Abstract
Background
This phase 3 trial is the first to evaluate the efficacy and safety of treatment with the systemic TNF‐α inhibitor, adalimumab, for Chinese patients with moderate‐to‐severe plaque psoriasis.
Methods
In the 12‐week, double‐blind, placebo‐controlled Period A, patients were randomized 4 : 1 to receive adalimumab 40 mg every‐other‐week (following a single 80 mg dose), or placebo every‐other‐week. In the subsequent 12‐week, open‐label, Period B, all patients received adalimumab 40 mg every‐other‐week starting at week 13, following a single, blinded dose at week 12 of adalimumab 80 mg or matching placebo (for patients receiving placebo or adalimumab in Period A respectively). In Period A, efficacy was analysed for all randomized patients and safety for all patients receiving ≥1 dose of the study drug.
Results
For the 425 patients in this study (87 placebo; 338 adalimumab), a higher percentage randomized to adalimumab achieved the primary endpoint of ≥75% improvement from baseline in PASI score (PASI 75) at week 12: placebo 11.5% (10/87); adalimumab 77.8% (263/338; P < 0.001). Physician's Global Assessment of clear to minimal was achieved at week 12 by 14.9% placebo (13/87) and 80.5% adalimumab (272/338; P < 0.001). For patients who received adalimumab at any time during the study (All‐adalimumab Population), treatment‐emergent adverse events (AEs) were reported by 63.4%; the most common was upper respiratory infection (16.1%). Serious AEs were reported by 3.5% of the All‐adalimumab Population, and serious infectious AEs by 1.2%, which include lung infection, pneumonia and tuberculosis [2 (0.5%) patients each]. There was one death (chronic heart failure).
Conclusion
In these Chinese patients with moderate‐to‐severe psoriasis, a significantly greater percentage treated with adalimumab compared with placebo achieved efficacy endpoints at week 12 and efficacy was sustained to week 24. Safety results were consistent with the known adalimumab safety profile; no new safety signals were identified in the 24 weeks of treatment.
Introduction
Psoriasis is a chronic inflammatory skin disease, characterized by scaly, erythematous, well‐defined papules and plaques, and is associated with comorbidities1, 2, 3, 4 including psoriatic arthritis, depression, cardiovascular disease, obesity, diabetes and Crohn's disease. Psoriasis together with its attendant comorbidities, markedly impairs quality‐of‐life for these patients. The most common type of psoriasis is plaque psoriasis.5 A major role in plaque formation is played by cytokines released from cutaneous antigen‐presenting cells, T cells and keratinocytes.6 A key pro‐inflammatory cytokine involved with the pathogenesis of psoriasis is tumour necrosis factor alpha (TNF‐α).
The prevalence of psoriasis in China is approximately 0.12–0.47%, and has increased fourfold over the past 25 years.7 Treatment options in China for chronic plaque psoriasis include topical agents, phototherapy, traditional Chinese medicine and systemic agents such as methotrexate, cyclosporine, corticosteroids,8, 9, 10 as well as etanercept and infliximab, which are the only biologic agents approved by the State Food and Drug Administration (SFDA) for treatment of psoriasis in China. Adalimumab, a fully human, IgG1 monoclonal antibody specific for TNF‐α, is a systemic biologic agent currently approved in the United States, Europe and Japan for the treatment of a wide range of inflammatory diseases, including plaque psoriasis, and has been approved in China for the treatment of rheumatoid arthritis and ankylosing spondylitis.
We report results from a phase 3, randomized, placebo‐controlled, double‐blind trial that is the first to evaluate the efficacy and safety of adalimumab treatment for Chinese patients with moderate‐to‐severe plaque psoriasis (clinicaltrials.gov NCT01646073).
Methods
An independent ethics committee or institutional review board, in compliance with Good Clinical Practice, reviewed and approved the study protocol and other study‐related documents, and the ethical, scientific and medical appropriateness of the study prior to its conduct. The study was conducted according to the protocol, International Conference on Harmonisation guidelines, applicable regulations and guidelines governing clinical study conduct and the ethical principles originating in the Declaration of Helsinki. Prior to any study‐related events, patients reviewed and signed an informed consent.
Patients
Adult men and women (18 years of age or older) were included in the study if they had a diagnosis of psoriasis for at least 6 months, and stable plaque psoriasis for at least 2 months before the screening and baseline visits. At baseline, patients had moderate‐to‐severe disease, and had failed to respond to or were intolerant to previous systemic therapy. Prior to enrolment, patients were evaluated for latent tuberculosis by means of a purified protein derivative or a T‐spot test. Patients were excluded from the study if they had previous exposure to a biologic treatment or received other systemic therapies for psoriasis within 28 days of baseline. Details can be found in the Appendix.
