Summary
For socially monogamous species, breeder bond dissolution has important consequences for population dynamics, but the extent to which extrinsic or intrinsic population factors causes pair dissolution remain poorly understood, especially among carnivores.
Using an extensive life‐history data set, a survival analysis and competing risks framework, we examined the fate of 153 different wolf (Canis lupus) pairs in the recolonizing Scandinavian wolf population, during 14 winters of snow tracking and DNA monitoring.
Wolf pair dissolution was generally linked to a mortality event and was strongly affected by extrinsic (i.e. anthropogenic) causes. No divorce was observed, and among the pair dissolution where causes have been identified, death of one or both wolves was always involved. Median time from pair formation to pair dissolution was three consecutive winters (i.e. approximately 2 years). Pair dissolution was mostly human‐related, primarily caused by legal control actions (36·7%), verified poaching (9·2%) and traffic‐related causes (2·1%). Intrinsic factors, such as disease and age, accounted for only 7·7% of pair dissolutions. The remaining 44·3% of dissolution events were from unknown causes, but we argue that a large portion could be explained by an additional source of human‐caused mortality, cryptic poaching.
Extrinsic population factors, such as variables describing the geographical location of the pair, had a stronger effect on risk of pair dissolution compared to anthropogenic landscape characteristics. Population intrinsic factors, such as the inbreeding coefficient of the male pair member, had a negative effect on pair bond duration. The mechanism behind this result remains unknown, but might be explained by lower survival of inbred males or more complex inbreeding effects mediated by behaviour.
Our study provides quantitative estimates of breeder bond duration in a social carnivore and highlights the effect of extrinsic (i.e. anthropogenic) and intrinsic factors (i.e. inbreeding) involved in wolf pair bond duration. Unlike the effects of intrinsic and extrinsic factors that are commonly reported on individual survival or population growth, here we provide quantitative estimates of their potential effect on the social unit of the population, the wolf pair.
Keywords: anthropogenic, Canis lupus, extrinsic, inbreeding, intrinsic, pair bond duration
Introduction
Population regulation is often described through intrinsic or extrinsic population processes. Species with strong social structures are often more prone to experience some kind of intrinsic population regulation (Odden et al. 2014). In such social systems, extrinsic factors (e.g. predation or hunting mortality) may interact with intrinsic factors in such a way that total mortality increases beyond the effect of the actual direct mortality itself (i.e. causing a super‐additive effect) (Milner, Nilsen & Andreassen 2007; Rutledge et al. 2010; Andreassen et al. 2013; Borg et al. 2015). Due to this super‐additive effect, it is essential to understand the mechanisms involved in population regulation, as a few accidental deaths may have a disproportionally large effect on the population. For instance, many threatened large carnivore populations are exposed to human‐caused mortality events. If these species have strong social bonding between members of a social unit, or experience sexually selected infanticide, such human‐caused mortality can result in the social disruption of the group and/or the loss of dependent offspring (Brainerd et al. 2008; Rutledge et al. 2010; Gosselin et al. 2015).
Many species have evolved complex social systems, with a few dominant individuals monopolizing reproduction within the social unit (Macdonald 1983; Jennions & Macdonald 1994; Hatchwell 2009). This is the case for some threatened large carnivore species, from which several have developed a monogamous mating system. In theory, breeders from socially monogamous species repeatedly face the choice of whether to remain together with their current partner or divorce and find another partner. However, lifetime reproductive success of dominant individuals generally increases with the length of their dominance tenure (Hodge et al. 2008; Sánchez‐Macouzet, Rodriguez & Drummond 2014). The duration of pair bonds has also been suggested to have positive effects on reproductive performance of socially monogamous species by increasing pair familiarity (Sánchez‐Macouzet, Rodriguez & Drummond 2014).
Maintaining dominance tenure seems, therefore, to be a primary route to gain fitness in socially monogamous species. However, dominance tenure is threatened by a variety of factors that may vary in space. For instance, recolonization and expansion of large carnivore populations into human‐dominated landscapes is often directly affected by human‐caused mortality, for example through legal (hunting and trapping) and illegal (poaching and poisoning) actions (Mech 1995; Persson, Ericsson & Segerström 2009; Liberg et al. 2011). Indirect effects of human activities, for example habitat fragmentation, habitat loss (Delibes, Gaona & Ferreras 2001) and geographical or management boundaries (Bischof, Brøseth & Gimenez 2015), are also known to restrict large carnivores distribution, leading to genetic structuring and sometimes inbreeding depression with strong consequences for population viability (Keller & Waller 2002; Liberg et al. 2005), and possibly also dominance tenure (Kempenaers, Adriaensen & Dhondt 1998; Sparkman et al. 2012). In addition, large carnivore populations are also affected by extrinsic factors, such as food availability (Zedrosser, Dahle & Swenson 2006; Cubaynes et al. 2014) or population intrinsic factors, such as intraspecific competition (Cubaynes et al. 2014), which has also been found to affect dominance tenure (Hodge et al. 2008; Berger et al. 2015).
In this article, we used data from a long‐term monitoring programme of a social carnivore population, the wolf (Canis lupus) in Scandinavia (Liberg et al. 2012) to examine the causes and the length of an important population demographic trait, pair bond duration. The exhaustive genetic and demographic information collected on the recolonizing Scandinavian wolf population (Wabakken et al. 2001, 2012; Liberg et al. 2012) offers a unique opportunity to better understand the factors involved in pair dissolution in a large carnivore population that is under strong anthropogenic influence (Karlsson et al. 2007; Liberg et al. 2011).
Specifically, we aimed at dissociating the effect of intrinsic and extrinsic population factors involved in wolf pair dissolution.
First, we quantified pair dissolution and causes of pair dissolution and predicted pair dissolution to be mainly caused by extrinsic (i.e. anthropogenic) factors resulting in short wolf pair bond duration (H1).
Then, we quantified to which extent population intrinsic and extrinsic characteristics of the pairs explained risk of pair bond dissolution. We hypothesized that spatial variation in extrinsic factors (mainly anthropogenic) explained spatial variation in pair bond duration (H2). Because the population is still in a recolonization phase, with abundant food resources, we further predicted (H3) that there should be no or small effects of population intrinsic factors, such as intraspecific competition, through food availability or wolf density (Mattisson et al. 2013). Finally, (H4) we tested the hypothesis that inbreeding (i.e. intrinsic factor) had a negative role in pair bond duration (Kempenaers, Adriaensen & Dhondt 1998) in addition to the inbreeding depression previously observed in this population (Vila et al. 2003; Liberg et al. 2005).
Materials and methods
Study area
The study was conducted in the south‐central part of the Scandinavian Peninsula (Sweden and Norway 59°–62 °N, 11°–19 °E; Fig. 1). The landscape is dominated by boreal forest, interspersed with bogs and lakes. Agricultural and urbanized lands cover <5% of the study area. Due to extensive commercial logging and forest management practices, the average density of gravel forestry road is high (i.e. 0·88 km km−2 inside wolf territories, Zimmermann et al. 2014). However, the density of main roads (tarred public roads) is approximately four times lower than the gravel road density (Zimmermann et al. 2014). Human density is low and the study area encompasses large areas with less than one human per km2 (Wabakken et al. 2001). The climate is continental and snow covers the ground for 3–6 months annually, mainly during October–April. Moose are the main wolf prey in Scandinavia and are very abundant (average: 1·3 per km2; range 0·7–3·3) throughout the study area (Zimmermann et al. 2015).
Figure 1.
