Abstract
1. Scope
The SCFA acetate (Ac) and propionate (Pr) are major fermentation products of dietary fibers and provide additional energy to the host. We investigated short‐ and long‐term effects of dietary Ac and Pr supplementation on diet‐induced obesity and hepatic lipid metabolism.
2. Methods and results
C3H/HeOuJ mice received high‐fat (HF) diets supplemented with 5% SCFA in different Ac:Pr ratios, a high acetate (HF‐HAc; 2.5:1 Ac:Pr) or high Pr ratio (HF‐HPr; 1:2.5 Ac:Pr) for 6 or 22 weeks. Control diets (low‐fat (LF), HF) contained no SCFA. SCFA did not affect body composition but reduced hepatic gene and protein expression of lipogenic enzymes leading to a reduced hepatic triglyceride concentration after 22 weeks in HF‐HPr mice. Analysis of long‐chain fatty acid composition (liver and plasma phospholipids) showed that supplementation of both ratios led to a lower ω6:ω3 ratio. Pr directly led to increased odd‐chain fatty acid (C15:0, C17:0) formation as confirmed in vitro using HepG2 cells. Remarkably, plasma C15:0 was correlated with the attenuation of HF diet‐induced insulin resistance.
3. Conclusion
Dependent on the Ac:Pr ratio, especially odd‐chain fatty acid formation and insulin sensitivity are differentially affected, indicating the importance of Pr.
Keywords: Acetate, Energy metabolism, High‐fat diet, Obesity, Propionate, SCFA
Abbreviations
- Ac
acetate
- ANOVA
analysis of variance
- AUC
area under the curve
- FA
fatty acids
- HF
high fat
- HOMA‐IR
homeostatic model assessment value for insulin resistance
- LCFA
long‐chain fatty acid
- LF
low‐fat
- OCFA
odd‐chain fatty acid
- oGTT
oral glucose tolerance test
- Pr
propionate
- wks
weeks
1. Introduction
Over the last decades, numerous lines of evidence have suggested that the intestinal microbiota plays an important role in obesity development 1, 2, 3. It was shown repeatedly that microbiota transplantation from lean or obese subjects induces similar phenotypes in the acceptor mice 4, 5. A recent study additionally demonstrated that the microbiota from lean donors can invade obese recipient mice and reduce their adiposity during cohousing. Remarkably, this effect was only observed when the mice were fed a low‐fat (LF), high‐fiber diet 4.
The role of dietary fibers and their bacterial fermentation products, SCFA (mainly acetate (Ac), propionate (Pr), butyrate), in obesity development is controversially discussed. Data from human studies indicate that overweight subjects show higher fecal SCFA than lean individuals, possibly due to larger amounts of colonic SCFA production 6, 7. Thus, SCFA may contribute to an increased dietary energy extraction and thereby promote adiposity. On the other hand, SCFA are reported to stimulate peptide YY and glucagon‐like peptide‐1 secretion, which increase satiety and result in a leaner phenotype 8, 9, 10, 11.
Using gnotobiotic mice associated with a simplified human microbiota, we previously compared the effects of inulin (fermentable dietary fiber) and cellulose (nonfermentable dietary fiber) on high‐fat (HF) diet‐induced obesity 12. Inulin supplementation did not affect body weight and body fat gain after 6 weeks (wks). However, inulin reduced the hepatic gene expression of enzymes involved in lipogenesis and fatty acid elongation/desaturation and altered the phospholipid profile. Furthermore, it changed the intestinal microbiota, increased total SCFA production, and reduced the Ac:Pr ratio. Based on these data, we hypothesize that the Ac:Pr ratio is important for the changes in lipid metabolism and that a prolonged dietary intervention would lead to differences in body weight/fat.
To elucidate the role of Ac and Pr in obesity development we used conventional C3H/HeOuJ mice, which were fed semisynthetic, obesity promoting HF diets supplemented with either a high Ac or a high Pr ratio for 6 and 22 wks. For analysis of direct effects of Ac and Pr on hepatic lipid metabolism, HepG2 (human hepatic carcinoma) cells were incubated with either Ac or Pr.
2. Materials and methods
2.1. Animals and experimental setup
All experiments were approved by the ethics committee of the Ministry for environment, health, and consumer protection of Brandenburg, Germany (approval no. V3‐2347‐49‐2011). Conventional male C3H/HeOuJ mice (8 wks of age) were obtained from Charles River Laboratories (Wilmington, USA). They were kept individually in polycarbonate cages in a climate controlled room (22 ± 2°C, relative air humidity 55 ± 5%) with a 12‐h light:dark cycle and ad libitum access to food and water.
At 10 wks of age mice were switched from standard chow diet (Altromin fortified type 1314, Altromin GmbH, Lage, Germany) to semisynthetic diets (Table 1). Gross energy content of the diets was determined by bomb calorimetry as described 12. Experimental diets were mixed and pelleted in house. For 6 wks (experiment 1) or 22 wks (experiment 2), ten animals per group were fed a HF diet supplemented with 5% SCFA, either with a high Ac (Ac:Pr, 2.5:1, HF‐HAc) or a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr). A HF diet without SCFA served as control. In experiment 2, a LF diet was included as an additional control. Body weight and body composition were determined weekly. Fasting blood glucose and insulin for homeostatic model assessment value for insulin resistance (HOMA‐IR) were measured in wk 6 and an oral glucose tolerance test (oGTT) was conducted in wk 20 (both in experiment 2). At the end of dietary intervention, animals were euthanized with isoflurane between 8 and 11 am and peripheral blood was obtained by cardiac puncture. Tissues were removed, weighed, and immediately frozen in liquid nitrogen before storage at −80°C.
Table 1.
Composition of semisynthetic experimental diets
| Component | LF (g/kg) | HF (g/kg) | HF‐HAc (g/kg) | HF‐HPr (g/kg) |
|---|---|---|---|---|
| Caseina | 220 | 267 | 267 | 267 |
| Wheat starchb | 386.5 | 129.0 | 102.6 | 101.8 |
| Maltodextrinc | 100 | 100 | 100 | 100 |
| Dextrosec | 50 | 50 | 50 | 50 |
| Sucrosed | 100 | 100 | 100 | 100 |
| Coconut oile | 7.7 | 37.8 | 37.8 | 37.8 |
| Sunflower oild | 30.1 | 147 | 147 | 147 |
| Linseed oilf | 5.2 | 25.2 | 25.2 | 25.2 |
| Celluloseg | 50 | 50 | 50 | 50 |
| Acetateh | / | / | 51.4 | 20.6 |
| Propionatei | / | / | 18.5 | 46.3 |
| NaClh | / | 24.06 | / | 2.6 |
| CaCO3 h | / | 19.48 | / | 1.2 |
| Mineral mixc | 35 | 35 | 35 | 35 |
| Vitamin mixc | 10 | 10 | 10 | 10 |
| Choline bitartratei | 2.5 | 2.5 | 2.5 | 2.5 |
| l‐cysteinei | 3 | 3 | 3 | 3 |
| Total energy content (kJ/g)j | 17.5 | 20.5 | 20.7 | 20.9 |
LF diet and HF diets supplemented with 5% SCFA, either with a high Ac ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFA (HF).
