need for iridectomy or sclerectomy which cause inflammation, which can lead to cataract formation. In addition, the fluid dynamics of the MicroShunt could be controlled by the lumen diameter and length to minimize hypotony, yet provide pressure in the mid‐low teens. Still further, it was anticipated that this plateless device would eliminate motility problems associated with encapsulation of the plates on large drainage valves.39 And so began the development of the SIBS‐based InnFocus MicroShunt®.40
Pre‐clinical testing of the InnFocus MicroShunt
The InnFocus MicroShunt® is made by extruding a SIBS tube of the specified outer and inner diameters and then insert‐molding the fixation member onto the tube. The device is trimmed to length, packaged on a silicone rubber mount in a Mylar/Tyvek® pouch and ethylene oxide sterilized. The weight of each MicroShunt is ∼0.8 mg.
Pre‐clinical bench testing generally followed ISO 11979‐5:2006 § 5.3 and Annex A and/or B, used to test intraocular lenses. The required weight to solvent ratio required for many of the extraction and stability tests, of 10 g sample to 100 mL solvent was impractical due to the small weight of the MicroShunt. It would have required 12,500 MicroShunts for each extraction and hydrolysis test required (repeated with three different lots of material) which would have taken over 20 years to complete with the current molding equipment. If the ratio was changed to, for example, 1 mg sample to 10 µL solvent, the quantity of extract may be below the threshold sensitivity of the analytical instrumentation. Instead facsimiles of the MicroShunt were fabricated by extruding tubing of the same diameter and lumen size as the MicroShunt, cutting the tubes to 2″ lengths, positioning dozens of them next to each other to form clusters and then cross‐compression molding fin material, in the form of a filament, over each tube in the cluster, thereby binding them all together. The facsimiles therefore were manufactured using the same materials and heat history as the final product. Approximately 0.2 g of facsimile was then packaged separately in the final packaging used for the MicroShunt and ethylene oxide sterilized. Sufficient facsimile was made to test at the required ratio of 10 g sample to 100 mL solvent. The tests are summarized below. All extracts were analyzed by UV/VIS spectrophotometry and chromatographically using HPLC/PDA (photodiode array UV detection), GC/FID (flame ionization detection), and GC/MS techniques.
Physical stability test
Physical stability testing was performed as recommended by the ANSI Z80.27‐2001 Glaucoma Shunt Standard. Sterilized MicroShunt samples were incubated in distilled water at 37 ± 2°C for 14 days. Dimensional changes were slight (between 1.0% and 4.5%) and fell within specified tolerances. Flow rates through the device were measured at an entrance pressure of 20 mm. Changes before and after incubation were 7.95 ± 0.58 and 7.03 ± 0.55 µL/min (at 20 mm Hg using 11 mm long tubes), respectively representing an 11.6% drop in flow rate. Although significant, it was not considered an impairment to the performance of the device. Tensile strength testing of the bond joint between the fin and tube showed an 11.2% increase from baseline to after sterilization.
Hydrolysis testing
MicroShunt facsimiles were incubated in distilled water at either 55 ± 2°C, for 15 months; 85 ± 2°C, for 57 days, or 100 ± 2°C for 20 days, for the equivalent of 5 years of real‐time exposure. The aged samples were examined gravimetrically and by scanning electron microscopy (SEM). SEM analysis showed no changes in appearance (hydrolysis would have been evident by pitting or cracking of the material surface). Gravimetric analysis, as measured to 0.00001 g precision, showed no evidence of hydrolytic instability with an insignificant change in weight of < 0.1%. No significant analytes were detected in the hydrolysates by any of the spectrophotometric or chromatographic instruments. All of these tests consistently demonstrated the chemical hydrolytic stability of SIBS.
Exhaustive extraction testing in isopropyl alcohol
Sterilized MicroShunt facsimiles were aged in their package for an equivalence of 3 years and then subjected to Soxhlet extraction with isopropyl alcohol for 4 h. Weight changes were less than 0.2%. Analysis of the eluent showed only trace levels of low molecular weight siloxanes, ethylene glycol, benzophenone, 2‐phenylphenol, and a low molecular weight alkyl polyol contaminants; virtually all of these contaminates originated from the packaging materials and were considered insignificant as later confirmed by biocompatibility testing.
Leachable testing
Facsimiles were incubated in both water and isopropyl alcohol at a temperature of 35 ± 2°C for 72 ± 2 h. Trace amounts of packaging material contaminants were found as in the exhaustive extraction testing. Metallic contaminants were determined by Inductive Coupled Plasma/Mass Spectrophotometry. Although very low levels of metal were detected, subsequent biocompatibility testing confirmed physiological insignificance.
