Abstract
Although pressure therapy (PT) represents the standard care for prevention and treatment of hypertrophic scar (HS) from burns, its practice is largely based on empirical evidence and its effectiveness remains controversial. To clarify the effect of PT (15–25 mmHg) for HS, we performed the systematic review and meta-analysis. Several electronic databases were screened to identify related randomized controlled trials (RCTs). 12 RCTs involving 710 patients with 761 HS resulting from burn injuries were included. Compared with non/low-PT, cases treated with PT (15–25 mmHg) showed significant differences in Vancouver Scar Scale score (MD = −0.58, 95% CI = −0.78–−0.37), thickness (SMD = −0.25, 95% CI = −0.40–−0.11), brightness (MD = 2.00, 95% CI = 0.59–3.42), redness (MD = −0.79, 95% CI = −1.52–−0.07), pigmentation (MD = −0.16, 95% CI = −0.32–−0.00) and hardness (SMD = −0.65, 95% CI = −1.07–−0.23). However, there was no difference in vascularity (MD = 0.03, 95% CI = −0.43–0.48). Our analysis indicated that patients with HS who were managed with PT (15–25 mmHg) showed significant improvements. Due to limitations, more large and well-designed studies are needed to confirm our findings and the side-effects of the PT may also need to be evaluated.
Postnatal wound healing after dermal injury is an imperfect process, inevitably resulting in scar formation as the skin re-establishes its integrity1. Scar tissue is notably different from the surrounding healthy skin with respect to colour, pigmentation, vascularity, thickness and hardness which may lead to psychological complications such as stigmatization and poor self-esteem2,3. Scar also causes a range of symptoms including pain, pruritus, erythema and dryness. If it is located close to a joint, scar contracture may result in articular stiffness. So, scars may give rise to cosmetic, symptomatic, psychological and functional problems for patients thereby making a seriously impact on their quality of life4,5,6. Hypertrophic scar (HS) is a very common cutaneous complication following dermal wound, especially after severe burns7,8. Currently, there are a variety of therapeutic options for treatment of HS including pressure therapy (PT), silicone-based products, intralesional corticosteroids, laser therapy, bleomycin, fluorouracil, topical imiquimod and surgical excision2,9,10. However, no ideal or all-purpose method of scar control exists and HS management remains a problematic challenge for both patients and health care providers5,11.
PT has been the mainstay of HS treatment since the early 1970s12,13. Although it represents the standard care for prevention and treatment of HS from burns, there is no scientific evidence for its uses and its practice is largely based on empirical evidence14. Firstly, the exact optimal pressure required for effective treatment has never been scientifically established15,16,17. Some studies indicated that 15 mmHg pressure was required to achieve a therapeutic effect3,18. Pressure less than 15 mmHg (low-PT) may not show the desired effect and a pressure of more than 40 mmHg is more likely to cause severe discomfort and may be potentially harmful15. The application of 15–25 mmHg pressure is most commonly used in clinic practice2,19. Secondly, the effectiveness of PT in HS prevention and treatment remains controversial. Some studies showed that PT could promote HS maturation, restrain its formation, improve its appearance, minimize itch and pain10,20. However, others indicated that PT not only left the ultimate outcome of burn wounds unchanged but also increased the incidence of overheating, pruritus, blistering, wound breakdown and abnormal bone growth7,18.
In 2009, a standard meta-analysis was conducted including six randomized controlled trials (RCTs) involving 316 patients. The pooling of data from four studies suggested that PT could only mildly improve scar height but did not appear to alter global scar score (Vancouver Scar Scale score, VSS), pigmentation, vascularity, pliability and colour21. Based on the inconsistent study conclusions and the results of the meta-analysis, ambiguous recommendations were given by the recent international clinical guidelines22,23,24. Between 2009 and now, some new relevant RCTs were published. Therefore, we performed the updated systematic review and meta-analysis including all eligible RCTs to reappraise the effect of PT (15–25 mmHg) for HS in burns.
Methods
This meta-analysis was reported according to the PRISMA guidelines25.
Inclusion and exclusion criteria
A study was included if it met the following criteria: (1) Study type: RCT reported by original articles; (2) Patients: those with second degree burns or more or those with HS from burns; (3) Interventions: PT (15–25 mmHg); (4) Comparators: no pressure (non-PT) or low-PT; (5) Outcomes: VSS, thickness, color, pigmentation, hardness and vascularity. We excluded editorials, brief reports and data that had multiple publications.
Data sources and searches
We searched PubMed, Cochrane Library (Issue 1, 2016), CNKI (China National Knowledge Infrastructure) and Embase from their inception date up to January 25, 2016. The medical subject headings (MeSH) and free-text words were used. In order to identify grey literatures, we searched the ClinicalTrial.gov and OpenGrey (www.opengrey.eu). We used Google scholar to find additional records, which were not included in those databases. The reference lists from included studies, reviews and guidelines were also hand-searched. The detailed search strategy is shown in Supplementary File S1. In the course of literature search, no language or other restrictions were set.
Study selection
Eligible studies were selected by two independent investigators. First, the titles and abstracts were screened to identify all potentially eligible trials. Then, full text was reviewed to further confirm the studies, which met the inclusion criteria. Lastly, repetitive studies were excluded. Disagreements were resolved by discussion.
Data extraction
Two investigators independently extracted the following items from each eligible studies: (1) basic information: Surname of the first author, country of the investigation and year of publication; (2) characteristics of participants in each study group, including sample size, average age, ethnicity, percentage of total body surface area (%TBSA) and burn site; (3) treatment information: type of interventions, pressure dose, wear time, duration of treatment, follow-up period, number of cases that discontinued treatment and those that were lost during follow-up; (4) clinical outcomes. In case of any conflict, a discussion was carried out to achieve consensus.
Methodological quality assessment
The methodological quality of each included study was evaluated using the Cochrane Risk of Bias tool26. The items included random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. Two authors assessed the quality of eligible studies independently and discrepancies were resolved by discussion.
Data analysis
Statistical analysis was undertaken using RevMan5.3 software (Cochrane Collaboration, Copenhagen, The Nordic Cochrane Centre). Inverse variance method in random-effects model was applied in the meta-analysis. Heterogeneity was estimated using the I2 statistic and Q tests, I2 < 50% and P > 0.1 indicated low risk of heterogeneity. For each of the comparisons, effect sizes for studies using the same outcome measures were pooled using weighted mean difference (MD) and where not applicable, standardized mean difference (SMD) was used. 95% confidence interval (CI) was also calculated for each pooled result. The Z test was used to assess the statistical significance of the pooled MD/SMD and two-tailed P < 0.05 was considered significant. Sensitivity analyses were performed by using fixed-effect model to evaluate the stability of the result. We constructed funnel plot to assess for the possibility of publication bias. The resulting symmetrical funnel plot indicated low risk of publication bias.
