Abstract
Using picosecond excitation at 1064 nm, surface-enhanced hyper-Raman scattering (SEHRS) spectra of the nucleobases adenine, guanine, cytosine, thymine, and uracil with two different types of silver nanoparticles were obtained. Comparing the SEHRS spectra with SERS data from the identical samples excited at 532 nm and with known infrared spectra, the major bands in the spectra are assigned. Due to the different selection rules for the one- and two-photon excited Raman scattering, we observe strong variation in relative signal strengths of many molecular vibrations obtained in SEHRS and SERS spectra. The two-photon excited spectra of the nucleobases are found to be very sensitive with respect to molecule–nanoparticle interactions. Using both the SEHRS and SERS data, a comprehensive vibrational characterization of the interaction of nucleobases with silver nanostructures can be achieved.
Introduction
Raman spectroscopy is widely used for the sensitive characterization of molecular structure and interactions. In the structure elucidation of nucleic acids and their nucleotide building blocks, Raman spectroscopy, in resonance with electronic transitions in the UV, has been one of the most important tools.1−4 The possibility to enhance Raman signals of many chemical compounds in surface-enhanced Raman scattering (SERS)5−7 has been used to study the structure and interaction of nucleotides and nucleic acids off-resonance.8−13 Hyper-Raman scattering (HRS) is the two-photon excited analogue of Raman scattering and gives signals shifted relative to the second harmonic of the excitation wavelength.14−16
In the local fields of plasmonic materials the very low hyper-Raman cross-sections can be overcome,17−19 and sensitive probing of the interaction of the molecules with silver or gold nanoparticles, often used as plasmonic substrates, is enabled.20−22 SEHRS with its different selection rules compared to Raman and infrared absorption spectroscopy provides complementary chemical and structural information.15 HRS, due to the nonlinearity of the process, benefits even more from electromagnetic enhancement than Raman scattering, and it is possible to acquire spectra with cross-sections that are similar to two-photon fluorescence.23 While resonant hyper-Raman scattering in solutions of organic molecules is feasible,24 obtaining nonresonant hyper-Raman spectra from solutions of biomolecules without the help of surface enhancement is practically not possible because of the low cross-section of HRS. In addition to a higher electromagnetic contribution in SEHRS compared to SERS, also the chemical contribution in SEHRS enhancement can vary.22
SEHRS spectra of several chemical compounds such as pyridine,25,26 bipyridines,20 pyrazine,27 and adenine28 are known. Meanwhile, first SEHRS spectra of complex biological materials23 suggest a thorough assessment of the capabilities of SEHRS as a tool for microprobing of organic structures and materials. For example, it has been shown recently that the combination of SERS with SEHRS is more powerful for microenvironmental pH sensing than SERS alone.21,29
To explore further the potential of SEHRS for probing bioorganic samples, in this work, we report nonresonant SEHRS spectra of five important nucleobases, adenine, guanine, cytosine, thymine, and uracil. In order to characterize the nucleobases’ interaction with the plasmonic nanostructure used as SEHRS substrate, the spectra are obtained with different types of silver nanostructures and the SEHRS spectra excited with 1064 nm are compared with SERS data obtained at 532 nm excitation from the identical samples. The data are discussed before the extensive background of previous work on nucleobases carried out by means of Raman, SERS, and infrared spectroscopy.
Experimental Section
Synthesis of the Nanoparticles and Sample Preparation
Silver nitrate (99.9999%), hydroxylamine hydrochloride (99%), sodium hydroxide (p.a.), magnesium sulfate heptahydrate (99%), borax/sodium hydroxide buffer solution (pH 10), adenine (99%), guanine (99%), thymine (97%), uracil (99%), and cytosine (99%) were purchased from Sigma-Aldrich. Trisodium citrate dihydrate (99%) was purchased from Th. Geyer, and sodium chloride (99,6%) was purchased from J. T. Baker. All chemicals were used without further purification. All solutions were prepared using Milli-Q water (USF Elga Purelab Plus purification system).