Biocompatibility testing
Biocompatibility studies were completed in accordance with ISO 10993‐1‐2009 recommendations. In addition to the ISO 10993 biocompatibility studies, animal studies in rabbit eyes were conducted to demonstrate safety and function of the device in vivo. There were a total of four rabbit studies. The first two studies were conducted as non‐GLP studies at the University of Miami's Bascom Palmer Eye Institute (Miami, FL). The third study was a GLP study performed at NAMSA (Northwood, Ohio) contract facility. The first three studies were conducted on the MIDI‐Tube version of the device [Figure 4(A)]. The final chronic animal study was conducted as a non‐GLP study at the University of Miami's Bascom Palmer Eye Institute on the MIDI‐Ray [Figure 4(B)]. All biocompatibility testing suggested that the InnFocus devices were sufficiently safe to proceed to human testing.
Figure 4.
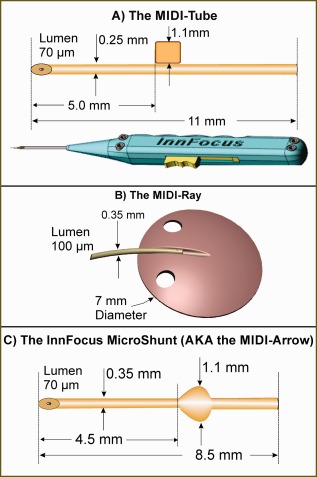
The three generations of glaucoma shunts: (A) the MIDI‐Tube used in the first Bordeaux study with the slotted inserter; (B) the MIDI‐Ray used in the first Dominican Republic study; and (C) the final design called the InnFocus MicroShunt® used thereafter.
The four iterations of the glaucoma device
There were three major iterations of shunt design as shown in Figure 4 and four clinical studies with varying procedural differences presented in Table 2.
Table 2.
Design Comparison of the MIDI‐Tube, MIDI‐Ray, and InnFocus MicroShunt®
| Device | MIDI‐Tube | MIDI‐Tube | MIDI‐Ray | InnFocus MicroShunt® (AKA, MIDI‐Arrow) |
|---|---|---|---|---|
| Study | Bordeaux I | Bordeaux II | DR I | DR II |
| Tube Outer Diameter (mm) | 0.25 | 0.25 | 0.35 | 0.35 |
| Tube Lumen Diameter (µm) | 70 | 70 | 100 | 70 |
| Total length (mm) | 11 | 11 | 12 | 8.5 |
| Migration restrictor type | Tab | Tab | Plate | Fin |
| Fixation member (mm) | 1 × 1 | 1 × 1 | 7 diam. | 1.1 Wingspan |
| Needle Tract Gauge | 27 | 25–27 | 27 | 25–27 |
| Introducer | Inserter | Inserter | Forceps | Forceps |
| MMC concentration, Time (min) | None | 0.2 mg/mL, 2 | None | 0.4mg/mL, 3 |
| Area of MMC applied | None | Sclera | None | Entire flap |
| Lumen tied off? | No | No | Yes | No |
The three design iterations were tested first in both acute and chronic rabbit eye studies at the University of Miami, Bascom Palmer Eye Institute OBC laboratory; and then in pilot feasibility studies over a period of four years to determine the best design as well as the best implantation technique. All animal studies were authorized by the University of Miami ACUC (Animal Care and Use Committee). All feasibility studies in humans were authorized by the appropriate government ethics committees. In France, approval was granted by AFSSAPS (Agence Française de Sécurité Sanitaire des Produits de Santé) and later by ANSM (Agence Nationale de Sécurité du Medicament et des Produits de Santé). In the Dominican Republic, approval was granted by CONABIOS (the Dominican Republic National Counsel of Bioethics and Health). Local hospital‐based ethics committee approvals were also obtained where required.
A brief summary of the baseline characteristics, change in IOP, and glaucoma medication use per patient at one year are tabulated in Table 3. The major criterion for qualified success, adopted from the Tube versus Trabeculectomy (“TVT”) Study publications,41, 42 is IOP ≤ 21 mm Hg with a reduction from baseline of ≥ 20% with or without glaucoma medication and with no further incisional procedure.
Table 3.