Results
Study selection
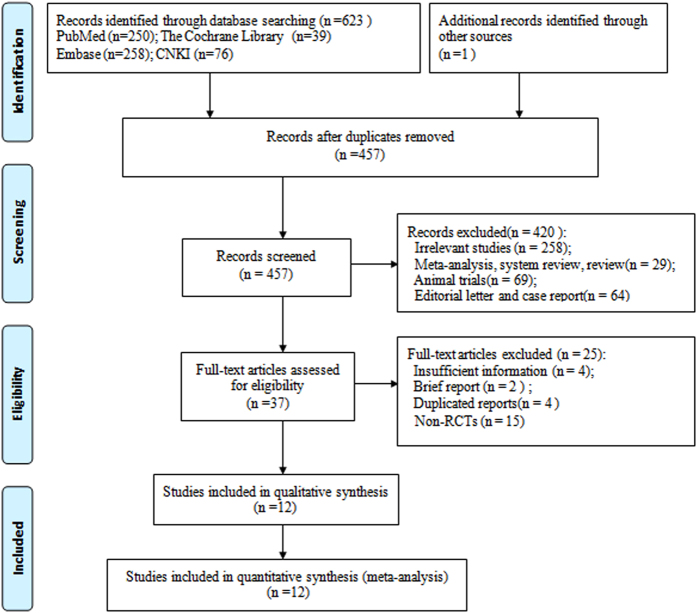
Our search results yielded 623 records. After excluding duplications, we screened 457 titles and abstracts. A total of 420 records were deleted. This included 258 irrelevant studies, 29 meta-analyses, systematic reviews and reviews, 69 animal studies, 64 editorials and case reports. 37 potentially relevant studies were left for further review of the full-text. We continued to exclude 25 articles: 15 studies for non-RCT study design, 4 studies for insufficient information, 2 brief reports and 4 duplicated reports. 12 studies27,28,29,30,31,32,33,34,35,36,37,38 were ultimately included for meta-analysis. The details of study selection is shown in Fig. 1.
Figure 1. Flow diagram of the selection process for eligible studies.
Study Characteristics and Quality Assessment
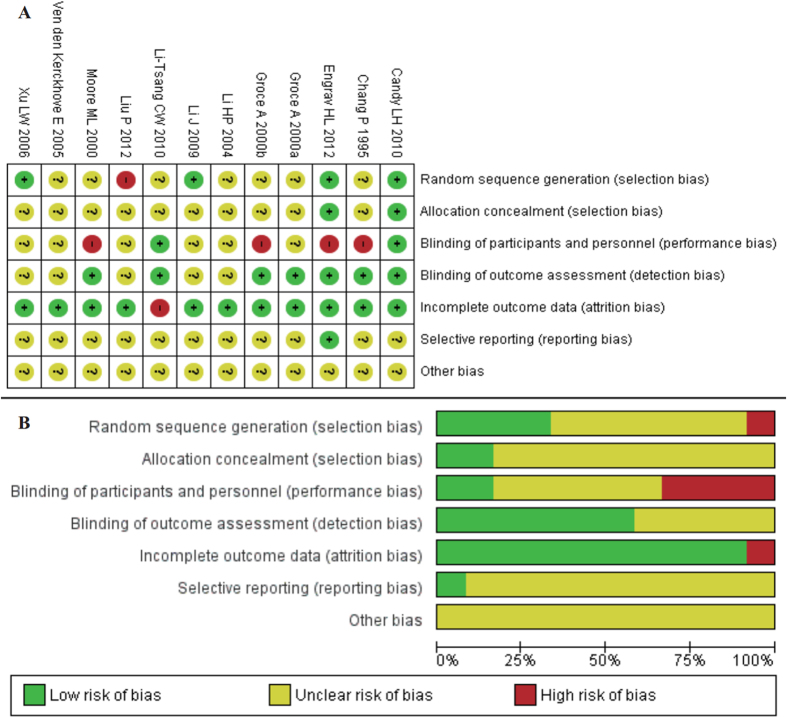
The general characteristics of the included studies are reported in Table 1. Eight27,28,29,30,31,32,33,34 out of the twelve studies were published in English and the others were in Chinese. Twelve studies involved 710 burn patients and the sample-size ranged from 1729 to 12231. Two studies27,30 used within-patient design where each patient had at least two wounds, some wounds were/one wound was treated with PT (15–25 mmHg), others wounds/one wound as a control; or in patients with only one wound, one-half of the wound applied PT (15–25 mmHg) and the other-half was considered as control. Ten studies28,29,31,32,33,34,35,36,37,38 used between-patient design where each patient was given a treatment. 441, 171, 226 HS were allocated into those treated with PT (15–25 mmHg), low-PT and non-PT groups respectively. Seven of these studies27,31,32,35,36,37,38 began PT for burn patients 1 to 3 weeks after wound closure or reepithelialisation. Two studies28,29 started PT for patients several months post-injury. And three trials33,34,36 did not report the time interval between PT and time of injury. Eleven27,28,29,31,32,33,34,35,36,37,38 of the twelve trials reported the age of patients, two trials33,34 included only pediatric patients, five27,29,31,32,37 studied adults (age ≥18 years) and four28,35,36,38 investigated both children and adults. Two studies34,36 did not mention the burn site while the others were on the limbs. Four studies27,28,29,36 reported burn surface area as size dimension, six31,32,33,34,35,37 used %TBSA, while it was not reported in two studies30,38. Different kinds of pressure garments such as: Medical Z Corporation®, Jobst®, Urgosyval®, Tricolast®, etc were used in those studies. Four studies31,36,37,38 did not clearly describe the manufacturer of pressure garment. Only five studies27,29,30,32,33 reported the pressure value (scar/garment interface) assessment method. The longest duration of treatment was 12 months30 and the shortest 2 months36, while one study31 did not report the duration of treatment. Endpoint outcomes included VSS score, thickness, color, pigmentation, hardness and vascularity. Three studies27,28,35 had cases lost in the follow-up period and one study28 inadequately reported the reasons for losing. The risk of bias figure and the risk of bias summery figure are shown in Fig. 2A,B. Overall, the quality of included studies was under moderate risk of bias.