Silver nanoparticles were prepared by chemical reduction of silver nitrate by citrate or hydroxylamine, respectively. For citrate reduced silver nanoparticles,30 46 mg of silver nitrate was dissolved in 245 mL of water and heated to boiling with extensive stirring. A 5 mL aliquot of a 0.04 M sodium citrate solution was added dropwise, and the reaction mixture was kept boiling for ca. 1 h. For hydroxylamine reduced silver nanoparticles,31 17 mg of silver nitrate, dissolved in 10 mL of water, was added rapidly to a 90 mL solution, containing 11 mg of hydroxylamine hydrochloride and 12 mg of sodium hydroxide. The reaction mixture was stirred for 30 min at room temperature.
For the SERS and SEHRS experiments, silver nanoaggregates were formed by the addition of sodium chloride or magnesium sulfate to the nanoparticle solutions and were mixed with stock solutions of the nucleobases to give a final sample concentration of 5 × 10–5 M. Due to its poor water solubility, the guanine stock solution contained 0.001 M hydrochloric acid.
Raman Experiments
The SERS and SEHRS spectra were measured using an imaging spectrometer by microprobe sampling (10× objective). The experimental setup was described previously in ref (21). Briefly, hyper-Raman excitation at 1064 nm was provided by a mode-locked laser producing 7 ps pulses at a 76 MHz repetition rate, and its second harmonic was used for Raman excitation at 532 nm. The liquid samples were placed in microcontainers, and the Raman and hyper-Raman scattering were collected in confocal and epi-illumination microscope configuration. Typically, SERS spectra were accumulated for 1 s with a photon flux density of 1.4 × 1027 photons cm–2 s–1, and SEHRS spectra for 40–60 s with 1.7 × 1028 or 5.1 × 1028 photons cm–2 s–1. Spectral resolution was 3–6 cm–1, considering the full spectral range. The hyper-Raman spectra were background corrected using an automatic algorithm provided by ref (32).
Results and Discussion
Using high repetition rate mode-locked picosecond excitation at 1064 nm with photon flux densities ranging from 3.4 × 1027 to 5.1 × 1028 photons cm–2 s–1 and silver nanostructures prepared according to two different protocols as plasmonic substrates, it was possible to obtain high quality nonresonant SEHRS spectra of the five nucleobases (chemical structures in Figure 1). The overall SEHRS signals yielded in the experiments with citrate reduced silver nanoparticles were 5–10 times higher than those obtained with the hydroxylamine reduced nanoparticles. This is consistent with the SEHRS enhancement factors in previous experiments with the same nanostructures,21 pointing to specific properties of the nanoaggregates that are formed by the different nanoparticles.
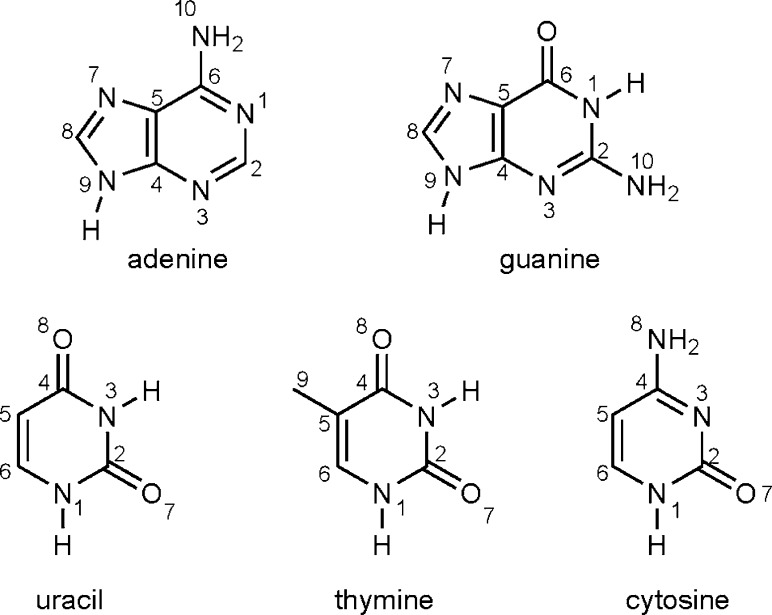
Figure 1.
