Abstract
The increasing usage of nanoparticles has caused their considerable release into the aquatic environment. Meanwhile, anthropogenic CO2 emissions have caused a reduction of seawater pH. However, their combined effects on marine species have not been experimentally evaluated. This study estimated the physiological toxicity of nano-TiO2 in the mussel Mytilus coruscus under high pCO2 (2500–2600 μatm). We found that respiration rate (RR), food absorption efficiency (AE), clearance rate (CR), scope for growth (SFG) and O:N ratio were significantly reduced by nano-TiO2, whereas faecal organic weight rate and ammonia excretion rate (ER) were increased under nano-TiO2 conditions. High pCO2 exerted lower effects on CR, RR, ER and O:N ratio than nano-TiO2. Despite this, significant interactions of CO2-induced pH change and nano-TiO2 were found in RR, ER and O:N ratio. PCA showed close relationships among most test parameters, i.e., RR, CR, AE, SFG and O:N ratio. The normal physiological responses were strongly correlated to a positive SFG with normal pH and no/low nano-TiO2 conditions. Our results indicate that physiological functions of M. coruscus are more severely impaired by the combination of nano-TiO2 and high pCO2.
Increasing production of engineered nanoparticles (NPs) has raised concern about their potential biological and ecological effects1. Among nanomaterials, nanosized titanium dioxide (nano-TiO2) is considered as one of the most frequently used NPs and the most extensively investigated NP so far in ecotoxicology2. Nano-TiO2 has been released in the aquatic environments, such as the estuarine and coastal waters, causing a health risk to marine organisms2,3,4. Nano-TiO2 has been shown to induce detrimental biological effects on aquatic animals in different in vitro and in vivo systems4,5. To date, the potential impact of nano-TiO2 on aquatic ecosystems and marine invertebrates has attracted special attention1,2,6,7.
Marine bivalves are important model organisms to assess the effect of NPs on aquatic biota, and may play a significant role in NPs uptake, biotransformation and trophic transfer through food chains8,9,10. In general, nano-TiO2 has been shown to exert toxicity mainly via inducing ROS production and oxidative stress in cells5,9,11,12,13,14. Nano-TiO2 has showed toxic effects in bivalve hemocytes15,16,17,18, stimulating different immune responses (e.g., oxidative stress and the release of hydrolytic enzymes). TiO2 nanoparticles also displayed embryotoxicity to Mytilus galloprovincialis19 and induced DNA damage in Lymnea luteola20. Previous studies have demonstrated adverse/toxic effects of nano-TiO2 on immune system and digestive gland function in marine mussels9,11,12,15,16,21. In bivalves the gills represent the first site of contacting particles from the surrounding environment due to their role in the feeding process22. Katsumiti et al.13 found that nano-TiO2 showed adverse effects to mussel gill cells13. As gill cells play important roles in food transport and respiration for mussels23, there is a unique interest in expanding studies on nano-TiO2 toxicity in mussel’s feeding and physiology. However, information on the effects of nano-TiO2 on bivalve feeding and physiology is still scarce.
The ocean surface pH now is approximately 0.1 units lower than the preindustrial epoch24 and a reduction of nearly 0.8 units within the next three centuries due to ocean acidification (OA) is predicted24,25. Some studies show that CO2-induced pH reduction impacts bivalve physiology by changing extracellular acid–base balance26,27, metabolic activities28 and feeding29,30. A rise in pCO2 levels can induce changes in the extracellular acid–base balance that can produce metabolic disturbances, adversely affecting relevant biological processes, such as calcification, metabolism, growth and fitness31,32. Many marine calcifying organisms have exhibited negative responses to high pCO2, such as disorder in metabolic rates26,28, reduction of food uptake29,30 and alteration in calcification and development33. Bivalve molluscs have a limited capacity for acid–base regulation due to the lack of developed ion-exchange and nonbicarbonate mechanisms32. Consequently, their ability to regulate the acid–base status of internal tissues during contact with low pH water is limited. In order to correct acid–base imbalances an organism must employ energetically costly active ion exchange mechanisms32. Furthermore, the extracellular alterations caused by exposure to elevated pCO2 are likely to affect processes such as energy partitioning and metabolism32,34.
Scope for growth (SFG), calculated from clearance rate, respiration rate and excretion rate, is a reliable tool for evaluating effects of environmental stressors on marine bivalves35,36,37,38. These physiological parameters not only present key processes related to energy budget, but also mirror the physiological plasticity of the animals to adapt stressful surrounding conditions37,38,39,40. However, toxic effects of nanomaterials on physiological responses in marine mussels receive little attention.
The bioaccumulation, environmental and biological impacts of nanoparticles are difficult to predict because of the complexity of marine ecosystem and lack of relevant information to date1. Further complexity is expected when interactions of metal oxide nanoparticles with seawater pH are present as pH change in water is predicted to influence metal speciation41 and nanoparticle behavior (e.g., aggregation and disaggregation)42. It is reported that the size and form of nanoparticles can influence the toxicity on aquatic organisms13. For instance, the bulk form of nano-TiO2 is more toxic with respect to histopathological and histochemical changes5. Thus, nano-TiO2 may exert different toxic effect on mussels if their behaviour was changed by CO2-induced pH reduction. Despite meriting considerable research effort in recent years, the biological impacts of nanoparticle or reduced seawater pH have been mostly considered with single factor exposure in marine environmental research, whereas their interactive effects are still poorly understood41. For instance, to date, whether the toxicity of nano-TiO2 on marine animals is affected by pH is still unclear. As nanoparticles and CO2-induced pH reduction may occur simultaneously and the physiological responses of the animals subjected to such multiple stressors are unclear, their interactive effects must be thoroughly clarified in marine bivalves41. Recent evidences suggest that interactions of nano-TiO2 with other chemical/physical factors may cause an increased toxicity or adverse effects in different marine species, for example, 2,3,7,8-TCDD, Cd2+ and hypoxia increased the toxicity of nano-TiO2 to marine mussels, raising attentions on ecotoxicological effects of nano-TiO2 on aquatic organisms3,4,21,43,44. Consequently, it is essential to explore how the combination of NP and reduced seawater pH affects marine organisms.
The thick shell mussel Mytilus coruscus is an ecologically important marine bivalve, widely inhabiting in the Changjiang estuary and coastal waters of the East China Sea. Our sampling area, the Shengsi island, is the largest area for mussel aquaculture in China, where in recent years mass mussel mortalities have been found due to hypoxia or some other environmental stressors, for example, large pH fluctuation in summer. A large amount of nutrients from estuaries of Changjiang and Qiantangjiang has entered this area, resulting in eutrophication and biological CO2 production. Consequently, in these regions, organisms can experience metal pollutants and pH fluctuations due to runoff events, eutrophication and biological CO2 production. Hence, this species is a typical organism for reflecting the biological effects of NP and pH change. Titanium particles have been reported to be released into some aquatic environments with concentrations of 0.1~3 mg/L, representing an environmental exposure and a threat for aquatic organisms and ecosystem health45,46. In our earlier studies, nano-TiO2 ranged from 2.5–10 mg/L in seawater caused some immune toxic effects on hemocytes in mussels44. Moreover, high concentrations of nano-TiO2 (10 mg/L) resulted in moderate gill damage5. Hence, the physiological functions, such as filtration and respiration, probably are affected by such TiO2 concentration. Moreover, whether high pCO2 can affect mussel’s ability to cope with nano-TiO2 has not yet been examined. We hypothesize that nano-TiO2 may be more toxic under reduced pH conditions compared to under normal pH conditions as pH reduction causes additional stress to the physiology of mussels. The purpose of this study was to assess the interactive effects of nano-TiO2 and high pCO2 on M. coruscus by integrating key eco-physiological parameters, i.e., clearance rate, food absorption efficiency, organic rate in faeces, respiration rate, ammonia excretion rate, oxygen to nitrogen (O:N) ratio as well as scope for growth (SFG). Such data are essential for the toxicity assessment of nano-TiO2 in coastal and estuarine areas (e.g., the Shengsi island), where much nutrients flow in and eutrophication and CO2-induced pH reduction frequently occur47.