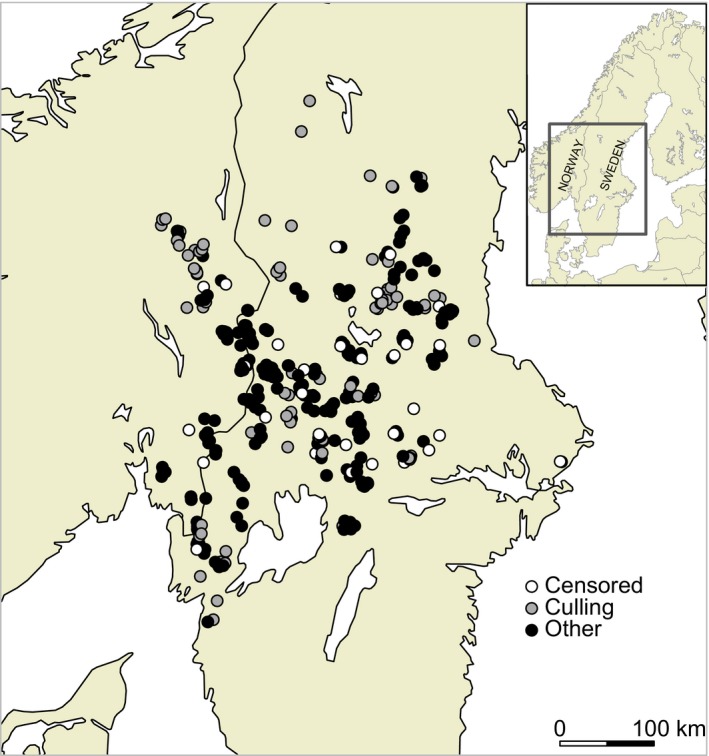
Centroid location of the 369 wolf territorial pair‐winters monitored in Scandinavia during 14 winters, 1998/1999–2011/2012. Grey circles represent pairs that have dissolved due to culling, black circles represent pairs that have dissolved for other reasons, and white circles are for pairs that were censored (i.e. dissolution not observed).
Identifying wolf territories and pairs
Monitoring of the Scandinavian wolf population was performed by the Norwegian and Swedish management authorities and consisted of tracking wolves on snow from October 1 to April 30 (Wabakken et al. 2001; Liberg et al. 2012) over a distance of 2200–5600 km each winter (see further details in Appendix S1, Supporting Information). We also utilized data collected by the cooperative Swedish–Norwegian Wolf Research Project (SKANDULV, Liberg et al. 2012) from 3 to 21 territorial wolves equipped with functioning radiocollars each winter. A near complete pedigree of the population has been reconstructed by a combination of individual DNA profiles (samples collected from scats, urine, blood, tissue and hair) and long‐term annual snow tracking of territorial individuals (Liberg et al. 2005, 2012; Bensch et al. 2006). Information on the spatial location of wolf territories during winter was gathered from snow tracking in combination with DNA‐analyses of collected samples, or by VHF/GPS location when available. The two main goals of the monitoring programme are (i) to register the annual number and spatial distribution of all reproduction events and territorial pairs (hereafter, we used the term territorial wolf pair for: a pair of two potential breeders or two breeders with their offspring, i.e. a pack) and (ii) maintain and continuously update the pedigree of the population. Special tracking efforts were, therefore, made every winter to detect and genetically identify new potential breeders within territorial pairs. This extensive and long‐term monitoring programme provided a near complete description of the annual distribution and dynamics of wolf territories in Scandinavia, including the identities of the territory‐marking individuals (Fig. 1). During our study period (1998/1999–2011/2012), the population increased by fourfold–sixfold from 10 to 60 pairs, and, on average, from 70 to 295 wolves (Wabakken et al. 1999, 2012).
Identifying causes of pair dissolution
All pair dissolutions were assigned to one of five classes: (i) death caused by culling (i.e. legal control actions or license hunting), (ii) verified poaching, (iii) natural causes of death (e.g. age and diseases), (iv) traffic mortality and (v) unknown causes (i.e. when a pair dissolution was verified (one or both individuals were missing), but could not be linked to any of the other four categories). After a pair dissolution event in which one of the pair members went missing, replacement was confirmed when a new wolf started territorial scent marking together with the remaining individual from the previous pair.
Extracting characteristics of the territory and the pair
In Scandinavia, wolf pair home ranges have an average size of approx. 1000 km2 (Mattisson et al. 2013). However, accurate home range boundaries (i.e. calculated using at least 9 months with location data, each with five or more locations) were unknown for the majority of pairs, which were not radiocollared (Mattisson et al. 2013). Instead, we used all available spatial information (i.e. VHF/GPS and/or snow‐tracking locations) to compute a centroid point location for each territory and year. We then extracted the large‐scale spatial characteristics (Table 1) of the wolf territories within an average circular wolf territory of 1000 km2 placed around this centroid point (Mattisson et al. 2013; Ordiz et al. 2015).
Table 1.
List of variables used to model wolf risk of pair bond dissolution in Scandinavia during the period 1998–2011. The name, description and related‐hypothesis of each variable used are mentioned. Time series shows whether the variables used varied with time or not. Quadratic effect shows whether a quadratic effect of the variable was tested or not
| Name | Description | Hypothesis | Time series | Quadratic effect | Sources |
|---|---|---|---|---|---|
| Road1 | Total length of paved roads (km per km2) | (H2) Reflects human activity | No | No | (1:100 000, Lantmäteriet, Sweden; N50 kartdata, Staten‐skartverk, Norway) |
| Road2 | Total length of gravel roads (km per km2) | (H2) Reflects human accessibility | No | No | (1:100 000, Lantmäteriet, Sweden; N50 kartdata Statens Kartverk, Norway) |
| RoadBuild | Per cent of roads stretches with ≤ 2 buildings per km | (H2) Reflects human accessibility & remoteness | No | No | (Lantmäteriet, Sweden; N50 kartdata, Statens Kartverk, Norway |
| Hum | Human density, number of inhabitants per km2 | (H2) Reflects human activity | No | No | www.scb.se, Sweden; www.ssb.no, Norway |
| Conf1 | Dogs depredation events | (H2) Reflects potential for conflicts | No | No | www.rovdjursforum.se, Sweden, www.rovbase.no, Norway |
| Conf2 | Sheep depredation events | (H2) Reflects potential for conflicts | No | No | www.rovdjursforum.se, Sweden, www.rovbase.no, Norway |
| TimePres | Number of winters that wolf pairs occupied the area | (H2) Increase tolerance through time | Yes | No | Wabakken et al. (1999, 2001, 2012) |
| Country | Country in which the wolf territory was located (Sweden/Norway/Cross‐border) | (H2) Human attitudes towards wolves differ between Sweden and Norway | No | No | Gangaas, Kaltenborn & Andreassen (2013) |
| LocEast | Location on the longitude scale | (H2) Longitude scale | Yes | Yes | WGS 84/UTM zone 33 |
| LocNorth | Location on the latitude scale | (H2) Latitude scale | Yes | Yes | WGS 84/UTM zone 33 |
| LocCore | Distance from core area of the wolf population | (H2) Effect of management | Yes | Yes | Wabakken et al. (1999, 2001, 2012) |
| Density | Number of wolf territories within a 40 km radius | (H3) Density dependence | Yes | No | Wabakken et al. (1999, 2001, 2012) |
| Moose | Annual number of moose shot per km2 used as an index for local moose density | (H3) Food availability | Yes | No | http://www.viltdata.se/, Sweden; www.ssb.no, Norway |
| Age_F Age_M | Proxy for the minimum age of Female and Male pair members | (H3) Effect of age of pair members | Yes | Yes | Wabakken et al. (1999, 2001, 2012) |
| F_male F_female F | Male, female, potential offspring inbreeding coefficients. | (H4) Inbreeding avoidance | No | No | Liberg et al. (2005) |
Extrinsic characteristics
We used human density (number of inhabitants per km2), density of gravel roads and main roads (km per km2) and an index that combined information on the spatial location of roads and buildings to quantify areas that were both highly accessible by humans yet remote (Table 1, Appendix S2). Wolf depredation on domestic animals and dogs is an important source of conflict with humans in Scandinavia (Herfindal et al. 2005; Liberg et al. 2010). We therefore quantified the spatial variation of wolf depredation events for domestic sheep and hunting dogs. (Table 1, Appendix S2).
We used descriptors of the geographical location of the territory, such as longitude, latitude and the distance from the core area of the wolf population (here defined as the annual centre of all estimated centroid points of wolf territories) as additional covariates. As tolerance (Gangaas, Kaltenborn & Andreassen 2013) and management of wolves differs between Sweden and Norway, we also included the country in which wolf pairs were located as a covariate in the models.
To map prey density, we created a moose density index based on harvest density (number of moose harvested per km2) at the municipality level in Norway and at the moose management unit (‘älgförvaltningsområde’) level in Sweden. Harvest density has been found to be a robust, but delayed, indicator of spatio‐temporal variation in moose density (Ueno et al. 2014). To account for this delay, we used harvest density figures from the year t + 1 to estimate a moose density index in year t.