Table 1. Characteristics of the studies included in this meta-analysis.
| Study | Study design | Sample size/(PT/Control) | Average age (year) | Burn area or %TBSA | Details in PT group | Time to PT | Type of PT used/Method of ssessment | Control group | Flow-up | With-draw | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Engrav 2012 | Within wounds | 54 (54/54) | 36 ± 14 | ≥4 cm in diameter | 25.00 ± 6.3 mmHg 23 h per day | ≥3 weeks to heal | Medical Z Corporation®/I-Scan system | 6.40 ± 6.2 mmHg 23 h per day | 9.5 | 11 | Thickness; Hardness; Color |
| Li-Tsang 2010 | Between patien | 104 (59/45) | 22 ± 19 | >16 cm2 | 15~25 mmHg 24 h per day except bathing time | 14.9 ± 30.8 months post-injury | Tailor-made pressure garment/Not report | Non-PT | 6 | 20 | Thickness; Hardness; Color; Pain; Pruritus |
| Candy 2010 | Between patient | 17 (53HS) (25/28) | 26 ± 8 | >4 cm × 4 cm | 23.23 ± 1.11 mmHg 23 h per day except for hygienic measures | 5.21 ± 1.91 months post-injury | Tailor-made plastazote paddings/Pliance X System | 14.53 ± 1.0 mmHg 23 h per day except for hygienic measures | 5 | 0 | Thickness; Hardness; Color |
| Moore 2000 | Within wounds | 23 (23/23) | Not report | Not report | Mean = 25 mmHg 23 h per day except bathing time | healed burn wound | Medical Z Corporation®/I-Scan system | Non-PT | 12 | 0 | Thickness; Hardness; Color |
| Chang 1995 | Between patient | 122 (64/58) | 31 ± 2 | (21.7 ± 2.2)% | 15~25 mmHg | healed burn wound | Pressure garment/Not report | Non-PT | Not report | 0 | Length of stay; Wound maturation time |
| Ven den Kerckhove 2005 | Between patient | 60 (75HS) (41/34) | 37.5 (19~6) | 8.5% (1~30%) | 19.75 ± 3.44 mmHg 23 h per day | 2 weeks after reepithelialisation | Tricolast® or Anvarex®/ENV 12718 | 11.85 ± 2.41 mmHg 23 h per day | 3 | 0 | Thickness; Color |
| Groce 2000a | Between patient | 50 (25/25) | 6.6 (1~7) | 48.3% (11~99%) | 24.7 ± 8.5 mmHg | Not report | Jobst®/Tek-Scan Matscan | 10.4 ± 7.6 mmHg | 6 | 0 | VSS; Thickness; Hardness; Pigmentation; Vascularity |
| Groce 2000b | Between patient | 28 (10/18) | 8.2 (1~6) | 11.2% (1~30%) | Mean = 21.8 mmHg | Not report | Jobst®/Not report | Non-PT | 6 | 0 | VSS; Thickness; Hardness; Pigmentation; Vascularity |
| Li 2009 | Between patient | 60 (30/30) | 37 (14~52) | 10~50% | 10~25 mmHg 23.5 h per day | 1 week after wound closure | Urgosyval®/Not report | 5~10 mmHg 23.5 h per day | 6 | 2 | Scar tissue perfusion |
| Li 2004 | Between patient | 43 (34/9) | 21 ± 19 | (7.13 ± 4.77) cm × (4.14 ± 2.94) cm | 24~25 mmHg 24 h per day except bathing time | Not report | Pressure garment/Not report | Non-PT | 2 | 0 | VSS; Thickness; Hardness; Color |
| Zhu 2012 | Between patient | 62 (31/31) | 34 ± 13 | 10~50% | 15~25 mmHg 24 h per day except bathing time | 2~ week after wound closure | Pressure garment/Not report | Non-PT | 6 | 0 | VSS |
| Xu 2006 | Between patient | 87 (45/42) | 5~50 | Not report | 15~25 mmHg 24 h per day except bathing time | 2~3 week after wound closure | Pressure garment/Not report | Non-PT | 6 | 0 | VSS |
HS: Hypertrophic scar; PT: Pressure therapy; Non-PT: Pressure therapy not using; %TBSA: percentage of total body surface area; VSS: Vancouver Scar Scale score.
Figure 2. The quality assessment of the included studies.
(A) Risk of bias summary; (B) Risk of bias graph.
Meta-analysis
The results of meta-analysis are displayed in Table 2.
Table 2. A summary results of the meta-analysis.
| Outcomes | NO. of study | Heterogeneity test |
Effect Model | Effect size |
|||
|---|---|---|---|---|---|---|---|
| I2 | P | MD/SMD | 95% CI | P | |||
| VSS | 5 | 37% | 0.17 | Random | −0.60 | −0.92, −0.28 | <0.01 |
| Fixed | −0.58 | −0.78, −0.37 | <0.01 | ||||
| Thickness | 7 | 48% | 0.09 | Random | −0.38 | −0.63, −0.12 | <0.01 |
| Fixed | −0.25 | −0.40, −0.11 | <0.01 | ||||
| L*a*b*: L | 4 | 0% | 0.59 | Random | 2.00 | 0.59, 3.42 | 0.01 |
| Fixed | 2.00 | 0.59, 3.42 | 0.01 | ||||
| L*a*b*: a | 5 | 0% | 0.57 | Random | −0.79 | −1.52, −0.07 | 0.03 |
| Fixed | −0.79 | −1.52, −0.07 | 0.03 | ||||
| L*a*b*: b | 4 | 50% | 0.11 | Random | 0.86 | −0.16, 1.87 | 0.10 |
| Fixed | 0.86 | 0.18, 1.55 | 0.01 | ||||
| Pigmentation | 3 | 4% | 0.35 | Random | −0.17 | −0.33, −0.00 | 0.05 |
| Fixed | −0.16 | −0.32, −0.00 | 0.04 | ||||
| Hardness | 5 | 65% | 0.02 | Random | −0.65 | −1.07, −0.23 | <0.01 |
| Fixed | −0.60 | −0.84, −0.37 | <0.01 | ||||
| Vascularity | 2 | 0% | 0.80 | Random | 0.03 | −0.43, 0.48 | 0.91 |
| Fixed | 0.03 | −0.43, 0.48 | 0.91 | ||||
VSS: Vancouver Scar Scale score; L*a*b*: L: Scar brightness; L*a*b*: a: Scar redness; L*a*b*: b: Scar yellowness; MD: mean difference; SMD: standardized mean difference.