Dauermilchwerk Peiting GmbH, Landshut, Germany.
Kröner Stärke, Ibbenbüren/Westfalen, Germany.
Altromin Spezialfutter GmbH & Co. KG, Lage, Germany (detailed composition see 12).
REWE Markt GmbH, Köln, Germany.
Ostthüringer Nahrungsmittelwerk Gera, Gera, Germany.
Kunella Feinkost, Cottbus, Germany.
Rettenmaier und Soehne GmbH, Rosenberg, Germany.
Carl Roth GmbH + Co. KG, Karlsruhe, Germany (Acetate mixture: sodium acetate and calcium acetate monohydrate, 1:1).
Sigma Aldrich, Steinheim, Germany (Pr mixture: sodium Pr and calcium Pr, 1:1).
Measured by bomb calorimetry.
2.2. Body composition
Body fat mass was measured with a nuclear magnetic resonance spectrometer EchoMRI™‐Analyzer (Echo Medical Systems, Houston, USA) as described previously 12.
2.3. Indirect calorimetry
Energy expenditure was determined by indirect calorimetry in individual mice using PhenoMaster System (TSE Systems GmbH, Homburg, Germany) as described before 13.
2.4. oGTT and HOMA‐IR
For oGTT and HOMA‐IR measurements, mice were fasted for 6 h. Blood samples were taken from tail vein. Glucose was measured using glucose test strips (Contour NEXT Sensoren, Bayer AG, Leverkusen, Germany) and plasma insulin concentrations were determined with Insulin Mouse Ultrasensitive ELISA (Alpco Diagnostics, Salem, USA). In wk 6, HOMA‐IR was calculated according to the following formula: fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5. oGTT was performed in wk 20 by oral glucose application (2 mg glucose/g body weight). Blood glucose was measured before and 15, 30, 60, 120, and 180 min after glucose challenge.
2.5. Triglyceride analysis
Triglyceride concentration was analyzed in liver (40 mg of tissue) and peripheral plasma (1:2 diluted in water) using Triglyceride Determination Kit (Sigma Aldrich, Steinheim, Germany) as previously described 12.
2.6. Quantitative PCR
One microgram RNA was used for cDNA preparation with random hexamer primers using RevertAid™ H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, USA). Quantitative PCR was performed with the Applied Biosystems 7900 HT Fast Real‐Time PCR System (Applied Biosystems, Foster City, USA) using TaqMan or SYBR® Green Universal PCR Master Mix (Applied Biosystems), gene‐specific primers (3 μM each), and 5 ng cDNA. Primer and probe sequences for murine samples were used as previously described 12; the primers for the human HepG2 samples are listed in Supporting Information Table 1. Gene expression in the animal samples was calculated according to the ΔΔCT method using hypoxanthine guanine phosphoribosyl transferase as reference gene and expressed relative to the HF group (without SCFA) normalized to a value of 1. In the HepG2 samples, the ribosomal protein L13a (Rpl13a) was used as reference gene and gene expression was calculated relative to control medium without SCFA.
2.7. Western blot analysis
Liver protein was extracted after RNA isolation from the phenol phase according to the manufacturers’ protocol (PEQLAB Biotechnologie GmbH, Erlangen, Germany). Immunoblotting was performed as described previously using 20 μg of protein 14. Signals were detected with the FUSION‐SL Advance 4.2 MP imaging system (PEQLAB Biotechnologie GmbH) and quantified using the BIO‐1D Advanced Analysis Software (PEQLAB Biotechnologie GmbH). Antibodies were used as previously described 12. Protein expression was normalized to α‐tubulin (Sigma‐Aldrich).
2.8. Cell culture experiments
Human hepatic carcinoma (HepG2) cells were cultured at 37°C (5% CO2) in RPMI 1640 growth medium (Gibco, CA, USA) supplemented with 10% v/v fetal calf serum (Biochrom GmbH, Germany), 100 U penicillin, streptomycin (100 μg/mL), and 2 mM l‐alanyl‐l‐glutamine (PSG; Gibco). HepG2 cells were seeded at 500 000 cells/well into standard six‐well plates. After 3 days, growth media was replaced by RPMI 1640 media supplemented with PSG (without fetal calf serum) and Ac or Pr. The total amount of SCFA for each condition was 500 μM; RPMI 1640 + PSG served as control; cells were incubated for 24 h (RNA isolation) or 48 h (long‐chain fatty acid (LCFA) analysis). After a washing step with PBS, cells were harvested by scraping. Cells were centrifuged (250 × g, 5 min) and the resulting cell pellet was resuspended in 1 mL H2O for LCFA analysis. For RNA isolation, the cells were directly lysed after the washing step by adding 1 mL peqGOLD TriFast (PEQLAB Biotechnologie GmbH) and the following steps were done according the manufacturers’ instructions.
2.9. LCFA analysis
LCFA composition was determined in the phospholipid fraction by GC as described 12.
2.10. Statistical analysis
Data are presented as mean ± SEM. Statistics were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, USA). Normal distribution and homogeneity of variances were tested using the Kolmogorov–Smirnov test. Normally distributed data were analyzed by one‐way analysis of variance (ANOVA) with Bonferroni's posttest; non‐normally distributed data were analyzed using nonparametric Kruskal–Wallis test. Differences with p < 0.05 were considered as statistically significant.