Intrinsic characteristics
Local density of wolf pairs was used as a proxy of density‐dependent effects on pair bond duration (Mattisson et al. 2013). Each winter, we counted the number of neighbouring territories having their centroid point within a 40‐km‐radius buffer (i.e. two times the radius of a large wolf home range) around the centroid location of each pair.
Human tolerance towards carnivores may sometimes increase with time of coexistence (Zimmermann, Wabakken & Dötterer 2001). Based on wolf monitoring data, the centroid location of each winter territory identified was used as the centre of a 1000‐km2 buffer zone (i.e. size of an average wolf home range) and a wolf territory was considered present in pixels covering the buffer. We then created a time series of maps showing the number of winters that territorial wolf pairs had been recorded in each pixel (200 × 200 m, Appendix S2, Supporting information) of the study area since the first wolf pair re‐establishment in 1982 (Wabakken et al. 2001).
Because age of the individuals forming the pair can affect pair bond duration, we assigned a year of birth to all individuals. However, due to our extensive data set, we could not assign exact year of birth to all individuals. We therefore estimated a latest possible year of birth (i.e. minimum possible age) to obtain a proxy for the age of individuals forming the pair. The latter was estimated using a combination of multiple sources of information, such as the year of first DNA capture and the last year that the parental pair was known to have successfully reproduced. We also assumed that the individual should be minimum 2 years old before the first detected breeding of the individual, and 1 year before first pairing.
Earlier studies have shown that the level of inbreeding may affect fitness traits among Scandinavian wolves (Liberg et al. 2005; Bensch et al. 2006). We used the reconstructed pedigree to calculate the individual inbreeding coefficient f (Liberg et al. 2005), which represents the amount of ancestry shared by parents of an individual (Keller & Waller 2002). To estimate the effect of inbreeding depression on pair bond duration, we used the inbreeding coefficient of the individuals in each pair (i.e. the male and female), and the inbreeding coefficient of their potential offspring as separate variables. Five different Finnish–Russian immigrants that formed a pair were assumed to be outbred (i.e. f = 0). For two individuals, with missing pedigree information, we randomly assigned a inbreeding coefficient that was derived from the distribution of inbreeding coefficients calculated from the potential mating of individuals available for mating at the time of birth of the two individuals.
Pair bond duration
We summarized data from individuals identified during tracking events for each winter. If pair members could not be directly detected during a winter, we used indirect information to confirm their presence, such as the genetic detection of offspring from the non‐detected pair member. These multiple sources of information to confirm presence of pair members were combined with a survival analysis framework to quantify the pair bond duration of territorial wolf pairs. Survival analysis refers to statistical procedures for which the outcome variable of interest is time until an event occurs (Kleinbaum & Klein 2011). In our case, each winter monitoring period (October–April) was set as the time unit. A pair detected within a specific territory during each winter was assumed to have been present during that entire winter, because the exact date of dissolution was unknown in most cases. The dissolution event was attributed to the winter in which one or both of the previously identified individuals were no longer detected within a previously defined territory. Thus, we counted the number of consecutive winters in which a specific pair was identified in its territory from the winter of establishment until the winter in which no signs of one or both individuals were found (i.e. pair dissolution). Hence, if a territorial female and male were found together for three consecutive winters, but not during the fourth winter, we considered that the dissolution occurred at the end of the third winter (i.e. the pair persisted for three consecutive winters and for approximately 2 years). Three different criteria for pair dissolution were used as follows: (i) evidence that one or both individuals were dead, (ii) replacement of one or both individuals by another individual the following winter and (iii) failure to record two scent‐marking individuals in a previously verified territory, despite large tracking efforts. Censoring (when monitoring stops without the event of interest having occurred; Kleinbaum & Klein 2011) only occurred at the end of our study in 2011/2012.
We used a Kaplan–Meier survivor function to quantify the probability that a specific pair will persist over time (Kleinbaum & Klein 2011). It is a step function that decreases from 1 (all wolf pairs are intact at time t) towards a minimum value of 0 (when dissolution of all pairs has occurred). To model the relative influence of covariates (Table 1) on risk of pair dissolution, we used semiparametric Cox proportional hazard (CPH) models (Kleinbaum & Klein 2011). These models provide hazard ratios (HR) of covariates on the baseline hazards (instantaneous potential of dissolution) for the event to occur at a time t per unit time (Kleinbaum & Klein 2011). We used a counting‐process style input, which allows time‐varying covariates to be used (Fieberg & DelGiudice 2009). Pair members were identified as correlated groups of observations and were clustered in order to obtain robust sandwich variance estimators (Kleinbaum & Klein 2011).
Cause‐specific pair dissolution
In the case of multiple causes of pair dissolution, a general approach such as Kaplan–Meier is not sufficient because it involves mutually exclusive events in time (i.e. if pair i splits up due to cause k, it is not available to split up from cause j). We therefore estimated specific causes of dissolution using a nonparametric cumulative incidence function estimator (Heisey & Patterson 2006).
To model the impact of covariates (Table 1) on the cause‐specific risk of pair dissolution, we re‐classified causes of pair dissolution into two main categories, (i) culling (i.e. all legal killing, including control and license hunting) and (ii) other causes (i.e. unknown, natural mortality, verified poaching and traffic related). We created the second category because we could not exclude natural mortality, poaching and traffic‐related causes of dissolution from unknown causes of dissolution. We followed methods described by Lunn & McNeil (1995) and Heisey & Patterson (2006) to account for competing risks. We first duplicated the data set as many times as the number of dissolution causes. Then, we used the ‘strata()’ function to compute different baseline hazard functions for each dissolution cause (Therneau 2014). Finally, we included interaction terms between important covariates obtained after model selection and strata to estimate the potential effects of covariates in relation to different causes of dissolution.
Model selection
To determine which factors (Table 1) influenced risk of pair dissolution, we performed CPH model selection based on corrected Akaike's information criterion (AICc) (Burnham & Anderson 2002; Liang & Zou 2008). Before running model selection, we checked for collinearity between all covariates (r < 0·6). Among two correlated variables, only the variable with the lowest AICc score in a simple model was retained in the model selection process (Appendix S3). We standardized all continuous covariates to 1 SD to facilitate interpretation and comparison of the relative strength of parameter estimates (Schielzeth 2010; Grueber et al. 2011). All combinations of additive variables were biologically plausible. Therefore, we considered all possible combinations of models (Table 1), using the ‘MuMIn’ r package (Barton 2014). We did not consider individual models with more than five variables to avoid over‐fitting models (Grueber et al. 2011). We considered the quadratic forms of some of the variables (Table 1) in the model selection process, but only included that transformation when a model containing both the linear and quadratic forms of the variable had a lower AICc (i.e. ∆AICc ≥ 2) than a model containing just the linear form. We also considered some interactive terms (age_F × age_M; age_F × F_female; age_M × F_male), but only if the interactive model had lower AICc (i.e. ∆AICc ≥ 2) compared to the inclusion of additive model. We then checked for hazard proportionality using the scaled Schoenfeld residuals (Kleinbaum & Klein 2011). We performed model averaging, based on AICc, and calculated confidence intervals for all models with ∆ AICc ≤ 2 (Burnham & Anderson 2002; Grueber et al. 2011; Barton 2014). In addition, we used 95% confidence intervals around averaged hazard ratio estimates to help interpret uncertainty in parameters estimation and variable importance (Fletcher & Dillingham 2011; Galipaud et al. 2014). Additionally, we also tested whether the replacement of one individual in the pair could be attributed to its degree of relatedness by comparing the inbreeding coefficient of the new individual to the inbreeding coefficient of the replaced individual, using a paired t‐test. We also tested the robustness of our method to extract landscape characteristics, by adding some noise to the centroid location of the territory (See Appendix S4 for further details). All analyses were performed using r version 3.0.3 (R Core Team 2014) and the Survival package (Therneau 2014).