Structure and atom labeling for adenine, guanine, uracil, thymine, and cytosine.
Both one- and two-photon excited spectra with the two nanoparticle solutions exhibit characteristic vibrational bands of the investigated compounds, and the SERS spectra obtained here (spectra B and D in Figures 2–5) are in good agreement with similar studies reported previously, for example in refs (11−13, 28, and 33−36). We obtain very similar SERS spectra and very similar SEHRS spectra respectively with citrate and hydroxylamine reduced silver nanoparticles. This verifies the high reproducibility of the spectra. Nevertheless, as will be discussed in the following sections, small differences were observed, specifically regarding the intensity ratios for some bands. As will be shown, the molecules display qualitatively very different SERS and SEHRS spectra (compare, for example, spectra A and B in each of the Figures 2–6). One very obvious difference between SERS and SEHRS spectra common to all five nucleobases is the signal of the symmetric ring breathing mode, which is particularly enhanced in SERS but weak or medium compared to other bands in the SEHRS spectra. This mode is also very strong in the Raman but medium in the IR absorption spectra of the solid compounds (Raman and IR absorption spectra of the nucleobases in ref (37)). Regarding the ring breathing mode, the SEHRS and the IR spectra show more similarity than SERS with IR spectra.
Figure 2.
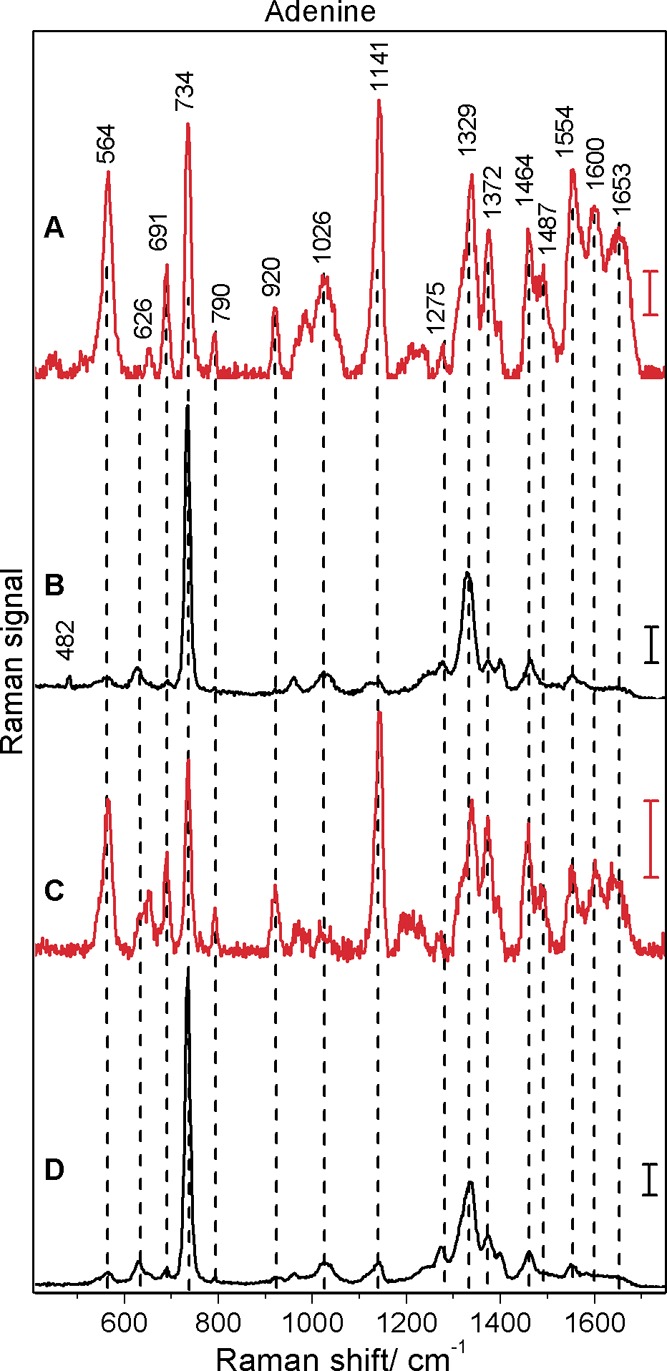
Surface-enhanced hyper-Raman (A, C) and surface-enhanced Raman (B, D) spectra of adenine obtained with citrate (A, B) and hydroxylamine (C, D) reduced silver nanoparticles: excitation, 1064 nm (A, C) and 532 nm (B, D); photon flux density, 5.1 × 1028 photons cm–2 s–1 (A, C) and 1.4 × 1027 photons cm–2 s–1 (B, D); acquisition time, 20 s (A), 60 s (C), and 1 s (B, D); scale bars, 5 cps (A), 1000 cps (B), 1 cps (C), and 1500 cps (D); adenine concentration, 5 × 10–5 M.