3. Results
3.1. SCFA change hepatic lipid metabolism without affecting body weight
Phenotypic characterization during dietary intervention did not reveal any differences in body weight gain, body fat/lean mass after 6 wks (Fig. 1A, Table 2) or 22 wks (Fig. 1B, Table 3). Also feed intake and energy expenditure were not affected (Tables 2 and 3). While circulating plasma triglyceride levels showed no differences, long‐term (22 wks) supplementation of SCFA decreased liver triglyceride content (Fig. 1D). This was only significant in HF‐HPr animals, leading to a reduction of about 40% (HF versus HF‐HPr). Although at wk 6 there were no differences in liver triglycerides yet (Fig. 1C), hepatic gene and protein expression was already affected. SCFA supplementation resulted in a diminished expression of lipogenic genes (Acly, Acacb, Fasn (where Acly is ATP citrate lyase; Acacb is acetyl‐CoA carboxylase beta; Fasn is fatty acid synthase)). These enzymes are mainly regulated by transcription factors, such as Srebf1 (where Srebf1 is sterol regulatory element binding transcription factor 1), which was reduced in tendency (Fig. 2A). Besides that, fatty acid desaturation and elongation enzymes were downregulated (Scd1, where Scd1 is stearoyl‐coenzyme A desaturase 1) or at least in tendency reduced (Elovl5, Elovl6 (where Elovl5 and 6 are elongation of long‐chain fatty acid family members 5 and 6)). This repression was confirmed at protein level by Western blot (Fig. 2B and C). Protein expressions of ACLY, ACAC, and SCD1 were much lower in SCFA‐supplemented mice compared to control (HF). The same trend was obvious in FASN protein expression. Long‐term (22 wks) effects of Ac and Pr on gene expression were similar to these short‐term effects (Supporting Information Table 2). Taken together, these results demonstrate that both, high Ac and high Pr supplementation, reduce hepatic gene and protein expression of lipogenic enzymes while only the high Pr ratio significantly diminished liver triglyceride content by about 40%.
Figure 1.
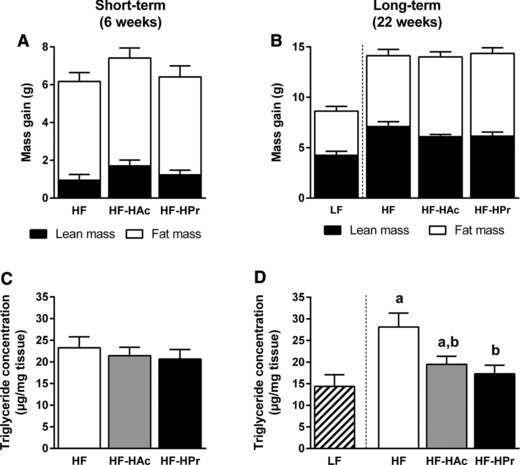
Effects of dietary SCFA on body composition change (A, B) and hepatic triglyceride concentration (C, D) after 6 wks (A, C) and 22 wks (B, D) of intervention. C3H mice were fed a LF diet or HF diet supplemented with 5% SCFA, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFA (HF). Data are mean + SEM, n = 7–10. Means with different letters are significantly different (ANOVA with Bonferroni post hoc test was performed between HF groups, p < 0.05).
Table 2.
No changes in body and plasma parameters of C3H mice after 6 wks of dietary SCFA supplementation
| Short‐term study (6 wks) | ||||
|---|---|---|---|---|
| HF | HF‐HAc | HF‐HPr | p‐Value | |
| Body weight start (g) | 27.4 ± 0.5 | 27.3 ± 0.4 | 27.4 ± 0.6 | ns |
| Body weight final (g) | 33.5 ± 0.8 | 34.6 ± 0.8 | 33.8 ± 0.8 | ns |
| Final fat mass (g) | 6.4 ± 0.6 | 7.5 ± 0.7 | 6.5 ± 0.7 | ns |
| Final lean mass (g) | 27.1 ± 0.6 | 27.2 ± 0.2 | 27.3 ± 0.3 | ns |
| Feed intake (g/d) | 3.4 ± 0.3 | 3.7 ± 0.1 | 3.5 ± 0.2 | ns |
| Energy expenditure (kJ/d) | 50.5 ± 1.8 | 52.2 ± 1.6 | 54.3 ± 1.6 | ns |
| Liver weight (g) | 1.53 ± 0.06 | 1.56 ± 0.05 | 1.57 ± 0.03 | ns |
| Plasma triglycerides (mmol/L) | 1.49 ± 0.20 | 1.90 ± 0.40 | 1.86 ± 0.27 | ns |
Mice were fed experimental HF diets supplemented with 5% SCFA, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFA (HF) for 6 wks. Data are mean ± SEM, n = 5–10. ns, not significant. ANOVA with Bonferroni post hoc test was performed, p < 0.05.
Table 3.
Dietary SCFA do not affect body and plasma parameters of C3H mice after 22 wks of intervention
| Long‐term study (22 wks) | |||||
|---|---|---|---|---|---|
| LF | HF | HF‐HAc | HF‐HPr | p‐Value | |
| Body weight start (g) | 28.7 ± 0.3 | 28.6 ± 0.4 | 28.7 ± 0.5 | 28.6 ± 0.5 | ns |
| Body weight final (g) | 37.0 ± 0.9 | 42.2 ± 1.2 | 42.4 ± 0.5 | 42.4 ± 0.9 | ns |
| Final fat mass (g) | 7.0 ± 0.5 | 9.5 ± 0.7 | 10.6 ± 0.4 | 10.2 ± 0.6 | ns |
| Final lean mass (g) | 29.7 ± 0.4 | 32.5 ± 0.6 | 31.4 ± 0.3 | 31.8 ± 0.6 | ns |
| Feed intake (g/d) | 2.8 ± 0.1 | 3.0 ± 0.1 | 3.2 ± 0.2 | 3.0 ± 0.1 | ns |
| Energy expenditure (kJ/d) | 45.2 ± 2.0 | 49.3 ± 2.2 | 47.4 ± 2.0 | 50.3 ± 2.6 | ns |
| Liver weight (g) | 1.77 ± 0.06 | 1.97 ± 0.07 | 2.00 ± 0.08 | 1.95 ± 0.06 | ns |
| Plasma triglycerides (mmol/L) | 0.96 ± 0.21 | 1.49 ± 0.24 | 1.58 ± 0.17 | 1.43 ± 0.18 | ns |
Mice were fed experimental LF diet or HF diets supplemented with 5% SCFA, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFA (HF) for 22 wks. Data are mean ± SEM, n = 8–10. ns, not significant. ANOVA with Bonferroni post hoc test was performed between HF groups, p < 0.05.
Figure 2.