Results
NPs characterization
X-ray diffraction patterns revealed the presence of both anatase (70%) and rutile (30%) phases (Supplementary Figure 1A), which was consistent with the product information nano-TiO2 showed different sizes under scanning electron microscope (SEM) and transmission electron microscope (TEM), the morphology was typical of grinded particles, and some particles were agglomerated (Supplementary Figure 1B and C). A size histogram of particles based on TEM image showed a relatively non-uniform composition with a majority of 20–30 nm particles (Supplementary Figure 1D), basically in agreement with the declared size.
Seawater chemistry
Salinity of the water consistently was around 25‰, and dissolved oxygen in the exposure tanks consistently was maintained at about 7.0 mg l−1 during the experiment. Total alkalinity ranged from 2136 to 2276 μmol kg−1, and CO2 levels were maintained at ca. 350 μatm and ca. 2600 μatm in the normal (pH 8.1) and low pH (pH 7.3) treatments, respectively. The nano-TiO2 concentrations in seawater determined by atomic absorption spectrophotometer in different pH conditions, were in average 1.66 ± 0.18 mg l−1 and 7.88 ± 0.55 mg l−1 (pH 8.1) and 1.52 ± 0.14 mg l−1 and 7.37 ± 0.75 mg l−1 (pH 7.3) for the nominal 2.5 (low) and 10 mg l−1 (high) exposure concentration, respectively, and no significant effects of pH on nano-TiO2 concentrations were found. The detailed seawater chemistry in each treatment was summarized in Supplementary Table 1.
Physiological parameters
Clearance rates were significantly decreased with the increased nano-TiO2 concentration with the lowest value observed at 10 mg l−1 nano-TiO2 treatment during the whole experiment at each pH level (Supplementary Table 2; Fig. 1A); pH significantly affected clearance rates at day 1 and 3 under all nano-TiO2 treatments, with lower values at pH 7.3 than pH 8.1; however, low pH only negatively affected clearance rates at day 7 under nano-TiO210 mg l−1, and at day 14 under nano-TiO2 2.5 mg l−1 (Supplementary Table 2; Fig. 1A).
Figure 1.
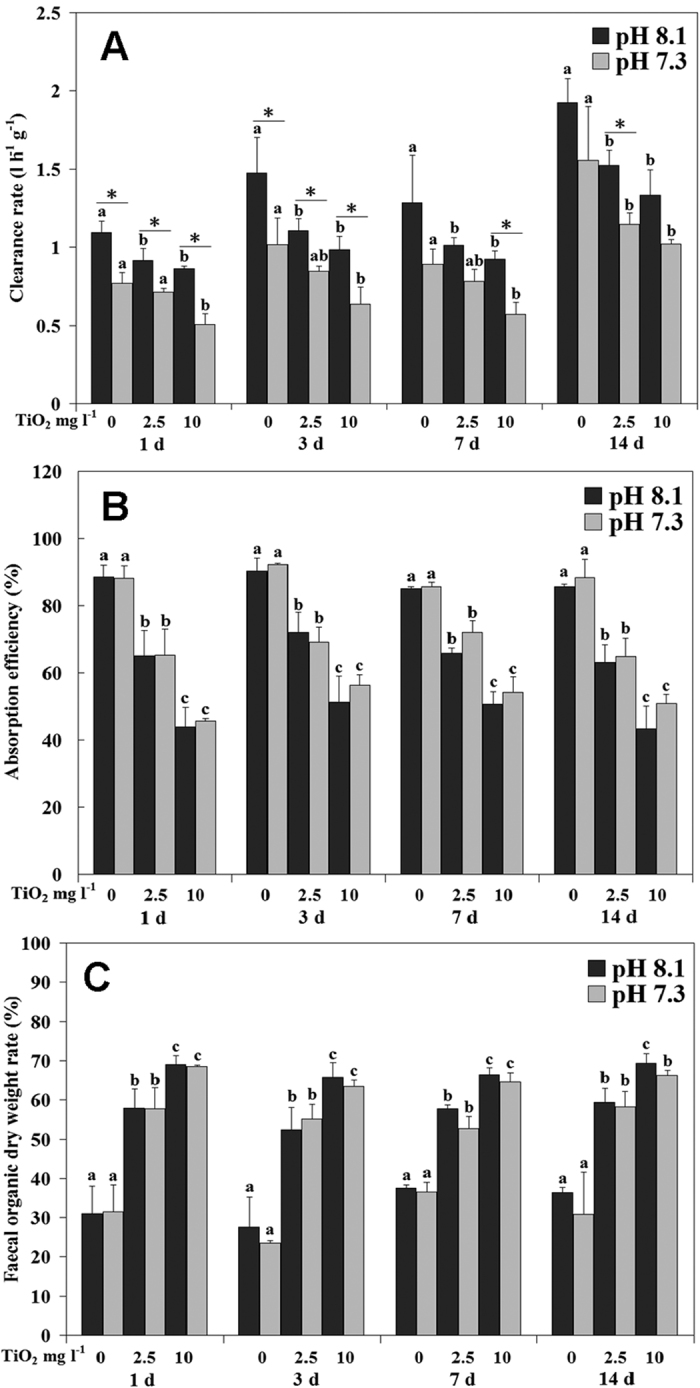
Clearance rate (CR, A), Absorption efficiency (AE, B) and faecal organic weight rate (E, C) of M. coruscus exposed to six treatments for 14 days. The values with different superscripts at each pH are significantly different among three nano-TiO2 treatments (P < 0.05). The values denoted by an asterisk between two pH groups at each n-TiO2 concentration are significantly different (P < 0.05).
Absorption efficiency significantly decreased with the increased nano-TiO2 concentration throughout the experiment at each pH level. However, pH showed no significant effect on AE during the whole experiment (Supplementary Table 2; Fig. 1B). Organic weight ratio in feces significantly increased with nano-TiO2 increment during the whole experiment at each pH level, but no pH effect was found on this parameter (Supplementary Table 2; Fig. 1C).
Respiration rates were significantly influenced by nano-TiO2 and pH during the entire experiment, and by their interactions at day 7 (Supplementary Table 2; Fig. 2A). Respiration rates decreased with nano-TiO2 increment during the experiment, with the lowest value observed under 10 mg l−1 nano-TiO2 condition. In general, low pH reduced the respiration rates, although sometimes there were no significant effects of low pH. At day 1, low pH only reduced respiration rates when nano-TiO2 was absent; at day 3 and day 14, low pH reduced respiration rates under nano-TiO2 0 and 2.5 mg l−1 treatments; at day 7, low pH reduced respiration rates under all nano-TiO2 conditions (Supplementary Table 2; Fig. 2A).
Figure 2.