VSS score
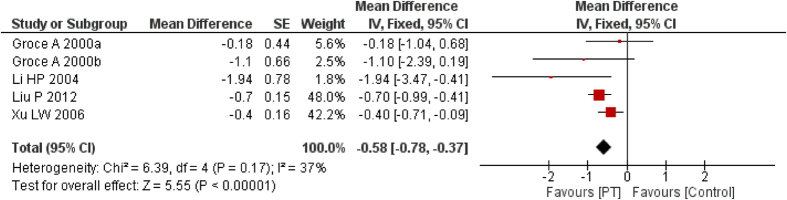
VSS score consists of four parameters–pliability, height, vascularity and pigmentation. It is a widely used tool to assess the severity of scar36. The total score is 15 and normal skin scores 0. The higher the score, the more severe the scar. Five studies33,34,36,38 involving 260 participants reported VSS score. The combined MD showed that PT (15–25 mmHg) could significantly reduce the VSS score (I2 = 37%, MD = −0.60, 95% CI = −0.92–−0.28, P < 0.01, random-effect model). The result varied little when a fixed-effect model was applied (MD = −0.58, 95% CI = −0.78–−0.37, P < 0.01) (Fig. 3), which suggested that the result was stable.
Figure 3. Forest plot of PT vs. non/low-PT in scar VSS score.
Thickness
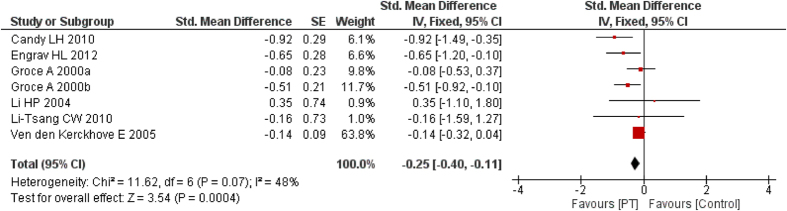
Eight studies27,28,29,30,32,33,34,36 compared PT (15–25 mmHg) with non/low-PT to assess the difference in thickness of the scar tissue. One study30 that provided no data compared PT (15–25 mmHg) with non-PT and the result was measured using subjective scar assesment (Seattle method) and Ultrasound. However, while Seattle method measured significant difference in scar thickness, ultrasound detected no differences. Of the other seven studies, two33,34 used VSS and five used ultrasound to assess scar thickness. Pooled result found PT (15–25 mmHg) could significantly reduce the thickness (I2 = 45%, SMD = −0.38, 95% CI = −0.63–−0.12, P < 0.01, random-effect model). The fixed-effect model produced a similar result (I2 = 45%, SMD = −0.25, 95% CI = −0.40–−0.11, P < 0.01) (Fig. 4).
Figure 4. Forest plot of PT vs. non/low-PT in scar thickness.
Colour
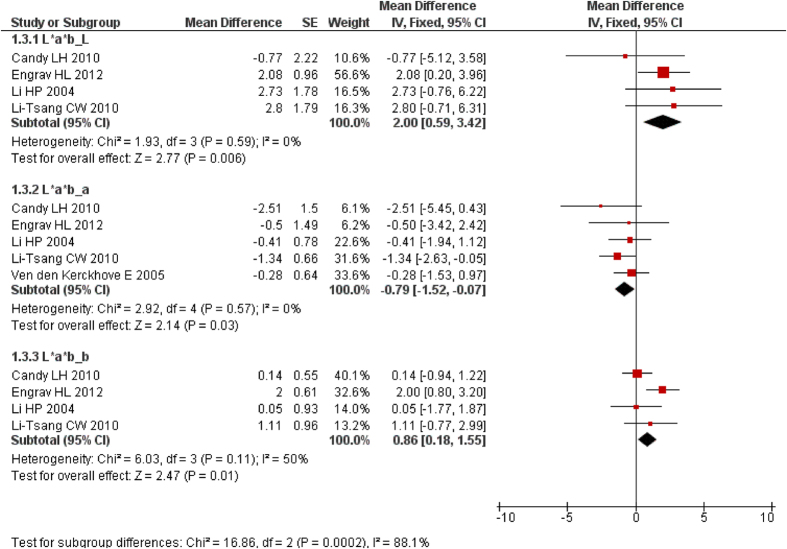
Five studies27,28,29,32,36 assessed the effect of PT (15–25 mmHg) on scar colour by using L*a*b* color space, in which “L” refers to the brightness, “a” the redness and “b” the yellowness27,29. Usually, scar tissue is less bright and yellow and redder than normal skin. The results of meta-analysis showed that PT (15–25 mmHg) could improve the cosmetic effect (Fig. 5). L*a*b*: L (I2 = 0%, MD = 2.00, 95% CI = 0.59–3.42, P = 0.01, random and fixed-effect model); a (I2 = 0%, MD = −0.79, 95% CI = −1.52–−0.07, P = 0.03, random and fixed-effect model); and b (I2 = 50%, MD = 0.86, 95% CI = −0.16–1.87, P = 0.10, random-effect model; and MD = 0.86, 95% CI = 0.18–1.55, P = 0.01, fixed-effect model). The pooled results were unchanged in sensitivity analysis for increasing brightness and decreasing redness using different effect model thereby indicating that the result is very credible. However, for increasing scar yellowness the result changed significantly showing that the effect of PT (15–25 mmHg) on yellowness needs further investigation.
Figure 5. Forest plot of PT vs. non/low-PT in scar colour.
L: brightness, (a) redness, (b) yellowness.
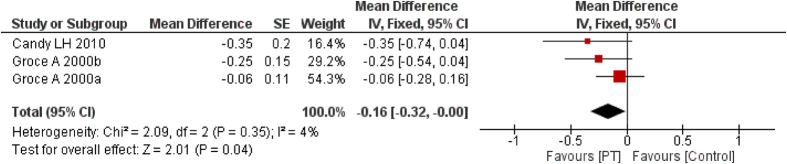
Pigmentation
Three studies29,33,34 reported pigmentation based on VSS. For pigmentation, as shown in Fig. 6, I2 = 4%, MD = −0.17, 95% CI = −0.33–−0.00, P = 0.05. The fixed-effect mode showed a similar result, MD = −0.16, 95% CI = −0.32–−0.00, P = 0.04.