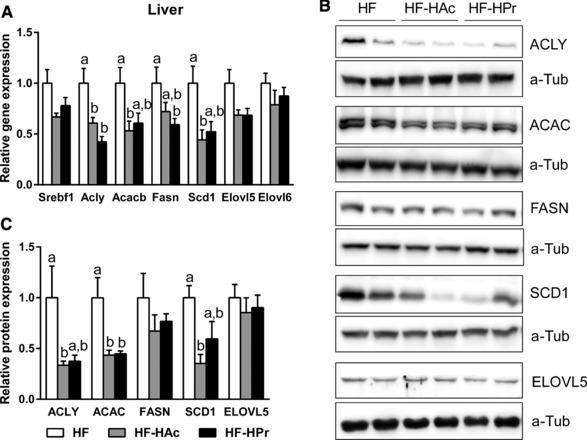
Hepatic mRNA expression of enzymes involved in lipid metabolism (A), representative bands (B), and corresponding hepatic protein expression (C) after 6 wks of intervention. C3H mice were fed a HF diet supplemented with 5% SCFA, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFA (HF). HF group was set to 1 and protein results are relative to α‐tubulin. Data are mean + SEM, n = 7–8. Means with different letters are significantly different (ANOVA with Bonferroni post hoc test, p < 0.05).
3.2. SCFA alter the formation of LCFAs
To investigate if the reduced expression of lipogenesis genes resulted in LCFA composition changes, GC was used to determine LCFAs in liver and plasma phospholipids (Fig. 3). SCFA supplementation for 6 wks resulted in a higher formation of omega‐3 fatty acids (ω3 FAs), especially in liver phospholipids, whereas the amount of ω6 FAs tended to decrease (compared to HF). Thus, SCFA‐supplemented groups exhibited a lower ω6/ω3 FA ratio than the HF group in liver and plasma phospholipids. In accordance with a rising proportion of Pr in the diet, the formation of odd‐chain FAs (OCFAs; C15:0 and C17:0) in plasma and liver phospholipids increased and reached the highest amount after HPr feeding (Fig. 3). Similar changes in LCFA composition were also detectable after 22 wks (Table 4). Here again, OCFA and ω3 FA contents were increased while the proportion of ω6 FAs was rather lower. To examine if the observed effects were directly and specifically modulated by Ac or Pr, we used HepG2 cells as an in vitro model for liver lipid metabolism. While incubation with either Ac or Pr did not influence mRNA expression of lipogenic enzymes (Fig. 4A), cellular phospholipid LCFA profiles changed similar to the in vivo data (Fig. 4B and C). In detail, ω3 FA formation was in tendency increased after Pr treatment, while ω6 FA level remained unaffected. Even more remarkable, OCFA production was increased fivefold after incubation with Pr, compared to control medium. Together, these data suggest that both Ac and Pr supplementation decrease the ω6/ω3 FA ratio, while Pr directly increases hepatic OCFA production.
Figure 3.
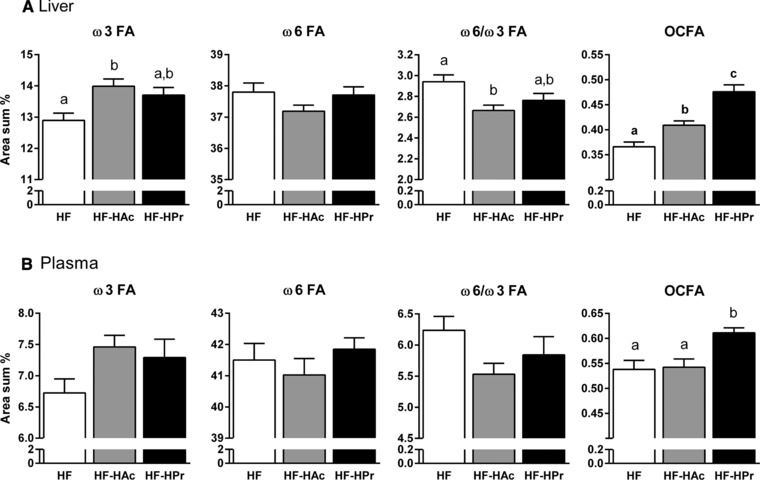
Effects of dietary SCFA on liver (A) and plasma (B) phospholipid LCFA profile. For detailed information on OCFA, ω3, and ω6 FA composition see Table 4. C3H mice were fed a HF diet with 5% SCFA, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFA (HF) for 6 wks. Data are mean + SEM, n = 9–10. Means with different letters are significantly different (ANOVA with Bonferroni post hoc test, p < 0.05).
Table 4.
Formation of OCFAs, ω3, and ω6 FA after 22 wks of dietary SCFA supplementation in liver and plasma phospholipids
| Long‐term study (22 wks) | |||||
|---|---|---|---|---|---|
| LF | HF | HF‐HAc | HF‐HPr | p‐Value | |
| Liver | |||||
| ω3 FA (area sum %) | 11.19 ± 0.30 | 12.86 ± 0.17a | 13.87 ± 0.18b | 13.36 ± 0.32a,b | <0.05 |
| ω6 FA (area sum %) | 35.38 ± 0.16 | 38.85 ± 0.16a | 38.19 ± 0.18b | 38.37 ± 0.15a,b | <0.05 |
| OCFA (area sum %) | 0.20 ± 0.01 | 0.30 ± 0.01a | 0.32 ± 0.00a | 0.40 ± 0.01b | <0.001 |
| Plasma | 0.26 ± 0.01 | 0.35 ± 0.01a | 0.38 ± 0.01a | 0.47 ± 0.01b | <0.05 |
| ω3 FA (area sum %) | 7.05 ± 0.18 | 7.37 ± 0.17a | 8.09 ± 0.25a,b | 8.09 ± 0.27b | <0.05 |
| ω6 FA (area sum %) | 36.38 ± 0.74 | 42.81 ± 0.45 | 42.23 ± 0.34 | 41.60 ± 0.48 | ns |
| OCFA (area sum %) | 0.26 ± 0.01 | 0.35 ± 0.01a | 0.38 ± 0.01a | 0.47 ± 0.01b | <0.05 |
OCFAs, odd‐chain fatty acids, sum of C15:0 and C17:0; ω3 FAs, sum of C18:3n3, C20:5n3, C22:5n3, and C22:6n3; ω6 FAs, sum of C18:2n6t, C18:2n6c, C18:3n6, C20:2n6, C20:3n6, C20:4n6, C22:4n6, and C22:5n6 fatty acids. C3H‐mice were fed experimental LF diet or HF diets supplemented with 5% SCFA, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFA (HF) for 22 wks. Data are mean ± SEM, n = 8–10. ns, not significant. Means with different letters within one row are significantly different (ANOVA with Bonferroni post hoc test was only performed between HF groups, p < 0.05).
Figure 4.