Figure 5.
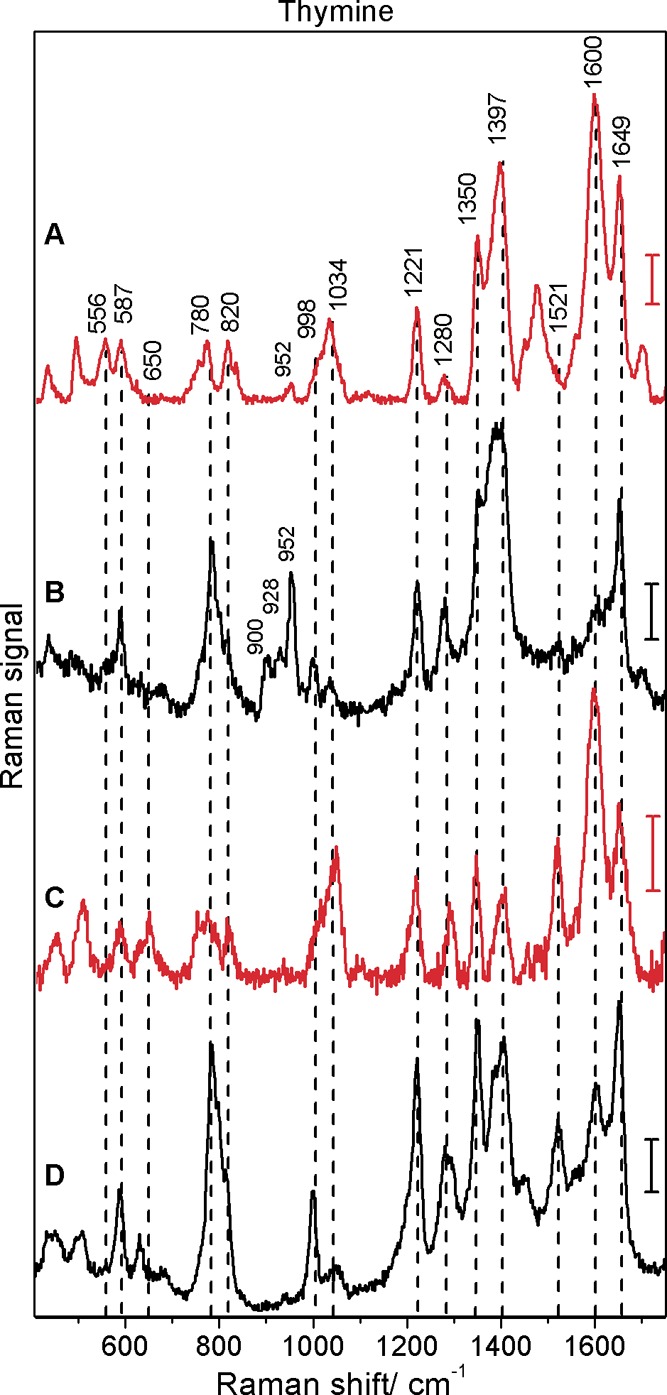
Surface-enhanced hyper-Raman (A, C) and surface-enhanced Raman (B, D) spectra of thymine obtained with citrate (A, B) and hydroxylamine (C, D) reduced silver nanoparticles: excitation, 1064 nm (A, C) and 532 nm (B, D); photon flux density, 1.7 × 1028 photons cm–2 s–1 (A, C) and 1.4 × 1027 photons cm–2 s–1 (B, D); acquisition time, 40 s (A), 100 s (C), and 1 s (B, D); scale bars, 5 cps (A), 300 cps (B), 1 cps (C), and 600 cps (D); thymine concentration, 5 × 10–5 M. Spectra with hydroxylamine reduced silver nanoparticles were obtained at pH 10.
