Abstract
The phase III Belatacept Evaluation of Nephroprotection and Efficacy as First‐Line Immunosuppression Trial–Extended Criteria Donors Trial (BENEFIT‐EXT) study compared more or less intensive belatacept‐based immunosuppression with cyclosporine (CsA)–based immunosuppression in recipients of extended criteria donor kidneys. In this post hoc analysis, patient outcomes were assessed according to donor kidney subtype. In total, 68.9% of patients received an expanded criteria donor kidney (United Network for Organ Sharing definition), 10.1% received a donation after cardiac death kidney, and 21.0% received a kidney with an anticipated cold ischemic time ≥24 h. Over 7 years, time to death or graft loss was similar between belatacept‐ and CsA‐based immunosuppression, regardless of donor kidney subtype. In all three donor kidney cohorts, estimated mean GFR increased over months 1–84 for belatacept‐based treatment but declined for CsA‐based treatment. The estimated differences in GFR significantly favored each belatacept‐based regimen versus the CsA‐based regimen in the three subgroups (p < 0.0001 for overall treatment effect). No differences in the safety profile of belatacept were observed by donor kidney subtype.
Keywords: clinical research/practice, kidney transplantation/nephrology, donors and donation: deceased, donors and donation: extended criteria, donors and donation: donation after circulatory death (DCD), immunosuppressant, calcineurin inhibitor: cyclosporine A (CsA)
Short abstract
Irrespective of the type of extended donor kidney transplanted, belatacept‐based immunosuppression is associated with similar death/graft loss and improved renal function at 7 years posttransplant versus cyclosporine‐ based immunosuppression, with no notable differences in the safety profile of belatacept by donor kidney subtype.
Abbreviations
- AE
adverse event
- AR
acute rejection
- BENEFIT
Belatacept Evaluation of Nephroprotection and Efficacy as First‐Line Immunosuppression Trial
- BENEFIT‐EXT
BENEFIT‐Extended Criteria Donors Trial
- CI
confidence interval
- CIT
cold ischemia time
- CNI
calcineurin inhibitor
- CsA
cyclosporine
- DCD
donation after cardiac death
- DGF
delayed graft function
- DSA
donor‐specific antibody
- ECD
expanded criteria donor
- HR
hazard ratio
- LI
less intensive
- MI
more intensive
- PRA
panel reactive antibody
- PTLD
posttransplant lymphoproliferative disorder
- SCD
standard‐criteria deceased donor
- SD
standard deviation
- UNOS
United Network for Organ Sharing
Introduction
Kidney transplantation is the preferred intervention for patients with end‐stage renal disease because it improves patient survival and quality of life 1, 2, 3, but the donor organ supply is limited 4, 5, 6. Expansion of the donor pool to include kidneys from older donors and/or donors with health issues (United Network for Organ Sharing [UNOS] expanded criteria donor [ECD] kidneys) has helped address growing demand. Nevertheless, recipients of kidneys that meet expanded donation criteria tend to have poorer rates of graft and patient survival compared with recipients of living donor or standard‐criteria deceased donor (SCD) kidneys 2, 7, 8, 9, 10. Moreover, patients transplanted with ECD kidneys are more likely to experience delayed graft function (DGF) and diminished allograft function 11, resulting in increased resource utilization and a higher risk of graft loss. Although donation after cardiac death (DCD; i.e. non–heart‐beating donors) kidneys are increasingly being used to help overcome organ shortages 12, better understanding of outcomes in recipients of this donor kidney subtype is needed.
Impaired renal function, particularly GFR, at 1 year after transplant is strongly associated with reduced graft survival and a higher incidence of cardiac death 13, 14, 15, 16, 17, 18, 19. Calcineurin inhibitors (CNIs) have been linked to declining renal function and chronic allograft nephropathy, which may eventually lead to allograft failure 20. There is a need for effective non–CNI‐based immunosuppressive regimens; however, data on non–CNI‐based regimens in recipients of ECD kidneys are limited.
Belatacept is a fusion protein composed of the Fc fragment of a human IgG1 immunoglobulin linked to the modified extracellular domain of CTLA4. Belatacept selectively inhibits T cell activation through costimulation blockade 21, 22, 23, 24 and is indicated for the prophylaxis of organ rejection in adult kidney transplant recipients. Its approval was based in part on the results of two phase III trials: Belatacept Evaluation of Nephroprotection and Efficacy as First‐Line Immunosuppression Trial (BENEFIT), which enrolled recipients of living donor or SCD kidneys 25, and the BENEFIT‐Extended Criteria Donors Trial (BENEFIT‐EXT) 26. BENEFIT‐EXT is the largest prospective study conducted to date examining non–CNI‐based immunosuppression in recipients of kidneys meeting extended donation criteria.
At 3 years after transplant in BENEFIT and BENEFIT‐EXT, belatacept was associated with superior renal function to cyclosporine (CsA) and similar patient and graft survival despite higher grades (BENEFIT and BENEFIT‐EXT) and higher rates (BENEFIT only) of early acute rejection (AR) 27, 28. Post hoc analyses performed at 7 years after transplant demonstrated a significant reduction in the risk of death or graft loss among belatacept‐ versus CsA‐treated patients in BENEFIT 29, whereas rates of death or graft loss were similar across treatment arms in BENEFIT‐EXT 30. In BENEFIT and BENEFIT‐EXT, renal function at 7 years after transplant was significantly greater in belatacept‐ versus CsA‐treated patients 29, 30. Over 7 years, the probability of AR was greater for belatacept‐ versus CsA‐based immunosuppression in BENEFIT 29; the risk of AR was similar in belatacept‐ and CsA‐treated patients in BENEFIT‐EXT 30. To better understand the efficacy and safety of belatacept across donor kidney subtypes, we conducted a post hoc analysis of 7‐year data from BENEFIT‐EXT.
Patients and Methods
Design and patients
BENEFIT‐EXT (ClinicalTrials.gov identifier NCT00114777) was a 3‐year international, randomized, partially blinded, phase III trial of de novo kidney transplant recipients aged ≥18 years. If approved by the treating physician, patients were eligible to continue study treatment beyond 3 years if they provided additional written informed consent. To remain in the study beyond 3 years, patients were required to continue with the immunosuppressive regimen to which they had been randomized. As described previously 26, patients were transplanted with an extended criteria donation kidney, which was protocol defined as a UNOS ECD kidney, a DCD kidney or a kidney with an anticipated cold ischemic time (CIT) ≥24 h. UNOS ECD kidneys were defined as kidneys from donors aged ≥60 years or aged 50–59 years with two or more other risk factors (cerebrovascular accident, hypertension or serum creatinine >1.5 mg/dL). Because donor kidneys could have met more than one extended donation criterion, patients were placed into one of three mutually exclusive cohorts according to the following hierarchy: (i) DCD, (ii) UNOS ECD, (iii) anticipated CIT ≥24 h. Consequently, patients in the DCD cohort could have received a kidney that also met UNOS ECD criteria and/or had an anticipated CIT ≥24 h. Patients in the UNOS ECD cohort could have received a kidney that also had an anticipated CIT ≥24 h. It is important to note that CIT ≥24 h was only anticipated; therefore, patients could have received a kidney with CIT <24 h.