Results
Pair dissolution
General
Genetic identity of both territorial wolf pair members (i.e. the scent‐marking female and male) was determined in 98% of the 442 winter territories documented during 14 consecutive winters from 1998/1999 to 2011/2012. In total, we detected 179 different pairs representing 429 monitored pair‐winters and 295 different individuals (140 females, 155 males). Among our 13 winter to winter pair bond duration estimates, we determined the fate of 153 different pairs and documented a total of 119 dissolution events. The winter following most of these dissolution events, a replacement occurred for 70 pairs (58·8%), with one (72·9%, n = 51) or both pair members (27·1%, n = 19) replaced (Fig. 2). For the remaining 49 (41·2%) dissolution events, no replacement occurred before or during the next winter, and we detected one individual being left alone in 32·7% (n = 16) of the cases. However, we could not detect any individuals or pairs within the territory previously occupied by the dissolved pair in 67·3% (n = 33) of the cases (Fig. 2). Our proxy for minimum age showed that mean (±SD) age at pair establishment was 2 (±1·61) and 2·4 (±1·90) years old, 3·7 (±2·37) and 4·1 (±2·60) at pair dissolution, and mean age of wolves observed in a pair was 3·2 (±2·21) and 3·64 (±2·50) for males and females, respectively.
Figure 2.
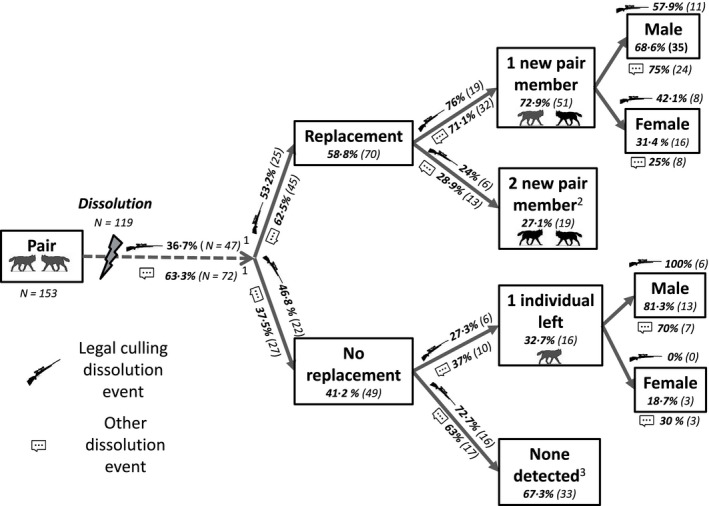
Flow chart of the consequences of pair dissolution in the Scandinavian wolf population during the period 1998–2011. Among the 153 different wolf pairs included in this study, 47 dissolved due to legal culling ( ) and 72 pairs due to others causes (
) and 72 pairs due to others causes ( ; i.e. natural, traffic‐related, poaching and unknown causes). The winter following a dissolution event, we identified either: (i) a replacement of two individuals (i.e. both the male and the female were replaced) or one individual (i.e. the male or the female was replaced); or (ii) no replacement, meaning that we detected one individual left alone (i.e. the male or the female) or no pairs could be confirmed within the territory. Percentages and number of events are presented to show the extent to which culling and other dissolution events were followed by a replacement or not. 1Percentages were estimated using nonparametric cumulative incidence function estimator (see methods). 2At least one new pair (two new individuals) detected with a territory overlapping the territory of the previously dissolved pair. 3No pair could be detected overlapping with the territory previously occupied by the dissolved pair.
; i.e. natural, traffic‐related, poaching and unknown causes). The winter following a dissolution event, we identified either: (i) a replacement of two individuals (i.e. both the male and the female were replaced) or one individual (i.e. the male or the female was replaced); or (ii) no replacement, meaning that we detected one individual left alone (i.e. the male or the female) or no pairs could be confirmed within the territory. Percentages and number of events are presented to show the extent to which culling and other dissolution events were followed by a replacement or not. 1Percentages were estimated using nonparametric cumulative incidence function estimator (see methods). 2At least one new pair (two new individuals) detected with a territory overlapping the territory of the previously dissolved pair. 3No pair could be detected overlapping with the territory previously occupied by the dissolved pair.
Causes of pair dissolution
Altogether, the survival curve indicated that half of the pairs (i.e. median persistence of pairs) have dissolved after three [95% CI = (3–4)] consecutive winters (i.e. after approximately 2 years) (Fig. 3). The overall probability of a wolf pair bond persisting from one winter to the next (i.e. approximately 1 year) was 0·68 (0·63–0·73). No pair lasted for more than eight consecutive winters, except one that lasted for 12 consecutive winters, with both male and female being at least 13 years old when the pair dissolved. Dissolution due to unknown causes was most common and occurred in 44·3% [95% CI = (37·8–50·8)] of the cases. Causes of dissolution were determined in 55·7% of the cases with 36·7% (25·9–47·5%) of the cases attributed to culling, 9·2% (0–20·1%) to confirmed poaching, 7·7% (0–20·6%) to natural causes of mortality such as disease and age and 2·1% (0–11·9%) to traffic‐related accidents.
Figure 3.
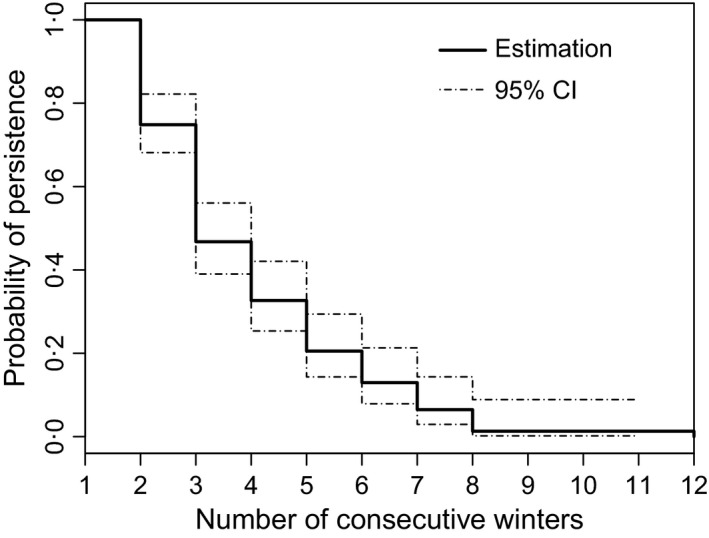
Kaplan–Meier cumulative survival curve with 95% confidence intervals showing the probability of wolf pair bond persistence in Scandinavia during the winters 1998/1999–2011/2012. On the x‐axis, winter 1 shows the first winter a pair was detected, winter 2 the second and so on.
Effect of intrinsic and extrinsic characteristics of the pair on risk of pair dissolution
According to the final CPH models based on all wolf pairs, the extrinsic variables Distance from the core area (LocCore) and Longitudinal gradient (LocEast), and intrinsic variables Inbreeding coefficient of the male (F_male) and Age of the males (Age_M) were the most important variables affecting pair bond duration. The low relative variable importance and 95% confidence interval of hazard ratios not overlapping with 1 (Tables 2 and 3) showed that all other variables had considerably less influence on pair bond duration. Risk of pair dissolution increased with the Distance from the core area, Age of the male and Inbreeding coefficient of the male, and pair bond duration was longer with increasing longitudinal gradient (Table 3).
Table 2.
Model inferences based on Cox proportional hazard regression models of factors affecting risk of pair dissolution in Scandinavia during the period 1998–2011. Best models based on AICc selection and the null model is also presented for comparison purposes. See Table 1 for variable descriptions
| Model set | K | logLik | AICc | ∆AICc | W i |
|---|---|---|---|---|---|
| AgeM + LocCore + LocEast + F_male | 4 | −475·66 | 959·37 | 0 | 0·27 |
| AgeM + LocCore + LocEast + F_male + AgeF | 5 | −474·86 | 959·81 | 0·43 | 0·22 |
| AgeM + LocCore + LocEast + F_male + Moose | 5 | −475·56 | 961·21 | 1·83 | 0·11 |
| AgeM + LocCore + LocEast + F_male + F | 5 | −475·58 | 961·25 | 1·88 | 0·10 |
| AgeM + Density + LocCore + RoadBuild + LocEast | 5 | −475·61 | 961·31 | 1·93 | 0·10 |
| AgeM + F_male + LocCore + RoadBuild + LocEast | 5 | −475·62 | 961·32 | 1·94 | 0·10 |
| AgeM + F_female + F_male + LocCore + LocEast | 5 | −475·63 | 961·34 | 1·97 | 0·10 |
| Null | 1 | −485·63 | 971·26 | 11·89 | 0 |
Only models with ∆ corrected Akaike's information criterion (AICc) < 2 are shown. K stands for number of parameters; W i for the model weight; logLik for log likelihood.