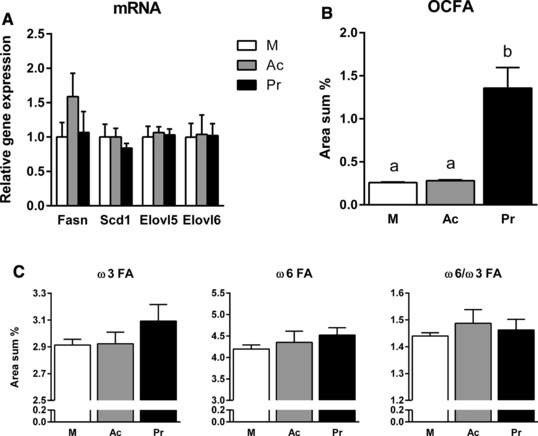
mRNA expression (A) and phospholipid fatty acid profile (B, C) of HepG2 cells after incubation with 500 μM acetate or propionate. Ac, acetate; Pr, propionate; M, control medium without SCFA. For detailed information on OCFAs, ω3, and ω6 FA composition see Table 4. Data are mean + SEM, n = 3–4. Means with different letters are significantly different (ANOVA with Bonferroni post hoc test, p < 0.05).
3.3. Pr attenuates HF diet‐induced insulin resistance
To elucidate if Ac and Pr affect HF diet‐induced insulin resistance, we determined HOMA‐IR in wk 6 and performed an oGTT in wk 20. Already after 6 wks, HPr supplementation significantly decreased HF diet‐induced insulin resistance compared to HF control while HAc had an intermediate effect (LF: 9.7 ± 1.1; HF: 24.6 ± 4.1a, HF‐HAc: 19.5 ± 3.9a,b, HF‐HPr: 13.5 ± 1.2b, p < 0.01). During oGTT in wk 20, there were no differences in fasting blood glucose and plasma insulin concentrations (t = 0) (Fig. 5A). In the HAc group, blood glucose was significantly increased after 15 min compared to HF (Fig. 5A) but the overall glucose response was not different between the groups (Fig. 5B). Although glucose tolerance was not affected by HF diet feeding, insulin sensitivity was decreased as evident from increased plasma insulin levels in HF mice compared to LF mice (Fig. 5A and B). Interestingly, plasma insulin levels were decreased in the SCFA‐supplemented groups compared to HF‐fed mice. This effect is particularly obvious in the area under the curve (AUC) of insulin (Fig. 5B), which shows that a high Pr ratio completely prevented the HF diet‐induced increase in insulin secretion. Since the OCFA pentadecanoic acid (C15:0) was shown to be inversely associated with type 2 diabetes in humans 15, we correlated C15:0 plasma levels with the AUC of insulin and found a significant negative association (Fig. 5C). In summary, these data suggest that especially Pr has beneficial effects on HF diet‐induced insulin resistance, potentially linked to its stimulating effect on OCFA production.
Figure 5.
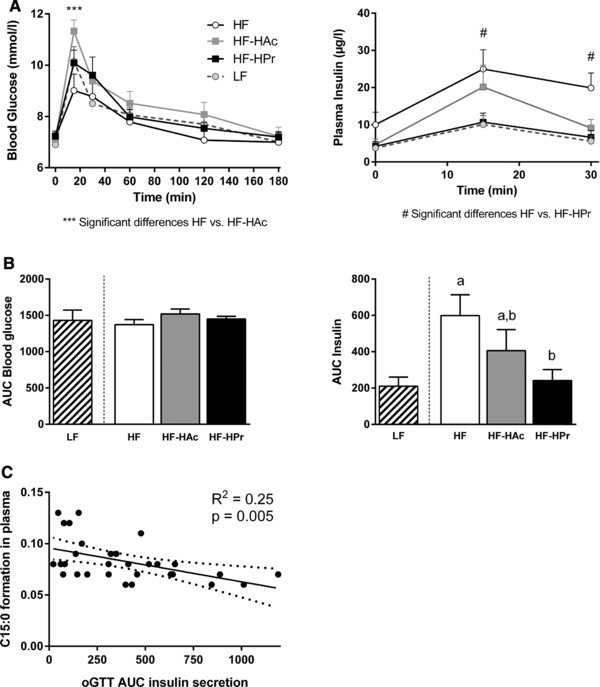
Oral glucose tolerance test in 6 h fasted C3H mice after 20 wks of intervention. Mice were fed a LF or HF diet with 5% SCFA, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFA (HF) for 20 wks. Blood glucose and plasma insulin concentrations before (t = 0 min) and after oral glucose application (t = 15, 30, 60, 120, 180 min) (A) and the corresponding total AUC (B). Data are mean + SEM, n = 9–10. Means with different letters are significantly different (ANOVA with Bonferroni post hoc test was performed between HF groups, p < 0.05). (C) Correlation of C15:0 in plasma phospholipids and calculated AUC of insulin secretion during oGTT.
4. Discussion
Here we discuss short‐ and long‐term metabolic effects of dietary SCFA supplementation in a HF diet‐induced obesity model. Our data indicate that different dietary Ac:Pr ratios (2.5:1 versus 1:2.5, Ac:Pr) influence hepatic lipid metabolism similarly, by reducing lipogenesis, which in the long term leads to lower hepatic triglyceride levels without affecting overall obesity. However, changes in liver and plasma LCFA composition are indirectly affected and not equally influenced by Ac and Pr. Furthermore, beneficial effects of dietary SCFA on insulin sensitivity are more pronounced with an increased dietary Pr concentration. Overall these results are in line with our data obtained after dietary supplementation of inulin 12. Hence, dietary SCFA supplementation and proximal intestinal absorption is comparable with effects achieved after microbial SCFA production accompanied by colonic absorption.
Dietary trials investigating the effects of SCFA on obesity development are rather controversial. Lin et al. 11 demonstrated that dietary administration of Pr or butyrate decreases feed intake via the induction of gut hormones and suppresses body weight gain in mice during 4 wks of intervention. By contrast, other studies could not confirm these differences in body weight after SCFA supplementation 16, 17. Based on our previous results 12 and additional literature data, showing that Pr can inhibit Ac‐dependent cholesterol and lipid synthesis in rats 18, 19 and humans 20, we suggest that Ac and Pr have antagonistic effects on the development of a HF diet‐induced obesity. More precisely, we hypothesize that a higher lipid synthesis in the high Ac group would lead to a higher body weight/fat gain and elevated liver triglyceride levels. However, neither after short‐term nor long‐term intervention we observed differences in body weight and body fat gain between the groups. Additionally, also feed intake and energy expenditure were not affected. Thus, our results rather support the hypothesis that SCFA do not influence the body weight gain during HF diet feeding. We speculate that the discrepancies in the studies are due to different model systems, showing that the effect of SCFA on obesity development is not consistent. Nevertheless, we have several indications that SCFA supplementation affects hepatic lipid metabolism. In both intervention groups (regardless of the Ac:Pr ratio) the expression of important lipogenic enzymes was reduced. Hence, our data indicate an attenuation of the lipogenic pathway in SCFA‐supplemented mice, which later on (after 22 wks) leads to lower liver triglyceride levels. Beneficial effects of Pr on hepatic triglyceride levels have already been shown in rodents 21 and human patients suffering from nonalcoholic fatty liver disease 22. Our effect on hepatic triglycerides was more prominent in HF‐HPr mice, indicating that not the Ac:Pr ratio but already the presence of Pr might be important in determining the physiological effects of SCFA on hepatic lipid metabolism. Of course this hypothesis should be confirmed by additional experiments, for instance by dietary supplementation of Ac alone.