Figure 6.
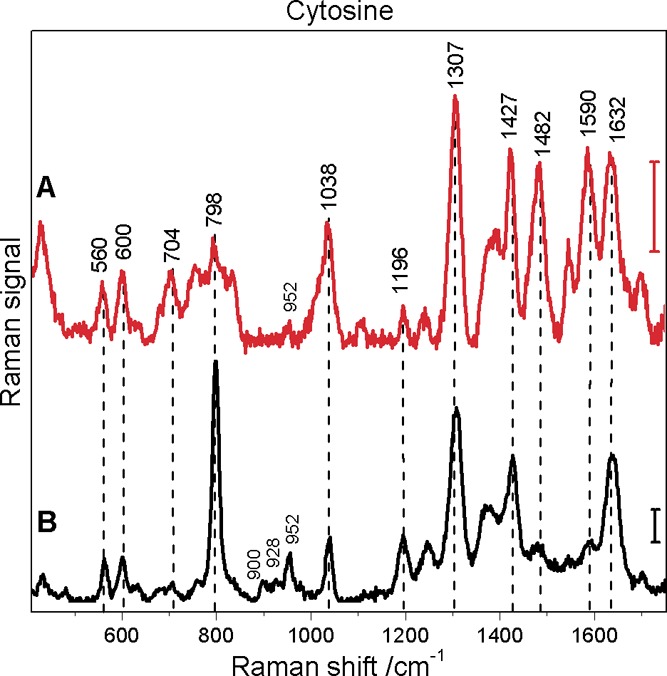
Surface-enhanced hyper-Raman (A) and surface-enhanced Raman (B) spectrum of cytosine obtained with citrate reduced silver nanoparticles: excitation, 1064 nm (A) and 532 nm (B); photon flux density, 4.7 × 1028 photons cm–2 s–1 (A) and 1.4 × 1027 photons cm–2 s–1 (B); acquisition time, 40 s (A) and 1 s (B); scale bars, 10 cps (A) and 500 cps (B); cytosine concentration, 5 × 10–5 M.
SEHRS Spectrum of Adenine
The SEHRS and SERS spectra of adenine are presented in Figure 2, and band assignments are given in Table 1. Both one- and two-photon excited spectra are in very good agreement with previously reported SERS8,33 and SEHRS spectra23,28 of adenine on silver substrates. In the SEHRS spectra (Figure 2A,C) the signal of the symmetric ring breathing mode (734 cm–1), which is dominating the SERS spectra (compare with spectra B and D of Figure 2), is similar to those of the other bands. This band is also very strong in the normal Raman spectrum, but medium in the IR absorption spectrum of solid adenine (see, e.g., ref (37)). Furthermore, the SEHRS data indicate a strong contribution from several bands associated with NH2 and N9–H deformation modes, specifically the NH2 rocking band at 1026 cm–1, in-plane NH2 scissoring vibrations at 1487, 1554, and around 1650 cm–1, and the three bands at 564, 1141, and 1600 cm–1, which can be associated with N9–H bending modes (see Table 1 for details).