Summary of Baseline Characteristics and 1 Year Results of the Three Iterations of Glaucoma Shunt Design and Four Clinical Trials in Bordeaux, France, and Santo Domingo, Dominican Republic (DR)
| Device | MIDI‐Tube | MIDI‐Tube | MIDI‐Ray | InnFocus MicroShunt® (AKA, MIDI‐Arrow) |
|---|---|---|---|---|
| Study | Bordeaux I | Bordeaux II | DR I | DR II |
| Baseline characteristics | ||||
| Number of patients | 24 | 16 | 12 | 23 |
| Average age | 65.2 ± 18.9 | 57.1 ± 13.5 | 56.8 ± 13 | 59.8 ± 15.3 |
| Race | Caucasian | Caucasian | Mixed | Mixed |
| Status of test eye: Phakic/Cataract/Pseudophakic | 9/1/14 | 10/0/6 | 4/7/1 | 10/11/2 |
| Glaucoma diagnosis POAG | 19 | 14 | 12 | 23 |
| Congenital | 3 | 0 | 0 | 0 |
| Plat. iris | 1 | 1 | 0 | 0 |
| Post steroid | 1 | 1 | 0 | 0 |
| Previous conjunctival surgeries | 12 | 11 | 0 | 0 |
| Baseline IOP (with full medication regimen) | 24.1 ± 7.8 | 21.1 ± 5.2 | 24.4 ± 4.4 | 23.8 ± 5.3 |
| Average glaucoma Medications | 2.9 | 1.7 | 1.7 | 2.4 ± 1.0 |
| Results at 1 year | ||||
| IOP (mmHg) | 16.2 | 12.8 | 14.4 ± 3.9 | 10.7 ± 2.8 |
| Glaucoma medication | 1.5 | 0.7 ± 0.5 | 1.2 ± 1.1 | 0.3 ± 0.8 |
| Surgical success | 42% | 67% | 58% | 100% |
The first generation product was a 250 µm outer diameter SIBS tube [Figure 4(A)] with a 1 mm × 1 mm × 250 µm thick SIBS tab jutting out of one side. It was called the MIDI‐Tube or “Minimally Invasive Drainage Implant.” The purpose of the tab was to serve as a fixation member to prevent migration of the shunt into the anterior chamber due to movement caused by globe rotation and blinking. The tab was attached to one wall of the shunt, rather than symmetrical about the tube, as the device was delivered through a slotted needle inserter [lower part of Figure 4(A)], where the tab jutted out of the slot in the needle.
Professor Isabelle Riss, formerly at the Hôpital Pellegrin in Bordeaux, France, and currently at Pôle Ophtalmologique de la Clinique Mutualiste, Pessac, Cedex, France, was the first surgeon to implant the MIDI‐Tube in humans in January 2006. Her hospital is a referral center for patients with severe glaucoma. Twenty‐four advanced cases, with about half of the eyes failing previous trabeculectomy, were used in the Bordeaux I study (see Table 3). Mitomycin C (MMC) was not used intraoperatively and the qualified success rate was 42% at one year. (MMC inhibits the proliferation of fibroblasts).43 In addition, there were two occurrences of erosion (successfully patched without secondary events) of the corner of the tab, which reoriented upward, through the conjunctiva in two patients who were extreme myopes (eye was elongated and the conjunctiva stretched thin). It was concluded that MMC would be required in this patient population to prevent fibrosis and sustain the bleb and that the tab should be redesigned to be less erosive. It was also found that the slotted needle inserter [Figure 4(A)] was unreliable, as the device, being very soft and somewhat sticky, often jammed in the inserter; it was more reliable to insert the device with a forceps through a pre‐formed needle tract than to push it through a non‐lubricated tube.
A second clinical study of the same MIDI‐Tube design (Bordeaux II study) was initiated in an additional 16 patients, 11 of which failed previous incisional procedures. This cohort of patients were treated intraoperatively with the application of low‐dose MMC in the sub‐conjunctival/Tenon's flap as a means of controlling healing of the conjunctiva to the sclera and loss of the bleb. MMC was applied to the scleral side of the flap only, using two to three Schirmer strips (sponge‐like strips used to absorb and measure tear volume). The MMC dose used a total of ∼0.6 mL of a 0.2 mg/mL concentration applied for 2–3 min and the MMC was flushed from the eye with 250 mL of sterile saline. The success rate increased to 67% at one year in these difficult patients. These data confirmed that MMC must be used in conjunction with a redesigned MIDI‐Tube tab in these late‐stage severe patients. (The Bordeaux II study was in progress when the aforementioned erosions were noted from the tab in the Bordeaux I study).