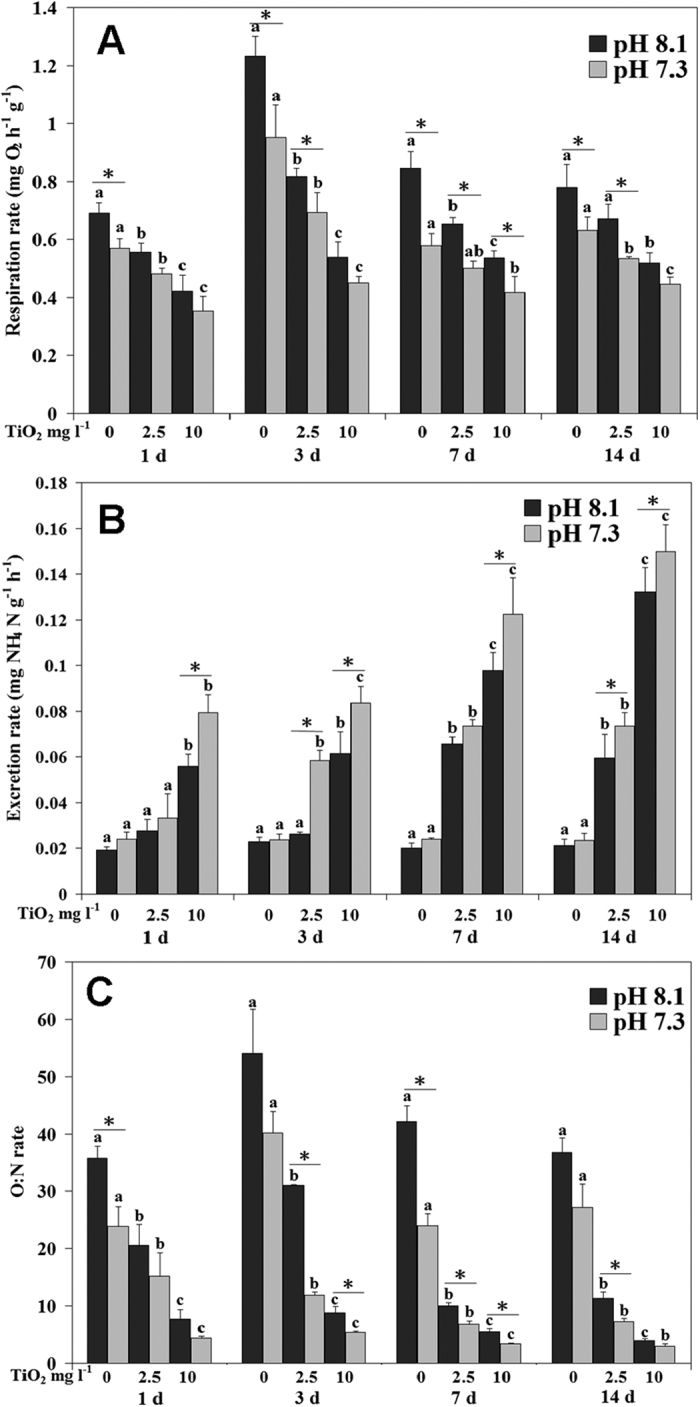
Ammonia excretion rate (ER, A), Respiration rate (RR, B) and O:N ratio (C) of M. coruscus exposed to six treatments for 14 days. The values with different superscripts at pH are significantly different among three nano-TiO2 treatments (P < 0.05). The values denoted by an asterisk between two pH groups at each nano-TiO2 concentration are significantly different (P < 0.05).
Ammonia excretion rates were significantly influenced by nano-TiO2 and pH during the entire experiment, and by their interactions at day 1 and 3 (Supplementary Table 2; Fig. 2B). Excretion rates generally increased with nano-TiO2 increment, with the highest values observed at nano-TiO210 mg l−1. For the pH effect, at day 1 and 7, excretion rate at pH 7.3 was significantly lower than pH 8.1 when mussels were exposed to nano-TiO2 10 mg l−1; at day 3 and 14, pH 7.3 increased excretion rates of mussels under two nano-TiO2 treatments, respectively.
O:N ratios were significantly influenced by nano-TiO2 and pH during the experiment, and by their interactions at day 3, 7 and 14 (Supplementary Table 2; Fig. 2C). Generally, O:N ratio decreased with nano-TiO2 increment, with the lowest values observed at 10 mg l−1 nano-TiO2. For the pH effect, at day 1, O:N ratio of pH 7.3 was significantly lower than pH 8.1 when nano-TiO2 was absent. At day 3, O:N ratio of pH 7.3 was significantly lower than pH 8.1 under two nano-TiO2 conditions. At day 7, pH 7.3 reduced O:N ratio when nano-TiO2 was present. At day 14, O:N ratio of pH 7.3 was significantly lower than pH 8.1 when nano-TiO2 was 10 mg l−1 (Supplementary Table 2; Fig. 2C).
The scope for growth (SFG) values were affected by pH at day 1 and day 3, and by nano-TiO2 during the entire experiment, but there was no interactive effect of them (Supplementary Table 2; Fig. 3). SFG decreased with nano-TiO2 increment, and even negative values were observed under the highest nano-TiO2 concentration. However, pH only affected SFG at day 1 and 3. At day 1, when nano-TiO2 was absent, SFG of pH 7.3 was lower than pH 8.1. At day 3, SFG of pH 7.3 was significantly lower than pH 8.1 when nano-TiO2 was 2.5 mg l−1.
Figure 3. Scope for growth (SFG) of M. coruscus exposed to six treatments for 14 days.
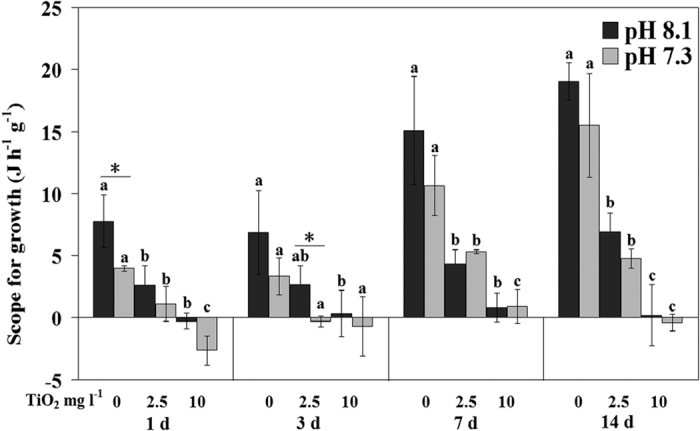
The values with different superscripts at each pH are significantly different among three nano-TiO2 treatments (P < 0.05). The values denoted by an asterisk between two pH groups at each nano-TiO2 concentration are significantly different (P < 0.05).
PCA showed that 85.64% of total variance was explained by PC1 (72.41%) and PC2 (13.23%) (Fig. 4). PC1 indicated a clear separation between non-nano-TiO2 and nano-TiO2 exposure treatments, showing a high convergence of most physiological parameters, especially high levels of SFG associated with CR and AE under non-nano-TiO2 treatments. PC2 separated two main experimental periods, i.e., day 1–7 and day 7–14. Generally, the reduced SFG with increased nano-TiO2 in this study was explained by inhibited physiological activities.
Figure 4. Principal component analysis (PCA) by integrating all measured parameters (CR, AE, RR, ER, O:N, SFG, E) for four times (days: 1, 3, 7 and 14) and six different treatments (▴–TiO2 0 × pH 8.1, △–TiO2 0 × pH 7.3, ▪–TiO2 2.5 × pH 8.1, ▫–TiO22.5 × pH 7.3, ♦–TiO210 × pH 8.1, ◊–TiO2 10 × pH 7.3) with a biplot.