BENEFIT‐EXT was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the ethics committee at each site. All patients provided written informed consent.
Interventions
Patients were randomized (1:1:1) to either more intensive (MI) or less intensive (LI) belatacept‐based immunosuppression or to CsA‐based immunosuppression. Dosing information has been published 26. All patients received basiliximab induction, mycophenolate mofetil and corticosteroids. Use of T cell–depleting agents was permitted for anticipated DGF in CsA‐treated patients at the discretion of the investigator.
The study was blinded to patients and study personnel with respect to the belatacept regimens and was open label with respect to allocation to belatacept or CsA (because of the need for therapeutic dose monitoring in CsA‐treated patients). Placebo infusions were used to maintain blinding between belatacept regimens.
Statistical analysis
Analyses were performed according to intent‐to‐treat principles at 7 years (84 mo) after transplant for all evaluable patients. The evaluable population was composed of patients who were alive and observable at 84 mo after randomization or who had died or experienced graft loss by month 84. The statistical approaches used for this subgroup analysis mirror those used for the overall population at 7 years after transplant 30. Briefly, time to death or graft loss was examined for each donor kidney subtype and compared for belatacept‐ and CsA‐based treatment using a log‐rank test; data were summarized using Kaplan–Meier curves and event rates. Cox regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) at month 84. The interaction between treatment and donor kidney subtype was also examined, with CsA‐treated patients in the UNOS ECD cohort serving as the reference (no adjustment for multiplicity testing). Time to death and time to death‐censored graft loss were assessed as sensitivity analyses; the same statistical methods were used as for the composite analysis of time to death or graft loss, with no adjustment for multiplicity testing 30.
Mean GFR and corresponding CIs were estimated from months 1–84 using a repeated‐measures model with an unstructured covariance matrix. The model included treatment, time and a time–treatment interaction; no further adjustment was made for other potentially confounding covariates. Time was regarded as a categorical variable (intervals of 3 mo up to month 36 and intervals of 6 mo thereafter). A slope‐based model without imputation was also used to determine whether there was a difference between each of the belatacept slopes and the CsA slope. The difference between slopes was tested using contrasts. In the slope‐based analysis, time was regarded as a continuous variable, treatment as a fixed effect, and intercept and time as random effects; no further adjustment was made for other potentially confounding covariates. Sensitivity analyses were performed in which GFR values that were missing due to death or graft loss were imputed as zero; the same models were used as for the analyses without imputation 30.
AR was defined as central biopsy–proven rejection that was clinically suspected either for protocol‐defined reasons or for other reasons and treated. Adverse events (AEs) were mapped to terms from the Medical Dictionary for Regulatory Activities (version 17.0) and expressed as incidence rates adjusted per 100 person‐years of treatment exposure. The presence of de novo donor‐specific antibodies (DSAs) was assessed centrally by solid‐phase flow cytometry (FLowPRA; One Lambda, Inc., Canoga Park, CA). HLA class specificity (class I or II) was determined by LABScreen single antigen beads (One Lambda, Inc.).
Results
Patient population
Patients are summarized by donor kidney subtype and treatment allocation in Figure 1. In total, 68.9% of all randomized transplanted patients received a UNOS ECD kidney, 10.1% received a DCD kidney and 21.0% received a kidney with anticipated CIT ≥24 h. Because CIT of ≥24 h was only anticipated, 16 of the 114 patients (14.0%) in this cohort actually received a kidney with CIT <24 h. Baseline demographics and disease characteristics for each subgroup are shown in Table 1. Data on treatment exposure and duration of follow‐up are summarized in Table S1.
Figure 1.
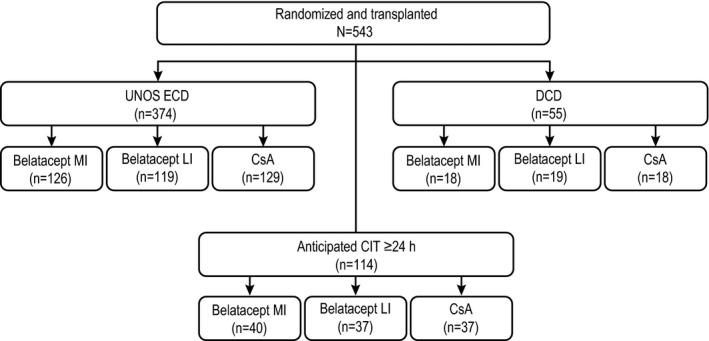
Patient numbers by donor kidney subgroup and treatment allocation. CIT, cold ischemia time; CsA, cyclosporine; DCD, donation after cardiac death; ECD, expanded criteria donor; LI, less intensive; MI, more intensive; UNOS, United Network for Organ Sharing.
Table 1.
