Abstract
Background
Abnormal cardiac morphology is a risk factor for cardiovascular complications in kidney transplant patients. A supraphysiologic level of fibroblast growth factor 23 (FGF-23) has been associated with myocardial hypertrophy in this patient population. Our aim was to evaluate the change in cardiac morphology and function following kidney transplantation and to evaluate the association between the change in FGF-23 concentrations and cardiac morphology.
Methods
We performed a longitudinal, prospective cohort study of 143 kidney transplant recipients (73% male, 75% white) measuring left ventricular (LV) mass index, left atrial (LA) volume index, and ejection fraction (EF) by echocardiography at months 1, 12, and 24 post-transplant. FGF-23 levels were measured at months 1 and 24 post-transplant.
Results
Unadjusted and adjusted linear mixed effects models were used to examine changes in outcomes over time. In the adjusted model, LV mass index (P<0.001) and LA volume index (P<0.001) decreased and EF (P=0.009) increased significantly over time. There was a significant association between decreasing FGF-23 levels and improving LV mass index following transplant (P=0.036) in the unadjusted model; however, there was no significant relationship in the adjusted model (0.195).
Conclusion
Understanding the progression of unique cardiovascular risk factors associated with kidney transplantation may provide potential opportunities to improve survival.
Keywords: Left ventricle hypertrophy, left ventricle mass, kidney transplant, cardiovascular risk factors
Introduction
Patients with end stage renal disease (ESRD) requiring maintenance hemodialysis have a high incidence and prevalence of cardiovascular disease, which is consequently a leading cause of mortality in this patient population (1). Successful kidney transplantation is associated with lower cardiovascular morbidity and mortality compared to patients who remain on the transplant waiting list (2–4). Despite improvements in certain traditional cardiovascular risk factors following kidney transplantation, the death rate from cardiovascular disease in patients with a functioning graft remains high compared to the general population (1).
Left ventricular (LV) hypertrophy is an independent risk factor for morbidity and mortality in both the general population and in patients with ESRD (5) and is present in 75% of patients with ESRD receiving maintenance hemodialysis (6). This high prevalence is multifactorial resulting from comorbid conditions, such as hypertension and diabetes, and the frequent hemodynamic changes experienced during maintenance hemodialysis (5). The increased LV mass in ESRD patients secondary to myocardial hypertrophy is a function of an enlargement of the LV end-diastolic diameter and increased LV wall thickness. While several reports indicate that LV mass improves following kidney transplant (7–13), this has not been universally observed in all kidney transplant patients (14–18).
Left ventricular mass has been shown to be directly correlated with Fibroblast Growth Factor (FGF-23) (19–23). An increased circulating level of FGF-23 is an independent risk factor for cardiovascular disease and mortality (24, 25) and a supraphysiologic level of FGF-23 has been observed to be independently associated with increased LV mass in patients with chronic kidney disease (19–23, 26). Following kidney transplantation, FGF-23 levels decrease concurrently with improved kidney function. Persistently elevated levels of FGF-23 following transplant have been reported to be independently associated with all cause mortality and allograft loss in kidney transplant patients (24). Limited data regarding the relationship between decreasing FGF-23 levels and changes in LV mass following kidney transplantation exist.
Our aim was to evaluate the longitudinal changes in cardiac morphology and function in a cohort of patients following kidney transplantation. In addition, we evaluated FGF-23 concentration following transplant to investigate a potential association between FGF-23 levels and the evolution of cardiac morphology and function. We hypothesized that cardiac morphology and function assessed by echocardiogram would improve following kidney transplantation in patients with functioning allografts, and longitudinal reduction in FGF-23 levels would be associated with these improvements.
Methods
Study Design
We conducted a single-center, prospective cohort study. De novo kidney transplant recipients were recruited from the VUMC Renal Transplant Clinic from August 2009 through May 2013. Inclusion criteria included patients aged ≥ 18 years who were undergoing or had recently undergone kidney transplantation. Exclusion criteria included patients with echocardiogram images that were inadequate to be used for interpretation. Patients in the cohort were followed up at one month following kidney transplantation, which served as the initial visit for documentation regarding demographic, medical and social history, laboratory values, and echocardiography evaluation for the longitudinal study. Follow up visits were performed at 12 and 24 months. All patients gave informed consent and the Vanderbilt University Medical Center (VUMC) Institutional Review Board approved the study protocol.
Echocardiography
A single, licensed sonographer blinded to clinical details obtained the ultrasound images. All echocardiograms were digitally acquired and archived for review. The initial evaluation was obtained at one month following transplant. Follow up examinations were performed at 12 and 24 months.
Two-dimensional, transthoracic echocardiograms (high-resolution ultrasound S5-1 MHz Sector Array transducer, Philips iU 22, Andover, MA) were obtained using the standard American Society of Echocardiography (ASE) protocols (27). The following cardiac parameters were evaluated to describe cardiac morphology and function: (1) Left ventricular (LV) mass index and LV mass (2) Left atrial (LA) volume index (3) relative wall thickness (RWT) and (4) ejection fraction.
A cardiac anesthesiologist (AH), board-certified in quantitative echocardiography, reviewed the images for interpretation and quality control. This reviewer was blinded to the clinical data and prior echocardiograms for the study cohort.
Left Ventricular Mass and Left Ventricular Mass Index
Ventricular measurements were obtained in the parasternal, long axis view. Left ventricular measurements were obtained at end-diastole (LV end diastolic dimension (LVEDD), posterior wall thickness (PWT), and interventricular septum thickness (IVS)) and end-systole (LV end systolic dimension (LVESD)). Left ventricular mass was calculated using the 2-dimensional (2D) linear formula recommended by the ASE as follows: LV mass = 0.8 × 1.04[(LVEDD+ PWT + IVS)3 − (LVEDD) 3]+ 0.6 grams. The calculated LV mass was indexed by the body surface area to grams/meter2 (g/m2). LV hypertrophy was defined when LV mass index was >95 g/m2 in women and >115 g/m2 in men(27).
Left Atrial Volume Index
Left atrial volume was measured at the end of systole when the LA chamber is at its greatest dimension. Based on the ASE recommendation, evaluation of LA volume was determined by the biplane-area-length method. Orthogonal apical views, apical four and two-chamber views were obtained for determination of LA area and length. Left atrial volume was calculated on the basis of the algorithm: 0.85 × A1 × A2/L; where A1 and A2 are the areas of the LA in four and two chamber views, respectively, and L is the shortest of the lengths obtained from the orthogonal views. The calculated LA volume was indexed by the body surface area to milliliters/meter2 (ml/m2). An abnormal LA volume index was defined as >29 ml/m2 (27).