Nine months into the Bordeaux II study, InnFocus decided to test in parallel an alternate model called the MIDI‐Ray [see Figure 4(B)] which was a SIBS tube (outer diameter 350 µm, lumen diameter 100 µm) with a 7 mm diameter SIBS plate that was 350‐µm thick. The device resembled a stingray, hence its name. The hypothesis was that the lack of encapsulation of the SIBS plate would facilitate fluid percolation through the sclera, as well as reduce problems associated with motility and diplopia, often encountered with the large plate valves.42 It could also obviate the need to use MMC. A twelve‐subject clinical study was initiated by Dr. Juan Batlle in September 2007 at Centro Laser, Santo Domingo, Dominican Republic. Unfortunately, the small plate and lack of capsule formation around the MIDI‐Ray SIBS plate resulted in a constrained drainage field which led to cystic‐type blebs and a qualified success rate of only 58%. In addition, the 100‐µm lumen resulted in a high incidence of acute hypotony (all cases resolved spontaneously). Following this discovery, the investigator tied off the tube with a suture until the device healed‐in.
The data accumulated from the Bordeaux II study indicated a 67% success rate without a plate, with low‐dose MMC and no hypotony. It was thus decided to continue with a plateless tube, to modify the tab to make it more atraumatic and, under the advisement of Drs. Francisco Fantes and Paul Palmberg, to use the Peng Khaw method of applying MMC, which was shown to be safe in the long‐term.44 This initial work demonstrated that (1) the SIBS tube provoked insignificant inflammation and encapsulation, (2) it was safe in human eyes, (3) it could be removed by simply pulling it out, and (4) more than one device could be placed in the same quadrant to provide additional outflow to the bleb (Figure 5).
Figure 5.
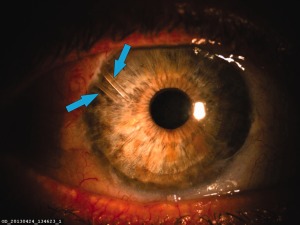
Photograph of two glaucoma shunts in the same quadrant of the eye. The lower arrow points to a MIDI‐Tube (250 μm outer diameter). The upper arrow points to an InnFocus MicroShunt® (350 μm outer diameter) that was implanted 4 years later to further reduce intraocular pressure (courtesy Prof. Isabelle Riss).
The new design of the micro‐shunt was initially called the MIDI‐Arrow [Figure 4(C)] as the fixation member was changed to an atraumatic, planar symmetrical fin‐like structure which resembled the feathers on an arrow. The MIDI‐Arrow name was later changed to the InnFocus MicroShunt® to avoid any association of an arrow in the eye. The surgical protocol was changed to provide a shallow 1 mm diameter wide, 1 mm long pocket in the sclera, in line with the needle tract to seat the fins. The purpose of the pocket and fins was to: (1) serve as a “cork” to seal the device in the pocket and prevent leakage around the tube; (2) serve as a fixation mechanism to prevent the device from migrating into the eye; (3) to prevent the fins from turning and eroding the conjunctiva (the aforementioned problem with the MIDI‐Tube) and; (4) serve as a mechanism of orienting the device such that the bevel in the anterior chamber was facing the cornea. The lumen of the InnFocus MicroShunt® remained at 70 µm in order to eliminate the need to tie off the tube during the healing phase and minimize hypotony. Figure 6 shows a graph of flow rate versus pressure of the InnFocus MicroShunt. Flow through the MicroShunt tends to subside at pressures <5 mm Hg.
Figure 6.
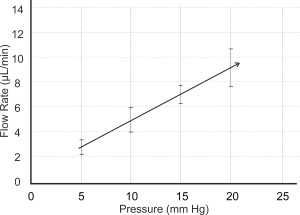
Measured flow rate versus pressure of the InnFocus MicroShunt, which is 8.5 mm long with a lumen diameter of 70 μm. It requires approximately 5 mm Hg pressure to initiate flow through the MicroShunt.
A feasibility trial with 0.4 mg/mL MMC applied for 3 min was initiated in the Dominican Republic. Twenty‐three mixed race (African, Caucasian and Native Indian) patients who suffered from primary open angle glaucoma (POAG), with no previous conjunctival surgery and who had failed maximum tolerated glaucoma medication were used in the study. The aforementioned changes to the device and the procedure led to an unprecedented qualified success rate of 100% with a large 50% drop in IOP from baseline at one year with 80% of patients totally off of glaucoma medication. There were two cases of transient hypotony (hypotony denotes IOP <6 mm) from this study that cleared spontaneously without pathological sequelae and there were no sight‐treating long‐term adverse events.
The Qualified Success rates of the one‐year feasibility studies of the MIDI‐Tube without MMC (Bordeaux I), MIDI‐Tube with low‐dose MMC (Bordeaux II), MIDI‐Ray (Dominican Republic I), and InnFocus MicroShunt® (Dominican Republic II) are plotted in the Kaplan–Meier curve shown in Figure 7. Based upon these excellent results, the team decided to freeze the InnFocus MicroShunt® design.