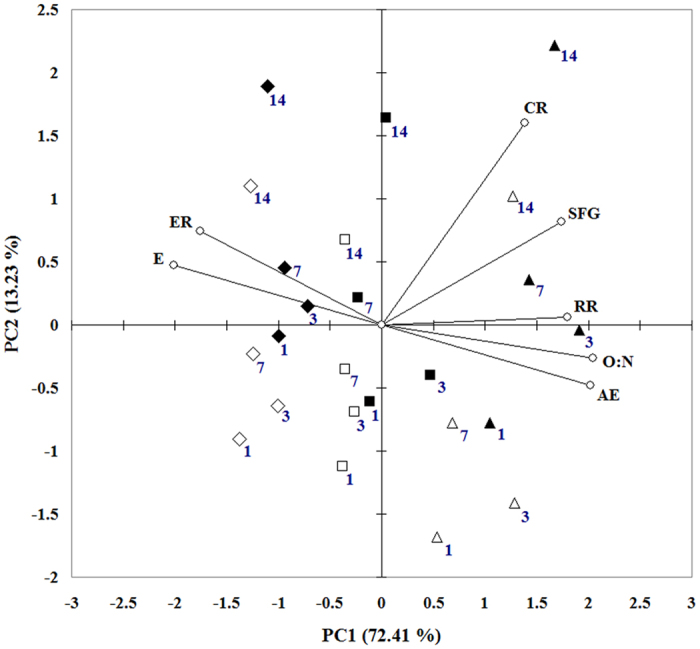
Both the scores of the experimental conditions and the loadings of the parameters (○) were present.
Discussion
Doyle et al.7 found measurable concentrations of nano-TiO2 in the gills of mussel and oyster following exposure7. Adsorption of NPs on gill surfaces results in a number of sublethal effects, e.g., gill pathology (such as hyperplasia and edema), respiratory toxicity, oxidative stress and dietary stress48,49, which subsequently impair filtration and ingestion of mussels. In the present study, nano-TiO2 exposure reduced CR, hence the food ingestion rate. The food ingestion rate in the zebra mussels Dreissena polymorpha decreased greatly in nano-TiO2 exposure media50. In our experiment, the mussel valve opening, distinguishing whether M. coruscus was filtering was lower if nano-TiO2 was present, which was similar to M. edulis exposed to nano-polystyrene51. This indicates that M. coruscus was able to recognize nano-TiO2 and hence reduced its filtration. A decreased filtering activity might cause severe consequences if the mussels were exposed to such concentrations in natural environments. Continuous limitation of CR would impair food intake thereby growth if the mussels were subjected to long term exposure.
Some bivalve species have showed different CR responses to CO2-induced low pH. Fernández-Reiriz et al.30 reported a decreased CR in the clam Ruditapes decussates under high pCO2 conditions30. Liu and He (2012) found reduced CRs in both the mussel Perna viridis and the clam Chlamys nobilis under high pCO2 conditions52. In our study, CO2-induced low pH reduced the CR of M. coruscus during the first few days, afterwards CR was only negatively affected by low pH under nano-TiO2 conditions sometimes, probably because the mussels showed some adaptation to low pH along with time, but could not overcome the additional stressor, nano-TiO2. Similarly, the CR of M. galloprovincialis was unaffected by pH 7.537. Sanders et al.53 also found that there was no significant effect of CO2-induced low pH on the filtering activity of king scallop Pecten maximus53. Thus, some bivalves can adapt reduced pH conditions, but additional stressors may change this situation based on the present study. It is known that some bivalves are sensitive to reduced pH under food limiting conditions53,54. Thus, in our study, nano-TiO2 first influenced the feeding and absorption of the mussels, which would further reduce the tolerance to high pCO2 because of the reduced energy uptake. Although the purpose of our study was not to address food limiting effects, nano-TiO2 posed a sub-optimal dietary state for the mussels during the exposure.
High pCO2 did not show significant effect on AE, implying the normal performance of the digestive systems of the mussels exposed to CO2-induced low pH conditions. This was in agreement with the gastropod N. conoidalis in which AE was also insensitive to low pH55. In such situations, the digestive enzymes may be relatively stable under the low pH conditions in this study. In the present study, reduced AE probably is caused by the decreased CR under nano-TiO2 exposure. NPs can attach to algal cells and form clusters, which can settle readily, resulting in a large decline of the algal concentration56,57,58. Hereby, the intake of algal cells was subsequently impaired. Moreover, exposure to nano-TiO2 induced oxidative stress and lysosomal membrane alteration in the digestive gland of mussels9,10. In addition, some other molecular and functional parameters of digestive gland can be affected by nano-TiO2 in mussels12,59. These results further verify the hypothesis that NPs can enter the digestive system which is a typical target for NP toxic effect in mussels. AE impairment, representing a significant stress response suggests that nano-TiO2 exposure may cause a serious harm to mussel health.
Some authors reported metabolic decline under high pCO2 and suggested that altered extracellular pH could cause these reductions26,34. Similar metabolic depressions have been observed in the clam R. decussatus30,60, the scallop Chlamys nobilis52, the mussel M. chilensis61, and the scavenging gastropod Nassarius conoidalis55 exposed to high pCO2 conditions. The above reports are consistent with the present study, where the respiration of mussel was significantly lowered at high pCO2 levels. Because carbon dioxide interacts with intra- and extracellular fluids, internally elevated CO2 levels may result in a respiratory acidosis62. In our study, lower respiration rates under nano-TiO2 exposure indicated that the metabolic activity was weakened by nano-TiO2. More particularly, the oxygen consumption of M. coruscus showed a strong sensitivity to nano-TiO2 during the entire experiment, indicating a limited capacity to adapt to NP exposure. Reduction in RR can be indirectly due to the disruption of ventilation by nano-TiO2. In mussels, CR and RR are linked as both occur by filtering water over the gills when their valves are open. Mussels are known to spend less time opening and filtering when there are contaminants present in the water as a behavioural response to avoid uptake of the contaminant. Hence the reduction in CR and RR could also be due to this behavioural response to nano-TiO2 rather than direct gill toxicity. Limited respiratory efficiency results in a growing mismatch between basic oxygen demand and oxygen supply and finally causes hypoxia and anaerobic metabolism63, which may be harmful to the mussels.
ER is considered as an index of high pCO2 stress in marine mussels30,37. An increase in ER may imply a drastic increment in the consumption of amino acids. Higher ER values under high pCO2 conditions were observed when nano-TiO2 was present, indicating nano-TiO2 affects the CO2 effect on ER. Under nano-TiO2 treatments, the inverse correlation between ER and O:N ratios indicates the enhanced protein catabolism and subsequently inhibited growth.
The O:N ratio is considered as an index of the nutritional state of the mussels by showing the metabolism of substrates. According to Widdows36 and Fernández-Reiriz et al.30, in mussels, O:N ratios of more than 30 usually indicate a catabolism of carbohydrates and lipids, whereas less than 30 suggest a protein catabolism mainly. Hereby, low O:N ratios are generally a sign of a stressed condition64. In this experiment, high pCO2 and nano-TiO2 treatments showed lower O:N ratios owing to the decreased respiration and enhanced protein metabolism as a consequence of energy demands.
In our study, the growth of M. coruscus was not negatively affected by high pCO2 at the end in terms of SFG. Similar to our study, SFG was stable or even increased under moderate pCO2 level in M. galloprovincialis30. The SFG values became negative when M. coruscus was exposed high nano-TiO2, probably as a result of a significant reduction in CR. Given that most of the other parameters measured would be affected by reduced filtration activity (valve opening time) of the mussels and hence time spent for feeding or respiration, nano-TiO2 obviously would impair the growth of mussels. The SFG results suggest that M. coruscus is not able to grow when nano-TiO2 is more than 10 mg l−1 in seawater, and nano-TiO2 (above 10 mg l−1) is thus stressful to the mussels.