Procedures
This phase 3, multicentre study included a 30‐day screening period followed by a 12‐week, double‐blind, placebo‐controlled Period A, a subsequent 12‐week, open‐label Period B, followed by a 70‐day follow‐up visit after the final dose of the study drug. Patients in Period A were randomized 4 : 1 to receive adalimumab 40 mg every‐other‐week (following a single 80 mg dose), or matching placebo. All patients in Period B received adalimumab 40 mg every‐other‐week starting at week 13, following a single, blinded dose at week 12 of adalimumab 80 mg (for patients randomized to placebo in Period A) or matching placebo (for patients randomized to adalimumab in Period A). Dose groups in Period B are identified by the drug received in Periods A and B; that is, adalimumab/adalimumab or placebo/adalimumab.
The primary efficacy endpoint was the proportion of patients achieving at least a 75% reduction in Psoriasis Area Severity Index score (PASI 75) at week 12 relative to the baseline score. Scores for PASI can range from 0 to 72, where higher scores indicate greater psoriasis severity. Secondary endpoints included the proportion of patients achieving PASI 75 at other time points, PASI 90 or PASI 100, and the proportion achieving Physicians Global Assessment (PGA) score of 0 or 1 on a disease severity scale from 0 to 5, where 0 is clear, 1 minimal, 2 mild and ≥3 moderate to severe. Other secondary endpoints included the mean change from baseline in Dermatology Life Quality Index (DLQI) scores, which ranged from 0 to 30, where effect on a patient's life was scored as follows: 0–1 no effect, 2–5 small, 6–10 moderate, 11–20 very large, 21–30 extremely large.11 Achievement of PASI 75 by subgroups of bodyweight and body mass index (BMI) was also analysed. Effect of bodily pain due to psoriasis on activities of daily living, measured in the SF‐36 Health Status Survey (0 great effect to 100 no effect), was also reported.
Statistical analyses
Efficacy was analysed in Period A for all randomized patients [intent‐to‐treat (ITT_A Population)], and in Period B, for all patients receiving at least one dose of study drug in Period B (ITT_B Population). Comparison between treatment groups was performed in Period A. Discrete secondary efficacy variables were based on Pearson's chi‐squared test except when ≥25% of the cells had expected counts of <5, where a Fisher's exact test was used. Continuous secondary efficacy variables were analysed by analysis of covariance including baseline as a covariate.
All patients who received at least one dose of study drug were analysed for safety in Period A (Safety Population A) and for patients who received at least one dose of adalimumab in Periods A or B of the study (All‐adalimumab Population). Treatment‐emergent adverse events (referred to hereon as ‘adverse events’) were those with onset or worsening after the first study‐drug injection, and within 70 days after the last study‐drug injection. The number of adverse events was tabulated using the Medical Dictionary for Drug Regulatory Activities (MedDRA®) system organ class and preferred term. Comparisons of adverse events between treatment groups in Period A were performed using Fisher's exact tests and one‐way analysis of variance.
All statistical tests were two‐tailed with a significance level of 0.05. Statistical analyses were performed using SAS® (SAS Institute Inc., Cary, NC, USA). Missing data were handled using non‐responder imputation (NRI) for categorical variables and last‐observation‐carried‐forward (LOCF) for continuous variables.
Results
This study was conducted from 14 August 2012 to 21 December 2013. A total of 425 patients from 16 sites in China were randomized in Period A (87 and 338 to placebo and adalimumab respectively); 418 (98.4%) patients completed Period A, and of those, 404 (96.7%) completed Period B (Fig. A1). The main reasons for discontinuation of patients were adverse events (2, 0.6% adalimumab in Period A; 7, 2.1% adalimumab in Period B) and withdrawal of consent (1, 1.1% placebo and 1, 0.3% adalimumab in Period A; 1, 1.2% placebo and 5, 1.5% adalimumab in Period B) (Fig. A1). At baseline (Table 1), the majority of patients (73.4%) were men. Patients' mean age was 43.2 years and mean BMI was 24.3 kg/m.2 Mean duration of psoriasis was 15.0 years, mean affected body surface area was 41.9% and mean score for effect of bodily pain due to psoriasis on activities of daily living was 49.4. Mean PASI score was 27.7 and mean DLQI score was 14.5. Demographics and baseline disease characteristics were similar between the two treatment groups.
Table 1.