Figure 7.
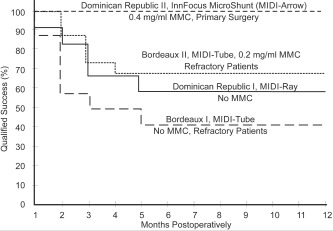
Kaplan–Meier survival curves showing the qualified success rate of the four iterations of the glaucoma shunt and procedure. The final device using the InnFocus MicroShunt® with 0.4 mg/mL MMC provided near perfect results.
The final and current implantation procedure for the MicroShunt [Figure 4(C)] is shown in Figure 8. More comprehensive descriptions of the clinical studies and outcomes have been submitted to peer‐reviewed ophthalmic journals.
Figure 8.
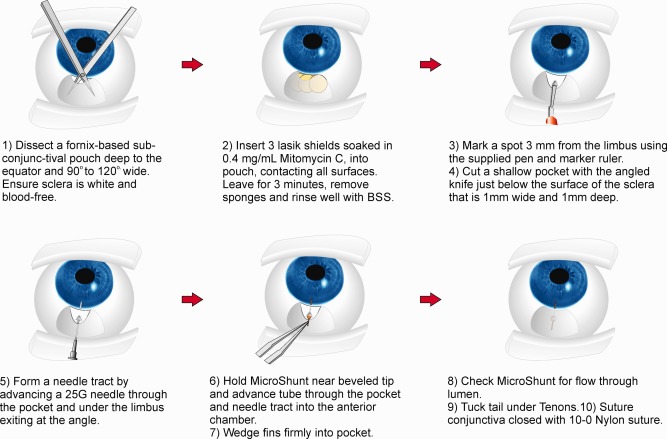
The final procedural iteration used to implant the InnFocus MicroShunt®.
DISCUSSION AND CONCLUSIONS
The development of the InnFocus MicroShunt® was an educated iterative process that occurred over the course of ten years. The process required sophisticated chemistry, engineering, and medicine including controlling the foreign body reaction with SIBS, designing the shunt to be atraumatic with a lumen size that minimized hypotony, developing a design and implantation procedure that protected the conjunctiva from being eroded by the device and using the correct placement of MMC. Fins on the shunt are held firmly in the shallow pocket formed in the sclera and act as a cork to divert aqueous humor into the lumen of the device; in addition, the hydrodynamic design of the device minimized hypotony. Draining to a bleb, as does the gold standard trabeculectomy, is important as the shunt bypasses the high resistances that can be anywhere in the drainage path for aqueous humor, for example, the trabecular meshwork, Schlemm's Canal, the collector channels, the suprachoroidal space, the aqueous veins, and the episcleral veins; that is, if flow resistance through the microcysts is less than into the episcleral veins.
The feasibility studies presented above with the final design of the InnFocus MicroShunt show a qualified success rate of 100% with a drop in intraocular pressure of ∼50% at one year. In addition, over 80% of the patients who received this device are off of glaucoma medication. The control of IOP to a level below 14 mm in over 80% of patients suggests that glaucomatous progression of vision loss will be unlikely.33 The results of this study are comparable to recent results published for trabeculectomy in the TVT study40, 41 as well as trabeculectomy alone and in combination with the ExPress Shunt (Alcon, Ft. Worth TX) as reported by Netland et al.45
The advantages of the MicroShunt procedure include: (1) no need for gonioscopy; (2) the ability to place the device in any quadrant of the eye; (3) the ability to cauterize bleeding blood vessels, which limits subconjunctival/subTenon fibrosis; (4) no dissection of the sclera; (5) no sclerectomy or iridectomy; (6) ease of procedure without the need for special equipment; and (7) minimal need for post‐operative interventions (such as suture lysis). In addition, the soft, conforming, non‐inflammatory nature of the SIBS material and use of a 3‐mm long translimbal needle track require no patch graft to prevent conjunctival erosion as required for the large drainage devices.39
This article follows and explains the development of the InnFocus MicroShunt. The intended use of the device is to provide a simple alternative to primary trabeculectomy. Once safety and effectiveness of the MicroShunt are well established, it is expected that this device will be used in the treatment of earlier stage patients as an alternative to long‐term glaucoma medication where the drugs, or rather the preservatives in the drugs,30 can wreak havoc on the cornea and the conjunctiva severely limiting effectiveness in the future.