Table 1. Raman Shift Values from SEHRS and SERS Spectra of Adenine with Hydroxylamine (AgHA) and Citrate (AgCit) Reduced Silver Nanoparticles and Assignment to Vibrations of the Adenine Molecule (Based on Reference (33)).
| Raman shift/cm–1a |
|||||
|---|---|---|---|---|---|
| SEHRS |
SERS |
||||
| AgHA | AgCit | AgHA | AgCit | plane | assignmentsb |
| 1641 m | 1653 m | in | sciss NH2, str C6–N10, C5–C6 | ||
| 1603 m | 1600 m | in | str N3–C4, N1–C6, C5–N7, N7–C8, bend N9–H | ||
| 1554 m | 1554 m | 1551 w | 1553 w | in | sciss NH2 |
| 1489 m | 1487 m | in | str N7–C8, bend C8–H, sciss NH2 | ||
| 1457 s | 1461 s | 1461 m | 1464 m | in | str C2–N3, N1–C6, bend C2–H, sciss NH2 |
| 1397 m | 1398 m | in | str C4–N9, C4–C5, C6–N10, N7–C8, bend C2–H | ||
| 1371 s | 1374 s | 1372 m | 1372 m | in | bend C2–H, N9–H, str C8–N9, C4–N9 |
| 1338 s | 1335 s | 1332 s | 1329 s | in | str C5–N7, N1–C2, C2–N3, C5–C6, bend C2/8-H |
| 1270 vw | 1275 vw | 1274 m | 1275 m | in | bend C8–H, N9–H, str N7–C8 |
| 1249 vw | 1251 vw | in | rock NH2, str C5–N7, N1–C2, C2–N3 | ||
| 1215 w? | 1220 w? | in | bend C8–H, N10–H11, str C4–N9, N3–C4, C6–N10 | ||
| 1141 s | 1140 s | 1136 m | 1135 m | in | str C8–N9, bend N9–H, C8–H |
| 1026 vw | 1025 br | 1026 w | 1026 w | in | rock NH2 |
| 965 w | 964 w | 960 w | 960 m | in | 5-ring def |
| 920 m | 920 m | 924 w | 920 vw | in | 6-ring def |
| 792 w | 790 m | 792 vw | 792 vw | out | 6-ring def, wag C8–H |
| 734 s | 735 s | 734 vs | 734 vs | in | ring breath |
| 689 s | 689 m | 689 w | 691 w | out | 5-ring def |
| 649 m | 652 m | out | 5-ring def, wag C8–H, N9–H | ||
| 631 m | 626 m | in | 6-ring def | ||
| 564 s | 564 s | 563 w | 560 w | out | wag C2–H, N9–H |
| 482 vw | out | wag N9–H, wag NH2 | |||
vs, very strong; s, strong; m, medium; w, weak; vw, very weak; br, broad.
Bend, bending; breath, breathing; def, deformation; rock, rocking; sciss, scissoring; str, stretching; wag, wagging; 5-ring, five-membered ring; 6-ring, six-membered ring.
The respective SEHRS (Figure 2A,C) and SERS (Figure 2B,D) spectra obtained with the two different silver nanostructures are very similar. Comparing the SEHRS spectra obtained with the different silver nanostructures (Figure 2A,C), we can observe small differences in the intensity ratios of the same bands. The differences in the SERS (Figure 2B,D) are very weak; a small band at 482 cm–1 associated with an out-of-plane N9–H and NH2 wagging appears only in the spectrum with citrate reduced silver nanoparticles (Figure 2B).