Baseline demographics by donor kidney subtype and treatment
| Characteristic | Belatacept MI | Belatacept LI | CsA |
|---|---|---|---|
| Age, years, mean (SD) | |||
| UNOS ECD | 59.1 (11.36) | 58.9 (10.97) | 58.9 (10.74) |
| DCD | 56.7 (13.35) | 53.7 (14.33) | 49.9 (11.92) |
| Anticipated CIT ≥24 h | 49.1 (13.27) | 48.2 (12.42) | 47.0 (12.30) |
| Male, n/n (%) | |||
| UNOS ECD | 85/126 (67.5) | 87/119 (73.1) | 78/129 (60.5) |
| DCD | 13/18 (72.2) | 14/19 (73.7) | 11/18 (61.1) |
| Anticipated CIT ≥24 h | 21/40 (52.5) | 28/37 (75.7) | 27/37 (73.0) |
| Region, n/n (%) | |||
| North America | |||
| UNOS ECD | 33/126 (26.2) | 24/119 (20.2) | 32/129 (24.8) |
| DCD | 8/18 (44.4) | 10/19 (52.6) | 7/18 (38.9) |
| Anticipated CIT ≥24 h | 9/40 (22.5) | 7/37 (18.9) | 7/37 (18.9) |
| South America | |||
| UNOS ECD | 23/126 (18.3) | 23/119 (19.3) | 25/129 (19.4) |
| DCD | 1/18 (5.6) | 3/19 (15.8) | 1/18 (5.6) |
| Anticipated CIT ≥24 h | 21/40 (52.5) | 21/37 (56.8) | 24/37 (64.9) |
| Europe | |||
| UNOS ECD | 69/126 (54.8) | 71/119 (59.7) | 72/129 (55.8) |
| DCD | 9/18 (50.0) | 6/19 (31.6) | 10/18 (55.6) |
| Anticipated CIT ≥24 h | 10/40 (25.0) | 8/37 (21.6) | 6/37 (16.2) |
| CIT, h, mean (median, range) | |||
| UNOS ECD | 16.9 (16.6, 0.7–32.8) | 19.0 (18.6, 5.8–42.7) | 17.1 (16.8, 4.5–36.8) |
| DCD | 17.4 (15.3, 4.3–38.0) | 21.2 (21.0, 7.8–43.0) | 18.4 (18.0, 6.0–29.9) |
| Anticipated CIT ≥24 h | 29.5 (29.1, 0.0–43.3) | 27.9 (27.8, 12.3–44.2) | 27.9 (26.3, 15.1–42.7) |
| Pretransplant dialysis, n/n (%) | |||
| UNOS ECD | 118/126 (93.7) | 112/119 (94.1) | 119/129 (92.2) |
| DCD | 15/18 (83.3) | 16/19 (84.2) | 17/18 (94.4) |
| Anticipated CIT ≥24 h | 39/40 (97.5) | 37/37 (100.0) | 34/37 (91.9) |
| Pretransplant diabetes, n/n (%) | |||
| UNOS ECD | 28/126 (22.2) | 23/119 (19.3) | 36/129 (27.9) |
| DCD | 4/18 (22.2) | 2/19 (10.5) | 11/18 (61.1) |
| Anticipated CIT ≥24 h | 6/40 (15.0) | 3/37 (8.1) | 6/37 (16.2) |
| Categorized PRA,a n/n (%) | |||
| <20% | |||
| UNOS ECD | 123/126 (97.6) | 113/119 (95.0) | 118/129 (91.5) |
| DCD | 17/18 (94.4) | 15/19 (78.9) | 17/18 (94.4) |
| Anticipated CIT ≥24 h | 37/40 (92.5) | 37/37 (100.0) | 33/37 (89.2) |
| ≥20% | |||
| UNOS ECD | 0/126 (0) | 0/119 (0) | 4/129 (3.1) |
| DCD | 0/18 (0) | 1/19 (5.3) | 0/18 (0) |
| Anticipated CIT ≥24 h | 0/40 (0) | 0/37 (0) | 2/37 (5.4) |
CIT, cold ischemia time; CsA, cyclosporine; DCD, donation after cardiac death; ECD, expanded criteria donor; LI, less intensive; MI, more intensive; PRA, panel reactive antibody; SD, standard deviation; UNOS, United Network for Organ Sharing.
PRA data missing for the remaining patients.
Patient and graft survival
The percentages of patients assessed for death or graft loss at 84 mo after transplant in the UNOS ECD, DCD and anticipated CIT ≥24 h cohorts were 68.4% (256 of 374), 63.6% (35 of 55) and 72.8% (83 of 114), respectively. In the UNOS ECD cohort, Kaplan–Meier estimated rates of death or graft loss at month 84 for MI belatacept, LI belatacept and CsA were 35.8%, 40.1% and 41.2%, respectively. The HR for the comparison of MI belatacept with CsA was 0.839 (95% CI 0.539–1.306; p = 0.44), and the HR for the comparison of LI belatacept with CsA was 0.944 (95% CI 0.614–1.452; p = 0.79) (Figure 2A). The corresponding Kaplan–Meier estimated rates in the DCD cohort were 39.4%, 7.7% and 48.7%, respectively. The HR for the comparison of MI belatacept with CsA was 0.722 (95% CI 0.242–2.157; p = 0.56), and the HR for the comparison of LI belatacept with CsA was 0.095 (95% CI 0.012–0.778; p = 0.0282) (Figure 2B). In the anticipated CIT ≥24 h cohort, Kaplan–Meier estimated rates of death or graft loss at month 84 for MI belatacept, LI belatacept and CsA were 27.9%, 30.2% and 14.9%, respectively. The HR for the comparison of MI belatacept with CsA was 1.906 (95% CI 0.651–5.576; p = 0.24), and the HR for the comparison of LI belatacept with CsA was 2.095 (95% CI 0.716–6.132; p = 0.18) (Figure 2C). The Kaplan–Meier curves for the sensitivity analyses in which time to death and time to graft loss were examined separately can be found in Figures S1 and S2, respectively. HRs and 95% CIs deriving from the interaction analysis of treatment and donor kidney subtype on time to death and/or graft loss are summarized in Table S2.
Figure 2.
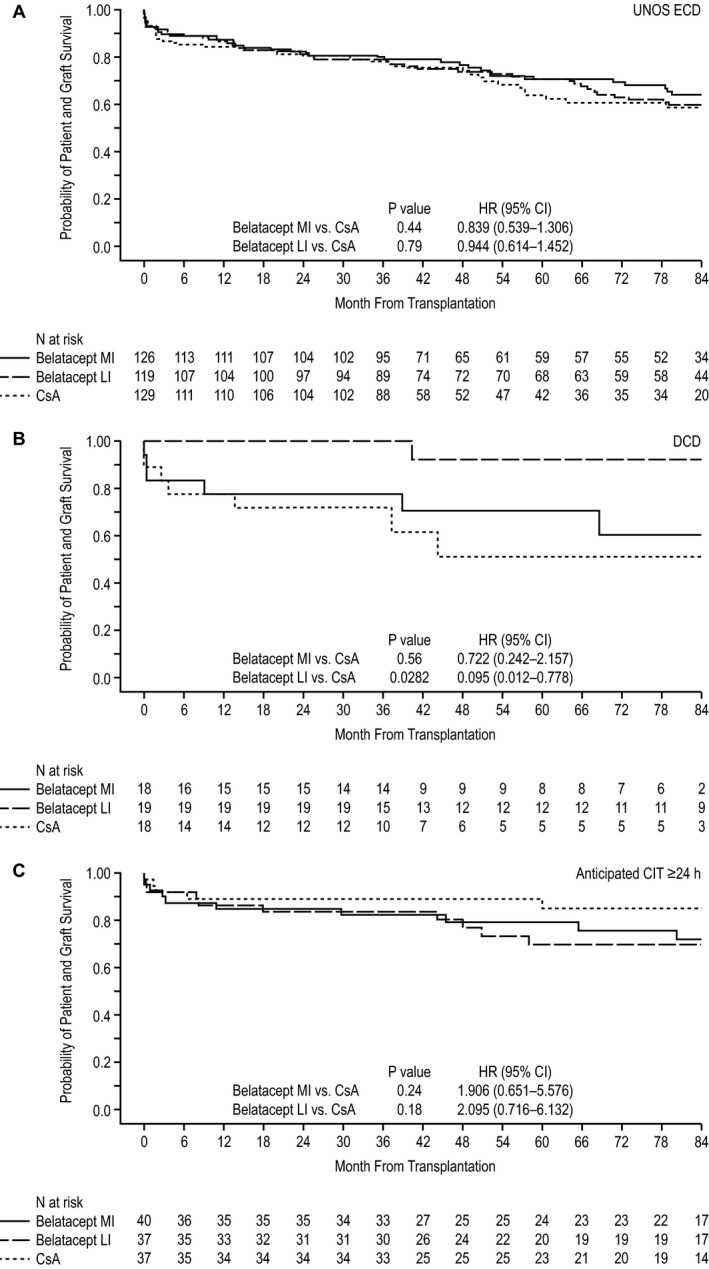
Kaplan–Meier curve for the composite end point of time to death or graft loss in the (A) UNOS ECD, (B) DCD, and (C) anticipated CIT ≥24 h cohorts. CI, confidence interval; CIT, cold ischemia time; CsA, cyclosporine; DCD, donation after cardiac death; ECD, expanded criteria donor; HR, hazard ratio; LI, less intensive; MI, more intensive; UNOS, United Network for Organ Sharing.