Figure 6. Forest plot of PT vs. non/low-PT in scar pigmentation.
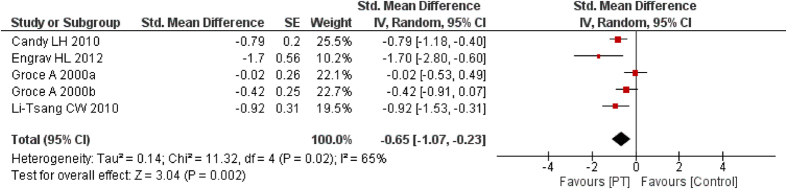
Hardness
Six studies27,28,29,30,33,34 observed changes in scar hardness in different treatment groups. Two33,34 of them measured scar hardness using VSS and the others used durometer. Five27,28,29,33,34 studies’ data can be pooled and as shown in Fig. 7, PT (15–25 mmHg) decreased the scar hardness (I2 = 65%, SMD = −0.65, 95% CI = −1.07–−0.23, P < 0.01, random-effect model). The result was not significantly changed in fixed-effect model (SMD = −0.60, 95% CI = −0.84–−0. 73, P < 0.01). One study30 that did not present data indicated that the scars that received PT (15–25 mmHg) were softer than non-PT (P < 0.01) at 9 months post-burn. But the difference disappeared at 12 months.
Figure 7. Forest plot of PT vs. non/low-PT in scar hardness.
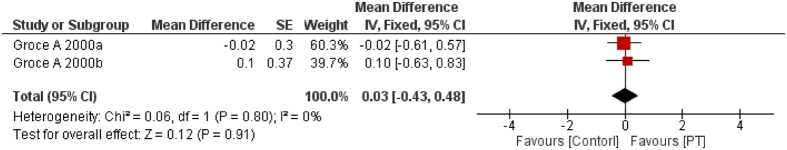
Vascularity
Three studies33,34,35 evaluated scar vascularity. The data of one of these studies35 which cannot be pooled measured the changes of blood perfusion at the scar tissue by laser Doppler perfusion imaging and found PT (15–25 mmHg) could significantly reduce the blood perfusion of scar tissue compared to low-PT (P < 0.05). The other two studies33,34 assessed the scar vascularity using VSS and the combined result found no significant difference in vascularity (I2 = 0%, MD = 0.03, 95% CI = −0.43–0.48, P = 0.91, random and fixed-effect model) Fig. 8.
Figure 8. Forest plot of PT vs. non/low-PT in scar vascularity.
Other outcomes
One of the studies31 with 122 burn patients (64 in PT group and 58 in non-PT) that was undertaken to determine the efficacy of PT (15–25 mmHg) showed no significant differences between the two groups when the length of hospital stay and time for wound maturation were compared. Another study28 concerned with the scar pain and itch (using Visual Analog Scale, VAS) found that PT had more effectiveness in reducing scar pain than non-PT (P = 0.02). However, there was no significant difference in alleviating itching between the two groups28.
Additional analysis
An unpublished trial was included in previous meta-analysis21, but we did not obtain the trial in all of our available databases. So, we performed an additional analysis, extracting the trial’s data from the previous study21 and recalculating the results. As shown in Supplementary File S2, all related clinical outcomes were unchanged.
Publication bias
Publication bias was assessed by funnel plot. All funnel plots were generally symmetrical (See Supplementary Fig. S1), indicating low risk of publication bias in our study.
Discussion
PT represents the standard care for prevention and treatment of HS from burns, but there is no scientific evidence for using it22,23. Practice is largely based on empirical evidence and its effectiveness remains controversial in current studies22. This makes it unfavorable for clinicians to choose effective treatment measures. In this systematic review and meta-analysis, we included all relevant RCTs to compare the therapeutic effects of PT (15–25 mmHg) and non/low-PT. The results indicated that PT (15–25 mmHg) could improve clinical effects including decreasing VSS score, pigmentation, redness and increasing scar brightness. Our results are reliable given that pooled results were unaltered in sensitivity and additional analyses and there was no publication bias in the included studies.
However, for increasing scar yellowness, the result was not stable enough in sensitivity analysis and needs to be investigated further. Similarly, the results of scar thickness, hardness and vascularity may be influenced by the study with no data or inconsistent outcome indicators. One study30 that provided no data gave results on scar thickness and hardness in patients who were treated with PT and non-PT for 12 months. The study indicated that PT had positive therapeutical effect at 9 months post-burn, but showed no difference between PT and non-PT at 12 months. The combined result found no significant difference between PT and non/low -PT in vascularity (MD = 0.03, 95% CI = −0.43–0.48, P = 0.91). One study35 measured the changes of scar tissue blood perfusion by laser Doppler perfusion imaging and it showed that PT (15–25 mmHg) could significantly reduce the blood perfusion of scar tissue and thus reduce the scar vascularity35. However, this study data cannot be pooled.
The overall methodological quality of the included studies was moderate. Eight of the twelve studies did not report the method of randomization and ten had inadequate reports of allocation concealment. There was inadequate blinding in most studies and as previous researches have demonstrated inadequate blinding maybe associated with exaggerated treatment effects21. One study28 inadequately reported the reasons for withdrawal from treatment. It has been previously shown that the treatment efficacy in patients who withdrew from researches were different from those that completed the trial. Studies that failed to provide details regarding withdrawals were at an increasing risk of producing invalid results26. Future investigations should ensure adequate randomization, concealment of allocation, blinding of patients and outcome assessors and descriptions of withdrawals and losing.
To be effective, pressure garment should be worn for at least 23 hours a day and the treatment should be continued for a period of 6–12 months or until the scar matures9,22,39,40,41. However, the exact optimal pressure required for effective treatment has never been scientifically established15. 15–25 mmHg pressure is used mostly in clinical practice. Higher pressures increase the effect and it has also been claimed to give more rapid results in the time of scar maturation19. However, pressure above 40 mmHg induces discomfort and potential harm, such as blistering, paresthesia, abnormal bone growth, limb necrosis, etc12,42. Moreover, higher pressure may also cause higher risk of pressure loss over time and increase the incidence of incompliance6,43. On the contrary, pressure less than 15 mmHg may appear to have poor/no effect2,28,29. A 12-year long-term high-quality study27 demonstrated that 15mmHg was considered to be the minimum effective “dose”. And other previous studies29,32 suggested that the pressure level required might need to be higher than 15 mmHg. Generally, it is recommended that pressure should be maintained between 15 to 25 mmHg which being above capillary pressure, diminishes the supply of blood and nutrients to the scar tissue and is safer and more effective2,27. Thus, in this study, we took the non-PT and low-PT as one treatment group to assess the effect of PT (15–25 mmHg) for hypertrophic burn scars.