Table 3.
Summary of parameter estimates after model averaging the hazard ratios of each parameter on wolf pair bond duration in Scandinavia during the period 1998–2011. A hazard ratio > 1·0 corresponds to an increased risk of pair dissolution for each additional unit of the covariate. All covariates were scaled to 1 SD for comparison purposes. Estimates were calculated from the best models selected after AICc selection (Table 3). See Table 1 for variable descriptions
| Parameter | Hazard ratio | 95% CI | Relative variable importance |
|---|---|---|---|
| LocCore | 1·30 | 1·10–1·52 | 1·00 |
| LocEast | 0·82 | 0·70–0·96 | 1·00 |
| Age_M | 1·30 | 1·09–1·54 | 1·00 |
| F_male | 1·35 | 1·12–1·63 | 1·00 |
| Age_F | 1·16 | 0·95–1·42 | 0·22 |
| Moose | 1·05 | 0·87–1·26 | 0·11 |
| F_female | 0·98 | 0·84–1·14 | 0·10 |
| Density | 1·03 | 0·86–1·24 | 0·10 |
| F | 1·04 | 0·87–1·25 | 0·10 |
| RoadBuild | 0·97 | 0·81–1·16 | 0·10 |
On average, pair dissolution tended to occur earlier when dissolution was due to culling compared to other causes (Fig. 4). The competing risk analysis and the 95% confidence intervals revealed that the risk of pair dissolution found for the intrinsic variables; Inbreeding coefficient and Age of the male were more important for dissolution due to other causes [HRF_male = 1·35, 95% CI = (1·04–1·76); HRAge_M = 1·32 (0·99–1·76), respectively] than due to culling [HRF_male = 1·38 (0·96–1·98); HRAge_M = 1·27 (0·89–1·81), respectively]. Concerning the extrinsic variables, the effect of Longitudinal gradient was more important for dissolution due to other causes (HR = 0·77, 95% CI = 0·61–0·97) than due to culling (HR = 0·90, 95% CI = 0·69–1·18). Conversely, the effect of Distance from the core area was more important for dissolution due to culling (HR = 1·52, 95% CI = 1·12–2·06) than due to other causes (HR = 1·16, 95% CI = 0·91–1·47).
Figure 4.
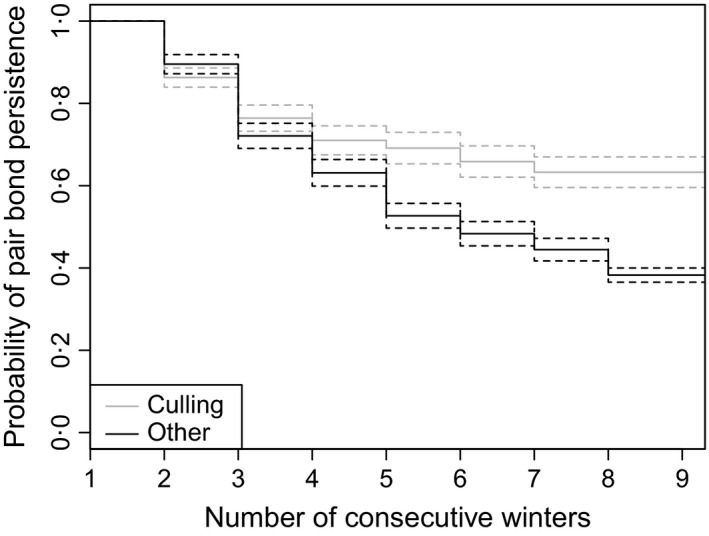
Nonparametric cumulative incidence estimates with 95% confidence intervals showing the probability of wolf pair bond persistence between the winters of 1998/1999 and 2011/2012, which dissolved due to either legal culling in grey (median pair persistence = 3 winters) and all other causes in black (median pair persistence = 4 winters). On the x‐axis, winter 1 shows the first winter a pair was detected, winter 2 the second and so on.
Inbreeding coefficient of the new replaced males
Since the males inbreeding coefficient was retained as an important variable, we checked whether inbreeding coefficient of the new male would be lower after a new replacement. However, new males were on average as inbred as the replaced males (average f new male = 0·266; average f old male = 0·241; t = 0·96, d.f. = 24, P = 0·35), and consequently, the arrival of a new male in the pair had no effect on the inbreeding coefficient of their pups (average f after new male = 0·285; average f before new male = 0·298; t = −0·54, d.f. = 24, P = 0·60).
Discussion
Importance of extrinsic factors in causes of pair dissolution
According to our hypothesis (H1), causes of pair bond dissolution were mainly due to extrinsic (i.e. anthropogenic) factors. The death of one or both partners was the typical proximate cause of pair dissolution (Hinton et al. 2015), which was supported by data from 98 radio‐marked pair members (Liberg et al. 2008). No divorces were observed, that is cases in which both individuals were still alive after a pair dissolution event. The cause of pair dissolution could be determined in 55·7% of the cases, and all involved the death of one or both wolves, most of which were caused by humans (culling: 36·7%, verified poaching: 9·2%, traffic: 2·1%) and 7·7% could be attributed to natural factors. Almost half of the dissolution events could not be assigned to any specific cause. If all or most of the unknown causes of pair dissolution were undetected mortality events, there are only two main possibilities: natural deaths or cryptic poaching. Legal culling is by definition reported in all cases, and it is likely that nearly all un‐intended traffic mortalities are also reported. Liberg et al. (2008) showed that natural causes made up 5·5% of all mortality of radiocollared breeding pair members in the Scandinavian wolf population. In our study, natural causes of pair dissolution amounted to 7·7% suggesting that a large proportion of the dissolution events caused by natural causes were detected, assuming GPS collared individuals were a representative sample of the population. As a consequence, a cryptic source of mortality, such as poaching, could be the main explanation for the remaining part of the unknown cases of dissolution. Poaching could, theoretically, be responsible for approximately half of all dissolution events which would be of the same magnitude as individual mortality caused by poaching in Scandinavia (Liberg et al. 2011).
We cannot entirely rule out the possibility that false absences, that is pairs that were considered dissolved but were actually intact, might explain a large number of dissolution events due to unknown causes. However, the continuously updated pedigree of the population reconstructed from DNA profiles, in combination with the comprehensive tracking effort (e.g. in the winter 2008/2009 approx. 100 field workers tracked wolves for >5400 km; Liberg et al. 2012) mean that very few reproducing pairs could have remained undetected for more than 1 year. Furthermore, the joined annual survival probability of female and male pair members (survfemale × survmale = 0·82 × 0·77 = 0·63) obtained from GPS collared animals (Liberg et al. 2008), falls within the confidence interval of our estimate of winter to winter pair bond duration (0·68; 95% CI: 0·63–0·73). This gives support to the estimates of pair bond duration obtained in our study.
Large carnivore mortality in human‐dominated landscapes is often human‐induced, both in Scandinavia (e.g. Bischof et al. 2009 for brown bears, Andrén et al. 2006 for lynx, Persson, Ericsson & Segerström 2009 for wolverines), and elsewhere (e.g. Jęodrzejewska et al. 1996; Falcucci et al. 2009 in Europe, Smith et al. 2010 in North America). Although the wolf is the most studied large carnivores, we are only aware of one study explicitly quantifying pair bond duration (Hinton et al. 2015). In this study, mean breeding pair bond duration of red wolves (Canis rufus) was estimated to 2 years (mean life span of wolf was 3·2 years) and >65% of pair bond dissolutions were caused by anthropogenic factors (Hinton et al. 2015). These estimates are comparable with the estimates obtained in our study and are quite different from the long wolf pair bond duration that seems to be perceived for wolf (e.g. Mech 1997). Adult wolf mortality rates are generally low in the absence of human offtake (Creel et al. 2015), which suggests that a median pair bond duration of three consecutive winters is relatively short for a long‐lived species such as the wolf (e.g. reported to have reached up to 15 years in the wild Carey & Judge 2000) and may reflect the strong impact of human‐related mortality in this population.