Relative Wall Thickness (RWT)
Ventricular measurements of the left ventricular posterior wall diameter (LVPWD) and left were ventricular end diastolic (LVEDD) dimension were obtained in the parasternal, long axis view. Relative wall thickness was calculated using the following ratio: 2 × LVPWD/LVEDD. A value of 0.42 was defined as a cutoff limit to define concentric (RWT > 0.42) or eccentric (RWT ≤0.42) LV hypertrophy(27). Concentric hypertrophy was defined as both an elevated LV mass index and RWT. Concentric remodeling was defined as an elevated RWT and a normal LV mass index. Eccentric hypertrophy was defined as an elevated LV mass index and normal RWT while normal geometry was normal LV mass index and a normal RWT (27).
Ejection Fraction
The cardiac function was evaluated by the biplane method by measuring the end diastolic volume (EDV) and end systolic volume (ESV). Ejection fraction was calculated by the following formula: (EDV-ESV)/EDV. An abnormal ejection fraction was defined as <55%.
Fibroblast Growth Factor-23 Measurement
Serum concentrations of FGF-23 were measured in duplicate after a single thaw of stored blood specimens using an intact FGF-23 ELISA kit (Immutopics, San Clemente, CA). Measurements in blood samples obtained at one month following kidney transplant served as the baseline FGF-23 measurements. The longitudinal assessment of FGF-23 levels was performed on blood samples obtained at the 24-month follow-up. The coefficient of determination from the primary standard deviation curve for the FGF-23 measurements was 0.9999, indicating high reproducibility.
Outcomes
The primary outcome was defined as the change in LV mass index from baseline (one month post-transplant) to the 12-month follow-up. Secondary outcomes included the change in LV mass index from baseline to 24 months, the change in LV mass, LA volume index, RWT, and ejection fraction from baseline, 12 months and 24 months following kidney transplant, and the association of FGF-23 levels with changes in cardiac morphology (LV mass index, LV mass, and LA volume index) and function (ejection fraction) from baseline to 24 months post-renal transplant.
Statistical analysis
Descriptive statistics were presented as mean with standard deviation or as median with interquartile range (IQR) for continuous variables and as percentages for categorical variables. Based on baseline information at one month post-transplant, we compared the differences between patients who followed up at 12 months post transplant and those patients who did not return for evaluation using either Pearson’s chi-square test (for categorical variables) or Wilcoxon rank sum test (for continuous variables). Linear mixed effects models with random intercepts were used to assess changes in cardiac function and morphology over time without and with adjustment for clinical covariates selected a priori due to their possible influence on echocardiographic findings. Selected covariates included age, race, sex, body mass index (BMI, kg/m2), months on dialysis, cardiovascular disease, mean arterial pressure (MAP), diabetes, and estimated glomerular filtration rate (eGFR). Months on dialysis prior to transplant, eGFR, MAP, and age were included in the models as non-linear terms using restricted cubic splines. Cardiovascular disease was defined as a baseline history of coronary artery disease, myocardial infarction, reperfusion (coronary artery bypass or percutaneous stent), congestive heart failure, arrhythmia, stroke, or peripheral vascular disease.
A linear mixed effects model with random intercepts was also used to examine the change in FGF-23 concentrations over time with and without adjustment for age, race, sex, MAP, cardiovascular disease, and eGFR. In order to meet the normality assumptions of the residuals, FGF-23 concentrations were natural logarithmically transformed. All covariates for models were chosen a priori. Analyses were performed using R, version 3.1.2 (http://www.r-project.org/). The 5% significance level (2 sided) was used.
Results
Patient Demographic and Other Characteristics
The study cohort included 143 patients who underwent one-month evaluation following kidney transplantation. Demographic and other patient characteristics are presented in Table 1. The cohort (mean age 49±13 years) was 73% male and 75% white. One hundred thirty six patients (95%) reported to have hypertension, and 39% and 38% of the cohort had a history of cardiovascular disease and diabetes respectively. Three percent of the cohort was taking angiotensin converting enzyme inhibitors, 3% angiotensin receptor blockers, and 57% of beta blockers at the initial evaluation. At 12 months, 6% were taking angiotensin converting enzyme inhibitors, 11% angiotensin receptor blockers, and 46% beta-blockers. At 24 months 5% were taking angiotensin converting enzyme inhibitors, 24% angiotensin receptor blockers, and 45% beta blockers. The median eGFR at one month, 12 month and 24 month follow up was 57 ml/min/m2, 59 ml/min/m2, and 57 ml/min/m2, respectively. The MAP was 96 mmHg at both the initial evaluation and 12 month evaluation, and was 98 mmHg at the 24 month follow up.
TABLE 1.
Baseline characteristics of the kidney transplant cohort at one-month post-transplantation
| Characteristic | N=143 |
|---|---|
| Age at transplant (years) | 49 (40, 58) |
|
| |
| Race | |
| White | 107 (75%) |
| Black | 33 (23%) |
| Other | 3 (2%) |
|
| |
| Sex (male) | 105 (73%) |
|
| |
| BMI | 27.1 (23.3, 30.5) |
|
| |
| Tobacco use | |
| Never | 75 (53%) |
| Current | 12 (8%) |
| Former | 56 (39%) |
|
| |
| Pack Years | 10 (3, 25) |
|
| |
| Cardiovascular disease1 | 55 (39%) |
|
| |
| Diabetes | 54 (38%) |
|
| |
| Hypertension | 136 (95%) |
|
| |
| Hyperlipidemia | 81 (57%) |
|
| |
| Months on Dialysis pre-transplant | 26.4 (13.5, 51.3) |
|
| |
| Primary cause of end-stage renal disease | |
| Diabetic nephropathy | 42 (29%) |
| Hypertensive nephropathy | 32 (22%) |
| Glomerular Disease | 30 (21%) |
| Tubulintersitial Disease | 8 (6%) |
| Cystic Disease | 19 (13%) |
| Structure Disease | 1 (1%) |
| Renal Neoplasia | 1 (1%) |
| Other | 10 (7%) |
|
| |
| Estimated Glomerular Filtration Rate (ml/min/m2) | 57 (48, 69) |
|
| |
| Mean Arterial Pressure (mm Hg) | 95.6 (87.5, 103.8) |
|
| |
| Induction Immunosuppression | |
| Alemtuzumab | 129 (90%) |
| Basiliximab | 13 (9%) |
| Anti-Thymocyte Globulin | 1 (1%) |
|
| |
| Maintenance Immunosuppression | |
| Tacrolimus | 140 (98%) |
| Everolimus | 4 (3%) |
| Sirolimus | 0 (0%) |
| Mycophenolate | 137 (96%) |
| Prednisone | 80 (56%) |
|
| |
| Anti-Hypertensive Medication | |
| ACE2 Inhibitors | 4 (3%) |
| Beta Blockers | 82 (57%) |
| Angiotensin Receptor Blocker | 4 (3%) |
|
| |
| Statin Therapy | 27 (19%) |
|
| |
| Slow Graft Function3 | 21 (15%) |
|
| |
| Delayed Graft Function | 9 (6%) |
Values expressed as median (25th, 75th percentiles), number (percent), or mean (standard deviation).