The InnFocus MicroShunt received a CE Mark on January 9, 2012 in Europe. Several clinical studies are currently under way in Europe, the Dominican Republic, Canada, and Japan to increase the number of patients as well as to investigate the treatment efficacy at different stages of the disease and for other forms of glaucoma. In addition, a U.S. Investigational Device Exception (IDE) was granted by the FDA in May 2013 and a multicenter clinical trial is under way comparing the MicroShunt to primary trabeculectomy in patients who are refractory to medication.
ACKNOWLEDGMENTS
The authors wish to acknowledge Dr. R. Alburquerque and Dr. A. Corona Peralta who assisted Dr. Batlle in the Dominican Republic, and Ms. Maria Consuelo Varela in the Dominican Republic and Ms. Sophie Albrespy in Bordeaux, France, who were the clinical coordinators in these studies. The authors dedicate this publication to the late Dr. Francisco Fantes, co‐inventor of the MicroShunt and the inspiration behind its development. Sadly, Dr. Fantes passed away in the third year of this study. The Bascom Palmer Eye Institute's team is supported in part by the Florida Lions Eye Bank, an unrestricted grant from Research to Prevent Blindness, NIH Center Grant and the Henri and Flore Lesieur Foundation (JMP). Authors LP, YPK, JBM, YK, and BAW are employees of InnFocus, Inc., the company that is developing the InnFocus MicroShunt® and own equity in the company. Co‐author JMP is a co‐inventor of the InnFocus MicroShunt® and will earn a royalty on the product. Co‐author RKP is on the Medical Advisory Board of InnFocus, Inc., and as such has stock options in InnFocus. Co‐authors JFB, IR, EA, and PP who conducted the actual implants (human and animal) and collated the clinical results have no financial interest related to the contents of this manuscript.
How to cite this article: Pinchuk L, Riss I, Batlle JF, Kato YP, Martin JB, Arrieta E, Palmberg P, Parrish RK, Weber BA, Kwon Y, Parel J‐M. 2017. The development of a micro‐shunt made from poly(styrene‐block‐isobutylene‐block‐styrene) to treat glaucoma. J Biomed Mater Res Part B 2017:105B:211–221.
REFERENCES
- 1. Pinchuk L. A review of the biostability and carcinogenicity of polyurethanes in medicine and the new generation of ‘biostable’ polyurethanes. J Biomater Sci Polym Edn 1994;6:225–267. [DOI] [PubMed] [Google Scholar]
- 2. Lamba NM, Woodhouse KA, Cooper SL. Polyurethanes in Biomedical Applications. CRC press; 1997. [Google Scholar]
- 3. Vondráček P, Doležel B, Biostability of medical elastomers: A review. Biomaterials 1984;5:209–214. [DOI] [PubMed] [Google Scholar]
- 4. Wolf CJ, Jerina KL, Brandon HJ, Young VL. The transport of octamethylcyclotetrasiloxane (D4) and polydimethylsiloxane (PDMS) in lightly cross‐linked silicone rubber. J Biomater Sci Polym Edn 2001;12:801–815. [DOI] [PubMed] [Google Scholar]
- 5. Portney GL. Silicone elastomer implantation cyclodialysis. A negative report. Arch Ophthalmol 1973;89:10–12. [DOI] [PubMed] [Google Scholar]
- 6. Pinchuk L. Crack‐resistant polycarbonate urethane polymer prostheses. US patent 5,133,742; 1992.