Because ω3‐ and ω6 FA are not synthesized de novo, they belong to the group of essential LCFAs. High circulating ω6 FA levels are associated with a higher risk for cardiovascular diseases 23 while overweight adults supplemented with ω3 FAs displayed reduced inflammation 24. Furthermore, ω3 FAs are associated with several other health benefits in the onset of autoimmune and cardiovascular diseases 25, 26, 27. The most abundant ω3 FA in plasma and liver phospholipids is docosahexaenoic acid (Supporting Information Fig. 1 or 2). In this present study, the dietary supplementation of SCFA for 6 and 22 wks resulted in a higher formation of ω3 FAs, particularly of docosahexaenoic acid, while the formation of ω6 FAs was rather low, but not significantly different. On the basis of these data, dietary SCFA supplementation led to a lower ω6/ω3 FA ratio, compared to HF feeding in liver phospholipids of conventional C3H mice. This is desirable in order to reduce the risk of chronic diseases 28. To investigate the reason for the observed differences in the ω6/ω3 FA ratio in more detail, we measured the hepatic mRNA expression of important desaturases and elongases involved in ω3 and ω6 FA biosynthesis. However, neither gene levels of Fads1 and Fads2 (where Fads1 and 2 are fatty acid desaturase 1 and 2) (data not shown), nor Elovl5 and Elovl6 were different among the groups. As indicated by Tu et al. 29, our data also support their evidence that the endogenous synthesis of LCFAs is controlled independently from the expression of biosynthetic enzymes and is more likely regulated by other factors, e.g. substrate competition.
Pr has often been described as a product derived from the catabolism of OCFAs 30, 31. However, some studies have shown that it is more likely the other way around in bacteria. Microbial OCFAs are mainly formed through elongation of Pr 32, 33. With the present mouse experiments we support the latter. Our results clearly show an increased formation of OCFAs in vivo, proportionally rising with a higher Pr ratio in the diet. This is obvious in plasma and liver phospholipids after 6 and 22 wks of intervention. Besides that, treatment of human HepG2 cells with Pr resulted in a fivefold higher formation of OCFAs, suggesting a hepatic production of OCFAs from Pr. Interestingly, OCFAs, especially C15:0, is inversely associated with type 2 diabetes 34, 35, which might be an additional link between Pr and glucose homeostasis.
Besides the reported effects on lipid metabolism, SCFA are often described to play an important role in glucose metabolism. Several studies have proven the Pr‐induced formation of glucose in humans 20 and animal models 36, 37, supporting the role of Pr as a gluconeogenic substrate. Furthermore, Pr is suggested to reduce postprandial and fasting blood glucose levels 22, 38, 39, 40. In the present study, we did not observe significant differences in fasting blood glucose levels after 6 and 20 wks of intervention, but overall they tended to be lower in LF‐fed animals. Importantly, during oGTT in wk 20, fasting mean insulin values (after 6 h of fasting) in the SCFA‐supplemented groups were about half of the HF group but not significantly different due to high SDs (t = 0 min). Nevertheless, insulin secretion after oral glucose load was reduced in HF‐HPr animals after 15 and 30 min. This reduction was stronger with an increasing proportion of dietary Pr, demonstrating that the suppression of HF diet‐induced insulin resistance appears to be mediated by Pr, which is in line with other animal studies 41, 42. Pr is hypothesized to increase insulin sensitivity by stimulating intestinal gluconeogenesis 41. Here, Pr acts as direct gluconeogenic substrate and the resulting glucose is detected by a portal vein glucose sensor that transmits the signal to the brain and promotes beneficial effects on food intake and glucose metabolism 43. However, Santaren et al. 15 described an inverse association of pentadecanoic acid with incident type 2 diabetes and indeed we could confirm an inverse correlation of plasma C15:0 formations with the AUC insulin.
Thus, our data indicate that the improvement of HF diet‐induced insulin resistance by Pr could also be linked to its stimulation of C15:0 production. Nevertheless, this association could be of correlative nature only, because so far there is no experimental evidence for a possible causal relationship.
In conclusion, our data confirm the regulatory role of SCFA on lipid and glucose metabolism, resulting in decreased hepatic lipid accumulation and improved fatty acid profile while they do not affect overall energy metabolism and obesity development. They thus lead to a healthier obese phenotype. Especially the effects of the high Pr ratio on the reduction of hepatic triglycerides, the increase in OCFA formation and the improvement of insulin sensitivity point to an important role of Pr in reducing obesity associated metabolic disturbances.
All authors read and approved the final manuscript and declared no conflicts of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
sup‐0001‐Figure S1
sup‐0002‐Figure S2
Table S1: Oligonucleotides used for the quantification of mRNA expression in HepG2 cells.
Table S2: Hepatic mRNA expression of lipid metabolism related enzymes after 22 wks of SCFA supplementation. C3H mice were fed a low‐fat diet or high‐fat diet supplemented with 5% SCFAs, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFAs (HF). Data are mean ± SEM, n = 8–10 ns, not significant. Means with different letters within one row are significantly different (ANOVA with Bonferroni post hoc test was performed between HF, HF‐HAc, and HF‐HPr group, p < 0.05).
Figure S1: Changes in liver and plasma phospholipid profile after 6 wks of dietary SCFA supplementation. C3H mice were fed a high‐fat diet supplemented with 5% SCFAs, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFAs (HF). Data are mean + SEM, n = 8–10. Values are expressed as area percentage of each fatty acid relative to the total area of all detected fatty acids. Means with different letters are significantly different (ANOVA with Bonferroni post hoc test, p < 0.05).