A combined end point of time to first occurrence of death, graft loss or estimated GFR <20 mL/min per 1.73 m2 was also examined. Kaplan–Meier estimated rates for this combined end point at month 84 in the UNOS ECD cohort were 41.6%, 43.1% and 54.0% for the MI belatacept, LI belatacept and CsA treatment arms, respectively. The HR for the comparison of MI belatacept with CsA was 0.695 (95% CI 0.471–1.024; p = 0.07), and the HR for the comparison of LI belatacept with CsA was 0.694 (95% CI 0.471–1.022; p = 0.06) (Figure S3A). The corresponding Kaplan–Meier estimated rates in the DCD cohort were 39.4%, 7.7% and 43.3%, respectively. The HR for the comparison of MI belatacept with CsA in the DCD cohort was 0.680 (95% CI 0.227–2.040; p = 0.49), and the HR for the comparison of LI belatacept with CsA was 0.093 (95% CI 0.011–0.757; p = 0.0265) (Figure S3B). In the anticipated CIT ≥24 h cohort, Kaplan–Meier estimated rates of the combined end point at month 84 for MI belatacept, LI belatacept and CsA were 27.9%, 30.2% and 20.0%, respectively. The HR for the comparison of MI belatacept with CsA was 1.338 (95% CI 0.509–3.516; p = 0.55), and the HR for the comparison of LI belatacept with CsA was 1.477 (95% CI 0.562–3.882; p = 0.43) (Figure S3C). HRs and 95% CIs deriving from the interaction analysis of treatment and donor kidney subtype on time to this combined end point are summarized in Table S2.
Renal function
Estimated mean GFR at month 84 ranged from 49.9 to 67.6 mL/min per 1.73 m2 in patients treated with MI belatacept, from 50.5 to 67.6 mL/min per 1.73 m2 in patients treated with LI belatacept and from 32.2 to 50.2 mL/min per 1.73 m2 in patients treated with CsA (Figure 3). Estimated differences in GFR significantly favored each belatacept‐based regimen versus the CsA‐based regimen in all three subgroups (p < 0.0001 for overall treatment effect).
Figure 3.
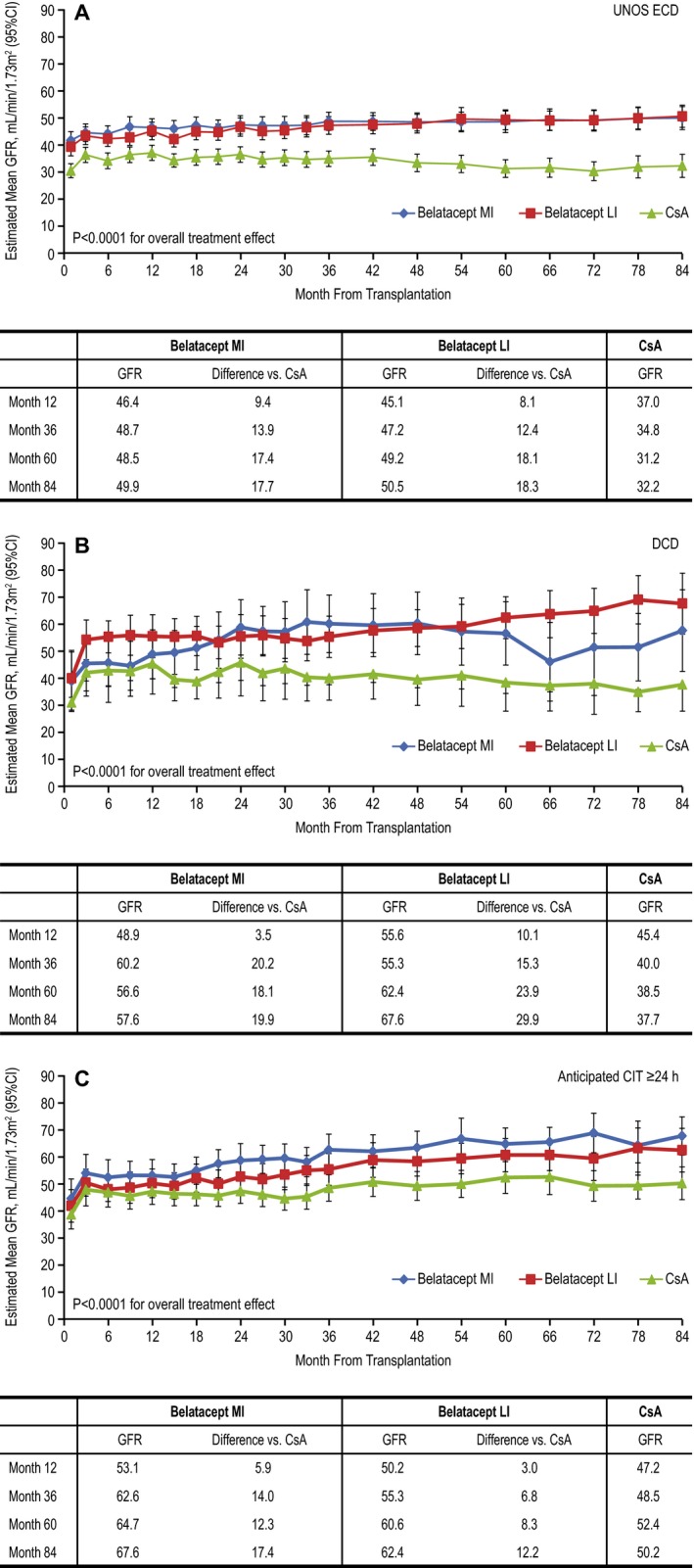
Estimated mean GFR for months 1–84 (mixed‐effects modeling without imputation) in the (A) UNOS ECD, (B) DCD and (C) anticipated CIT ≥24 h cohorts. Data represent means and 95% CIs. CI, confidence interval; CIT, cold ischemia time; CsA, cyclosporine; DCD, donation after cardiac death; ECD, expanded criteria donor; LI, less intensive; MI, more intensive; UNOS, United Network for Organ Sharing.