Cardiovascular disease includes coronary artery disease, myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, congestive heart failure, arrhythmia, stroke, and peripheral vascular disease.
Angiotensin Converting Enzyme
Slow graft function was defined as a less than a 50% drop in serum creatinine over 72 hours after transplant without the need for hemodialysis.
The flow chart of the longitudinal follow up of the study cohort including the detailed reasons for study drop out is shown in Figure 1. An analysis (For details, see Table 1 of the Supplemental Appendix) comparing selected characteristics of the patients who returned for follow up and the 41% of patients who did not remain in the study through the 12-month evaluation showed no significant differences between the groups.
Figure 1.
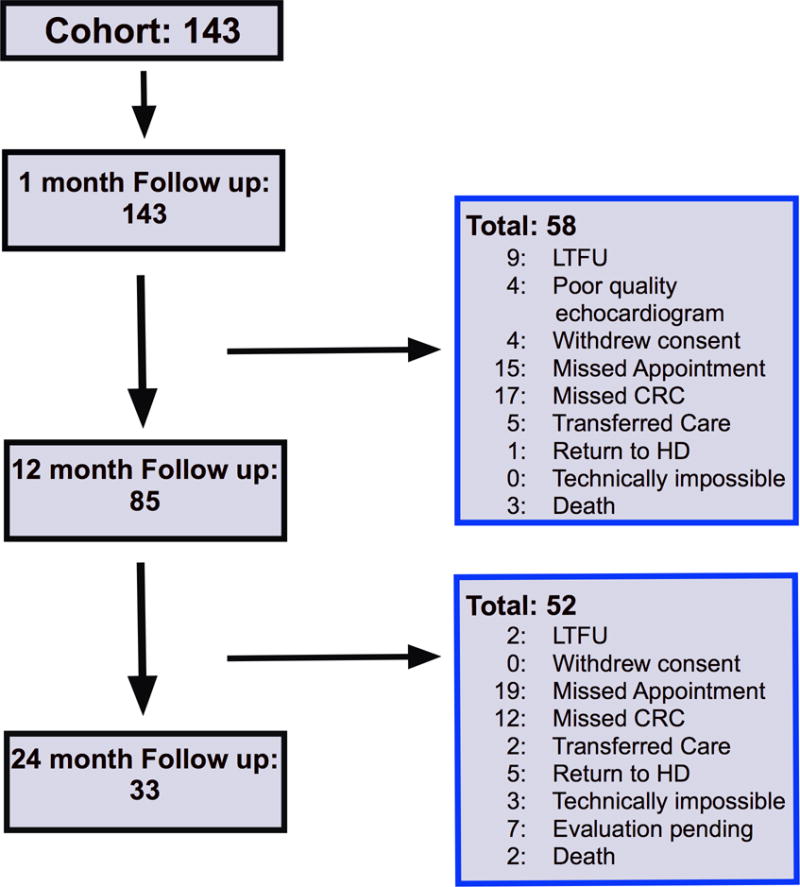
The induction and maintenance immunosuppression regimens were relatively standardized at VUMC with minimal immunosuppression changes during the study period. Ninety percent of the study cohort received alemtuzumab for induction. Tacrolimus and mycophenolate were used in 98% and 96% of the cohort, respectively. No patients in the study cohort were taking sirolimus at the one month follow up, however one patient was transitioned to sirolimus at the 12 month follow up and one patient was transitioned at the 24 month follow up. Four patients (3%) took everolimus during the study and had no changes in their immunosuppression regimen during the follow up period. There were four patients that took cyclosporine at the one-month follow up. Three of those patients continued cyclosporine throughout their follow up, while one patient was transitioned to tacrolimus at the month 12 follow up. One patient transitioned from tacrolimus to cyclosporine at the month 24 follow up.
Minimal acute rejection was observed. Two biopsy confirmed acute rejection episodes occurred by the one-month follow up period. In addition, two other biopsies confirmed acute rejection episodes were treated between the month 1 and 12-month follow up, and one biopsy confirmed acute rejection episode treated between the 12 and 24-month follow up.
Throughout the two-year study period, there was one major cardiac event (myocardial infarction) that occurred between the 12 and 24-month follow up. There were a total of six reported events of angina during the data collection period. Three events occurred in the perioperative period prior to the 1 month evaluation, while two events occurred between the one month and 12 month follow up. There was one event reported between the 12 month and 24 month follow up, and one dysrhythmic event which occurred between the one month and 12 month follow up.
Primary Outcome
The median LV mass index at 1 month and 12 months (Table 2) for the cohort was 102 g/m2 (IQR: 82,123) and 92 g/m2 (IQR: 76, 113), respectively (Figure 2). Thus, based on the prior definition, 38% of men (40/105) and 39% of women (15/38) had an elevated LV mass index at baseline. Seventeen percent (10/63) of men and 27% (6/22) of women had an elevated LV mass index at both 1 month and 12 months, while 21% (13/63) of men and 9% of women (2/22) had an elevated LV mass index at 1 month but a normal LV mass index at 12 months. Nine percent of both men (6/63) and women (2/22) had a normal LV mass index at baseline, but developed de novo LV hypertrophy at 12 months.
TABLE 2.