Figure S2: Effects of dietary SCFAs on liver and plasma phospholipid profile after 22 wks of intervention. C3H mice were fed a low‐fat (LF) diet or high‐fat diet supplemented with 5% SCFAs, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFAs (HF) for 22 wks. Values are expressed as area percentage of each fatty acid relative to the total area of all detected fatty acids. Data are mean + SEM, n = 8–10. Means with different letters are significantly different (ANOVA with Bonferroni post hoc test was performed between HF groups, p < 0.05).
Acknowledgments
K.W. performed experiments, analyzed data, and wrote the manuscript. S.S. performed cell culture experiments, analyzed data and provided input into the manuscript. S.K. designed and supervised the study. D.N. performed quantitative PCR in the long‐term study, K.A.K. performed cell culture experiments together with A.P.K. K.J.P. analyzed LCFA data. M.B. and S.K. interpreted data and had primary responsibility for the final content.
We gratefully thank Carolin Borchert, Antje Sylvester, Andrea Teichmann, Stefanie Deubel, and Andreas Wernitz for technical assistance and Ute Lehmann and Christin Jungnickel for taking care of the animals. This work was supported by funding to S.K. from the Deutsche Forschungsgemeinschaft, Bonn, Germany (DFG, KL613/18‐1).
5 References
- 1. Zhao, L. , The gut microbiota and obesity: from correlation to causality. Nat. Rev. Microbiol. 2013, 11, 639–647. [DOI] [PubMed] [Google Scholar]
- 2. Hartstra, A. V. , Bouter, K. E. , Backhed, F. , Nieuwdorp, M. , Insights into the role of the microbiome in obesity and type 2 diabetes Diabetes Care 2015, 38, 159–165. [DOI] [PubMed] [Google Scholar]
- 3. Ley, R. E. , Backhed, F. , Turnbaugh, P. , Lozupone, C. A. et al., Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ridaura, V. K. , Faith, J. J. , Rey, F. E. , Cheng, J. et al., Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turnbaugh, P. J. , Backhed, F. , Fulton, L. , Gordon, J. I. , Diet‐induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rahat‐Rozenbloom, S. , Fernandes, J. , Gloor, G. B. , Wolever, T. M. , Evidence for greater production of colonic short‐chain fatty acids in overweight than lean humans. Int. J. Obes. (Lond.) 2014, 38, 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwiertz, A. , Taras, D. , Schafer, K. , Beijer, S. et al., Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010, 18, 190–195. [DOI] [PubMed] [Google Scholar]
- 8. Zhou, J. , Martin, R. J. , Tulley, R. T. , Raggio, A. M. et al., Dietary resistant starch upregulates total GLP‐1 and PYY in a sustained day‐long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1160–E1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wren, A. M. , Bloom, S. R. , Gut hormones and appetite control. Gastroenterology 2007, 132, 2116–2130. [DOI] [PubMed] [Google Scholar]
- 10. Freeland, K. R. , Wilson, C. , Wolever, T. M. , Adaptation of colonic fermentation and glucagon‐like peptide‐1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br. J. Nutr. 2010, 103, 82–90. [DOI] [PubMed] [Google Scholar]
- 11. Lin, H. V. , Frassetto, A. , Kowalik, E. J. Jr. , Nawrocki, A. R. et al., Butyrate and propionate protect against diet‐induced obesity and regulate gut hormones via free fatty acid receptor 3‐independent mechanisms. PLoS One 2012, 7, e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weitkunat, K. , Schumann, S. , Petzke, K. J. , Blaut, M. et al., Effects of dietary inulin on bacterial growth, short‐chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J. Nutr. Biochem. 2015, 26, 929–937. [DOI] [PubMed] [Google Scholar]
- 13. Klaus, S. , Rudolph, B. , Dohrmann, C. , Wehr, R. , Expression of uncoupling protein 1 in skeletal muscle decreases muscle energy efficiency and affects thermoregulation and substrate oxidation. Physiol. Genomics 2005, 21, 193–200. [DOI] [PubMed] [Google Scholar]
- 14. Noatsch, A. , Petzke, K. J. , Millrose, M. K. , Klaus, S. , Body weight and energy homeostasis was not affected in C57BL/6 mice fed high whey protein or leucine‐supplemented low‐fat diets. Eur. J. Nutr. 2011, 50, 479–488. [DOI] [PubMed] [Google Scholar]
- 15. Santaren, I. D. , Watkins, S. M. , Liese, A. D. , Wagenknecht, L. E. et al., Serum pentadecanoic acid (15:0), a short‐term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am. J. Clin. Nutr. 2014, 100, 1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berthelot, V. , Bas, P. , Schmidely, P. , Duvaux‐Ponter, C. , Effect of dietary propionate on intake patterns and fatty acid composition of adipose tissues in lambs. Small Ruminant Res. 2001, 40, 29–39. [DOI] [PubMed] [Google Scholar]
- 17. Boillot, J. , Alamowitch, C. , Berger, A. M. , Luo, J. et al., Effects of dietary propionate on hepatic glucose‐production, whole‐body glucose‐utilization, carbohydrate and lipid‐metabolism in normal rats. Br. J. Nutr. 1995, 73, 241–251. [DOI] [PubMed] [Google Scholar]
- 18. Wright, R. S. , Anderson, J. W. , Bridges, S. R. , Propionate inhibits hepatocyte lipid synthesis. Proc. Soc. Exp. Biol. Med. 1990, 195, 26–29. [DOI] [PubMed] [Google Scholar]
- 19. Demigne, C. , Morand, C. , Levrat, M. A. , Besson, C. et al., Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br. J. Nutr. 1995, 74, 209–219. [DOI] [PubMed] [Google Scholar]
- 20. Wolever, T. M. , Spadafora, P. , Eshuis, H. , Interaction between colonic acetate and propionate in humans. Am. J. Clin. Nutr. 1991, 53, 681–687. [DOI] [PubMed] [Google Scholar]
- 21. Cheng, H. H. , Lai, M. H. , Fermentation of resistant rice starch produces propionate reducing serum and hepatic cholesterol in rats. J. Nutr. 2000, 130, 1991–1995. [DOI] [PubMed] [Google Scholar]
- 22. Chambers, E. S. , Viardot, A. , Psichas, A. , Morrison, D. J. et al., Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Oliveira Otto, M. C. , Wu, J. H. , Baylin, A. , Vaidya, D. et al., Circulating and dietary omega‐3 and omega‐6 polyunsaturated fatty acids and incidence of CVD in the Multi‐Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan, Y. , Jiang, W. , Spinetti, T. , Tardivel, A. et al., Omega‐3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [DOI] [PubMed] [Google Scholar]