Using the slope‐based model (and relative to month 1), patients in the UNOS ECD cohort randomized to MI or LI belatacept experienced an estimated mean gain in GFR of 0.88 mL/min per 1.73 m2 (95% CI 0.34–1.43) and 1.24 mL/min per 1.73 m2 (95% CI 0.70–1.77) per year, respectively, whereas CsA‐treated patients had an estimated mean decline in GFR equivalent to −0.49 mL/min per 1.73 m2 (95% CI −1.07 to 0.09) per year. Compared with CsA, the interaction of the treatment versus time effect deriving from the repeated‐measures model significantly favored MI belatacept (p = 0.0008) and LI belatacept (p = 0.0000).
All patients in the DCD cohort had an estimated mean gain in GFR; the estimated gains per year in patients treated with MI belatacept, LI belatacept and CsA were 4.23 mL/min per 1.73 m2 (95% CI 1.16–7.29), 1.52 mL/min per 1.73 m2 (95% CI −1.02 to 4.06) and 1.06 mL/min per 1.73 m2 (95% CI –2.10 to 4.22), respectively. Relative to CsA‐treated patients, the slope estimates did not differ for patients treated with either MI belatacept (p = 0.16) or LI belatacept (p = 0.83).
As in the DCD cohort, all patients in the anticipated CIT ≥24 h cohort experienced an estimated mean gain in GFR; the estimated gains per year in patients treated with MI belatacept, LI belatacept and CsA were 2.56 mL/min per 1.73 m2 (95% CI 1.61–3.52), 2.33 mL/min per 1.73 m2 (95% CI 1.34–3.32) and 1.06 mL/min per 1.73 m2 (95% CI 0.10–2.01), respectively. Relative to CsA‐treated patients, the slope estimate differed significantly for patients treated with MI belatacept (p = 0.0284) but not LI belatacept (p = 0.07). Sensitivity analyses in which GFR values that were missing because of patient death or graft loss were imputed as zero yielded largely similar results (Figure S4).
Rates of DGF in patients treated with MI belatacept, LI belatacept and CsA in the UNOS ECD cohort were 44.4% (56 of 126), 46.2% (55 of 119) and 42.6% (55 of 129), respectively. The corresponding rates in the DCD cohort were 55.6% (10 of 18), 47.4% (9 of 19), and 83.3% (15 of 18), respectively. In the anticipated CIT ≥24 h cohort, 50.0% (20 of 40), 51.4% (19 of 37), and 54.1% (20 of 37) of patients treated with MI belatacept, LI belatacept and CsA, respectively, experienced DGF.
Acute rejection
In the UNOS ECD cohort, Kaplan–Meier cumulative event rates of AR at month 84 for MI belatacept, LI belatacept and CsA were 24.9%, 21.8% and 15.5%, respectively. The HR for the comparison of MI belatacept with CsA was 1.650 (95% CI 0.912–2.983; p = 0.10), and the HR for the comparison of LI belatacept with CsA was 1.454 (95% CI 0.789–2.680; p = 0.23) (Figure S5A). In total, 28.6% (8 of 28) of MI belatacept‐treated, 37.5% (9 of 24) of LI belatacept‐treated and 55.6% (10 of 18) of CsA‐treated patients who experienced AR also experienced death and/or graft loss. In the DCD cohort, Kaplan–Meier cumulative event rates of AR at month 84 for MI belatacept, LI belatacept and CsA were 11.9%, 21.1% and 26.3%, respectively. The HR for the comparison of MI belatacept with CsA was 0.481 (95% CI 0.088–2.629; p = 0.40), and the HR for the comparison of LI belatacept with CsA was 0.926 (95% CI 0.231–3.707; p = 0.91) (Figure S5B). In this cohort, 75% (3 of 4) of CsA‐treated patients who experienced AR also experienced death and/or graft loss; no belatacept‐treated patient experienced both AR and death/graft loss. The Kaplan–Meier cumulative event rates for MI belatacept, LI belatacept, and CsA in the anticipated CIT ≥24 h cohort were 13.3%, 11.4% and 19.3%, respectively. The HR for the comparison of MI belatacept with CsA was 0.642 (95% CI 0.204–2.024; p = 0.45), and the HR for the comparison of LI belatacept with CsA was 0.546 (95% CI 0.160–1.867; p = 0.34) (Figure S5C). The only patient in this cohort to experience both AR and death or graft loss was randomized to LI belatacept (25%, 1 of 4). HRs and 95% CIs deriving from the interaction analysis of treatment and donor kidney subtype on time to AR are summarized in Table S2.
Donor‐specific antibodies
In the UNOS ECD cohort, the Kaplan–Meier cumulative event rate of de novo DSAs at month 84 was 7.9%, 5.3% and 25.6% in patients treated with MI belatacept, LI belatacept and CsA, respectively. De novo DSA development occurred significantly less frequently with belatacept‐ versus CsA‐based immunosuppression. The HR for the comparison of MI belatacept with CsA was 0.336 (95% CI 0.130–0.867; p = 0.0241), and the HR for the comparison of LI belatacept with CsA was 0.209 (95% CI 0.069–0.631; p = 0.0055). No belatacept‐treated patient in the DCD cohort developed de novo DSAs compared with three CsA‐treated patients (Kaplan–Meier cumulative event rate at month 84 of 19.8%). In the anticipated CIT ≥24 h cohort, the Kaplan–Meier cumulative event rate of de novo DSAs at month 84 was 4.0%, 5.0% and 21.7% in patients treated with MI belatacept, LI belatacept and CsA, respectively. The HR for the comparison of MI belatacept with CsA was 0.133 (95% CI 0.016–1.081; p = 0.06), and the HR for the comparison of LI belatacept with CsA was 0.145 (95% CI 0.018–1.177; p = 0.07). HLA class specificities of de novo DSAs are described in Table S3.
Safety
In the UNOS ECD cohort, serious AEs occurred in 86.5% (109 of 126) of MI belatacept‐treated patients, 92.4% (110 of 119) of LI belatacept‐treated patients and 87.6% (113 of 129) of CsA‐treated patients. The corresponding values in the DCD cohort were 88.9% (16 of 18), 78.9% (15 of 19), and 83.3% (15 of 18), respectively. In the anticipated CIT ≥24 h cohort, serious AEs occurred in 87.5% (35 of 40), 83.8% (31 of 37), and 73.0% (27 of 37) of patients treated with MI belatacept, LI belatacept and CsA, respectively (Table S4). Incidence rates of serious infections, any‐grade viral infections, any‐grade fungal infections and any‐grade malignancies per 100 person‐years of treatment exposure were generally similar across donor kidney subgroups and treatment arms, although some numerical differences were observed (Table 2). Of the nine cases of posttransplant lymphoproliferative disorder (PTLD) reported prior to month 84, six occurred in patients transplanted with a kidney meeting UNOS ECD criteria, none occurred in the DCD cohort and three occurred in patients who received a kidney with an anticipated CIT ≥24 h (Table 3).