PCA distinguished non-nano-TiO2 treatments from exposed treatments since non-nano-TiO2 treatments were grouped together at positive side whereas exposed treatments were grouped at negative side by PC1, reflecting higher values of AE, O:N, RR, CR and SFG were achieved under non-nano-TiO2 conditions whereas nano-TiO2 induced high ER and E. PC2 reflected the time change of most physiological parameters, as higher values of E, ER, CR and SFG were positive, corresponding to the experimental time, namely the later period, day 7 and day 14. By integrating ANOVA and PCA results, the characteristics of physiological responses to nano-TiO2 exposure were lower CR, AE, RR, O:N ratio and SFG associated with higher E and ER. CR, RR and AE can be reflected by filtration activity. In the present study, the filtration activity was impaired by nano-TiO2, hence the CR, RR and AE were reduced. SFG is mostly dependent on absorption rate, which is mostly determined by ingestion rate and AE. Thus, if CR was reduced, SFG was also reduced (Fig. 4). In contrast, higher E and ER under high nano-TiO2 indicate the low absorption rate and subsequently high protein catabolism, which can also impair the growth of mussels.
Nanoparticles exert toxicity via mainly oxidative stress mechanisms65. M. edulis increased oxyradical production and antioxidant enzyme activities when they were exposed to nano-TiO211,15. In our previous study, the hemocyte functions of M. coruscus exposed to nano-TiO2 and high pCO2 were impaired associated with an increase of ROS production, indicating an oxidative stress and fitness impairment66. Hence, in the present study, some physiological functions, such as clearance, respiration and absorption were all impaired when mussels were exposed to nano-TiO2, especially the combined treatment. The CO2-induced low pH in seawater could change the physiochemical properties of nano-TiO2 and lead to a slightly greater aggregation of nano-TiO266. In the present study, although the exposed nano-TiO2 concentration under low pH was lower than under normal pH, no significant difference was found in each nano-TiO2 concentration (Supplementary Table 1). It is reported that mussels are able to capture and ingest aggregates of nanoparticles more efficiently compared with those freely suspended6. Thereby, under CO2-induced low pH conditions, M. coruscus may accumulate more nano-TiO2 than pH 8.1, accordingly causing more severe toxic effects. Mussels usually lower their metabolic rate after exposure to acidified water26. In the present study, mussels closed their valves when nano-TiO2 was present, which further decreased their filtration and respiration. Thus, both high pCO2 and nano-TiO2 exerted negative effects on the physiological functions of mussels with more severe effects than the single stressor.
Conclusions
High pCO2 showed minor effect on the physiology of M. coruscus as most physiological parameters were almost unaffected by CO2-induced low pH. In contrast, nano-TiO2 showed significant negative effects on the feeding and physiology of mussels, suggesting that nano-TiO2 may present a health threat to the mussels, as depressions in feeding and digestion may reduce the ability of mussels to grow and defense predators. Above all, most physiological functions of M. coruscus were more severely impaired by the combination of nano-TiO2 and high pCO2, suggesting a synergistic effect on mussels.
Methods
Experimental animals
Experimental mussels (30.0 ± 2.0 mm shell length, 70.0 ± 5.0 mg dry tissue weight) were collected from the Shengsi island of Zhejiang Province, China (water temperature: 25.0 °C; salinity: 25.0‰; and pH: 8.1). The experimental mussels were wild, and the handling of them was conducted in accordance with the guidelines set by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Ocean University, Shanghai, China. After transportation to aquarium of the university, the mussels were held in fibre-glass tanks (500 l) with a filtering apparatus in laboratory. The laboratory conditions were maintained according to sampling area in September: temperature, salinity, dissolved oxygen and pH were kept constant at 25 ± 0.5 °C, 26 ± 1‰, 7 ± 0.5 mg O2 l−1 and 8.10 ± 0.02. Mussels were fed with the microalgae Isochrysis galbana daily (25,000 cells ml−1, ca. 3% of the tissue dry weight). Two weeks were allowed for mussels to acclimatize to the above laboratory conditions.
Characterization of nano-TiO2
Preparation of nano-TiO2 solution
Nano-TiO2 P25 particles (anatase/rutile, 7/3, declared purity of >99.5%, ~21 nm, spec. surface area 35–65 m2/g) were purchased from Sigma Aldrich. Stock suspensions (10.0 g l−1) were prepared daily with sterile filtered seawater (0.45 μm Teflon filter, pH 8.1, salinity 25‰) by sonication at 100 W, 50% on/off cycle, for 15 min in a cooling ice bath-type sonicator (frequency 40 kHz, UP200S Hielscher Ultrasonic Technology, Teltow, Germany).
TiO2 characterization by X-ray diffraction and electron microscope
X-ray powder diffractometer (Siemens D500, Karlsruhe, Germany) was applied to exam the crystal structures of nano-TiO2. Scanning electron microscope (FEI/Philips XL30 Esem-FEG, Netherlands) and transmission electron microscope (FEI/Philips Tecnai 12 BioTWIN, the Netherlands) were used to observe the surface morphology, primary size and shape of nano-TiO244. The diameter of 1000 NPs was measured using an image analysis program (Image J, v1.44; National Institute of Health, USA).
Experimental design and system
The experimental mussels were randomly divided to six treatments with three nano-TiO2 concentrations (0 (control), 2.5 and 10.0 mg l−1) under two pH values (7.3, 8.1(control)). The pH 7.3 was selected as an extreme pH value expected by the year 2300 and relevant for some coastal waters25,47,67. The low pH treatment was achieved by aerating pure carbon dioxide. The experimental system included a computer, pH regulator (DAQ-M; Loligo® Systems Inc., Tjele, Denmark), pH meter, solenoid valve, CO2 and air supplies, water pump, filtering system, temperature regulator, head tank and exposure tank. The CO2 flow from the pure CO2 cylinder to the header tank was controlled by the pCO2/pH regulator which can open or close the solenoid valve when the seawater pH values deviated from the preset values by ±0.1 pH units. Water temperature was kept at 25 °C by temperature regulators. The tanks were covered with acrylic sheets to prevent external interference. To achieve exposed concentrations of 2.5 and 10 mg l−1 nano-TiO2 in the experimental tanks, each test solution was prepared in a water tank (500 l) by adding relevant volume of 10.0 g l−1 sonicated stock solution to the seawater, respectively. The seawater in the exposure tank was renewed and re-dosed everyday with new working solutions of nano-TiO2 to keep the exposure at relatively consistent nano-TiO2 concentrations. In each treatment, there were three flow-through tanks (30 l) as three replicates (30 mussels per replicate), and mussels were fed with microalgae Isochrysis galbana (2.5 × 104 cells ml−1) at 1st, 3rd, 5th, 7th, 9th,11th and 14th day during the whole experiment. The mussels were exposed to six treatments for 14 days, and examinations on all physiological parameters of M. coruscus were carried out on the 1st, 3rd, 7th and 14th day.
Monitoring of carbonate chemistry of seawater
Gran titration was applied to measure total alkalinity (TA) with a total alkalinity titrator system68. The pH and TA were used to calculate other carbonate system parameters, i.e., partial pressure of CO2 (pCO2) and dissolved inorganic carbon (DIC), and the saturation state of omega calcite and aragonite using CO2SYS software as described previously37. Nanoparticle concentrations in water were measured using the standard test method for determination by atomic absorption spectroscopy of titanium dioxide content69.
Physiological measurements
Clearance rate
Clearance rate (CR) was measured using all individuals as a whole in each replicate tank. Prior to CR measurement, the mussels were fasting for at least twelve hours to evacuate their digestive tracts. The initial microalgae concentration was 2.5 × 104 cells ml−1, and no mussels produced pseudo-faeces at this concentration. Three identical tanks without experimental mussels were used as the control. An initial 20 ml water was sampled from the tank center with a syringe at the beginning, and then 20 ml aliquots were collected at 30 min intervals for 120 min, without being influenced by large decrease of algal concentration (<30%). The algal concentration in each sample was counted by a particle analyzer (Multisizer 3 Coulter Counter, Beckman, Irvine, USA). Cell concentrations in control tanks did not show any significant variation during the measurements. CR was calculated according to the formula of Coughlan70:
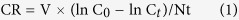 |
where CR represents the clearance rate (l h−1), V represents the water volume in the tank (l), C0 represents the initial microalgae concentration (cells ml−1), Ct represents the microalgae concentration at time t (cells ml−1), N represents the number of experimental mussels in the tank, t represents the sampling time (h). CR and the rest physiological parameters were standardized to unit dry weight (see below scope for growth).