Baseline demographics and clinical characteristics
| ITT_A Population | |||
|---|---|---|---|
| Placebo N = 87 | Adalimumab N = 338 | All ITT_A N = 425 | |
| Age, years, mean [±SD] | 43.8 [12.45] | 43.1 [11.91] | 43.2 [12.01] |
| Male, n (%) | 58 (66.7) | 254 (75.1) | 312 (73.4) |
| Female, n (%) | 29 (33.3) | 84 (24.9) | 113 (26.6) |
| Weight, kg, mean [±SD] | 67.0 [10.62] | 69.7 [12.43] | 69.2 [12.12] |
| BMI,a kg/m,2 mean [±SD] | 23.6 [2.86] | 24.4 [3.48] | 24.3 [3.38] |
| BMI categories, n (%) | |||
| <25 (normal weight or less) | 61 (70.1) | 193 (51.4) | 254 (59.8) |
| 25 to <30 (overweight) | 25 (28.7) | 126 (37.3) | 151 (35.5) |
| ≥30 (obese) | 1 (1.1) | 19 (5.6) | 20 (4.7) |
| Nicotine user, n (%) | 32 (36.8) | 111 (32.9) | 143 (33.7) |
| Duration of psoriasis, years, mean [±SD] | 15.8 [10.31] | 14.8 [10.11] | 15.0 [10.15] |
| History of PsA, n (%) | 10 (11.5) | 43 (12.7) | 53 (12.5) |
| Swollen, stiff or tender joints,b n (%) | 8/10 (80.0) | 34/43 (79.1) | 42/53 (79.2) |
| BSA with psoriasis, %, mean [±SD] | 39.3 [22.50] | 42.6 [21.75] | 41.9 [21.92] |
| PASI score (range 0–72), mean [±SD] | 25.6 [10.98] | 28.2 [12.0] | 27.7 [11.83] |
| PGA categoryc (range 0–5), n (%) | |||
| Moderate (3) | 57 (65.5) | 214 (63.5) | 271 (63.8) |
| Marked (4) | 28 (32.2) | 110 (32.5) | 138 (32.5) |
| Severe (5) | 2 (2.3) | 14 (4.1) | 16 (3.8) |
| Bodily paind | 50.1 [8.14] | 49.3 [9.28] | 49.4 [9.05] |
| DLQI score,e mean [±SD] | 13.4 [7.10] | 14.7 [7.10] | 14.5 [7.11] |
| Prior medications for psoriasis; >10% of patients in Period A, n (%) | |||
| Acitretin | 54 (62.1) | 208 (61.5) | 262 (61.6) |
| Methotrexate | 23 (26.4) | 99 (29.3) | 122 (28.7) |
| Unspecified herbal | 30 (34.5) | 100 (29.6) | 130 (30.6) |
Obesity classification based on World Health Organization 2000 obesity tables.
Present at baseline. Percentage was calculated based on all patients with PsA.
No patient had PGA clear, minimal or mild (0,1,2) at baseline.
Measured in the SF‐36 Health Status Survey. Scale 0 great effect of pain on health status to 100 no effect.
Range 0–30. Effect on a patient's life scores: 0–1 no effect, 2–5 small, 6–10 moderate, 11–20 very large, 21–30 extremely large.
BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; ITT_A, intent‐to‐treat population in Period A; PASI, Psoriasis Area Severity Index; PGA, Physician's Global Assessment; PsA, psoriatic arthritis; SD, standard deviation.
In Period A, the majority of patients (60.7% overall; 59.5% adalimumab; 65.5% for placebo) received concomitant medications. All patients in Period A reported using at least one prior medication for treatment of psoriasis, most frequently (≥10% of patients in any group) acitretin, methotrexate and unspecified herbal medications.
Efficacy
Treatment response rates are shown in Fig. 1. In Period A, a statistically significantly higher proportion of patients in the adalimumab group (77.8%) compared with placebo (11.5%) achieved the primary endpoint of PASI 75 at week 12 (P < 0.001). The rate for achieving PASI 90 and PASI 100 at week 12 was also statistically significantly higher in the adalimumab group than the placebo group (PASI 90: 55.6% vs. 3.4%; PASI 100: 13.3% vs. 1.1%). For patients receiving adalimumab in Period A, PASI 75, 90 and 100 response rates further increased across Period B to week 24 after continuing adalimumab treatment (87.7%, 76.3% and 35.7% respectively), and rates at week 24 for patients who switched from placebo to adalimumab were 90.6%, 75.3% and 34.1% respectively. PASI 75 response at week 12 was achieved by more than 75% of patients in the adalimumab group across patient subgroup categories of bodyweight (<69 vs. ≥69 kg) and BMI (<25 vs. ≥25 kg/m2). Statistically significant differences (P < 0.001) were observed between adalimumab and placebo in each of these subgroups (Fig. 2a), as well as in other patient subgroups of age, gender, disease duration and psoriatic arthritis at baseline (Fig. 2b).