Table 2.
Cumulative incidence rates of selected adverse events adjusted per 100 person‐years of treatment exposure
| Cohort | Serious infectiona | Any‐grade viral infectionb | Any‐grade fungal infectionb | Any‐grade malignancya |
|---|---|---|---|---|
| UNOS ECD | ||||
| Belatacept MI | 22.56 | 22.65 | 10.84 | 4.46 |
| Belatacept LI | 18.06 | 16.26 | 6.46 | 3.77 |
| CsA | 23.48 | 22.57 | 11.06 | 4.46 |
| DCD | ||||
| Belatacept MI | 19.81 | 19.34 | 9.81 | 3.78 |
| Belatacept LI | 13.37 | 12.64 | 6.50 | 1.99 |
| CsA | 24.79 | 18.76 | 8.12 | 4.86 |
| Anticipated CIT ≥24 h | ||||
| Belatacept MI | 24.21 | 17.60 | 7.20 | 2.02 |
| Belatacept LI | 14.30 | 25.09 | 8.71 | 2.26 |
| CsA | 12.47 | 11.95 | 11.99 | 1.06 |
CIT, cold ischemia time; CsA, cyclosporine; DCD, donation after cardiac death; ECD, expanded criteria donor; LI, less intensive; MI, more intensive; UNOS, United Network for Organ Sharing.
The duration (patient‐years) of patient exposure to assigned study drug was calculated from the randomization date to the event date, to the date of last follow‐up or to month 84, whichever was earliest.
The duration (patient‐years) of patient exposure to assigned study drug was calculated from the randomization date to the event date, to the date of last dose of study medication plus 56 days or to month 84, whichever was earliest.
Table 3.
Cumulative incidence rates of posttransplant lymphoproliferative disorder adjusted per 100 person‐years of treatment exposure by donor kidney subtype and time of onset
| n (incidence rate) | |||
|---|---|---|---|
| Belatacept MI | Belatacept LI | CsA | |
| UNOS ECD | |||
| Months 0–12 | 1 (0.82) | 2 (1.74) | 0 |
| Months 12–24 | 1 (0.87) | 1 (0.91) | 0 |
| Months 24–48 | 0 | 0 | 0 |
| Months 48–60 | 0 | 0 | 1 (2.05) |
| Months 60–84 | 0 | 0 | 0 |
| Overall | 2 (0.34) | 3 (0.50) | 1 (0.19) |
| DCD | |||
| Overall | 0 | 0 | 0 |
| Anticipated CIT ≥24 h | |||
| Months 0–36 | 0 | 0 | 0 |
| Months 36–48 | 0 | 1 (3.89) | 0 |
| Months 48–60 | 0 | 2 (9.59) | 0 |
| Months 60–84 | 0 | 0 | 0 |
| Overall | 0 | 3 (1.60) | 0 |
CIT, cold ischemia time; CsA, cyclosporine; DCD, donation after cardiac death; ECD, expanded criteria donor; LI, less intensive; MI, more intensive; UNOS, United Network for Organ Sharing.
Discussion
This subgroup analysis of BENEFIT‐EXT shows that the efficacy and safety of belatacept‐based immunosuppression did not differ in recipients of different extended criteria donor kidney subtypes. Time to death or graft loss was similar between belatacept‐ and CsA‐treated patients in all subgroups. Nevertheless, a statistically significant difference in favor of LI belatacept versus CsA was observed in the DCD cohort (HR 0.095, 95% CI 0.012–0.778; p = 0.0282), but the numbers of patients for this post hoc analysis were small. A study focused on recipients of DCD kidneys may be warranted to determine whether this improvement in survival might be observed in a larger cohort. In general, these findings mirror those derived from the analysis of the overall BENEFIT‐EXT population at 7 years after transplant, in which time to death or graft loss was similar for the comparison of CsA with both MI belatacept (HR 0.915, 95% CI 0.625–1.339; p = 0.65) and LI belatacept (HR 0.927, 95% CI 0.634–1.356; p = 0.70) 30.
Similar to the intent‐to‐treat analyses performed at 1, 3 and 7 years after transplant for the BENEFIT‐EXT 26, 28, 30 and BENEFIT populations 25, 27, 29, renal function over months 1–84 was significantly greater for belatacept‐ versus CsA‐treated patients across all donor kidney subtypes (p < 0.0001 for overall treatment effect). The preservation of renal function in belatacept‐treated recipients of UNOS ECD kidneys is clinically significant because patients who receive this donor kidney subtype typically have poorer allograft and patient outcomes than recipients of SCD kidneys 31. In fact, renal dysfunction is an important risk factor for cardiovascular disease, which is the leading cause of death with a functioning graft in kidney transplant recipients 16, 19. Although it is tempting to speculate that the improvements in renal function seen with belatacept‐based immunosuppression may have a positive effect on patient survival, the results of sensitivity analyses examining time to death showed no statistically significant differences between belatacept‐ and CsA‐based immunosuppression in either the overall study population 30 or any of the donor kidney subtypes. BENEFIT‐EXT, however, was not powered to uncover survival differences between treatment regimens.
Acceptable outcomes with DCD kidneys are possible (and, at best, are similar to those seen in recipients of SCD kidneys) 32, but recipients of DCD kidneys may experience an increased risk of DGF; however, this has been linked to older donor age (e.g. >50 years) and CIT > 12 h and does not necessarily compromise longer term renal function or patient/graft survival (relative to patients receiving donation after brain death donor kidneys) 32, 33, 34. Regardless, DCD kidneys are marginalized, with stigma surrounding their use (i.e. prospective recipients of DCD kidneys are required to sign additional consent forms). Notably, in the present analysis, rates of DGF in belatacept‐treated patients transplanted with a DCD kidney (47.4–55.6%) were comparable to those seen in belatacept‐treated patients who received a UNOS ECD kidney (44.4–46.2%) or a kidney with an anticipated CIT ≥24 h (50.0–51.4%). Among CsA‐treated patients, rates of DGF were highest in the DCD cohort (83.3%; UNOS ECD, 42.6%; anticipated CIT ≥24 h, 54.1%), but patient numbers in this subgroup were small.
In the three subgroups, Kaplan–Meier cumulative event rates of AR over the total study period were similar between belatacept‐ and CsA‐based immunosuppression. In addition, Kaplan–Meier cumulative events rates of AR at month 84 were comparable across donor kidney subgroups (UNOS ECD, 15.5–24.9%; DCD, 11.9–26.3%; anticipated CIT ≥24 h, 11.4–19.3%).