Ingestion rate (IR) was computed by multiplying POM (particulate organic matter, mg l−1) by CR71, i.e., the food intake per hour. The POM concentration was transformed to joules using a conversion factor of 23.5 J mg−1 for Isochrysis galbana72.
Absorption efficiency
Absorption efficiency (AE) was determined according to Conover73. AE was determined by collecting the feces after CR measurements in each replicate tank. The organic content in microalgae was measured by filtering 5 L seawater with 2.5 × 104 cell ml−1 algae using glass fiber filters (Whatman® GF/C). Ammonium formate solution (0.5 M) was used to rinse these fulter papers which were then dried at 110 °C for 24 hours and weighed, and then ashed in a muffle furnace (450 °C for 6 hours) and reweighed. Blank GF/C filters were also treated using the same procedure to correct weight change caused by daily humidity variations. Faeces were carefully collected using a pipette from the tanks 12 hours after the CR measurements, and the organic content of faeces was determined as above. AE was calculated according to Conover73:
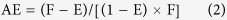 |
where AE represents the absorption efficiency (%), F represents the ratio of ash-free dry weight:dry weight in the microalgae, and E represents the ratio of ash-free dry weight:dry weight in the faeces.
Respiration rate
Fifteen mussels were randomly sampled from each tank and divided averagely into three replicates. The respiration rate (RR) was determined for five mussels in a closed glass respirometer (1000 ml) filled with air-saturated seawater from the corresponding experimental tank for one hour. To ensure the mussels started to respire in the chamber, the measurement began 20 minutes later when the mussels opened their valves. DO concentrations in three chambers without mussels were also recorded as the control. The oxygen consumption rate within the chamber was measured by oxygen meters (model YSI 58). The oxygen concentrations at the beginning and the end in each chamber were recorded.
The RR was calculated according to the following formula:
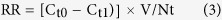 |
where RR represents the respiration rate (mg O2 h−1), Ct0 and Ct1 represent the initial and final DO concentrations in the chamber (mg O2 l−1), V (l) represent the volume of the seawater in the chamber, N represents the number of mussels in the chamber, t (h) represents the time elapsed. RR values were converted into J/h using a conversion coefficient of 13.98 J mg O2−1 74.
Ammonia excretion rate
When RR measurements were finished, ammonia excretion rates (ER) of the same mussels were examined. Water was sampled from each chamber and stored at −20 °C until analysis. The concentration of ammonia produced by mussels was measured by the phenol-hypochlorite method75. ER was obtained from the difference of ammonia concentrations between the chamber containing mussels and the control chamber according to the equation:
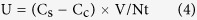 |
where U represents the rate of ammonia excretion (mg NH4–N h−1), Cs represents the ammonia concentration (mg l−1) in the experimental sample, Cc represents the ammonia concentration in the control sample, V represents the volume (l) of seawater in the chamber, N represents the number of mussels and t represents the time elapsed (h). Values of excretion rate were converted into J h−1 using a conversion coefficient of 25 J mg NH4–N−1 76. The ratio of oxygen consumption to ammonia excretion expressed as atomic equivalents (O:N) was calculated to assess the utilization of different biochemical compositions for energy metabolism36.
Scope for growth
At the end of the experiment, soft tissues of the mussels were collected and dried at 90 °C for 24 h to calculate their tissue dry weight. CR (l h−1), RR (mg O2 h−1) and ER (mg NH4–N h−1) were converted to mass specific rates as a ‘standard mussel’ of 1 g dry weight using the formula: Ys = (Ws/We)b × Ye, where Ys is the physiological value for a mussel of standard weight, Ws is the standard weight (1 g), We is the measured weight of the mussel (g), Ye is the uncorrected (measured) physiological value, and b is the weight exponent for the physiological value (b = 0.67). Each physiological parameter was transformed to energy equivalents (J h−1 g−1) to calculate the scope for growth (SFG), showing the difference between the energy acquired from the food and the energy depleted by respiration and excretion.
SFG was calculated according to Smaal and Widdows74,76,77:
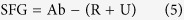 |
where SFG represents scope for growth (J h−1g−1), Ab represents absorption rate which derives from IR × AE (J h−1 g−1), R represents the energy loss in respiration (J h−1g−1), and U represents the energy loss in ammonia excretion (J h−1g−1).
Statistical analyses
Normality of the data was checked by Shapiro-Wilk’s W test and homogeneity of variances was evaluated by Levene’s test using SPSS 16.0. To evaluate the interactive effects of pH and nano-TiO2 on physiological parameters, two-way repeated measures analysis of variance (ANOVA) were used. When there was a significant interaction, one-way ANOVA followed by Tukey’s HSD test or student t test was conducted for each factor separately in each level of the other factor. Principal component analysis (PCA) was conducted for multivariate analysis using XLSTAT®2014. A biplot was graphed with both the measured variables and the observations. All data were expressed as the mean ± standard deviations, and the results were statistically significant at p < 0.05.
Additional Information
How to cite this article: Hu, M. et al. CO2-induced pH reduction increases physiological toxicity of nano-TiO2 in the mussel Mytilus coruscus. Sci. Rep. 7, 40015; doi: 10.1038/srep40015 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The work described in this paper was supported by a research grant (project no. 31302207) from Natural Science Foundation of China and a grant (project no. 13ZR1455700) from Shanghai Municipal Natural Science Foundation. This work was also supported by the Key Laboratory of Organic Pollution Process and Control, Zhejiang Province. We would like to thank two anonymous reviewers for their helpful comments.
Footnotes
Author Contributions Y.J. designed and led the study, Y.Y., X.Z., Y.H., K.N. and Y.M. performed the experiments, M.H., D.H., W.Q. and Y.J. analyzed data and wrote the manuscript.