Figure 1.
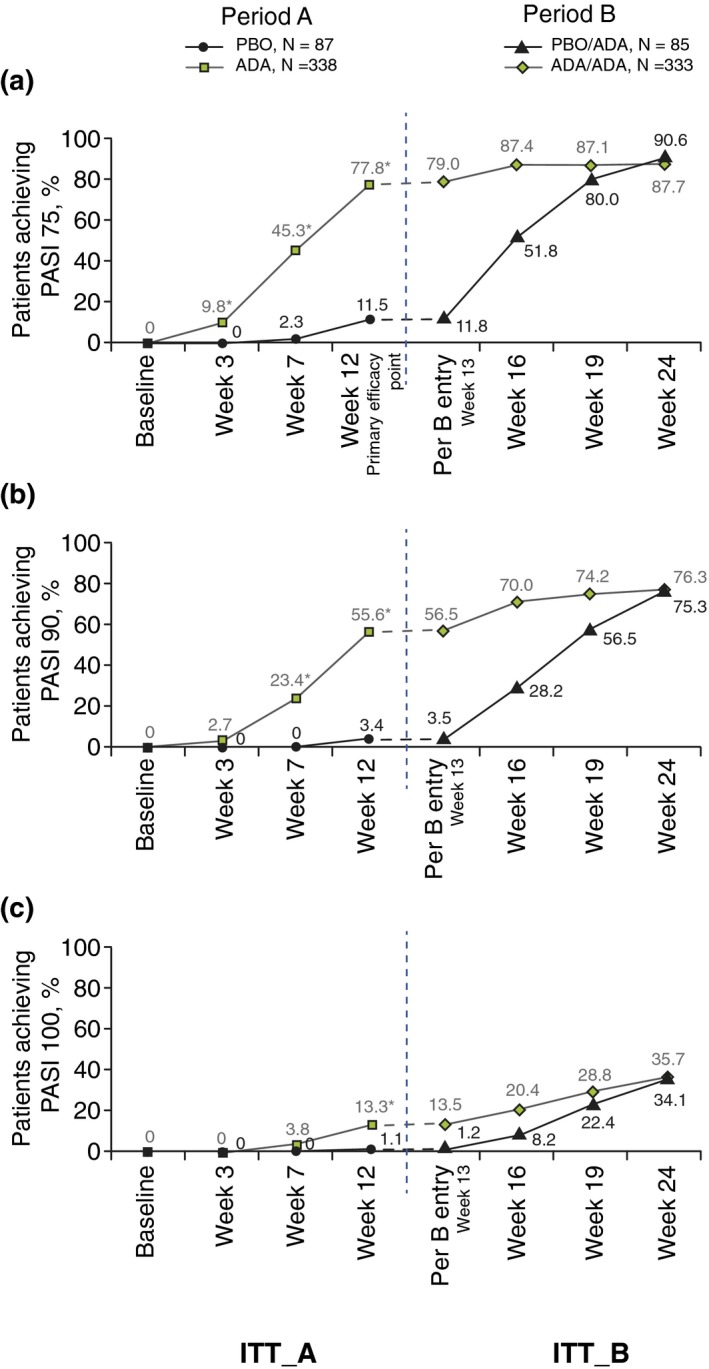
PASI 75, 90 and 100 Response Rate. (a) PASI 75. *P = 0.002 week 3; P < 0.001 weeks 7 and 12. 95% CIs: week 3 [6.6, 12.9]; week 7 [36.8, 49.1]; week 12 [58.3, 74.4]. (b) PASI 90. *P < 0.001. 95% CIs: week 7 [18.9, 27.9]; week 12 [45.6, 58.7]. (c) PASI 100. *P = 0.001. 95% CI: week 12 [7.9, 16.4]. Non‐responder imputation. ADA, adalimumab 40 mg every‐other‐week dosing; CIs, confidence intervals; ITT_A, all randomized patients; ITT_B, all patients receiving at least one dose of study drug in Period B; PASI, Psoriasis Area Severity Index; PBO, placebo.
Figure 2.