Changes in cardiac function, cardiac morphology and other clinical characteristics during the study period
| Parameters | Month 1 | Month 12 | Month 24 |
|---|---|---|---|
| Left Ventricular Mass Index (grams/meter2) | 102 (82, 123) | 92 (76, 113) | 96 (73, 108) |
| Left Ventricular Mass (grams) | 200 (160, 251) | 189 (151, 234) | 198 (147, 238) |
| Ejection Fraction (percentage) | 61 (56, 64) | 63 (59, 66) | 63 (59, 66) |
| Left Atrial Volume Index (milliliters/meters2) | 27 (22, 37) | 27 (21, 32) | 25 (21, 30) |
| Estimated Glomerular Filtration Rate (ml/min/m2) | 57 (48, 70) | 58 (48, 71) | 57 (44, 68) |
| Body Mass Index (kilograms/meter2) | 27 (23, 30) | 29 (25, 32) | 29 (25, 33) |
| Mean Arterial Pressure (mm Hg) | 96 (87, 104) | 96 (91, 104) | 99 (92, 105) |
Values expressed as median (25th, 75th percentiles)
Figure 2.
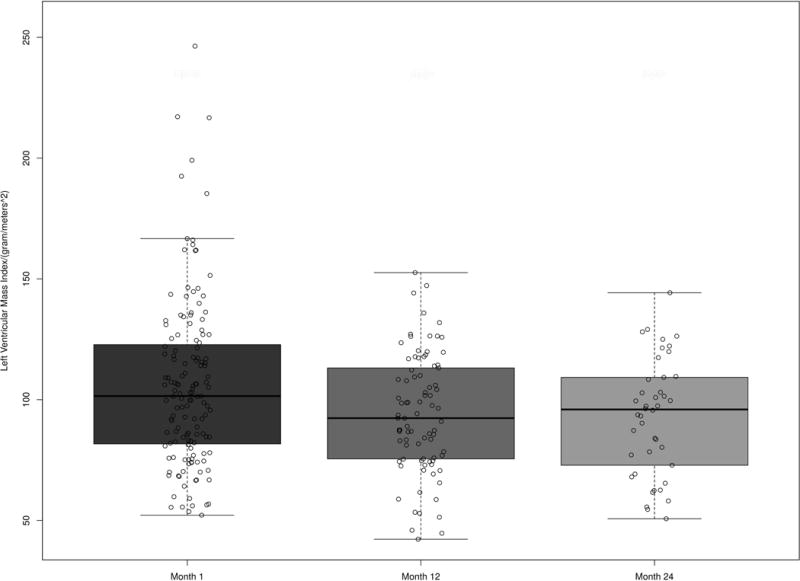
The unadjusted and adjusted models examining the change in LV mass index over time are presented in Table 3. After adjusting for age, race, sex, months on dialysis, cardiovascular disease, MAP, diabetes, BMI, and eGFR, LV mass index changed significantly over time (P<0.001). Compared to month 1, LV mass index decreased by 10.82 g/m2 (95% CI: −16.28, −5.36, P<0.001) at month 12 (Table 3). The covariates that were significant in the adjusted model were months since transplant (ref: 1 month, effect from 1 month to 12 months: −10.84, 95% CI: −16.29, −5.38, P=<0.001), BMI (non-linear effect: −2.78, 95% CI: −9.26, 3.67, P=0.043) and sex (ref: males, effect −14.150, 95% CI: −24.19, −4.11, P=0.006). Age (P=0.334), MAP (P=0.447), cardiovascular disease (P=0.566), diabetes (P=0.183), race (P=0.110), eGFR (P=0.288) and months on dialysis prior to transplant (P=0.072) did not significantly impact the change in LV mass index during the study period.
Table 3.
Unadjusted and adjusted change in echocardiogram morphology and function at 12 and 24 months compared to the baseline evaluation at 1 month following kidney transplant
| Unadjusted Outcome | 12 month effect | 95% CI | P Value | 24 month effect | 95% CI | P Value | Overall P |
|---|---|---|---|---|---|---|---|
| LV mass index | −10.21 | −15.52, −4.90 | <0.001 | −16.21 | −23.37, −9.04 | <0.001 | <0.001 |
| LV mass | −12.61 | −22.71, −2.51 | 0.016 | −22.64 | −36.30, −8.98 | 0.001 | 0.002 |
| Ejection fraction | 1.57 | 0.18, 2.97 | 0.029 | 2.23 | 0.39, 4.07 | 0.019 | 0.021 |
| LA volume index | −2.95 | −4.80, −1.09 | 0.002 | −4.87 | −7.34, −2.40 | <0.001 | <0.001 |
|
| |||||||
|
Adjusteda Outcome |
12 month effect | 95% CI | P Value | 24 month effect | 95% CI | P Value | Overall P |
|
| |||||||
| LV mass index | −10.82 | −16.28, −5.36 | <0.001 | −15.59 | −22.90, −8.27 | <0.001 | <0.001 |
| LV mass | −13.96 | −24.48, −3.43 | 0.010 | −21.47 | −35.59, −7.35 | 0.003 | 0.003 |
| Ejection fraction | 1.54 | 0.09, 2.98 | 0.039 | 2.62 | 0.72, 4.52 | 0.008 | 0.013 |
| LA volume index | −3.31 | −5.19, −1.44 | <0.001 | −5.12 | −7.60, −2.64 | <0.001 | <0.001 |
95% Confidence Interval: Lower bound, Upper bound
Each outcome was adjusted for age at transplant, race, gender, time on dialysis, cardiovascular disease, Mean arterial pressure, estimated glomerular filtration rate, diabetes, and body mass index
Secondary Outcomes
LV mass index at 24 months
The median LV mass index at 24 months for the cohort was 96 g/m2 (IQR: 73, 108) (Figure 2). After the adjustment for risk factors, LV mass index changed significantly over time with a decrease of 15.59 g/m2 (95% CI: −22.90, −8.27, P<0.001) from month 1 compared to month 24.
LV mass, Ejection fraction, and LA volume index
The median measurements for LV mass, ejection fraction, and LA volume index at baseline, 12 months and 24 months following kidney transplant are described in Table 2. The change in LV mass, ejection fraction, and LA volume index over time in unadjusted and adjusted models are shown in Table 3. In the adjusted model, LV mass changed significantly at both 12 months and 24 months (both p values <0.05). Compared to baseline, LV mass decreased by 13.96 grams (95% CI: −24.48, −3.43, P=0.010) at 12 months and 21.47 grams (95% CI: −35.59, −7.35, P=0.003) at 24 months.
Based on our definition for elevated LA volume index (>29 ml/m2), 46% (60/131) of the population had an elevated LA volume index at baseline. In the adjusted model, LA volume index changed significantly at both 12 months and 24 months. Compared to baseline, LA volume index decreased by 3.31 ml/m2 (95% CI: −5.19, −1.44, P<0.001) at 12 months, and by 5.12 ml/m2 (95% CI: −7.60, −2.64, P<0.001) at 24 months.