Importance of spatial variation in extrinsic factors on risk of pair dissolution
Our survival analysis revealed that spatial variation in extrinsic factors was an important factor influencing risk of pair dissolution (H2). However, the geographical location of pairs in Scandinavia better explained pair bond duration than the anthropogenic‐related variables. Although a consensus exists among scientists to apply management and conservation actions at a relevant biological unit, administrative or jurisdiction boundaries are often used as a basis for management decisions (Bischof, Brøseth & Gimenez 2015). According to official policy in both Norway and Sweden, wolves are not allowed to establish in all areas of the peninsula. For example, Scandinavian born wolves that move into the reindeer husbandry area (i.e. covering approximately the northern half of Scandinavia) and outside the specific Norwegian management zone established for breeding wolves (i.e. along the southern Swedish–Norwegian border) are promptly killed legally. As a consequence, the wolf breeding area is constrained to central Scandinavia (Fig. 1) which likely explains the higher risk of mortality due to culling observed at the periphery of the population. Furthermore, a greater tolerance for poaching exists in Norway than in Sweden (Gangaas, Kaltenborn & Andreassen 2013), which could be the causal mechanism to the longitudinal trend found in pair bond dissolution. Therefore, this could suggest that risk of pair dissolution may not be related to spatial variation in anthropogenic characteristics of the landscape, but rather to variation in tolerance towards carnivores and poaching.
Importance of intrinsic factors on risk of pair dissolution
The Scandinavian wolf population currently suffers from severe inbreeding depression that reduces individual fitness (Liberg et al. 2005; Bensch et al. 2006). We found a negative effect of the male pair member inbreeding coefficient on pair duration (H4), but only for dissolution events caused by ‘other’ causes. The ‘incompatibility hypothesis’ suggests that the pairing of two individuals that are of intrinsically good quality, but when paired together result in reduced fitness, would benefit from pairing with a new partner (Choudhury 1995). Thus, the replacement of pair members with a new individual resulting in relatively less inbred offspring could be a mechanism reflecting inbreeding avoidance (Choudhury 1995; Sparkman et al. 2012). Interestingly, this pattern could not be confirmed in this population, since no cases of divorce were detected (i.e. where both pair members were observed as a new pair after a dissolution event). In addition, replaced males were not less inbred than their predecessor. However, we could not directly test for the ‘incompatibility hypothesis’ since this required explicit data on the reproductive success for each pair, and our monitoring did not provide accurate estimates of litter size but only whether reproduction could be confirmed or not. Once wolf pairs started to reproduce, their subsequent reproduction rate was high with >95% of pairs with a confirmed positive reproductive status (SKANDULV unpublished). However, since the proportion of the genome identical by descent, under some circumstances, can vary substantially among individuals with identical pedigree‐based ancestry (e.g. full siblings), true differences in inbreeding and fitness between individuals may not have been captured entirely by using pedigree information (Kardos, Allendorf & Luikart 2014). An alternative explanation is that inbreeding depression may cause increased mortality of highly inbred males (Keller & Waller 2002). However, there has not been any effect of inbreeding on adult mortality detected in this population so far.
Although the Scandinavian wolf population has increased fourfold to sixfold during our study, we did not find evidence of density‐dependent pair dissolution through an increase in local wolf density or through changes in the density of their main prey (moose), as we hypothesized (H3). This is supported by the lack of home range size response to density‐related factors (Mattisson et al. 2013) and, so far, there is only one confirmed observation of intraspecific killing among collared Scandinavian wolves (Liberg et al. 2008; Wabakken et al. 2009). The age of the male was more important than the age of the female for explaining variation in wolf pair bond duration (Table 3). This could be explained by the fact that males tend to have a generally lower survival rate than females in the population (Liberg et al. 2008). In another study, males also showed body mass to decline after approximately 5 years, which could be explained by intense intrasexual competition between males causing weak selection for male longevity (MacNulty et al. 2009).
Consequences of wolf pair dissolution
In a socially monogamous species, the maintenance of the family‐based social structure can have important fitness benefits associated with the adaptive evolution of kinship (Lukas & Clutton‐Brock 2013). For instance, pair bond duration (Sánchez‐Macouzet, Rodriguez & Drummond 2014) and the presence of helpers (Sparkman et al. 2011) can have positive effects on reproductive success. Moreover, wolf breeder loss can result in lower pup survival, abandonment of territories, dissolution of social groups (Brainerd et al. 2008) or unusual behaviour such as incestuous mating (Vonholdt et al. 2008). Although the impact of wolf pair dissolution on population growth is context‐dependent (Brainerd et al. 2008; Borg et al. 2015), the high dissolution rate observed in our study suggests that extrinsic factors (i.e. anthropogenic) could have an impact on the recolonization of the population and would deserve further attention (Liberg et al. 2011). While consequences of human impact on populations usually focuses on numerical response (i.e. population size estimates; but see Rutledge et al. 2010), we provided quantitative estimates of anthropogenic influence on the dynamics of the social unit of the population, the wolf pair. Additionally, intrinsically linked population factors, such as the high levels of inbreeding observed in this population, also negatively affect the duration of wolf pair bonds and may contribute to inbreeding depression. The mechanisms behind this result are still unclear and further research could help to distinguish whether inbreeding could act on the divorce rate of pairs or lower the survival of highly inbred males. Identifying sources of spatial variation on estimates of fitness related measures, such as pair bond duration, is strongly needed to understand how intrinsic and extrinsic population factors interact to shape the demography of large carnivore populations. This type of information is also essential to provide appropriate recommendations for a conservation‐oriented management (Falcucci et al. 2009; Gaillard et al. 2010).
Data accessibility
Data available from Dryad Digital Repository http://dx.doi.org/10.5061/dryad.242t8 (Milleret et al. 2016).
Supporting information
Appendix S1. Summary of winter tracking efforts.
Appendix S2. Expanded methods description
Appendix S3. Coefficient of correlation between highly correlated covariates (r > 0.60).
Appendix S4. Test of the robustness of the centroid and buffer method.
Acknowledgements
We are grateful to B. Zimmermann, J. Mattisson and J. Månsson who kindly helped to join maps across country borders. We warmly thank J.V. López‐Bao, A. Ordiz, Anne Loison and two anonymous reviewers who provided valuable comments on earlier versions of the manuscript and J. Milner and A. Tallian for language check. We thank all people involved in field work, E. Maartmann, T. H. Strømseth and Å. Aronsson in particular. The field work was coordinated by the Swedish County Administration boards, Wildlife damage centre, Sweden, and Hedmark University of Applied Sciences, Norway. Financially, the study was supported by HUC, the Swedish University of Agricultural Sciences, The Swedish Research Council Formas, the Swedish Environmental Protection Agency, The Norwegian Research Council, the Norwegian Environment Agency, the Norwegian Institute for Nature Research and the County Governor of Hedmark.