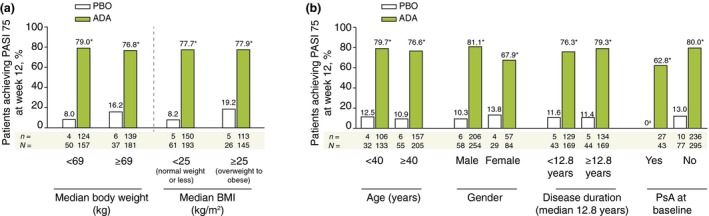
PASI 75 Response at week 12 for Patient Subgroups (ITT_A Population). (a) *P < 0.001. 95% CIs: median bodyweight (kg) <69 [61.1, 80.8] and ≥69 [47.2, 74.0]; median BMI (kg/m2) <25 [60.5, 78.6] and ≥25 [42.1, 75.3]. Non‐responder imputation. (b) *P < 0.001. 95% CIs: age (years) <40 [53.9, 80.5] and ≥40 [55.6, 75.8]; male [61.6, 80.0] and female [38.0, 70.1]; median duration of psoriasis (years) <12.8 [53.2, 76.2] and ≥12.8 [56.7, 79.1]; psoriatic arthritis at baseline, yes [48.3, 77.2] and no [58.2, 75.8]. Non‐responder imputation. a N = 10. ADA, adalimumab 40 mg every‐other‐week dosing; BMI, body mass index; CI, confidence intervals; PASI, Psoriasis Area Severity Index; PBO, placebo; PsA, psoriatic arthritis.
The adalimumab group experienced significantly greater PGA 0/1 response rates at week 12 (80.5%) than the placebo group (14.9%), and rates further increased in the adalimumab group by week 24 (83.8%). Patients in the placebo group who switched to adalimumab in Period B reached a response rate of 89.4% by week 24 (Fig. A2a). The adalimumab group experienced a significantly greater mean reduction in DLQI scores from baseline at week 12 (−9.07) than the placebo group (−4.17), and the reduction continued for the adalimumab group to week 24 (−9.95). Patients in the placebo group who switched to adalimumab in Period B reached a reduction in DLQI score of −9.68 by week 24 (Fig. A2b).
Patients in the adalimumab group experienced a significantly greater increase (mean) from baseline to week 12 in SF‐36 Health Status Survey scores for body pain due to psoriasis (8.34) than the placebo group (2.50; mean difference 5.84, P < 0.001; LOCF), and the increase continued for the adalimumab group to week 24 (9.82). Patients in the placebo group who switched to adalimumab in Period B reached a score increase of 9.01 by week 24. Increased scores indicate improvement.
Safety
Adverse events (Table 2) were reported by 37.9% and 46.7% in the Period A placebo and adalimumab groups, respectively, and by 63.4% in the All‐adalimumab Population (Table 2) [269.3 events per 100 patient years (E/100PY)]. The most common adverse event was upper respiratory tract infection (Period A: 6.9% and 10.1% in the placebo and adalimumab groups respectively; All‐adalimumab Population: 16.1%).
Table 2.
Proportion of patients with treatment‐emergent adverse events
| Period A, n (%) | All‐adalimumab Populationa, n (%) N = 423 | ||
|---|---|---|---|
| Placebo N = 87 | Adalimumab N = 338 | ||
| Any AE | 33 (37.9) | 158 (46.7) | 268 (63.4) |
| AE leading to study‐drug discontinuation | 0 | 2 (0.6) | 9 (2.1) |
| AE leading to death | 0 | 0 | 1 (0.2) |
| Serious AEb , c | 3 (3.4) | 4 (1.2) | 15 (3.5) |
| Arrhythmia | 0 | 0 | 1 (0.2) |
| Arthralgia | 0 | 0 | 1 (0.2) |
| Cancerd | 0 | 0 | 2 (0.5) |
| Cardiac failure | 0 | 0 | 1 (0.2) |
| Cerebral infarction | 0 | 1 (0.3) | 1 (0.2) |
| Erythema multiforme | 0 | 0 | 1 (0.2) |
| Fracturee | 0 | 1 (0.3) | 2 (0.5) |
| Gastric ulcer | 0 | 0 | 1 (0.2) |
| Haematuria | 0 | 1 (0.3) | 1 (0.2) |
| Hypertension | 0 | 1 (0.3) | 1 (0.2) |
| IVD protrusion | 0 | 0 | 1 (0.2) |
| Myocardial infarction | 0 | 1 (0.3) | 1 (0.2) |
| Myocarditis | 0 | 0 | 1 (0.2) |
| Pleural effusion | 0 | 0 | 1 (0.2) |
| Pleural fibrosis | 0 | 0 | 1 (0.2) |
| Psoriasis exacerbation | 1 (1.1) | 0 | 0 |
| Psoriatic arthropathy | 1 (1.1) | 0 | 0 |
| Pyloric stenosis | 0 | 0 | 1 (0.2) |
| Skin injury | 0 | 0 | 1 (0.2) |
| Retinal detachment | 1 (1.1) | 0 | 0 |
| Infection | 14 (16.1) | 59 (17.5) | 128 (30.3) |
| Serious infection | 0 | 0 | 5 (1.2) |
| Lung infection | 0 | 0 | 2 (0.5) |
| Pneumonia | 0 | 0 | 2 (0.5) |
| Tuberculosisf | 0 | 0 | 2 (0.5) |
Received at least one dose of adalimumab during the study. Events [E/100PY] for any AE was 657 [269.3], AE leading to study‐drug discontinuation 18 [7.4], AE leading to death 1 [0.4], Serious AE 22 [9.0], infections 162 [66.4] and serious infections 6 [2.5].