Twenty-three percent (30/132) of the population had depressed cardiac function (ejection fraction <55%) at baseline. In the adjusted model, ejection fraction changed significantly at both 12 months and 24 months. Compared to baseline, ejection fraction improved by 1.54 percent (95% CI: 0.09, 2.98, P=0.039) at 12 months and 2.62 percent (95% CI: 0.72, 4.52, P=0.008) at 24 months.
RWT and LV mass index
Based on our defined cutoff limit (RWT > 0.42), there was not a significant regression of concentric cardiac wall thickness over time (P=0.096). Based on our prior definitions, 36% of men (38/105, LV mass index>115 and RWT>0.42) and 37% of women (14/38, LV mass index>95 and RWT>0.42) had concentric hypertrophy at baseline, and 41% (43/105, LV mass index<115 and RWT>0.42) of men and 55% of women (31/38, LV mass index<95 and RWT>0.42) had concentric remodeling at baseline. At month 12, 25% (35/63) of men and 32% (7/22) of women had concentric hypertrophy, while 56% (35/63) of men and 68% (15/22) of women had concentric remodeling.
FGF-23 Levels
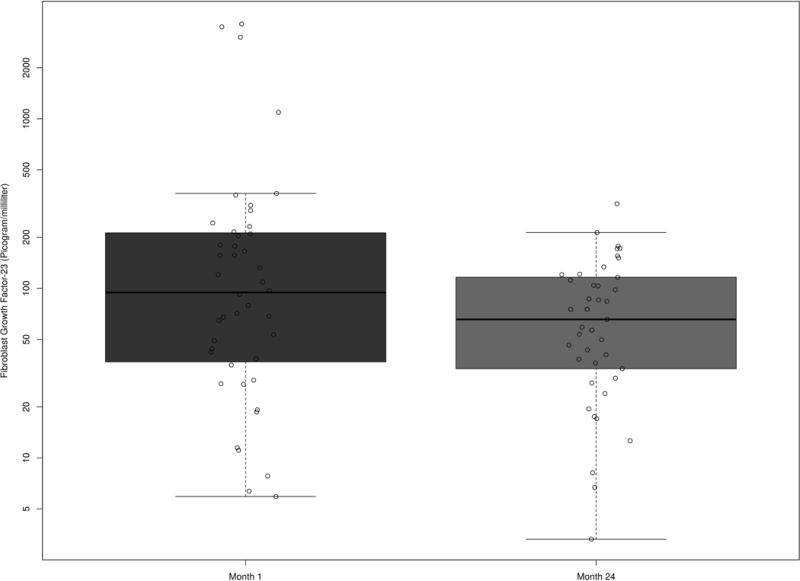
Forty-five patients were included in the analysis that measured FGF-23 at 1 month and 24 months follow-up after kidney transplantation (Figure 3). FGF-23 decreased significantly over time (P=0.024). In particular, compared to month 1, FGF-23 decreased by 36% at month 24 (95% CI: 7%, 56%).
Figure 3.
Cardiac morphology and FGF-23 level
The relationships between FGF-23 and cardiac morphology and function over time can be reviewed in Table 4. In the unadjusted model, there was a significant association between FGF-23 and LV mass index (Figure 4). With one IQR (~100 picograms per milliliters) decrease in FGF-23, LV mass index decreased by 8.83 g/m2 (95% CI: −17.10, −0.56, P= 0.036). However, adjusting for age, race, gender, MAP, cardiovascular disease, and eGFR, there was no statistically significant relationship between the decrease in FGF-23 and LV mass index over time from 1 month to 24 months following kidney transplant (effect= −5.82, 95% CI: −14.62, −2.98, P=0.195). The covariates that were themselves significant in the model were race and time since transplant (Table 5).
Table 4.
The effect of one interquartile range decrease of FGF-23 on cardiac morphology and function with and without adjustment for co-variants
| Outcome | Unadjusted effect | 95% CI | P Value | Adjusteda effect |
95% CI | P Value |
|---|---|---|---|---|---|---|
| LV mass index | −8.83 | −17.10, −0.56 | 0.036 | −5.82 | −14.62, 2.98 | 0.195 |
| LV mass | −20.84 | −37.20, −4.47 | 0.013 | −15.01 | −32.26, 2.24 | 0.088 |
| Ejection fraction | −0.38 | −2.03, 1.27 | 0.655 | −1.51 | −3.23, 0.22 | 0.086 |
| LA volume index | −1.07 | −3.88, 1.74 | 0.455 | 1.22 | −1.88, 4.33 | 0.440 |
Each outcome was adjusted for age at transplant, race, gender, cardiovascular disease, Mean arterial pressure, and estimated glomerular filtration rate.
95% Confidence Interval: Lower bound, Upper bound
Figure 4.
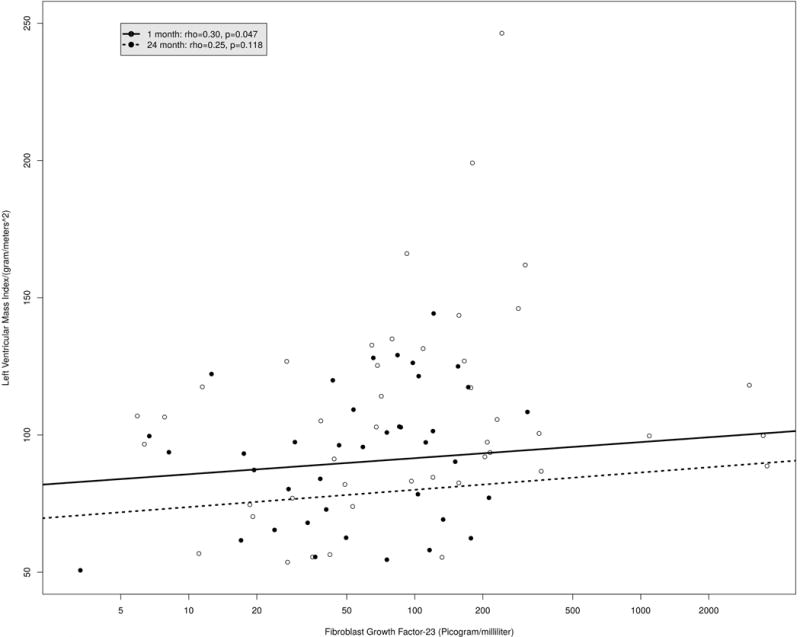
Table 5.