Although we found that PT (15–25 mmHg) had positive impacts on HS, some confounding factors could influence the therapeutic effects. Firstly, the time to begin PT39,40,44,45,46. Different time interval between PT and burn-injury may have different PT effects, for example a recently healed burn wound and an established HS may respond differently to PT (15–25 mmHg). Previous studies recommended that PT should be used as soon as the healing skin can tolerate the pressure39,40,44 and/or shear force generated by the intervention45,46. The earlier the treatment begins the better the outcomes44. Secondly, the age of the burn patient47,48,49. Different life stages may have different healing processes and treatment compliances, which may have a great influence on assessing the effect of PT48. Thirdly, the location of burn12,50. Burn in different parts of the body may need different treatment requirements. And pressure is difficult to apply evenly across the body, particularly in concave areas and flexor joints12. Fourthly, methods of measuring the outcomes. Inequable validity may be found in different evaluation methods or scales36. Last but not least, pressure monitoring. In the course of PT, pressure losing along with therapy could also impact the final treatment effect and thus more attention should be paid on pressure monitoring29,32. However, in our included studies, the confounding factors were different or unreported and due to insufficient information we could not consider their influence. The effect of PT at different terms post-injury, life stage of patients, parts of the body burned, depth of burn, etc., should be further investigated in future studies. In addition, recognized and/or objective evaluation methods should be utilized and the pressure in scar/garment interface should be monitored in the future investigations.
Several investigations48,51,52 showed that PT had effectiveness in enhancing HS maturation and controlling the itch associated with HS because PT could reduce collagen synthesis and prevent HS formation and contracture. However, others studies49,53 found PT may cause skin breakdown/ulceration and aggravate the level of itching, especially in summer months. Prolonged wear of pressure garment may affect the skin perspiration and thus create heat and pruritus49. Only one of the included studies31 focused on length of hospital stay and time of wound maturation for which no significant differences were found between the PT and non-PT group. Another study28 found that PT had no more effectiveness in alleviating scar itching than non-PT where VAS score of itching changed from 4.47 ± 2.45 to 2.63 ± 1.91 and from 4.78 ± 3.35 to 3.09 ± 2.34 in PT and non-PT group respectively. Due to limited number of studies the efficacy of PT in scar maturation and itching could not be assessed to the best of its potential.
Some side-effects of PT such as blistering, ulceration, scar breakdown, limb swelling, etc. which are usually caused by too much pressure being applied were reported in previous studies12,54,55. In all of our included studies, 15–25 mmHg pressure, which was considered as safety pressure level was used in PT group. However, other adverse effects such as skeletal deformity, heterotopic ossification, muscle atrophy, joint stiffness47,56, etc. that could be caused by long-term usage of pressure garment may not have occurred or were unreported in our included studies. Thus, we did not assess the safety of PT. Future studies may need to pay more attention on the adverse-effects of PT. In addition, some studies12,53 declared that poor appearance of pressure garments made patients feel self-conscious, causing embarrassment and other problems to patients. More RCTs may also need to investigate the impact of PT in patients’ quality of life. In this way, the effects of PT may be investigated more comprehensively and accurately.
The exact mechanisms of how pressure positively influences the scar outcome following burns are not fully understood7,8,9. However, it is widely believed that the pressure can control collagen synthesis, facilitate scar maturation and reduce scar redness by limiting the supply of blood, oxygen and nutrients to the scar tissue28,29. Pressure has been also postulated to reduce the levels of collagen production more rapidly than the natural maturation process15. Mechanical loading induces alteration in collagen fiber turnover, remodeling and realignment and the reduced development of whorled collagen nodules result in the thinning and softening of scar tissues7,12,16. In addition, it is accepted that application of pressure commonly alleviates the itch and pain associated with active hypertrophic scars42,43.
In 2009, a standard meta-analysis21, included six RCTs, involving 316 patients, pooling four studies’ data suggested that PT when compared non/low-PT could only mildly improve scar height (SMD = −0.31, 95% CI = −0.63 to 0.00, P = 0.05), but could not to alter global scar scores (VSS), pigmentation, vascularity, pliability and colour. Based on the current inconsistent study conclusions and the results of Meta-analysis, an international clinical recommendation23 in 2014 concluded: “The long-time standard care for prevention and treatment of hypertrophic scars from burns is largely based on empirical evidence; no change in global scar scores and only small improvement in scar height was reported in meta-analysis; low pressure is less effective than high-pressure treatments; and patients with moderate or severe scarring experienced greater clinical benefit”23. And another useful guide22 commented: “PT alleviates itching and pain associated with abnormal scars but with no scientific evidence in the use of pressure garments; given current lack of evidence, well-designed clinical trials are required to examine the effectiveness, risks and costs of PT”22.
The sample-size of previous meta-analysis21 was relatively small and the conclusions were not stable enough. From 2009 to now, five new relevant RCTs were carried out. Therefore, we reappraised the effect of PT (15–25 mmHg) for HS in burns. The results of our study were significantly different from the previous meta-analysis. The sample-size was larger, the pooled results were unaltered in sensitivity analysis and there was no obvious publication bias in the included studies. More interestingly, we added an unpublished trial data in our study and all related clinical outcomes were found to be unchanged. So, our results are more reliable.
However, it is important to note the limitations of our study. Firstly, our results may be influenced by the small number of included studies, the limited sample-size and inconsistent clinical outcomes of each study. Secondly, due to insufficient data, our study did not consider the %TBSA, burn degree and burn site although the different %TBSA, burn degree and burn site may have varying efficacy. Thirdly, since long-term follow-up studies were rare, our study failed to analyze the prospective efficacy of PT. Fourthly, none of the included studies studied adverse effects and we were unable to assess the safety of PT. Last but not least, our analysis suffered in quality of included studies because most of the studies did not describe the allocation concealment and blinding method, which may exaggerate the treatment effects, especially in subjective outcomes.
Conclusions
Our meta-analysis demonstrated that burn patients managed with PT (15–25 mmHg) showed significant improvements. Due to the limitations of the current studies, larger and well-designed studies are needed to confirm our findings. Furthermore, the side-effects of PT may also need to be evaluated in the future.