References
- Andreassen, H.P. , Glorvigen, P. , Rémy, A. & Ims, R.A. (2013) New views on how population‐intrinsic and community‐extrinsic processes interact during the vole population cycles. Oikos, 122, 507–515. [Google Scholar]
- Andrén, H. , Linnell, J.D.C. , Liberg, O. , Andersen, R. , Danell, A. , Karlsson, J. et al (2006) Survival rates and causes of mortality in Eurasian lynx (Lynx lynx) in multi‐use landscapes. Biological Conservation, 131, 23–32. [Google Scholar]
- Barton, K . (2014) MuMIn: Multi‐Model Inference. R package version 1.10.5. http://CRAN.R-project.org/package=MuMIn. [Google Scholar]
- Bensch, S. , Andrén, H. , Hansson, B. , Pedersen, H.C. , Sand, H. , Sejberg, D. et al (2006) Selection for heterozygosity gives hope to a wild population of inbred wolves. PLoS One, 1, e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, V. , Lemaitre, J.‐F. , Allainé, D. , Gaillard, J.‐M. & Cohas, A. (2015) Early and adult social environments have independent effects on individual fitness in a social vertebrate. Proceedings of the Royal Society of London B: Biological Sciences, 282, 20151167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof, R. , Brøseth, H. & Gimenez, O. (2015) Wildlife in a politically divided world: insularism inflates estimates of brown bear abundance. Conservation Letters, 9, 122–130. [Google Scholar]
- Bischof, R. , Swenson, J.E. , Yoccoz, N.G. , Mysterud, A. & Gimenez, O. (2009) The magnitude and selectivity of natural and multiple anthropogenic mortality causes in hunted brown bears. Journal of Animal Ecology, 78, 656–665. [DOI] [PubMed] [Google Scholar]
- Borg, B.L. , Brainerd, S.M. , Meier, T.J. & Prugh, L.R. (2015) Impacts of breeder loss on social structure, reproduction and population growth in a social canid. Journal of Animal Ecology, 84, 177–187. [DOI] [PubMed] [Google Scholar]
- Brainerd, S.M. , Andrén, H. , Bangs, E.E. , Bradley, E.H. , Fontaine, J.A. , Hall, W. et al (2008) The effects of breeder loss on wolves. The Journal of Wildlife Management, 72, 89–98. [Google Scholar]
- Burnham, K.P. & Anderson, D.R . (2002) Model Selection and Multi‐Model Inference: A Practical Information‐Theoretic Approach. Springer‐Velag, New York, NY, USA. [Google Scholar]
- Carey, J.R. & Judge, D.S . (2000) Longevity Records: Life Spans of Mammals, Birds, Amphibians, Reptiles, and Fish. Odense University Press, Odense, Denmark. [Google Scholar]
- Choudhury, S. (1995) Divorce in birds: a review of the hypotheses. Animal Behaviour, 50, 413–429. [Google Scholar]
- Creel, S. , Becker, M. , Christianson, D. , Dröge, E. , Hammerschlag, N. , Hayward, M.W. et al (2015) Questionable policy for large carnivore hunting. Science, 350, 1473–1475. [DOI] [PubMed] [Google Scholar]
- Cubaynes, S. , MacNulty, D.R. , Stahler, D.R. , Quimby, K.A. , Smith, D.W. & Coulson, T. (2014) Density‐dependent intraspecific aggression regulates survival in northern Yellowstone wolves (Canis lupus). Journal of Animal Ecology, 83, 1344–1356. [DOI] [PubMed] [Google Scholar]
- Delibes, M. , Gaona, P. & Ferreras, P. (2001) Effects of an attractive sink leading into maladaptive habitat selection. The American Naturalist, 158, 277–285. [DOI] [PubMed] [Google Scholar]
- Falcucci, A. , Ciucci, P. , Maiorano, L. , Gentile, L. & Boitani, L. (2009) Assessing habitat quality for conservation using an integrated occurrence‐mortality model. Journal of Applied Ecology, 46, 600–609. [Google Scholar]
- Fieberg, J. & DelGiudice, G.D. (2009) What time is it? Choice of time origin and scale in extended proportional hazards models. Ecology, 90, 1687–1697. [DOI] [PubMed] [Google Scholar]
- Fletcher, D. & Dillingham, P.W. (2011) Model‐averaged confidence intervals for factorial experiments. Computational Statistics & Data Analysis, 55, 3041–3048. [Google Scholar]
- Gaillard, J.‐M. , Hebblewhite, M. , Loison, A. , Fuller, M. , Powell, R. , Basille, M. et al (2010) Habitat–performance relationships: finding the right metric at a given spatial scale. Philosophical Transactions of the Royal Society B: Biological Sciences, 365, 2255–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galipaud, M. , Gillingham, M.A.F. , David, M. & Dechaume‐Moncharmont, F.‐X. (2014) Ecologists overestimate the importance of predictor variables in model averaging: a plea for cautious interpretations. Methods in Ecology and Evolution, 5, 983–991. [Google Scholar]
- Gangaas, K.E. , Kaltenborn, B.P. & Andreassen, H.P. (2013) Geo‐spatial aspects of acceptance of illegal hunting of large carnivores in Scandinavia. PLoS One, 8, e68849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin, J. , Zedrosser, A. , Swenson, J.E. & Pelletier, F. (2015) The relative importance of direct and indirect effects of hunting mortality on the population dynamics of brown bears. Proceedings of the Royal Society B: Biological Sciences, 282, 1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber, C.E. , Nakagawa, S. , Laws, R.J. & Jamieson, I.G. (2011) Multimodel inference in ecology and evolution: challenges and solutions. Journal of Evolutionary Biology, 24, 699–711. [DOI] [PubMed] [Google Scholar]
- Hatchwell, B.J. (2009) The evolution of cooperative breeding in birds: kinship, dispersal and life history. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 3217–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisey, D.M. & Patterson, B.R. (2006) A review of methods to estimate cause‐specific mortality in presence of competing risks. Journal of Wildlife Management, 70, 1544–1555. [Google Scholar]
- Herfindal, I. , Linnell, J.D.C. , Moa, P.F. , Odden, J. , Austmo, L.B. & Andersen, R. (2005) Does recreational hunting of lynx reduce depredation losses of domestic sheep? Journal of Wildlife Management, 69, 1034–1042. [Google Scholar]
- Hinton, J.W. , Brzeski, K.E. , Rabon, D.R. Jr & Chamberlain, M.J. (2015) Effects of anthropogenic mortality on critically endangered red wolf Canis rufus breeding pairs: implications for red wolf recovery. Oryx, FirstView, 1–8. [Google Scholar]
- Hodge, S.J. , Manica, A. , Flower, T.P. & Clutton‐Brock, T.H. (2008) Determinants of reproductive success in dominant female meerkats. Journal of Animal Ecology, 77, 92–102. [DOI] [PubMed] [Google Scholar]
- Jennions, M.D. & Macdonald, D.W. (1994) Cooperative breeding in mammals. Trends in Ecology & Evolution, 9, 89–93. [DOI] [PubMed] [Google Scholar]
- Jęodrzejewska, B. , Jęodrzejewski, W. , Bunevich, A.N. , Minkowski, L. & Okarma, H. (1996) Population dynamics of wolves Canis lupus in Bialowieza Primeval Forest (Poland and Belarus) in relation to hunting by humans, 1847–1993. Mammal Review, 26, 103–126. [Google Scholar]
- Kardos, M. , Allendorf, F.W. & Luikart, G. (2014) Evaluating the role of inbreeding depression in heterozygosity‐fitness correlations: how useful are tests for identity disequilibrium? Molecular Ecology Resources, 14, 519–530. [DOI] [PubMed] [Google Scholar]
- Karlsson, J. , Brøseth, H. , Sand, H. & Andrèn, H. (2007) Predicting occurrence of wolf territories in Scandinavia. Journal of Zoology, 272, 276–283. [Google Scholar]
- Keller, L.F. & Waller, D.M. (2002) Inbreeding effects in wild populations. Trends in Ecology & Evolution, 17, 230–241. [Google Scholar]
- Kempenaers, B. , Adriaensen, F. & Dhondt, A.A. (1998) Inbreeding and divorce in blue and great tits. Animal Behaviour, 56, 737–740. [DOI] [PubMed] [Google Scholar]
- Kleinbaum, D.G. & Klein, M. (2011) Survival Analysis: A Self‐Learning Text, Third Edition. Springer, New York, NY, USA. [Google Scholar]
- Liang, H. & Zou, G. (2008) Improved AIC selection strategy for survival analysis. Computational Statistics & Data Analysis, 52, 2538–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberg, O. , Andrén, H. , Pedersen, H.‐C. , Sand, H. , Sejberg, D. , Wabakken, P. et al (2005) Severe inbreeding depression in a wild wolf Canis lupus population. Biology Letters, 1, 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberg, O. , Sand, H. , Wabakken, P. & Pedersen, H.C . (2008) Dödlighet och illegal jakt i den skandinaviska vargstammen. Viltskadecenter Rapport, 1, 1–42. [Google Scholar]