Multivariable linear regression model of the relationship between FGF-23 and LV mass index in kidney transplant recipients.
| Covariate | Effect | S.E. | 95% CI1 | P |
|---|---|---|---|---|
| Predictors of LV mass index | ||||
| Age at transplant | −8.59 | 7.58 | −23.45, 6.27 | 0.507 |
| Race (reference: white) | 34.84 | 11.41 | 12.48, 57.21 | 0.009 |
| Sex (reference: males) | −12.15 | 10.91 | −33.53, 9.22 | 0.265 |
| Cardiovascular Disease2 | −12.76 | 8.83 | −30.07, 4.55 | 0.149 |
| Glomerular filtration rate | −1.59 | 4.53 | −10.47, 7.30 | 0.446 |
| Mean arterial pressure | −0.93 | 4.37 | −9.50, 7.64 | 0.924 |
| Change over time | −11.42 | 5.29 | −21.79, −1.04 | 0.031 |
95% Confidence Interval: Lower bound, Upper bound
Cardiovascular disease includes coronary artery disease, myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, congestive heart failure, arrhythmia, stroke, and peripheral vascular disease.
Discussion
The aim of our study was to evaluate the longitudinal change in cardiac morphology and function following kidney transplantation along with FGF-23 levels to gain an improved understanding of the evolution of cardiovascular risk factors in patients with a functioning allograft. In our prospective cohort, our results show that there was a significant improvement in LV mass index, LA volume index and ejection fraction from baseline to 12 and 24 months following transplantation. We also observed a significant decrease in FGF-23 levels, but this was not associated with LV mass index in adjusted analyses.
In contrast to our primary finding, several prior studies have failed to show a significant improvement in LV mass following kidney transplant (14–18). For instance, Patel et al evaluated 25 patients who underwent kidney transplant and 25 patients who remained on the waiting list with cardiac magnetic resonance over a mean time period of 2.4 to 2.8 years and saw no significant difference in the change in LV mass index between groups. However, consistent with our findings, other studies support that LV mass decreases following kidney transplant (8, 10, 12, 29–31). Rigatto et al performed a prospective study evaluating LV mass index in 143 transplant recipients over a four-year period and observed that LV mass index decreased significantly over the first two years after transplant, but plateaued over the third and fourth year. Other reports that have described an improvement in LV mass following transplant have a limited cohort size (<45 patients) (8, 10, 30, 31) or are retrospective in study design (29). Given the long duration of follow up in our study evaluating a large prospective cohort, our findings strengthen the evidence describing an improvement in LV mass after kidney transplantation. In addition, the conversion from calcineurin inhibitors to sirolimus or everolimus has been shown to be associated with improved LV mass (32). This potential confounder was mitigated in our study by the minimal use of everolimus (4 patients) and sirolimus (2 patients) in our study cohort.
The majority of our cohort had either concentric hypertrophy (elevated LV mass index and elevated RWT) or concentric remodeling (normal LV mass index and elevated RWT) at baseline. However, 25% of males and 32% of females had concentric hypertrophy at month 12, while 56% of males and 68% of females had concentric remodeling. The physiologic shift from LV hypertrophy to LV remodeling is supported by the significant improvement in LV mass index following transplant observed in our analysis. The study cohort also had a low percentage (9%) of patients who developed de novo LV hypertrophy during the follow up period. Overall, this suggests improvement in cardiac morphology post kidney transplant. The importance of these findings is highlighted by a recent prospective, cohort study that showed that the regression of left ventricular hypertrophy following transplant resulted in a lower incidence of mortality and cardiovascular events regardless of the therapeutic strategy used to control cardiovascular risk factors (28).
A unique aspect of our study is that we also evaluated the longitudinal change in the LA volume index in the cohort. Left atrial volume index is a reflection of long-standing hemodynamic conditions and diastolic function providing a good correlate to predict cardiovascular outcomes. An increased LA volume has been shown to be associated with an increased risk of stroke and death in both the general population, ESRD patients and kidney transplant recipients (34–37). Patel et al evaluated 119 kidney transplant recipients with cardiac magnetic resonance and reported that the presence of an elevated LA volume prior to transplant was an independent risk factor for reduced post-transplant survival (38). Our report is the first study describing the longitudinal change in LA volume index in kidney transplant receipts. The significant reduction in LA volume index over time following transplant could be a possible mechanism explaining the improved survival experienced by transplant recipients compared to patients who remain on the waiting list.
The specific factors related to chronic kidney disease that lead to a high prevalence of increased LV mass prior to transplant remain poorly defined. By evaluating the relationship of FGF-23 and cardiac morphology, we aimed to evaluate one potential mechanism leading to improved cardiovascular risk in the setting of a functioning allograft. Due to altered metabolism and clearance, FGF-23 levels in patients with chronic kidney disease are elevated and have been shown to be an independent risk factor for LV mass index, LV hypertrophy, cardiovascular events, and increased risk of death (20, 23, 25, 41, 42), though less is known for kidney transplant recipients. Data suggest that FGF-23 may directly inducing cardiac hypertrophy by FGF receptor dependent activation (26).
This is the first prospective report describing the relationship between FGF-23 and cardiac morphology and function following kidney transplant. We observed a significant association between LV mass index and FGF-23 over time following transplant, however the association became non-significant after adjustment for several risk factors. The lack of association observed may potentially be due to our cohort being underpowered; thus further investigation of a potential relationship between FGF-23 and the change in LV mass index following kidney transplant warrants additional larger studies. However, it could also suggest that other metabolic changes related to transplant potentially provides the survival advantage associated with improved LV mass seen in transplant patients compared to patients with end stage renal disease. We also observed that BMI at transplant and male sex were predictive of decreased LV mass index. Given the stable eGFR and MAP throughout the study, these factors do not seem to be responsible for ongoing LV mass reduction after the initial effect of transplant.
A limitation of our study is the substantial percentage of patients who did not complete the follow up, although for approximately one-third of patients this was due to logistical or practical difficulties in scheduling an echocardiogram. A sensitivity analysis did not show any significant demographic or clinical differences between patients who did not return for the 12 month follow up compared to those who completed the study, suggesting that any bias is likely to be minimal. However, differences between these two groups in characteristics not examined cannot be ruled out. Furthermore, the echocardiography in the evaluation of LV mass in patients with ESRD can be inaccurate. Cardiac magnetic resonance imaging (cMRI) has been shown to be a superior imaging modality to evaluate cardiac morphology than echocardiograms (43)(44). The limitation for echocardiography compared to cMRI in assessing LV mass after transplant is that the observed reduction in LV mass seen with echocardiography may be an artifact related to improved intravascular volume (45). This limitation is minimized since our initial evaluation was performed at one month, at which time fluid status should be normalized with a functioning allograft. Furthermore, the reliability of the echocardiogram measurements could have been strengthened by taking the average of the measurements if two ultrasonographers performed the studies rather than one specialist.