Additional Information
How to cite this article: Ai, J.-W. et al. The effectiveness of pressure therapy (15–25 mmHg) for hypertrophic burn scars: A systematic review and meta-analysis. Sci. Rep. 7, 40185; doi: 10.1038/srep40185 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We are grateful to Mr. Manish Pradhananga for checking the English spelling, grammar and language expression.
Footnotes
Author Contributions B.P. and J.W.A. designed this article. J.T.L. and S.D.P. searched studies and collected data. Y.L., D.S.L. and H.M.L. analysed and explained the data. J.W.A. and S.D.P. drafted the manuscript. J.W.A. and J.T.L. reviewed the manuscript. All authors read and approved the final manuscript.
References
- Sidgwick G. P., McGeorge D. & Bayat A. A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring. Arch Dermatol Res 307, 461–477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhalil A. et al. A translational animal model for scar compression therapy using an automated pressure delivery system. Eplasty 15, e29 (2015). [PMC free article] [PubMed] [Google Scholar]
- Park T. H., Seo S. W., Kim J. K. & Chang C. H. Outcomes of surgical excision with pressure therapy using magnets and identification of risk factors for recurrent keloids. Plast Reconstr Surg 128, 431–439 (2011). [DOI] [PubMed] [Google Scholar]
- Son D. & Harijan A. Overview of Surgical Scar Prevention and Management. J Korean Med Sci 29, 751–757 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widgerow A. D. & Chait L. A. Scar management practice and science: a comprehensive approach to controlling scar tissue and avoiding hypertrophic scarring. Adv Skin Wound Care 24, 555–561 (2011). [DOI] [PubMed] [Google Scholar]
- Song C. Hypertrophic scars and keloids in surgery current concepts. Annals Plastic Surg 73, S108–S118 (2014). [DOI] [PubMed] [Google Scholar]
- Macintyre L. & Baird M. Pressure garments for use in the treatment of hypertrophic scars–an evaluation of current construction techniques in NHS hospitals. Burns 31, 11–14 (2005). [DOI] [PubMed] [Google Scholar]
- Harte D., Gordon J., Shaw M., Stinson M. & Porter-Armstrong A. The use of pressure and silicone in hypertrophic scar management in burns patients: A pilot randomized controlled trial. J Burn Care Res 30, 632–642 (2009). [DOI] [PubMed] [Google Scholar]
- Kim S. et al. Update on scar management guidelines for treating Asian patients. Plast Reconstr Surg 132, 1580–1589 (2013). [DOI] [PubMed] [Google Scholar]
- Carvalhaes S. M., Petroianu A., Ferreira M. A. T., Barros V. M. D. & Lopes R. V. Assesment of the treatment of earlobe keloids with triamcinolone injections, surgical resection, and local pressure. Rev Col Bras Cir 42, 9–13 (2015). [DOI] [PubMed] [Google Scholar]
- Williams F. N., Herndon D. N. & Branski L. K. Where we stand with human hypertrophic and keloid scar models. Exp Dermatol 23, 811–812 (2014). [DOI] [PubMed] [Google Scholar]
- Macintyre L. & Baird M. Pressure garments for use in the treatment of hypertrophic scars—a review of the problems associated with their use. Burns 32, 10–15 (2006). [DOI] [PubMed] [Google Scholar]
- Reno F. et al. In vitro mechanical compression induces apoptosis and regulates cytokines release in hypertrophic scars. Wound Repair Regen 11, 331–336 (2003). [DOI] [PubMed] [Google Scholar]
- Kim J. Y. et al. Burn scar biomechanics after pressure garment therapy. Plast Reconstr Surg 136, 572–581 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiyeh B. S., El K. A. M. & Dibo S. A. Pressure garment therapy (PGT) of burn scars: evidence-based efficacy. Ann Burns Fire Disasters 26, 205–212 (2013). [PMC free article] [PubMed] [Google Scholar]
- Huang D., Shen K. H. & Wang H. G. Pressure therapy upregulates matrix metalloproteinase expression and downregulates collagen expression in hypertrophic scar tissue. Chin Med J (Engl) 126, 3321–3324 (2013). [PubMed] [Google Scholar]
- Meaume S., Le Pillouer-Prost A., Richert B., Roseeuw D. & Vadoud J. Management of scars: updated practical guidelines and use of silicones. Eur J Dermatol 24, 435–443 (2014). [DOI] [PubMed] [Google Scholar]
- Steinstraesser L. et al. Pressure garment therapy alone and in combination with silicone for the prevention of hypertrophic scarring: randomized controlled trial with intraindividual comparison. Plast Reconstr Surg 128, 306e–313e (2011). [DOI] [PubMed] [Google Scholar]
- Chrisostomidis C. et al. Management of external ear keloids using form-pressure therapy. Clin Exp Dermatol 33, 273–275 (2008). [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Dougherty M. E. & Kagan R. J. The Effect of Positioning Devices and Pressure Therapy on Outcome After Full-Thickness Burns of the Neck. J Burn Care Res 28, 451–459 (2007). [DOI] [PubMed] [Google Scholar]
- Anzarut A., Olson J., Singh P., Rowe B. H. & Tredget E. E. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. J Plast Reconstr Aesthet Surg 62, 77–84 (2009). [DOI] [PubMed] [Google Scholar]
- Arno A. I., Gauglitz G. G., Barret J. P. & Jeschke M. G. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burns 40, 1255–1266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. H. et al. Updated international clinical recommendations on scar management: Part 1 — evaluating the evidence. Dermatol Surg 40, 812–824 (2014). [DOI] [PubMed] [Google Scholar]
- Gold M. H. et al. Updated international clinical recommendations on scar management: part 2 — algorithms for scar prevention and treatment. Dermatol Surg 40, 825–831 (2014). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Libeti T. Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 89, 873–880 (2009). [PubMed] [Google Scholar]
- Zeng X. et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 8, 2–10 (2015). [DOI] [PubMed] [Google Scholar]
- Engrav L. H. et al. 12-Year within-wound study of the effectiveness of custom pressure garment therapy. Burns 36, 975–983 (2010). [DOI] [PubMed] [Google Scholar]