SEHRS Spectrum of Guanine
The SEHRS and SERS spectra of guanine with citrate and hydroxylamine reduced silver nanoparticles are shown in Figure 3. As in adenine, the respective SEHRS and SERS spectra of guanine with the two types of silver nanostructures are very similar (compare Figure 3A with Figure 3C for SEHRS and Figure 3B with Figure 3D for SERS). The SEHRS spectra of guanine differ from the SERS spectra in the region between 1200 and 1700 cm–1, e.g., in a pronounced SEHRS signal of the NH2 scissoring at around 1570 cm–1 and in the absence of the in-plane NH bending mode at 1351 cm–1 (Figure 3A,C). The most prominenent differences, however, are found in the region below 800 cm–1: The ring breathing mode at 660 cm–1 is very strong in SERS (Figure 3B,D) but very weak in the SEHRS spectra (Figure 3A,C). Vice versa, the ring deformation modes at 572 and 517 cm–1 are very strong in SEHRS and weak in the SERS spectra (see Table 2 for detailed band assignments). Unlike adenine, the SEHRS spectra of guanine (Figure 3) do not show comparable signals for the ring breathing mode and the ring deformation modes below 700 cm–1 (compare, e.g., Figure 3A with Figure 2A).
Figure 3.
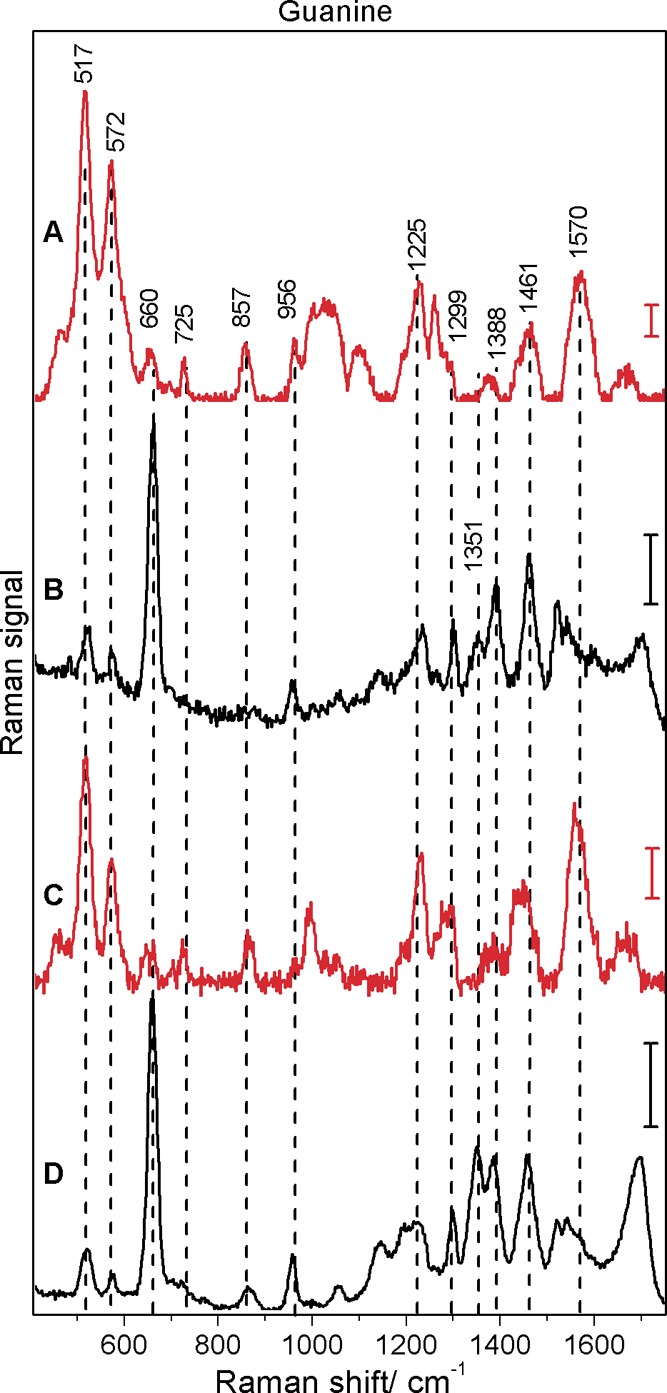
Surface-enhanced hyper-Raman (A, C) and surface-enhanced Raman (B, D) spectra of guanine obtained with citrate (A, B) and hydroxylamine (C, D) reduced silver nanoparticles: excitation, 1064 nm (A, C) and 532 nm (B, D); photon flux density, 5.1 × 1028 photons cm–2 s–1 (A, C) and 1.4 × 1027 photons cm–2 s–1 (B, D); acquisition time, 20 s (A), 60 s (C), and 1 s (B, D); scale bars, 5 cps (A), 500 cps (B), 1 cps (C), and 2500 cps (D); guanine concentration, 5 × 10–5 M.