In conclusion, our findings extend beyond what has previously been reported for echocardiogram measurements following transplant. In a prospective study of new kidney transplant recipients, we observed a significant improvement in LV mass index, and additionally show an improvement in LA volume index and ejection fraction following transplant. Our results add further data to support the observation that cardiac morphology improves following kidney transplant. The clinical relevance is related to prior observations that a regression in LV mass and LA volume has been shown to be associated with improved survival following renal transplant (28, 37). We were unable to show an association of LV mass and FGF-23 levels, though BMI and male sex may be important clinical factors. Additional prospective research regarding cardiac morphology and function following kidney transplant is needed to understand the possible mechanisms whereby transplantation impacts cardiovascular health.
Supplementary Material
Acknowledgments
Supported in part by Vanderbilt CTSA grant UL1TR000445 from the National Center for Advancing Translational Sciences and by Award Number S10RR027033 from the National Center for Research Resources. Other grant support includes K24 DK62849 (Ikizler) and P30 DK079341 from the National Institute of Diabetes, Digestive and Kidney Diseases; R01 HL070938 (Ikizler) from the National Heart, Lung, and Blood Institute; K23 GM100183 (Birdwell) from the National Institute of General Medical Sciences. Clark Kensinger received salary support from the Renal Biology and Disease Training Program grant: NIH/NIDDK T32DK007569. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 2D
2-dimensional
- ASE
American Society of Echocardiography
- BMI
body mass index
- eGFR
estimated glomerular filtration rate
- EDV
end diastolic volume
- ESRD
end stage renal disease
- ESV
end systolic volume
- FGF-23
Fibroblast Growth Factor
- g/m2
grams/meter2
- IVS
interventricular septum thickness
- IQR
interquartile range
- LA
Left atrial
- LV
Left ventricular
- LVEDD
Left ventricular end diastolic dimension
- LVPWD
left ventricular posterior wall diameter
- LVESD
Left ventricular end systolic dimension
- MAP
mean arterial pressure
- ml/m2
milliliters/meter2
- ml/min/1.73 m2
milliliters/minute/meter2
- PWT
posterior wall thickness
- RWT
relative wall thickness
- VUMC
Vanderbilt University Medical Center
Footnotes
The authors of this manuscript have no conflicts of interest to disclose.
This work was presented at the 2015 American Society of Nephrology conference.
1Clark D. Kensinger MD
Participated in research design, writing of the paper, performance of the research, and data analysis, and editing of the final manuscript.
2Antonio Hernandez MD
Participated in performance of the research, editing of the final manuscript and approval of article.
3Aihua Bian MPH
Participated in research design, writing of the paper, data analysis, contributed to analytic tools, and editing of the final manuscript.
4Meagan Fairchild
Participated in data acquisition, performance of the research, editing and approval of the final manuscript.
5Guanhua Chen PhD
Participated in research design, writing of the paper, data analysis, contributed to analytic tools, editing and approval of the final manuscript.
6Loren Lipworth-Elliot ScD
Participated in research design, editing of the manuscript and approval of the article.
7T. Alp Ikizler MD
Participated in research design, editing of the manuscript, and approval of the article.
8Kelly Birdwell MD, MSCI
Participated in research design, writing of the paper, performance of the research, and data analysis, and editing of the final manuscript.
References
- 1.United States Renal Data System, 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. 2014 Available from: [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 3.Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant. 2004;4(10):1662–1668. doi: 10.1111/j.1600-6143.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 4.Pilmore H, Dent H, Chang S, McDonald SP, Chadban SJ. Reduction in cardiovascular death after kidney transplantation. Transplantation. 2010;89(7):851–857. doi: 10.1097/TP.0b013e3181caeead. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47(1):186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 7.Murdoch CE, Zhang M, Cave AC, Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res. 2006;71(2):208–215. doi: 10.1016/j.cardiores.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Montanaro D, Gropuzzo M, Tulissi P, Vallone C, Boscutti G, Mioni R, et al. Effects of successful renal transplantation on left ventricular mass. Transplant Proc. 2005;37(6):2485–2487. doi: 10.1016/j.transproceed.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Lai KN, Barnden L, Mathew TH. Effect of renal transplantation on left ventricular function in hemodialysis patients. Clin Nephrol. 1982;18(2):74–78. [PubMed] [Google Scholar]
- 10.Osorio JM, Perez Marfil A, Ferreyra C, Perez Abud R, Ruiz Fuentes MC, Galindo P, et al. Evolution of left ventricular mass in renal transplant recipients: the influence of glucose homeostasis and oxidative stress. Transplant Proc. 2012;44(7):2063–2066. doi: 10.1016/j.transproceed.2012.07.086. [DOI] [PubMed] [Google Scholar]
- 11.Peteiro J, Alvarez N, Calvino R, Penas M, Ribera F, Castro Beiras A. Changes in left ventricular mass and filling after renal transplantation are related to changes in blood pressure: an echocardiographic and pulsed Doppler study. Cardiology. 1994;85(5):273–283. doi: 10.1159/000176695. [DOI] [PubMed] [Google Scholar]
- 12.Rigatto C, Foley RN, Kent GM, Guttmann R, Parfrey PS. Long-term changes in left ventricular hypertrophy after renal transplantation. Transplantation. 2000;70(4):570–575. doi: 10.1097/00007890-200008270-00006. [DOI] [PubMed] [Google Scholar]
- 13.Parfrey PS, Harnett JD, Foley RN, Kent GM, Murray DC, Barre PE, et al. Impact of renal transplantation on uremic cardiomyopathy. Transplantation. 1995;60(9):908–914. [PubMed] [Google Scholar]