Does not include serious infections which are listed separately.
A patient reporting ≥2 different preferred terms within the same organ class is counted only once.
Endometrial cancer and gastric cancer.
Vertebral fracture (adalimumab) and tibia fracture (adalimumab/adalimumab).
Tuberculosis (placebo/adalimumab) and lymph node tuberculosis (adalimumab/adalimumab).
There were no reports of opportunistic infection, malignancy or demyelinating disorder in Safety Population A. There were no reports of opportunistic infections excluding TB, no reports of lymphoma, non‐melanoma skin cancer, hepatosplenic T‐cell lymphoma, leukaemia, melanoma or demyelinating disorder in Safety Population B.
AE, treatment‐emergent adverse event; E/100PY, events per 100 patient years.
Serious adverse events rates in Period A were 3.4% and 1.2% in the placebo and adalimumab groups, respectively, and 3.5% (9.0 E/100PY) in the All‐adalimumab Population. Serious infections occurred in 1.2% of the All‐adalimumab Population (2.5 E/100PY) and in no patient in Period A. These included lung infection, pneumonia and tuberculosis, each reported by two patients (Table 2). One of the two patients with serious infection of tuberculosis received placebo/adalimumab and experienced active tuberculosis on Day 196 post study. This patient had an abnormal baseline chest X‐ray with no evidence of calcified granulomas, or pleural thickening or scarring, and had a negative purified protein derivative test. The second patient received adalimumab/adalimumab and experienced active, lymph node tuberculosis on Day 155 in Period B. At baseline, this patient had a normal chest X‐ray and a negative purified protein derivative test. Both patients received treatment and completed the study, and both events were considered to be possibly related to the study drug. Two patients in the adalimumab/adalimumab group each experienced a serious adverse event of malignancy in Period B (gastric cancer not related to adalimumab, and endometrial cancer, possibly related to adalimumab). No cases of non‐melanoma skin cancer, lymphoma or demyelinating disease were reported during the study. There was one death during the study, attributed to chronic heart failure in a 37‐year‐old male non‐smoker with no known risk factors, who was in the adalimumab/adalimumab group. On day 109, the patient experienced a non‐serious upper respiratory infection and by day 113, also had a fever, tachycardia and asthma‐like symptoms. On day 114, he experienced a pulmonary infection, severe myocarditis and arrhythmia along with chronic heart failure (all were considered possibly related to adalimumab), was admitted to the hospital and treated with medications. The following day, he experienced arrhythmia, which was unsuccessfully treated, and died.
Discussion
This study is the first to evaluate adalimumab treatment for Chinese patients with psoriasis. This phase 3 study demonstrated efficacy and rapid onset of therapeutic response as measured by achievement of PASI 75/90/100, PGA 0 or 1, as well as by improvement in DLQI. Disease burden at baseline was relatively higher than populations of psoriasis patients in other adalimumab trials.12, 13, 14 At the beginning of this trial, patients had a mean PASI score of 27.7, an affected mean body surface area of 42% and mean DLQI score of 14.5. While bodyweight was lower than for psoriasis patients in other adalimumab trials,12, 14 a substantial fraction (40.2%) in this trial were nonetheless overweight or obese (BMI ≥25 kg/m2).