References
- Klaine S. J. et al. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 27, 1825–1851 (2008). [DOI] [PubMed] [Google Scholar]
- Menard A., Drobne D. & Jemec A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 159, 677–684 (2011). [DOI] [PubMed] [Google Scholar]
- Matranga V. & Corsi I. Toxic effects of engineered nanoparticles in the marine environment: model organisms and molecular approaches. Mar. Environ. Res. 76, 32–40 (2012). [DOI] [PubMed] [Google Scholar]
- Canesi L., Ciacci C. & Balbi T. Interactive effects of nanoparticles with other contaminants in aquatic organisms: Friend or foe? Mar. Environ. Res. 111, 128–134 (2015). [DOI] [PubMed] [Google Scholar]
- D’Agata A. et al. Enhanced toxicity of ‘bulk’ titanium dioxide compared to ‘fresh’ and ‘aged’ nano-TiO2 in marine mussels (Mytilus galloprovincialis). Nanotoxicology 8, 549–558 (2014). [DOI] [PubMed] [Google Scholar]
- Ward J. E. & Kach D. J. Marine aggregates facilitate ingestion of nanoparticles by suspension feeding bivalves. Mar. Environ. Res. 68, 137–142 (2009). [DOI] [PubMed] [Google Scholar]
- Doyle J. J., Ward J. E. & Mason R. Exposure of bivalve shellfish to titania nanoparticles under an environmental-spill scenario: Encounter, ingestion and egestion. J. Mar. Bio. Assoc. UK 110, 137–149 (2016). [Google Scholar]
- Montes M. O., Hanna S. H., Lenihan H. S. & Keller A. A. Uptake, accumulation, and biotransformation of metal oxide nanoparticles by a marine suspension feeder. J. Hazard. Mater. 225–226, 139–145 (2012). [DOI] [PubMed] [Google Scholar]
- Canesi L. et al. Bivalve molluscs as a unique target group for nanoparticle toxicity. Mar. Environ. Res. 76, 16–21 (2012). [DOI] [PubMed] [Google Scholar]
- Hull M. S., Vikesland P. J. & Schultz I. R. Uptake and retention of metallic nanoparticles in the Mediterranean mussel (Mytilus galloprovincialis). Aquat. Toxicol. 140–141, 89–97 (2013). [DOI] [PubMed] [Google Scholar]
- Canesi L. et al. In vitro effects of suspensions of selected nanoparticles (C60 fullerene, TiO2, SiO2) on Mytilus hemocytes. Aquat. Toxicol. 96, 151–158 (2010). [DOI] [PubMed] [Google Scholar]
- Barmo C. et al. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 132–133, 9–18 (2013). [DOI] [PubMed] [Google Scholar]
- Katsumiti A. et al. Cytotoxicity of TiO2 nanoparticles to mussel hemocytes and gill cells in vitro: Influence of synthesis method, crystalline structure, size and additive. Nanotoxicology 9, 543–553 (2015). [DOI] [PubMed] [Google Scholar]
- Farkas J. et al. The impact of TiO2 nanoparticles on uptake and toxicity of benzo(a)pyrene in the blue mussel (Mytilus edulis). Sci. Total Environ. 511, 469–476 (2015). [DOI] [PubMed] [Google Scholar]
- Canesi L. et al. Biomarkers in Mytilus galloprovincialis exposed to suspensions of selected nanoparticles (Nano carbon black, C60 fullerene, Nano-TiO2, Nano-SiO2). Aquat. Toxicol. 100, 168–177 (2010). [DOI] [PubMed] [Google Scholar]
- Canesi L. et al. Interactive effects of n-TiO2 and 2,3,7,8-TCDD on the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 153, 53–65 (2014). [DOI] [PubMed] [Google Scholar]
- Ciacci C. et al. Immunomodulation by different types of n-oxides in the hemocytes of the marine bivalve Mytilus galloprovincialis. PLoS ONE 7, e36937 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marisa I. et al. In vitro exposure of haemocytes of the clam Ruditapes philippinarum to titanium dioxide (TiO2) nanoparticles: Nanoparticle characterisation, effects on phagocytic activity and internalisation of nanoparticles into haemocytes. Mar. Environ. Res. 103, 11–17 (2015). [DOI] [PubMed] [Google Scholar]
- Libralato G. et al. Embryotoxicity of TiO2 nanoparticles to Mytilus galloprovincialis (Lmk). Mar. Environ. Res. 92, 71–78 (2013). [DOI] [PubMed] [Google Scholar]
- Ali D. et al. Impairment of DNA in a freshwater gastropod (Lymnea luteola L.) after exposure to titanium dioxide nanoparticles. Arch. Environ. Contam. Toxicol. 68, 543–552 (2015). [DOI] [PubMed] [Google Scholar]
- Banni M. et al. Combined effects of n-TiO2 and 2,3,7,8-TCDD in Mytilus galloprovincialis digestive gland: A transcriptomic and immunohistochemical study. Environ. Res. 145, 135–144 (2016). [DOI] [PubMed] [Google Scholar]
- Chandurvelan R., Mardsen I. D., Gaw S. & Glover C. N. Impairment of green-lipped mussel (Perna canaliculus) physiology by waterborne cadmium: Relationship to tissue bioaccumulation and effect of exposure duration. Aquat. Toxicol. 124–125, 114–124 (2012). [DOI] [PubMed] [Google Scholar]
- Gómez-Mendikute A., Elizondo M., Venier P. & Cajaraville M. P. Characterization of mussel gill cells in vivo and in vitro. Cell Tissue. Res. 321, 131–140 (2005). [DOI] [PubMed] [Google Scholar]
- Orr J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (2005) [DOI] [PubMed] [Google Scholar]
- Caldeira K. & Wickett M. E. Anthropogenic carbon and ocean pH. Nature 425, 365 (2003). [DOI] [PubMed] [Google Scholar]
- Michaelidis B., Ouzounis C., Paleras A. & Pörtner H. O. Effects of long-term moderate hypercapnia on acid–base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 293, 109–118 (2005). [Google Scholar]
- Lewis C. et al. Ocean acidification increases copper toxicity differentially in two key marine invertebrates with distinct acid-base responses. Sci. Rep. 6, 21554 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen J. & Melzner F. Moderate seawater acidification does not elicit long-term metabolic depression in the blue mussel Mytilus edulis. Mar. Biol. 157, 2667−2676 (2010). [Google Scholar]
- Dupont S. & Thorndyke M. C. Impact of CO2-driven ocean acidification on invertebrates early life-history – What we know, what we need now and what we can do. Biogeosci. Disc. 6, 3109–3131 (2010). [Google Scholar]
- Fernández-Reiriz M. J., Range P., Álvarez-Salgado X. A. & Labarta U. Physiological energetics of juvenile clams Ruditapes decussatus in a high CO2 coastal ocean. Mar. Ecol. Prog. Ser. 433, 97–105 (2011). [Google Scholar]
- Fabry V. J., Seibel B. A., Feely R. A. & Orr J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432 (2008). [Google Scholar]
- Melzner F. et al. Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6, 2313–2331 (2009). [Google Scholar]
- Ross P., Parker L., O’Connor W. A. & Bailey E. A. The impact of ocean acidification on reproduction, early development and settlement of marine organism. Water 3, 1005–1030 (2011). [Google Scholar]
- Pörtner H. O., Langenbuch M. & Reipschläger A. Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J. Oceanogr. 60, 705–718 (2004). [Google Scholar]
- Bayne B. L. & Newell R. C. Physiological energetics of marine molluscs (ed. Saleuddin A. S. M. & Wilbur K. M.) 407–515 (Academic Press, 1983).
- Widdows J. Physiological measurements in The effects of stress and pollution on marine animals (ed. Bayne B. L. et al.) 3–40 (Praeger Scientific Publications, 1985).