- 25. Stone, N. J. , Fish consumption, fish oil, lipids, and coronary heart disease. Am. J. Clin. Nutr. 1997, 65, 1083–1086. [DOI] [PubMed] [Google Scholar]
- 26. Davidson, M. H. , Stein, E. A. , Bays, H. E. , Maki, K. C. et al., Efficacy and tolerability of adding prescription omega‐3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8‐week, randomized, double‐blind, placebo‐controlled study. Clin. Ther. 2007, 29, 1354–1367. [DOI] [PubMed] [Google Scholar]
- 27. Fernandes, G. , Bysani, C. , Venkatraman, J. T. , Tomar, V. et al., Increased TGF‐beta and decreased oncogene expression by omega‐3 fatty acids in the spleen delays onset of autoimmune disease in B/W mice. J. Immunol. 1994, 152, 5979–5987. [PubMed] [Google Scholar]
- 28. Simopoulos, A. P. , The importance of the ratio of omega‐6/omega‐3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [DOI] [PubMed] [Google Scholar]
- 29. Tu, W. C. , Cook‐Johnson, R. J. , James, M. J. , Muhlhausler, B. S. et al., Omega‐3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostaglandins Leukot. Essent Fatty Acids 2010, 83, 61–68. [DOI] [PubMed] [Google Scholar]
- 30. Sbai, D. , Narcy, C. , Thompson, G. N. , Mariotti, A. et al., Contribution of odd‐chain fatty acid oxidation to propionate production in disorders of propionate metabolism. Am. J. Clin. Nutr. 1994, 59, 1332–1337. [DOI] [PubMed] [Google Scholar]
- 31. Thompson, G. N. , Walter, J. H. , Bresson, J. L. , Ford, G. C. et al., Sources of propionate in inborn errors of propionate metabolism. Metabolism 1990, 39, 1133–1137. [DOI] [PubMed] [Google Scholar]
- 32. Ingram, L. O. , Chevalier, L. S. , Gabba, E. J. , Ley, K. D. et al., Propionate‐induced synthesis of odd‐chain‐length fatty acids by Escherichia coli . J. Bacteriol. 1977, 131, 1023–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Emmanuel, B. , The relative contribution of propionate, and long‐chain even‐numbered fatty acids to the production of long‐chain odd‐numbered fatty acids in rumen bacteria. Biochim. Biophys. Acta 1978, 528, 239–246. [DOI] [PubMed] [Google Scholar]
- 34. Forouhi, N. G. , Koulman, A. , Sharp, S. J. , Imamura, F. et al., Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC‐InterAct case‐cohort study. LancetDiabetes Endocrinol. 2014, 2, 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meikle, P. J. , Wong, G. , Barlow, C. K. , Weir, J. M. et al., Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS One 2013, 8, e74341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. den Besten, G. , Lange, K. , Havinga, R. , van Dijk, T. H. et al., Gut‐derived short‐chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910. [DOI] [PubMed] [Google Scholar]
- 37. Verbrugghe, A. , Hesta, M. , Daminet, S. , Polis, I. et al., Propionate absorbed from the colon acts as gluconeogenic substrate in a strict carnivore, the domestic cat (Felis catus). J. Anim. Physiol. Anim. Nutr. (Berl.) 2012, 96, 1054–1064. [DOI] [PubMed] [Google Scholar]
- 38. Todesco, T. , Rao, A. V. , Bosello, O. , Jenkins, D. J. , Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am. J. Clin. Nutr. 1991, 54, 860–865. [DOI] [PubMed] [Google Scholar]
- 39. Venter, C. S. , Vorster, H. H. , Cummings, J. H. , Effects of dietary propionate on carbohydrate and lipid metabolism in healthy volunteers. Am. J. Gastroenterol. 1990, 85, 549–553. [PubMed] [Google Scholar]
- 40. Darwiche, G. , Ostman, E. M. , Liljeberg, H. G. , Kallinen, N. et al., Measurements of the gastric emptying rate by use of ultrasonography: studies in humans using bread with added sodium propionate. Am. J. Clin. Nutr. 2001, 74, 254–258. [DOI] [PubMed] [Google Scholar]
- 41. De Vadder, F. , Kovatcheva‐Datchary, P. , Goncalves, D. , Vinera, J. et al., Microbiota‐generated metabolites promote metabolic benefits via gut‐brain neural circuits. Cell 2014, 156, 84–96. [DOI] [PubMed] [Google Scholar]
- 42. den Besten, G. , Bleeker, A. , Gerding, A. , van Eunen, K. et al., Short‐chain fatty acids protect against high‐fat diet‐induced obesity via a PPARgamma‐dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [DOI] [PubMed] [Google Scholar]
- 43. Delaere, F. , Duchampt, A. , Mounien, L. , Seyer, P. et al., The role of sodium‐coupled glucose co‐transporter 3 in the satiety effect of portal glucose sensing. Mol. Metab. 2012, 2, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
sup‐0001‐Figure S1
sup‐0002‐Figure S2
Table S1: Oligonucleotides used for the quantification of mRNA expression in HepG2 cells.
Table S2: Hepatic mRNA expression of lipid metabolism related enzymes after 22 wks of SCFA supplementation. C3H mice were fed a low‐fat diet or high‐fat diet supplemented with 5% SCFAs, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFAs (HF). Data are mean ± SEM, n = 8–10 ns, not significant. Means with different letters within one row are significantly different (ANOVA with Bonferroni post hoc test was performed between HF, HF‐HAc, and HF‐HPr group, p < 0.05).
Figure S1: Changes in liver and plasma phospholipid profile after 6 wks of dietary SCFA supplementation. C3H mice were fed a high‐fat diet supplemented with 5% SCFAs, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFAs (HF). Data are mean + SEM, n = 8–10. Values are expressed as area percentage of each fatty acid relative to the total area of all detected fatty acids. Means with different letters are significantly different (ANOVA with Bonferroni post hoc test, p < 0.05).
Figure S2: Effects of dietary SCFAs on liver and plasma phospholipid profile after 22 wks of intervention. C3H mice were fed a low‐fat (LF) diet or high‐fat diet supplemented with 5% SCFAs, either with a high acetate ratio (Ac:Pr, 2.5:1, HF‐HAc), a high Pr ratio (Ac:Pr, 1:2.5, HF‐HPr), or without SCFAs (HF) for 22 wks. Values are expressed as area percentage of each fatty acid relative to the total area of all detected fatty acids. Data are mean + SEM, n = 8–10. Means with different letters are significantly different (ANOVA with Bonferroni post hoc test was performed between HF groups, p < 0.05).