At 7 years after transplant in BENEFIT 29 and BENEFIT‐EXT 30, the cumulative incidence of de novo DSAs was statistically significantly lower for belatacept‐ versus CsA‐based treatment. Statistically significant differences in favor of belatacept‐based immunosuppression were seen in the subgroup of patients transplanted with a UNOS ECD kidney. This finding was not unexpected because the majority (68.9%) of patients in BENEFIX‐EXT received a UNOS ECD kidney. It was not possible to analyze de novo DSA incidence in the DCD cohort because of the low number of events (belatacept, n = 0; CsA, n = 3). In the group with anticipated CIT ≥24 h, there was a trend in favor of belatacept‐based immunosuppression, but statistical significance was not reached (MI belatacept vs. CsA, p = 0.06; LI belatacept vs. CsA, p = 0.07).
Safety was generally similar across treatment arms and donor kidney subgroups, with no new safety signals. The absolute incidence of serious AEs ranged from 86.5% to 92.4% in the UNOS ECD cohort, from 78.9% to 88.9% in the DCD cohort and from 73.0% to 87.5% in the anticipated ≥24 h cohort. The exposure‐adjusted incidences of serious infections, any‐grade viral infections, any‐grade fungal infections and any‐grade malignancies were largely similar, but some numerical differences were apparent. Of the nine cases of PTLD reported prior to month 84 in BENEFIT‐EXT, six occurred in the UNOS ECD cohort. This is likely due to the fact that the majority of patients in this study received a UNOS ECD kidney rather than a reflection of a predisposition of recipients of this donor kidney subtype to develop PTLD. This supposition is supported by results from a retrospective analysis of the U.S. Renal Data System, which found PTLD incidence to be comparable in patients receiving either a living or deceased donor kidney 35. Due to an increased risk of PTLD, belatacept is not approved for use in patients who are seronegative for Epstein‐Barr virus or whose serostatus is unknown prior to transplant.
In terms of limitations, the findings reported in this paper derive from post hoc analyses (p‐values are provided for illustrative purposes); therefore, any conclusions drawn should be considered hypothesis generating. Moreover, the DCD cohort was composed of a small number of patients (n = 55), and that limits the conclusions that can be drawn about this donor kidney subtype but highlights the need for additional studies. Despite these limitations, BENEFIT‐EXT is the largest prospective study of extended criteria donor kidney recipients treated with non–CNI‐based immunosuppression, and the results from this post hoc analysis demonstrate that belatacept is an effective and well‐tolerated treatment option for recipients of UNOS ECD kidneys, DCD kidneys or kidneys with an anticipated CIT ≥24 h.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. S. Florman and B. Bresnahan have received grant support from Bristol‐Myers Squibb. P.J. O'Connell has served as an advisory board member for Pfizer and a lecturer for Astellas. Dr. O'Connell has also received travel support to a scientific meeting from Novartis. U. Meier‐Kriesche is a salaried employee of Bristol‐Myers Squibb. T. Becker, A. Chevaile‐Ramos, D. Carvalho, G. Grannas, F. Muehlbacher, and C.P. Larsen have nothing to disclose.
Supporting information
Figure S1: Kaplan–Meier curve for time to death in the (A) United Network for Organ Sharing expanded criteria donor, (B) donation after cardiac death and (C) anticipated cold ischemia time ≥24 h cohort.
Figure S2: Kaplan–Meier curve for time to graft loss in the (A) United Network for Organ Sharing expanded criteria donor, (B) donation after cardiac death and (C) anticipated cold ischemia time ≥24 h cohorts.
Figure S3: Kaplan–Meier curve for time to first occurrence of death, graft loss or estimated GFR <20 mL/min per 1.73 m2 in the (A) United Network for Organ Sharing expanded criteria donor, (B) donation after cardiac death and (C) anticipated cold ischemia time ≥24 h cohorts.
Figure S4: Estimated mean GFR from months 1–84 (mixed‐effects modeling with imputation) in the (A) United Network for Organ Sharing expanded criteria donor, (B) donation after cardiac death and (C) anticipated cold ischemia time ≥24 h cohorts.
Figure S5: Kaplan–Meier estimates for acute rejection in the (A) United Network for Organ Sharing expanded criteria donor, (B) donation after cardiac death and (C) anticipated cold ischemia time ≥24 h cohorts.
Table S1: Duration of follow‐up and treatment exposure in randomized, transplanted and treated patients.
Table S2: Summary of the interaction between donor kidney subtype and treatment on time to various end points (randomization to month 84). Data are shown as hazard ratio (95% confidence interval).
Table S3: HLA class specificity of de novo donor‐specific antibodies.
Table S4: Cumulative incidence rates* of serious adverse eventss† occurring in >10% of patients in any treatment arm.
Acknowledgments
Professional medical writing and editorial assistance were provided by Jaimee Glasgow, Meredith Kalish, and Tiffany DeSimone of CodonMedical, an Ashfield Company, part of UDG Healthcare plc, and were funded by Bristol‐Myers Squibb.
Florman S, Becker T, Bresnahan B, Chevaile‐Ramos A, Carvalho D, Grannas G, Muehlbacher F, O'Connell PJ, Meier‐Kriesche HU & Larsen CP. Efficacy and Safety Outcomes of Extended Criteria Donor Kidneys by Subtype: Subgroup Analysis of BENEFIT‐EXT at 7 Years After Transplant. Am J Transplant 2017; 17: 180–190
[The copyright line for this article was changed on 28 October, 2016 after original online publication.]