- Fernández-Reiriz M. J., Range P., Álvarez-Salgado X. A., Espinosa J. & Labarta U. Tolerance of juvenile Mytilus galloprovincialis to experimental seawater acidification. Mar. Ecol. Prog. Ser. 454, 65–74 (2012). [Google Scholar]
- Albentosa M., Vinas L., Besada V., Franco A. & Gonzalez-Quijano A. First measurements of the scope for growth (SFG) in mussels from a large scale survey in the North-Atlantic Spanish coast. Sci. Total Environ. 435–436, 430–445 (2012). [DOI] [PubMed] [Google Scholar]
- Campillo J. A., Albentosa M., Valdés N. J., Moreno-González R. & León V. M. Impact assessment of agricultural inputs into a Mediterranean coastal lagoon (Mar Menor, SE Spain) on transplanted clams (Ruditapes decussatus) by biochemical and physiological responses. Aquat. Toxicol. 142–143, 365–379 (2013). [DOI] [PubMed] [Google Scholar]
- Duarte C. et al. Combined effects of temperature and ocean acidification on the juvenile individuals of the mussel Mytilus chilensis. J. S. Res. 85, 308–314 (2014). [Google Scholar]
- Kadar E. et al. The influence of engineered Fe2O3 nanoparticles and soluble (FeCl3) iron on the developmental toxicity caused by CO2-induced seawater acidification. Environ. Pollut. 158, 2490–2497 (2010). [DOI] [PubMed] [Google Scholar]
- Loosli F., Le Coustumer P. & Stoll S. TiO2 nanoparticles aggregation and disaggregation in presence of alginate and Suwannee River humic acids. pH and concentration effects on nanoparticle stability. Water Res. 47, 6052–6063 (2013). [DOI] [PubMed] [Google Scholar]
- Baker T. J., Tyler C. R. & Galloway T. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 186, 257–271 (2014). [DOI] [PubMed] [Google Scholar]
- Wang Y. J. et al. Immune toxicity of TiO2 under hypoxia in the green-lipped mussel Perna viridis based on flow cytometric analysis of hemocyte parameters. Sci. Total Environ. 470–471, 791–799 (2014). [DOI] [PubMed] [Google Scholar]
- Kiser M. A. et al. Titanium nanomaterial removal and release from wastewater treatment plants. Environ. Sci. Technol. 43, 6757–6763 (2009). [DOI] [PubMed] [Google Scholar]
- Westerhoff P., Song G., Hristovski K. & Kiser M. A. Occurrence and removal of titanium at full scale wastewater treatment plants: implications for TiO2 nanomaterials. J. Environ. Monit. 13, 1195–1203 (2011). [DOI] [PubMed] [Google Scholar]
- Wallace R. B. et al. Coastal ocean acidification: The other eutrophication problem. Estuar. Coast.Shelf Sci. 148, 1–13 (2014). [Google Scholar]
- Federici G., Shaw B. J. & Handy R. D. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat. Toxicol. 84, 415−430 (2007). [DOI] [PubMed] [Google Scholar]
- Croteau M. N., Dybowska A. D., Luoma S. N. & Valsami-Jones E. A novel approach reveals that zinc oxide nanoparticles are bioavailable and toxic after dietary exposures. Nanotoxicology 5, 79−90 (2011). [DOI] [PubMed] [Google Scholar]
- Bourgeault A. et al. The challenge of studying TiO2 nanoparticle bioaccumulation at environmental concentrations: crucial use of a stable isotope tracer. Environ. Sci. Technol. 49, 2451–2459 (2015). [DOI] [PubMed] [Google Scholar]
- Wegner A., Besseling E., Foekema E. M., Kamermans P. & Koelmans A. A. Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis L.). Environ. Toxicol. Chem. 11, 2490–2497 (2012). [DOI] [PubMed] [Google Scholar]
- Liu W. G. & He M. X. Effects of ocean acidification on the metabolic rates of three species of bivalve from southern coast of China. Chin. J. Oceanol. Limnol. 30, 206–211 (2012). [Google Scholar]
- Sanders M. B., Bean T. P., Hutchinson T. H. & Le Quesne W. J. F. Juvenile king scallop, Pecten maximus, is potentially tolerant to low levels of ocean acidification when food is unrestricted. PLoS ONE 8, e74118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen J., Casties I., Pansch C., Körtzinger A. & Melzner F. Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments. Glob. Change Biol. 19, 1017–1027 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang H. Y., Shin P. K. S. & Cheung S. G. Physiological responses and scope for growth upon medium-term exposure to the combined effects of ocean acidification and temperature in a subtidal scavenger Nassarius conoidalis. Mar. Environ. Res. 106, 51–60 (2015). [DOI] [PubMed] [Google Scholar]
- Campos B., Rivetti C., Rosenkranz P., Navas J. M. & Barata C. Effects of nanoparticles of TiO2 on food depletion and life-history responses of Daphnia magna. Aquat. Toxicol. 130–131, 174–183 (2013). [DOI] [PubMed] [Google Scholar]
- Li M., Czymmek K. J. & Huang C. P. Responses of Ceriodaphnia dubia to TiO2 and Al2O3 nanoparticles: a dynamic nano-toxicity assessment of energy budget distribution. J. Hazard. Mater. 187, 502–508 (2011). [DOI] [PubMed] [Google Scholar]
- Metzler D. M., Li M., Erdem A. & Huang C. P. Responses of algae to photocatalytic nano-TiO2 particles with an emphasis on the effect of particle size. Chem. Eng. J. 170, 538–546 (2011). [Google Scholar]
- Vale G., Franco C., Diniz M. S., dos Santos M. M. & Domingos R. F. Bioavailability of cadmium and biochemical responses on the freshwater bivalve Corbicula fluminea–the role of TiO2 nanoparticles. Ecotoxicol. Environ. Saf. 109, 161–168 (2014) [DOI] [PubMed] [Google Scholar]
- Range P. et al. Calcification, growth and mortality of juvenile clams Ruditapes decussatus under increased pCO2 and reduced pH: variable responses to ocean acidification at local scales? J. Exp. Mar. Biol. Ecol. 396, 177−184 (2011). [Google Scholar]
- Navarro J. M. et al. Impact of medium-term exposure to elevated pCO2 levels on the physiological energetics of the mussel Mytilus chilensis. Chemosphere 90, 1242–1248 (2013). [DOI] [PubMed] [Google Scholar]
- Seibel B. A., Maas A. E. & Dierssen H. M. Energetic plasticity underlies a variable response to ocean acidification in the pteropod, Limacina helicina antarctica. PLoS ONE 7, e30464 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederich M. & Pörtner H. O. Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, 1531–1538 (2000). [DOI] [PubMed] [Google Scholar]
- Anestis A. et al. Response of Mytilus galloprovincialis (L.) to increasing seawater temperature and to marteliosis: Metabolic and physiological parameters. Comp. Biochem. Physiol. A 156, 57–66 (2010). [DOI] [PubMed] [Google Scholar]
- Xia T. et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 6, 1794–1807 (2006). [DOI] [PubMed] [Google Scholar]
- Huang X. Z. et al. Hemocyte responses of the thick shell mussel Mytilus coruscus exposed to nano-TiO2 and seawater acidification. Aquat. Toxicol. 180, 1–10 (2016). [DOI] [PubMed] [Google Scholar]
- Cai W. J. et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 4, 766–770 (2011). [Google Scholar]
- Range P. et al. Impacts of CO2-induced seawater acidification on coastal Mediterranean bivalves and interactions with other climatic stressors. Regional Environ. Change 14 (Suppl 1), S19–S30 (2014). [Google Scholar]
- ASTM D4563-02. Standard Test Method for Determination by Atomic Absorption Spectroscopy of Titanium Dioxide Content of Pigments Recovered From Whole Paint, ASTM International, West Conshohocken, PA, www.astm.org (2015).
- Coughlan J. The estimation of filtering rate from the clearance of suspensions. Mar. Biol. 2, 356–358 (1969). [Google Scholar]
- Hawkins A. J. S. et al. Some general relationships in comparing the feeding physiology of suspension-feeding bivalve mollusks. J. Exp. Mar. Biol. Ecol. 219, 87–103 (1998). [Google Scholar]
- Bayne B. L. et al. The effects of stress and pollution on marine animals (ed. Bayne B. L. et al.) 1–84 (Praeger, 1985).
- Conover R. J. Assimilation of organic matter by zooplankton. Limnol. Oceanogr. 11, 338–354 (1966). [Google Scholar]
- Wang Y. J., Hu M. H., Wong W. H., Cheung S. G. & Shin P. K. S. The combined effects of oxygen availability and salinity on physiological responses and scope for growth in the green-lipped mussel Perna viridis. Mar. Pollut. Bull. 63, 255–261 (2011). [DOI] [PubMed] [Google Scholar]
- Solorzano L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol. Oceanogr. 14, 799–801 (1969). [Google Scholar]
- Elliott J. M. & Davison W. Energy equivalents of oxygen consumption in animals energetics. Oecologia 19, 195–201 (1975). [DOI] [PubMed] [Google Scholar]
- Smaal A. C. & Widdows J. Biomonitoring of coastal waters and estuaries in The scope for growth of bivalves as an integrated response parameter in biological monitoring (ed. Kramer K. J. M.) 247–267 (CRC Press, 1994).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