The treatment effect in this trial is consistent with other clinical trials for psoriasis conducted in North America or Europe.12, 14 In trials of western and Japanese patients (REVEAL and a phase 2/3 trial respectively),12, 15 PASI 75 was achieved by the majority of ADA every‐other‐week patients at weeks 12, 16 and 24 (Table A2). The response was significantly greater for all three studies at week 12, and at weeks 16 and 24 of REVEAL and the Japanese study (this study did not evaluate treatment response after week 12). These results were despite some baseline differences among the studies' populations (Table A1): the Japanese trial had more male patients compared with this study or REVEAL, and REVEAL patients had higher bodyweight and longer duration of psoriasis. Compared to the Western European and North American populations studied in prior adalimumab trials, the Chinese population in this study had a higher percentage of psoriasis‐affected body surface area, and higher PASI scores, which is more similar to the previously conducted Japanese study. Similar differences in baseline characteristics have been seen for Asian vs. western patients in trials of other biologic treatments for psoriasis.16
In this study, improvement in quality‐of‐life and reduction in pain, important aspects of response to psoriasis treatment especially for patients with moderate‐to‐severe disease,17 are consistent with other adalimumab trial results.12, 14
Safety outcomes in this population were generally favourable and consistent with those observed in previous trials of adalimumab treatment for moderate‐to‐severe plaque psoriasis.12, 14 The rates of serious adverse events, serious infections and discontinuation of study drug due to adverse events were low across all treatment groups. No cases of non‐melanoma skin cancer, lymphoma or demyelinating disease were reported during the study. The event of tuberculosis reported by two patients is noteworthy, as tuberculosis has been associated with TNF‐α therapy.18 Although it is generally a rare event globally,18 the high incidence of tuberculosis in China19 underscores the importance of tuberculosis screening and adequate prophylaxis when appropriate, prior to adalimumab treatment in Chinese patients.
Limitations of this study include that the duration of the treatment period did not span enough time to evaluate long‐term efficacy and safety, and that an active comparator consisting of other systemic treatments was not included in this trial.
In this 24‐week study of Chinese patients with moderate‐to‐severe plaque psoriasis, a significantly greater proportion of patients receiving adalimumab achieved PASI 75 and PGA 0 or 1 at week 12 compared with placebo, and efficacy was sustained for 24 weeks. Compared with placebo, adalimumab also demonstrated a significant and rapid improvement of psoriasis and a significantly greater improvement in quality‐of‐life and pain scores. Safety results were consistent with the known adalimumab safety profile, and no new safety signals were identified within the 24 weeks of adalimumab treatment.
Supporting information
Figure S1. Patient Disposition. Abbreviations: eow, every‐other‐week dosing.
Figure S2. PGA Response of clear (0) or minimal (1), and mean change from baseline in DLQI.
Table S1. Baseline demographics and clinical characteristics from this study and from studies in other patient populations.
Table S2. PASI 75 endpoint outcomes comparison.
Acknowledgements
The authors acknowledge Yue Kang, employed by AbbVie China, for contributions to the study design and conduct, protocol development and data interpretation, and Jody Bennett, employed by AbbVie, for medical writing support in the production of this manuscript.
Inclusion/Exclusion Criteria
Adult patients (≥18 years of age) were eligible for study participation if they had a clinical diagnosis of psoriasis for at least 6 months, and stable plaque psoriasis for at least 2 months before screening and baseline. At baseline, patients had moderate‐to‐severe disease, defined as ≥10% body surface area involvement, Physicians Global Assessment of at least moderate disease [score of 3 on a scale of 0 (clear) to 5 (severe)] and a Psoriasis Area Severity Index (PASI) score of ≥10. Patients also had failed to respond to or were intolerant to previous systemic therapy, and had a negative tuberculosis screening assessment. Patients were excluded from the study if they had a diagnosis of erythrodermic psoriasis, pustular psoriasis, psoriasis induced by medication or exacerbated by medication or new onset of guttate psoriasis, a diagnosis of other active skin diseases or skin infections that may have interfered with evaluation of psoriasis, could not discontinue topical therapies for the treatment of psoriasis, could not avoid UVB or PUVA phototherapies (for at least 14 or 28 days, respectively, before baseline), had previous exposure to a biologic treatment or received other systemic therapies for psoriasis (including adalimumab) within 28 days of baseline, took or required oral or injectable corticosteroids during the study, had a history of demyelinating disease or certain infections or cardiovascular events, or had certain malignancies or abnormal laboratory results, had active tuberculosis, had immune deficiency or was immunocompromised, or had clinically significant abnormal screening laboratory results. Female patients who were pregnant or breast‐feeding, or considered becoming pregnant during the study or for 150 days after the last dose of study medication were also excluded.
Sample Size and Blinding
The study was designed to enrol approximately 336 and 84 patients in the adalimumab and placebo arms, respectively, to adequately characterize the adalimumab safety profile and to provide over 95% power to detect the treatment difference with 0.05 two‐sided type I error. An interactive voice/web response system determined patient randomization. The randomization schedule was prepared by the Statistics Department of AbbVie, US. Randomization was performed using an adequate block size. All AbbVie personnel with direct oversight of the conduct and management of the trial (with the exception of the drug supply team), the investigator, study‐site personnel and the patient remained blinded to each patient's treatment throughout the 12 week blinded period of the study.