- 7. Wilson GJ, MacGregor DC, Klement P, Weber BA, Binnington AG, Pinchuk L. A compliant Corethane/Dacron composite vascular prosthesis: Comparison with 4‐mm EPTFE grafts in a canine model. Am Soc Artif Int Organs 1993;39:M526–M531. [PubMed] [Google Scholar]
- 8. Zhou Q, Cass‐Bejar I, Urbanski P, Stokes K. Glass wool‐H¬2O2/CoCl2 test system for in vitro evaluation of biodegradable stress cracking in polyurethane elastomers. J Biomed Mater Res 1995;29:467–475. [DOI] [PubMed] [Google Scholar]
- 9. Wu Y, Sellitti C, Anderson JM, Hiltner A, Lodoen GA, Payet CR. An FTIR‐ATR investigation of in vivo poly(ether urethane) degradation. J Appl Polym Sci 1992;46:201–211. [Google Scholar]
- 10. Anderson JM, Hiltner A, Zhao QA, Wu Y, Renier M, Schubert M, et al. Cell/polymer interactions in the biodegradation of polyurethanes In: Vert M, Feijen J, Albertson A, Scott G, Chiellini E, editors. Biodegradable Polymers and Plastics. Cambridge, England: Royal Society of Chemistry; 1992. pp 122–136. [Google Scholar]
- 11. Jasty M, Goetz DD, Bragdon CR, Lee KR, Hanson AE, Elder JR, et al. Wear of polyethylene acetabular components in total hip arthroplasty. An analysis of one hundred and twenty‐eight components retrieved at autopsy or revision operations. J Bone Joint Surg 1997;79:349–358. [PubMed] [Google Scholar]
- 12. Kurtz SM. The UHMWPE Handbook: Ultra‐High Molecular Weight Polyethylene in Total Joint Replacement. New York: Academic Press; 2004. [Google Scholar]
- 13. Wright TM, Goodman SB. Implant Wear. Rosemont, IL: American Academy of Orthopedic Surgeons; 2001. [Google Scholar]
- 14. Kurtz SM, Hozack WJ, Purtill JJ, Marcolongo M, Kraay MJ, Goldberg VM, Sharkey PF, Parvizi J, Rimnac CM, Edidin AA. 2006 Otto Aufranc Award article: Significance of in vivo degradation for polyethylene in total hip arthroplasty. Clin Orthop Relat Res 2006;453:47–57. [DOI] [PubMed] [Google Scholar]
- 15. Deacon J, Apple DJ. Further studies of IOL materials. In: Proceedings of the 11th annual meeting of the society for biomaterials. San Diego, CA: University of Alabama, Birmingham; 1985. p 205.
- 16. Goldberg EP, Yalon M, Stacholy J, Kurobe N, Longo WE, Osborn DC, et al. Biocompatibility of intraocular lens polymers. In: Proceedings of the 11th annual meeting of the society for biomaterials. San Diego, CA: University of Alabama, Birmingham; 1985. p 208.
- 17. Apple DJ, Mamalis N, Brady SE, Loftfield K, Kavka‐Van Norman D, Olson R J. Biocompatibility of implant materials: A review and scanning electron microscopy study. J Am Intraocul Implant Soc 1984;10:53–66. [DOI] [PubMed] [Google Scholar]
- 18. Kennedy JP. From thermoplastic elastomers to designed biomaterials. J Polym Sci [A1] 2005;43:2863–2951. [Google Scholar]
- 19. Pinchuk L. Biostable elastomeric polymers having quaternary carbons. US Patent no 5,741331; 1998.
- 20. Pinchuk L. Method of implanting biostable elastomeric polymers having quaternary carbons. US Patent no 6,102,939; 2000.
- 21. Pinchuk L. Method of device having biostable elastomeric polymers having quaternary carbons. US Patent no 6,197,240; 2001.
- 22. Wang B, Mishra MK, Kennedy JP. Living carbocationic polymerization XII. Telechelic polyisobutylenes by a sterically hindered bifunctional initiator . Polym Bull 1987;17:205–211. [Google Scholar]
- 23. Mishra MK, Kennedy JP. Living carbocationic polymerization VIII. Telechelic polyisobutylenes by the MeO(CH2)2C‐p‐C5H4‐C(CH3)2 Ome/BCl3 initiating system. Polym Bull 1987;17:7–13. [Google Scholar]
- 24. Liebhafsky HA. Silicones Under the Monogram: A Story of Industrial Research. New York: Wiley; 1978. pp 281–282. [Google Scholar]
- 25. Pinchuk L, Wilson GJ, Barry JJ, Schoephoerster RT, Parel J‐M and Kennedy JP. Medical applications of poly(styrene‐block‐isobutylene‐block‐styrene) (“SIBS”). Biomaterials 2008;29:448–460. [DOI] [PubMed] [Google Scholar]
- 26. Silber S, Colombo A, Banning AP, et al. Final 5‐year results of the TAXUS II Trial: A randomized study to assess the effectiveness of slow‐ and moderate‐release polymer‐based paclitaxel‐eluting stents for de novo coronary artery lesions.” Circulation 2009;120:1498–1504. [DOI] [PubMed] [Google Scholar]
- 27. Strickler F, Richard R, McFadden S. In vivo and in vitro characterization of poly(styrene‐b‐isobutylene‐b‐styrene) copolymer stent coatings for biostability, vascular compatibility and mechanical integrity. J Biomed Mater Res A 2010;92:773–782. [DOI] [PubMed] [Google Scholar]
- 28. Parel JM, Stoiber J, Fernandez V, et al., Optical properties and biocompatibility of a novel polymer for intraocular implants: Comparative study in the rabbit. (abstract) Ophthalmic Technologies XIV #5314‐45, San Jose CA, Jan 24–25, 2004.