- Liberg, O. , Aronson, Å. , Brainerd, S.M. , Karlsson, J. , Pedersen, H.C. , Sand, H. et al (2010) Integrating research into management of a recolonizing wolf population – the Scandinavian model The World of Wolves: New Perspectives on Ecology, Behaviour and Policy (eds Musiani M., Boitani L. & Paquet P.). University of Calgary Press, Calgary, AB, Canada. [Google Scholar]
- Liberg, O. , Chapron, G. , Wabakken, P. , Pedersen, H.C. , Hobbs, N.T. & Sand, H. (2011) Shoot, shovel and shut up: cryptic poaching slows restoration of a large carnivore in Europe. Proceedings of the Royal Society B: Biological Sciences, 279, 910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberg, O. , Aronson, Å. , Sand, H. , Wabakken, P. , Maartmann, E. , Svensson, L. et al (2012) Monitoring of wolves in Scandinavia. Hystrix, the Italian Journal of Mammalogy, 23, 29–34. [Google Scholar]
- Lukas, D. & Clutton‐Brock, T.H. (2013) The evolution of social monogamy in mammals. Science, 341, 526–530. [DOI] [PubMed] [Google Scholar]
- Lunn, M. & McNeil, D . (1995) Applying Cox regression to competing risks. Biometrics, 51, 524–532. [PubMed] [Google Scholar]
- Macdonald, D.W. (1983) The ecology of carnivore social behaviour. Nature, 301, 379–384. [Google Scholar]
- MacNulty, D.R. , Smith, D.W. , Mech, L.D. & Eberly, L.E. (2009) Body size and predatory performance in wolves: is bigger better? Journal of Animal Ecology, 78, 532–539. [DOI] [PubMed] [Google Scholar]
- Mattisson, J. , Sand, H. , Wabakken, P. , Gervasi, V. , Liberg, O. , Linnell, J.C. et al (2013) Home range size variation in a recovering wolf population: evaluating the effect of environmental, demographic, and social factors. Oecologia, 173, 1–13. [DOI] [PubMed] [Google Scholar]
- Mech, L.D. (1995) The challenge and opportunity of recovering wolf populations. Conservation Biology, 9, 270–278. [Google Scholar]
- Mech, L.D . (1997) The Arctic Wolf: Ten Years With the Pack. Voyageur Press, Stillwater, MN, USA. [Google Scholar]
- Milleret, C. , Wabakken, P. , Liberg, O. , Åkesson, M. , Flagstad, Ø. , Andreassen, H. P. et al (2016) Data from: Let's stay together? Intrinsic and extrinsic factors involved in pair bond dissolution in a recolonizing wolf population. Dryad Digital Repository, http://dx.doi.org/10.5061/dryad.242t8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, J.M. , Nilsen, E.B. & Andreassen, H.P. (2007) Demographic side effects of selective hunting in ungulates and carnivores. Conservation Biology, 21, 36–47. [DOI] [PubMed] [Google Scholar]
- Odden, M. , Ims, R. , Støen, O. , Swenson, J. & Andreassen, H. (2014) Bears are simply voles writ large: social structure determines the mechanisms of intrinsic population regulation in mammals. Oecologia, 175, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordiz, A. , Milleret, C. , Kindberg, J. , Månsson, J. , Wabakken, P. , Swenson, J.E. et al (2015) Wolves, people, and brown bears influence the expansion of the recolonizing wolf population in Scandinavia. Ecosphere, 6, 284. [Google Scholar]
- Persson, J. , Ericsson, G. & Segerström, P. (2009) Human caused mortality in the endangered Scandinavian wolverine population. Biological Conservation, 142, 325–331. [Google Scholar]
- R Core Team (2014) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: URL http://www.R-project.org/. [Google Scholar]
- Rutledge, L.Y. , Patterson, B.R. , Mills, K.J. , Loveless, K.M. , Murray, D.L. & White, B.N. (2010) Protection from harvesting restores the natural social structure of eastern wolf packs. Biological Conservation, 143, 332–339. [Google Scholar]
- Sánchez‐Macouzet, O. , Rodriguez, C. & Drummond, H. (2014) Better stay together: pair bond duration increases individual fitness independent of age‐related variation. Proceedings of the Royal Society of London B: Biological Sciences, 281, 20132843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schielzeth, H. (2010) Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution, 1, 103–113. [Google Scholar]
- Smith, D.W. , Bangs, E.E. , Oakleaf, J.K. , Mack, C. , Fontaine, J. , Boyd, D. et al (2010) Survival of colonizing wolves in the northern Rocky Mountains of the United States, 1982–2004. The Journal of Wildlife Management, 74, 620–634. [Google Scholar]
- Sparkman, A.M. , Adams, J. , Beyer, A. , Steury, T.D. , Waits, L. & Murray, D.L. (2011) Helper effects on pup lifetime fitness in the cooperatively breeding red wolf (Canis rufus). Proceedings of the Royal Society B: Biological Sciences, 278, 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman, A.M. , Adams, J.R. , Steury, T.D. , Waits, L.P. & Murray, D.L. (2012) Pack social dynamics and inbreeding avoidance in the cooperatively breeding red wolf. Behavioral Ecology, 23, 1186–1194. [Google Scholar]
- Therneau, T.M. (2014) A Package for Survival Analysis in S. R package version 2.37‐7. http://CRAN.R-project.org/package=survival. [Google Scholar]
- Ueno, M. , Solberg, E.J. , Iijima, H. , Rolandsen, C.M. & Gangsei, L.E . (2014) Performance of hunting statistics as spatiotemporal density indices of moose (Alces alces) in Norway. Ecosphere, 5, art13. [Google Scholar]
- Vila, C. , Sundqvist, A.‐K. , Flagstad, Ø. , Seddon, J. , Kojola, I. , Casulli, A. et al (2003) Rescue of a severely bottlenecked wolf (Canis lupus) population by a single immigrant. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonholdt, B.M. , Stahler, D.R. , Smith, D.W. , Earl, D.A. , Pollinger, J.P. & Wayne, R.K. (2008) The genealogy and genetic viability of reintroduced Yellowstone grey wolves. Molecular Ecology, 17, 252–274. [DOI] [PubMed] [Google Scholar]
- Wabakken, P. , Aronson, Å. , Sand, H. , Steinset, O.K. & Kojola, I . (1999) The wolf in Scandinavia: Status report of the 1998–1999 winter (in Norwegian with English summary). Hedmark University College, Oppdragsrapport, 19, 1–40. [Google Scholar]
- Wabakken, P. , Sand, H. , Liberg, O. & Bjärvall, A. (2001) The recovery, distribution, and population dynamics of wolves on the Scandinavian peninsula, 1978–1998. Canadian Journal of Zoology, 79, 710–725. [Google Scholar]
- Wabakken, P. , Aronson, Å. , Strømseth, T.H. , Sand, H. , Maartmann, E. , Svensson, L. et al (2009) The wolf in Scandinavia: Status report of the 2008–2009 winter (in Norwegian with English summary). Hedmark University College, Oppdragsrapport, 6, 1–52. [Google Scholar]
- Wabakken, P. , Svensson, L. , Kojola, I. , Maartmann, E. , Strømseth, T.H. , Flagstad, Ø. et al (2012) The wolf in Scandinavia and Finland: status report of wolf monitoring in the 2011–2012 winter (in Norwegian with English summary). Hedmark University College, Oppdragsrapport, 5, 1–46. [Google Scholar]
- Zedrosser, A. , Dahle, B. & Swenson, J.E. (2006) Population density and food conditions determine adult female size in brown bears. Journal of Mammalogy, 87, 510–518. [Google Scholar]
- Zimmermann, B. , Wabakken, P. & Dötterer, M. (2001) Human‐carnivore interactions in Norway: How does the re‐appearance of large carnivores affect people attitudes and levels of fear. Forest Snow and Landscape Research, 76, 1–17. [Google Scholar]
- Zimmermann, B. , Nelson, L. , Wabakken, P. , Sand, H. & Liberg, O. (2014) Behavioral responses of wolves to roads: scale‐dependent ambivalence. Behavioral Ecology, 25, 1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, B. , Sand, H. , Wabakken, P. , Liberg, O. & Andreassen, H.P. (2015) Predator‐dependent functional response in wolves: from food limitation to surplus killing. Journal of Animal Ecology, 84, 102–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Summary of winter tracking efforts.
Appendix S2. Expanded methods description
Appendix S3. Coefficient of correlation between highly correlated covariates (r > 0.60).
Appendix S4. Test of the robustness of the centroid and buffer method.
Data Availability Statement
Data available from Dryad Digital Repository http://dx.doi.org/10.5061/dryad.242t8 (Milleret et al. 2016).