- Li-Tsang C. W. P., Zheng Y. P. & Lau J. C. M. A randomized clinical trial to study the effect of silicone gel dressing and pressure therapy on posttraumatic hypertrophic scars. J Burn Care Res 31, 448–457 (2010). [DOI] [PubMed] [Google Scholar]
- Candy L. H., Cecilia L. T. & Ping Z. Y. Effect of different pressure magnitudes on hypertrophic scar in a Chinese population. Burns 36, 1234–1241 (2010). [DOI] [PubMed] [Google Scholar]
- Moore M. L. et al. Effectiveness of custom pressure garments in wound management a prospective trial within wounds with verified pressure. J Burn Care Res 21, S177 (2000). [Google Scholar]
- Chang P. et al. Prospective, randomized study of the efficacy of pressure garment therapy in patients with burns. J Burn Care Rehabil 16, 473–475 (1995). [DOI] [PubMed] [Google Scholar]
- Van den Kerckhove E. et al. The assessment of erythema and thickness on burn related scars during pressure garment therapy as a preventive measure for hypertrophic scarring. Burns 31, 696–702 (2005). [DOI] [PubMed] [Google Scholar]
- Groce A., Herndon D. N., McCauley R. L. & Chinkes D. The effect of high versus low pressure garments in the control of hypertrophic scars in the burned child: a preliminary 6 month report. Proceedings of the American Burn Association (2000). [Google Scholar]
- Groce A., Herndon D. N., McCauley R. L. & Chinkes D. The effects of pressure versus no pressure garments in the control of hypertrophic scarring in children with small burns: a preliminary 6 month report. Proceedings of the American Burn Association (2000). [Google Scholar]
- Li J., Bai Y. Q., Lü G. L., Du Y. R. & Zhao N. Influence of different pressure tension bandage on inhibiting scar proliferation(in Chinese). Zhongguo Zuzhi Gongcheng Yanjiu 13, 7583–7586 (2009). [Google Scholar]
- Li H. P. et al. The short term effect of pressure therapy and silicone gel on controlling the hypertrophic scar(in Chinese). Zhonghua Wuli Yixue Yu Kangfu Zazhi 26, 462–465 (2004). [Google Scholar]
- Liu P. et al. Sificon gel dressings and pressure therapy for treatment of hypertrophic burn scar in limbs(in Chinese). Linchuang Yixue Gongcheng 19, 1310–1311 (2012). [Google Scholar]
- Xu L. W., Cai S. P., Zou Y. X., Wang Y. Q. & Pang S. Q. Heparin sodium cream combined with pressure therapy to treat deep collapsed after 2 degree burn scar hyperplasia in 87 cases(in Chinese). Zhonghua Shaoshang Zazhi 22, 475 (2005). [Google Scholar]
- Bloemen M. C. et al. Prevention and curative management of hypertrophic scar formation. Burns 35, 463–475 (2009). [DOI] [PubMed] [Google Scholar]
- Davoodi P. & Fernandez JM O. S. J. Postburn sequelae in the pediatric patient: clinical presentations and treatment options. J Craniofac Surg 19, 1047–1052 (2008). [DOI] [PubMed] [Google Scholar]
- Berman B., Viera M. H., Amini S., Huo R. & Jones I. S. Prevention and management of hypertrophic scars and keloids after burns in children. J Craniofac Surg 19, 989–1006 (2008). [DOI] [PubMed] [Google Scholar]
- Rappoport K., Müller R. & Flores-Mir C. Dental and skeletal changes during pressure garment use in facial burns: A systematic review. Burns 34, 18–23 (2008). [DOI] [PubMed] [Google Scholar]
- Ripper S., Renneberg B., Landmann C., Weigel G. & Germann G. Adherence to pressure garment therapy in adult burn patients. Burns 35, 657–664 (2009). [DOI] [PubMed] [Google Scholar]
- Monstrey S. et al. Updated scar management practical guidelines: non-invasive and invasive measures. J Plast Reconstr Aesthet Surg 67, 1017–1025 (2014). [DOI] [PubMed] [Google Scholar]
- Gauglitz G. G., Korting H. C., Pavicic T., Ruzicka T. & Jeschke M. G. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 17, 113–125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latenser B. A. & Kowal-Vern A. Paediatric burn rehabilitation. Pediatr Rehabil 5, 3–10 (2002). [DOI] [PubMed] [Google Scholar]
- Hubbard M., Masters I. B., Williams G. R. & Chang A. B. Severe obstructive sleep apnoea secondary to pressure garments used in the treatment of hypertrophic burn scars. Eur Respir J 16, 1205–1207 (2000). [DOI] [PubMed] [Google Scholar]
- Williams F., Knapp D. & Wallen M. Comparison of the characteristics and features of pressure used in the management of burn scars. Burns 24, 322–335 (1998). [DOI] [PubMed] [Google Scholar]
- Johnson J., Greenspan B., Gorga D., Nagler W. & Goodwin C. Conservative treatment using compression suits for second and third degree burns in children. J Burn Care Rehabil 15, 180–188 (1994). [DOI] [PubMed] [Google Scholar]
- Giele H., Liddiard K., Booth K. & Wood F. Anatomical variations in pressures generated by pressure garments. Plast Reconstr Surg 10, 399–406 (1998). [DOI] [PubMed] [Google Scholar]
- Staley M., Richard R., Billmire D. & Warden G. Head/face/neck burns: therapist considerations for the pediatric patient. J Burn Care Rehabil 18, 164–171 (1997). [DOI] [PubMed] [Google Scholar]
- Ward R. S. Pressure therapy for the control of hypertrophic scar formation after burn injury. A history and review. J Burn Care Rehabil 12, 257–262 (1991). [DOI] [PubMed] [Google Scholar]
- Stewart R., Bhagwanjee A. M., Mbakaza Y. & Binase T. Pressure garment adherence in adult patients with burn injuries: an analysis of patient and clinician perceptions. Am J Occup Ther 54, 598–606 (2000). [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Pan B., Yakuboff K. P. & Rothchild D. Development of a Best Evidence Statement for the Use of Pressure Therapy for Management of Hypertrophic Scarring. J Burn Care Res 37, 255–264 (2016). [DOI] [PubMed] [Google Scholar]
- Macintyre L. & Baird M. Pressure garments for use in the treatment of hypertrophic scars-a review of the problems associated with their use. Burns 32, 10–15 (2006). [DOI] [PubMed] [Google Scholar]
- Fricke N. B., Omnell M. L., Dutcher K. A., Hollender L. G. & Engrav L. H. Skeletal and dental disturbances in children after facial burns and pressure garment use: a 4-year follow-up. J Burn Care Rehabil 20, 239–249 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.