- 14.De Lima JJ, Vieira ML, Viviani LF, Medeiros CJ, Ianhez LE, Kopel L, et al. Long-term impact of renal transplantation on carotid artery properties and on ventricular hypertrophy in end-stage renal failure patients. Nephrol Dial Transplant. 2002;17(4):645–651. doi: 10.1093/ndt/17.4.645. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez D, Lacalzada J, Rufino M, Torres A, Martin N, Barragan A, et al. Prediction of left ventricular mass changes after renal transplantation by polymorphism of the angiotensin-converting-enzyme gene. Kidney Int. 1997;51(4):1205–1211. doi: 10.1038/ki.1997.164. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez D, Gonzalez A, Rufino M, Laynez I, de la Rosa A, Porrini E, et al. Time-dependent changes in cardiac growth after kidney transplantation: the impact of pre-dialysis ventricular mass. Nephrol Dial Transplant. 2007;22(9):2678–2685. doi: 10.1093/ndt/gfm247. [DOI] [PubMed] [Google Scholar]
- 17.McGregor E, Stewart G, Rodger RS, Jardine AG. Early echocardiographic changes and survival following renal transplantation. Nephrol Dial Transplant. 2000;15(1):93–98. doi: 10.1093/ndt/15.1.93. [DOI] [PubMed] [Google Scholar]
- 18.Patel RK, Mark PB, Johnston N, McGregor E, Dargie HJ, Jardine AG. Renal transplantation is not associated with regression of left ventricular hypertrophy: a magnetic resonance study. Clin J Am Soc Nephrol. 2008;3(6):1807–1811. doi: 10.2215/CJN.01400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu HJ, Wu MS. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci. 2009;337(2):116–122. doi: 10.1097/MAJ.0b013e3181815498. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkpantur A, Balci M, Gurbuz OA, Afsar B, Canbakan B, Akdemir R, et al. Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol Dial Transplant. 2011;26(4):1346–1354. doi: 10.1093/ndt/gfq539. [DOI] [PubMed] [Google Scholar]
- 22.Jimbo R, Shimosawa T. Cardiovascular Risk Factors and Chronic Kidney Disease-FGF23: A Key Molecule in the Cardiovascular Disease. Int J Hypertens. 2014;2014:381082. doi: 10.1155/2014/381082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal I, Ide N, Ix JH, Kestenbaum B, Lanske B, Schiller NB, et al. Fibroblast growth factor-23 and cardiac structure and function. Journal of the American Heart Association. 2014;3(1):e000584. doi: 10.1161/JAHA.113.000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22(5):956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Paoletti E, Bellino D, Signori A, Pieracci L, Marsano L, Russo R, et al. Regression of asymptomatic cardiomyopathy and clinical outcome of renal transplant recipients: a long-term prospective cohort study. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv354. [DOI] [PubMed] [Google Scholar]
- 29.Vaidya OU, House JA, Coggins TR, Patil H, Vaidya A, Awad A, et al. Effect of renal transplantation for chronic renal disease on left ventricular mass. Am J Cardiol. 2012;110(2):254–257. doi: 10.1016/j.amjcard.2012.02.067. [DOI] [PubMed] [Google Scholar]
- 30.Dudziak M, Debska-Slizien A, Rutkowski B. Cardiovascular effects of successful renal transplantation: a 30-month study on left ventricular morphology, systolic and diastolic functions. Transplant Proc. 2005;37(2):1039–1043. doi: 10.1016/j.transproceed.2004.12.201. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira SR, Moises VA, Tavares A, Pacheco-Silva A. Cardiovascular effects of successful renal transplantation: a 1-year sequential study of left ventricular morphology and function, and 24-hour blood pressure profile. Transplantation. 2002;74(11):1580–1587. doi: 10.1097/00007890-200212150-00016. [DOI] [PubMed] [Google Scholar]
- 32.Paoletti E, Marsano L, Bellino D, Cassottana P, Cannella G. Effect of everolimus on left ventricular hypertrophy of de novo kidney transplant recipients: a 1 year, randomized, controlled trial. Transplantation. 2012;93(5):503–508. doi: 10.1097/TP.0b013e318242be28. [DOI] [PubMed] [Google Scholar]
- 33.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47(12):2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 34.Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79(8):1008–1014. doi: 10.4065/79.8.1008. [DOI] [PubMed] [Google Scholar]
- 35.Tripepi G, Benedetto FA, Mallamaci F, Tripepi R, Malatino L, Zoccali C. Left atrial volume monitoring and cardiovascular risk in patients with end-stage renal disease: a prospective cohort study. J Am Soc Nephrol. 2007;18(4):1316–1322. doi: 10.1681/ASN.2006080881. [DOI] [PubMed] [Google Scholar]
- 36.Patel RK, Jardine AG, Mark PB, Cunningham AF, Steedman T, Powell JR, et al. Association of left atrial volume with mortality among ESRD patients with left ventricular hypertrophy referred for kidney transplantation. Am J Kidney Dis. 2010;55(6):1088–1096. doi: 10.1053/j.ajkd.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kainz A, Goliasch G, Wiesbauer F, Binder T, Maurer G, Nesser HJ, et al. Left atrial diameter and survival among renal allograft recipients. Clin J Am Soc Nephrol. 2013;8(12):2100–2105. doi: 10.2215/CJN.04300413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel RK, Pennington C, Stevens KK, Taylor A, Gillis K, Rutherford E, et al. Effect of left atrial and ventricular abnormalities on renal transplant recipient outcome-a single-center study. Transplant Res. 2014;3(1):20. doi: 10.1186/s13737-014-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathew JS, Sachs MC, Katz R, Patton KK, Heckbert SR, Hoofnagle AN, et al. Fibroblast growth factor-23 and incident atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS) Circulation. 2014;130(4):298–307. doi: 10.1161/CIRCULATIONAHA.113.005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez Fructuoso AI, Maestro ML, Perez-Flores I, Valero R, Rafael S, Veganzones S, et al. Serum level of fibroblast growth factor 23 in maintenance renal transplant patients. Nephrol Dial Transplant. 2012;27(11):4227–4235. doi: 10.1093/ndt/gfs409. [DOI] [PubMed] [Google Scholar]
- 41.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25(2):349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112(6):855–861. doi: 10.1161/CIRCULATIONAHA.104.507723. [DOI] [PubMed] [Google Scholar]
- 44.Jakubovic BD, Wald R, Goldstein MB, Leong-Poi H, Yuen DA, Perl J, et al. Comparative assessment of 2-dimensional echocardiography vs cardiac magnetic resonance imaging in measuring left ventricular mass in patients with and without end-stage renal disease. Can J Cardiol. 2013;29(3):384–390. doi: 10.1016/j.cjca.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Stewart GA, Foster J, Cowan M, Rooney E, McDonagh T, Dargie HJ, et al. Echocardiography overestimates left ventricular mass in hemodialysis patients relative to magnetic resonance imaging. Kidney Int. 1999;56(6):2248–2253. doi: 10.1046/j.1523-1755.1999.00786.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