References
- 1. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Held PJ, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730. [DOI] [PubMed] [Google Scholar]
- 2. Ojo AO, Hanson JA, Meier‐Kriesche H, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait listed candidates. J Am Soc Nephrol 2001; 12: 589–597. [DOI] [PubMed] [Google Scholar]
- 3. Niu SF, Li IC. Quality of life of patients having renal replacement therapy. J Adv Nurs 2005; 51: 15–21. [DOI] [PubMed] [Google Scholar]
- 4. Cohen B, Smits JM, Haase B, Persijn G, Vanrenterghem Y, Frei U. Expanding the donor pool to increase renal transplantation. Nephrol Dial Transplant 2005; 20: 34–41. [DOI] [PubMed] [Google Scholar]
- 5. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) . OPTN/SRTR 2012 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration; 2014. [cited 2015 Feb 4]. Available from: http://onlinelibrary.wiley.com/doi/10.1111/ajt.v14.s1/issuetoc. [Google Scholar]
- 6. Solomon H. Opportunities and challenges of expanded criteria organs in liver and kidney transplantation as a response to organ shortage. Mo Med 2011; 108: 269–274. [PMC free article] [PubMed] [Google Scholar]
- 7. Danovitch GM, Cohen DJ, Weir MR, et al. Current status of kidney and pancreas transplantation in the United States, 1994–2003. Am J Transplant 2005; 5(Pt 2): 904–915. [DOI] [PubMed] [Google Scholar]
- 8. Collins MG, Chang SH, Russ GR, McDonald SP. Outcomes of transplantation using kidneys from donors meeting expanded criteria in Australia and New Zealand, 1991 to 2005. Transplantation 2009; 87: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 9. Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant 2003; 3(Suppl 4): 114–125. [DOI] [PubMed] [Google Scholar]
- 10. Saidi RF, Elias N, Kawai T, et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: Realities and costs. Am J Transplant 2007; 7: 2769–2774. [DOI] [PubMed] [Google Scholar]
- 11. Stallone G, Infante B, Gesualdo L. Older donors and older recipients in kidney transplantation. J Nephrol 2010; 23(Suppl 15): S98–S103. [PubMed] [Google Scholar]
- 12. Snoeijs MG, Schaubel DE, Hené R, et al. Kidneys from donors after cardiac death provide survival benefit. J Am Soc Nephrol 2010; 21: 1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lenihan CR, O'Kelly P, Mohan P, et al. MDRD‐estimated GFR at one year post‐renal transplant is a predictor of long‐term graft function. Ren Fail 2008; 30: 345–352. [DOI] [PubMed] [Google Scholar]
- 14. Salvadori M, Rosati A, Bock A, et al. Estimated one‐year glomerular filtration rate is the best predictor of long‐term graft function following renal transplant. Transplantation 2006; 81: 202–206. [DOI] [PubMed] [Google Scholar]
- 15. Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post‐transplant renal function in the first year predicts long‐term kidney transplant survival. Kidney Int 2002; 62: 311–318. [DOI] [PubMed] [Google Scholar]
- 16. Meier‐Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation 2003; 75: 1291–1295. [DOI] [PubMed] [Google Scholar]
- 17. Kasiske BL, Israni AK, Snyder JJ, Skeans MA; Patient Outcomes in Renal Transplantation (PORT) Investigators . The relationship between kidney function and long‐term graft survival after kidney transplant. Am J Kidney Dis 2011; 57: 466–475. [DOI] [PubMed] [Google Scholar]
- 18. Fellström B, Jardine AG, Soveri I, et al. Renal dysfunction as a risk factor for mortality and cardiovascular disease in renal transplantation: Experience from the Assessment of Lescol in Renal Transplantation trial. Transplantation 2005; 79: 1160–1163. [DOI] [PubMed] [Google Scholar]
- 19. Shlipak MG, Katz R, Kestenbaum B, et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol 2009; 20: 2625–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med 2003; 349: 2326–2333. [DOI] [PubMed] [Google Scholar]
- 21. Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high‐affinity variant of CTLA4‐Ig with potent immunosuppressive properties. Am J Transplant 2005; 5: 443–453. [DOI] [PubMed] [Google Scholar]
- 22. Bestard O, Campistol JM, Morales JM, et al. Advances in immunosuppression for kidney transplantation: New strategies for preserving kidney function and reducing cardiovascular risk. Nefrologia 2012; 32: 374–384. [DOI] [PubMed] [Google Scholar]
- 23. Grinyó JM, Bestard O, Torras J, Cruzado JM. Optimal immunosuppression to prevent chronic allograft dysfunction. Kidney Int Suppl 2010; 119: S66–S70. [DOI] [PubMed] [Google Scholar]
- 24. Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev 2014; 11: CD010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept‐based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010; 10: 535–546. [DOI] [PubMed] [Google Scholar]
- 26. Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT‐EXT study). Am J Transplant 2010; 10: 547–557. [DOI] [PubMed] [Google Scholar]
- 27. Vincenti F, Larsen CP, Alberu J, et al. Three‐year outcomes from BENEFIT, a randomized, active‐controlled, parallel‐group study in adult kidney transplant recipients. Am J Transplant 2012; 12: 210–217. [DOI] [PubMed] [Google Scholar]
- 28. Pestana JO, Grinyo JM, Vanrenterghem Y, et al. Three‐year outcomes from BENEFIT‐EXT: A phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant 2012; 12: 630–639. [DOI] [PubMed] [Google Scholar]
- 29. Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long‐term outcomes in kidney transplantation. N Engl J Med 2016; 374: 333–343. [DOI] [PubMed] [Google Scholar]
- 30. Durrbach A, Pestana JM, Florman S, et al. Long‐term outcomes in belatacept‐treated vs. cyclosporine‐treated recipients of extended criteria donor kidneys: Final results from BENEFIT‐EXT, a phase III randomized study. Am J Transplant 2016; doi: 10.1111/ajt.13830 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual data report: kidney. Am J Transplant 2014; 14(Suppl 1): 11–44. [DOI] [PubMed] [Google Scholar]
- 32. Locke JE, Segev DL, Warren DS, Dominici F, Simpkins CE, Montgomery RA. Outcomes of kidneys from donors after cardiac death: Implications for allocation and preservation. Am J Transplant 2007; 7: 1797–1807. [DOI] [PubMed] [Google Scholar]
- 33. Singh RP, Farney AC, Rogers J, et al. Kidney transplantation from donation after cardiac death donors: Lack of impact of delayed graft function on post‐transplant outcomes. Clin Transplant 2011; 25: 255–264. [DOI] [PubMed] [Google Scholar]
- 34. Hesse K, Aitken E, Clancy M, Vesey A. Expanded criteria donor and donation after circulatory death renal allografts in the west of Scotland: Their place in the kidney allocation process. Surgeon 2016; 14: 136–141. [DOI] [PubMed] [Google Scholar]
- 35. Caillard S, Dharnidharka V, Agodoa L, Bohen E, Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation 2005; 80: 1233–1243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Kaplan–Meier curve for time to death in the (A) United Network for Organ Sharing expanded criteria donor, (B) donation after cardiac death and (C) anticipated cold ischemia time ≥24 h cohort.
Figure S2: Kaplan–Meier curve for time to graft loss in the (A) United Network for Organ Sharing expanded criteria donor, (B) donation after cardiac death and (C) anticipated cold ischemia time ≥24 h cohorts.
Figure S3: Kaplan–Meier curve for time to first occurrence of death, graft loss or estimated GFR <20 mL/min per 1.73 m2 in the (A) United Network for Organ Sharing expanded criteria donor, (B) donation after cardiac death and (C) anticipated cold ischemia time ≥24 h cohorts.
Figure S4: Estimated mean GFR from months 1–84 (mixed‐effects modeling with imputation) in the (A) United Network for Organ Sharing expanded criteria donor, (B) donation after cardiac death and (C) anticipated cold ischemia time ≥24 h cohorts.
Figure S5: Kaplan–Meier estimates for acute rejection in the (A) United Network for Organ Sharing expanded criteria donor, (B) donation after cardiac death and (C) anticipated cold ischemia time ≥24 h cohorts.
Table S1: Duration of follow‐up and treatment exposure in randomized, transplanted and treated patients.
Table S2: Summary of the interaction between donor kidney subtype and treatment on time to various end points (randomization to month 84). Data are shown as hazard ratio (95% confidence interval).
Table S3: HLA class specificity of de novo donor‐specific antibodies.
Table S4: Cumulative incidence rates* of serious adverse eventss† occurring in >10% of patients in any treatment arm.