- 29. Acosta AC, Espana EM, Yamamoto H. A newly designed glaucoma drainage implant made of poly(styrene‐b‐isobutylene‐b‐styrene): Biocompatibility and function in normal rabbit eyes. Arch Ophthalmol 2006;124:1742–1749. [DOI] [PubMed] [Google Scholar]
- 30. Huang C, Wang H, Pan J, Zhou D, Chen W, Li W, Chen Y, Liu Z. Benzalkonium chloride induces subconjunctival fibrosis through the COX‐2–modulated activation of a TGFb1/Smad3 signaling pathway. Invest Ophthalmol Vis Sci 2014;55:8111–8122. [DOI] [PubMed] [Google Scholar]
- 31. Minckler DS, Baerveldt G, Alfaro MR, Francis BA. Clinical results with the trabectome for treatment of open‐angle glaucoma. Ophthalmology 2005;112:962–967 [DOI] [PubMed] [Google Scholar]
- 32. Imi K, Pederson JE, Toris CB, Hydrostatic pressure in the suprachoroidal space. Invest Ophthalmol Vis Sci 1989;30:233–238. [PubMed] [Google Scholar]
- 33. The AGIS Investigators . The advanced glaucoma intervention study (AGIS). VII. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000;130:429–440. [DOI] [PubMed] [Google Scholar]
- 34. Krohn J, Bertelsen T. Light microscopy of uveoscleral drainage routes after gelatine injections into the suprachoroidal space. Acta Ophthalmol Scand 1998;76:521–527. [DOI] [PubMed] [Google Scholar]
- 35. Morita K, Gao Y, Saito Y, Higashide T, Kobayashi A, Ohkubo S, Sugiyama K. In vivo confocal microscopy and ultrasound biomicroscopy study of filtering blebs after trabeculectomy: Limbus‐based versus fornix‐based conjunctival flaps. J Glaucoma 2012;21:383–391. [DOI] [PubMed] [Google Scholar]
- 36. Daugherty RL, Franzini JB. Fluid Mechanics with Engineering Applications, 7th ed. NY: McGraw‐Hill Book Company; 1965. [Google Scholar]
- 37. Arrieta EA, Aly M, Parrish R, Dubovy S. Clinicopathologic correlations of poly‐(styrene‐b‐isobutylene‐b‐styrene) glaucoma drainage devices of different internal diameters in rabbits. Ophthalmic Surg Lasers Imaging 2011;42:338–345. [DOI] [PubMed] [Google Scholar]
- 38. Fantes F, Acosta AC, Carraway J, Siddiq F, Pinchuk L, Weber BA. An independent GLP evaluation of a new glaucoma drain, the MIDI. Invest Ophthalmol Vis Sci 2006;47:E‐Abstract 3547. [Google Scholar]
- 39. Abdelaziz A, Capó H, Banitt M, Schiffman J, Feuer WJ, McKeown CA, Spencer NE, Parrish RK. Diplopia after glaucoma drainage device implantation. J AAPOS 2013;17:192–196. [DOI] [PubMed] [Google Scholar]
- 40. Pinchuk L, Parel J‐M, Fantes F. Glaucoma drainage device. US patent no. 7,431,709; 2008.
- 41. Gedde SJ, Heuer DK, Parrish RK II. Review of results from the tube versus trabeculectomy study. Curr Opin Ophthalmol 2010;21:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL; Tube versus trabeculectomy study group . Three‐year follow‐up of the tube versus trabeculectomy study. Am J Ophthalmol 2009;148:670–684. [DOI] [PubMed] [Google Scholar]
- 43. Khaw PT, Doyle J, Sherwood MB, Grierson I, Schultz G, McGorray S. Prolonged localized tissue effects from 5‐minute exposures to fluorouracil and Mitomycin C. Arch Ophthalmol 1993;111:263–267. [DOI] [PubMed] [Google Scholar]
- 44. Wells AP, Cordeiro MF, Bunce C, Khaw PT. Cystic bleb formation and related complications in limbus‐versus fornix‐based conjunctival flaps in pediatric and young adult trabeculectomy with Mitomycin C. Ophthalmology 2003;110:2192–2197. [DOI] [PubMed] [Google Scholar]
- 45. Netland P, Sarkisian S, Moster M, Ahmed I, Condon G, Salim S, Sherwood M, Siegfried C. Randomized, prospective, comparative trial of EX‐PRESS glaucoma filtration device versus trabeculectomy (XVT study). Am J Ophthalmol 2014;157:433–440. [DOI] [PubMed] [Google Scholar]