Conflicts of interest
L Cai, J Gu, J Zheng, M Zheng, G Wang, L‐Y Xi, F Hao, X‐M Liu, Q‐N Sun, Y Wang, W Lai, H Fang, Y‐T Tu, Q Sun, J Chen and X‐H Gao were investigators for this study, and J‐Z Zhang was the principal investigator for this study; all declare no financial, professional or personal relationships that might be perceived as a conflict of interest. Y Gu and HD Teixeira receive a salary as employees of AbbVie and may also receive stock, stock options and/or stock grants. MM Okun is a former AbbVie employee. The sponsor of this study, AbbVie, participated in the study design; study research; collection, analysis and interpretation of data; and writing, reviewing and approving of this publication. All authors had access to the data, and participated in the development, review and approval, and in the decision to submit this publication.
Funding sources
AbbVie Inc, North Chicago, Illinois, USA
References
- 1. Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology 2012; 225: 121–126. [DOI] [PubMed] [Google Scholar]
- 2. Farley E, Menter A. Psoriasis: comorbidities and associations. G Ital Dermatol Venereol 2011; 146: 9–15. [PubMed] [Google Scholar]
- 3. Kimball AB, Gladman D, Gelfand JM et al National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol 2008; 58: 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeung H, Takeshita J, Mehta NN et al Psoriasis severity and the prevalence of major medical comorbidity: a population‐based study. JAMA Dermatol 2013; 149: 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lebwohl M. Psoriasis. Lancet 2003; 361: 1197–1204. [DOI] [PubMed] [Google Scholar]
- 6. Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest 2004; 113: 1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding X, Wang T, Shen Y et al Prevalence of psoriasis in China: a population‐based study in six cities. Eur J Dermatol 2012; 22: 663–667. [DOI] [PubMed] [Google Scholar]
- 8. Lin YK, Wong WR, Chang YC et al The efficacy and safety of topically applied indigo naturalis ointment in patients with plaque‐type psoriasis. Dermatology 2007; 214: 155–161. [DOI] [PubMed] [Google Scholar]
- 9. Tran D, Kwok YK, Goh CL. A retrospective review of PUVA therapy at the National Skin Centre of Singapore. Photodermatol Photoimmunol Photomed 2001; 17: 164–167. [DOI] [PubMed] [Google Scholar]
- 10. Zhu X, Zheng M, Song M et al Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque‐type psoriasis: results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol 2013; 12: 166–174. [PubMed] [Google Scholar]
- 11. Hongbo Y, Thomas CL, Harrison MA et al Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol 2005; 125: 659–664. [DOI] [PubMed] [Google Scholar]
- 12. Menter A, Tyring SK, Gordon K et al Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2008; 58: 106–115. [DOI] [PubMed] [Google Scholar]
- 13. Papp KA, Signorovitch J, Ramakrishnan K et al Effects of adalimumab versus placebo on risk of symptom worsening in psoriasis and subsequent impacts on health‐related quality‐of‐life: analysis of pooled data from two randomized, double‐blind, placebo‐controlled, multicentre clinical trials. Clin Drug Investig 2011; 31: 51–60. [DOI] [PubMed] [Google Scholar]
- 14. Saurat JH, Stingl G, Dubertret L et al Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008; 158: 558–566. [DOI] [PubMed] [Google Scholar]
- 15. Asahina A, Nakagawa H, Etoh T, Ohtsuki M. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol 2010; 37: 299–310. [DOI] [PubMed] [Google Scholar]
- 16. Tsai YC, Tsai TF. A review of clinical trials of biologic agents and small molecules for psoriasis in Asian subjects. G Ital Dermatol Venereol 2016; 151: 412–431. [PubMed] [Google Scholar]
- 17. Krueger G, Koo J, Lebwohl M et al The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient‐membership survey. Arch Dermatol 2001; 137: 280–284. [PubMed] [Google Scholar]
- 18. Semble AL, Davis SA, Feldman SR. Safety and tolerability of tumor necrosis factor‐alpha inhibitors in psoriasis: a narrative review. Am J Clin Dermatol 2014; 15: 37–43. [DOI] [PubMed] [Google Scholar]
- 19. Global Tuberculosis Report 2014. World Health Organization WHO/HTM/TB/2014.08. Accessed 13 October 2015 at http://www.who.int/tb/publications/global_report/en/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient Disposition. Abbreviations: eow, every‐other‐week dosing.
Figure S2. PGA Response of clear (0) or minimal (1), and mean change from baseline in DLQI.
Table S1. Baseline demographics and clinical characteristics from this study and from studies in other patient populations.
Table S2. PASI 75 endpoint outcomes comparison.