Table 2. Raman Shift Values from SEHRS and SERS Spectra of Guanine with Hydroxylamine (AgHA) and Citrate (AgCit) Reduced Silver Nanoparticles and Assignment to Vibrations of the Guanine Molecule (Based on Reference (34)).
| Raman
shift/cm–1a |
|||||
|---|---|---|---|---|---|
| SEHRS |
SERS |
||||
| AgHA | AgCit | AgHA | AgCit | plane | assignmentsb |
| 1664 m | 1666 w | 1694 s | 1696 s | in | str C6=O, C5–C6, bend N1–H, sciss NH2 |
| 1562 vs | 1570 s | in | sciss NH2, str C2–N10 | ||
| 1543 w | 1541 w | in | ring str C–N, sciss NH2, bend N1–H | ||
| 1523 m | 1521 m | in | ring str C–N, bend N9–H | ||
| 1455 s | 1460 s | 1457 s | 1461 s | in | ring str C–N, bend C8–H, N1–H, N10–H |
| 1385 w | 1376 w | 1382 s | 1388 s | in | ring str C–N, C–C, rock NH2, bend N1/9–H- |
| 1351 m | 1352 m | in | bend N1–H, N10–H12, str C2–N10 | ||
| 1289 m | 1260 m | 1298 m | 1299 m | in | ring str C–N, C–C, bend C8–H, rock NH2 |
| 1229 m | 1225 m | 1211 m | 1231 m | in | bend C8–H, str N5–N7, N7–C8 |
| 1148 w | 1147 w | in | rock NH2, ring str C–N | ||
| 1051 vw | 1097 m | 1055 vw | 1057 w | in | str N1–C2, C2–N3 |
| 995 m? | 1016 br? | in | rock NH2, ring str C–N | ||
| 964 w | 956 w | 957 w | in | 5-ring def | |
| 865 m | 857 m | 864 w | in/out | 5-ring def, 6-ring def, wag N9–H, N1–H | |
| 725 w | 726 w | in | 6-ring def, bend C6=O | ||
| 653 w | 656 w | 658 vs | 660 vs | in/out | 6-ring breath, 5-ring def, wag NH2 |
| 572 s | 570 s | 575 w | 574 w | in | 6-ring def |
| 517 vs | 517 vs | 520 m | 519 m | in | 6-ring def |
| 459 w? | 463 w? | in | bend C2–N10 | ||
vs, very strong; s, strong; m, medium; w, weak; vw, very weak; br, broad.
Bend, bending; breath, breathing; def, deformation; rock, rocking; sciss, scissoring; str, stretching; wag, wagging; 5-ring, five-membered ring; 6-ring, six-membered ring.
SEHRS Spectra of Uracil and Thymine
Figure 4 and Figure 5 present the spectra of uracil and thymine, respectively. Previous SERS and DFT studies have shown that both pyrimidine bases interact with silver surfaces in their deprotonated forms, even at neutral pH.13,35,38−40 Therefore, also under the conditions of the experiments here, the spectra of uracil in Figure 4 and of thymine in Figure 5 must be those of the anions of the two nucleic acid bases. As for adenine and guanine discussed above, the SEHRS spectra of uracil (Figure 4A,C) and thymine (Figure 5A,C) differ greatly from their SERS spectra, this is observed for both types of silver nanoparticles. Tables 3 and 4 provide the band assignments for all spectra of uracil and thymine, respectively. In the SEHRS spectra we find strong signals due to the C=O stretching vibrations around 1600 cm–1 and a relatively low intensity ring breathing band at 802 cm–1 in uracil (Figure 4A,C) and around 780 cm–1 in thymine (Figure 5A,C).
Figure 4.
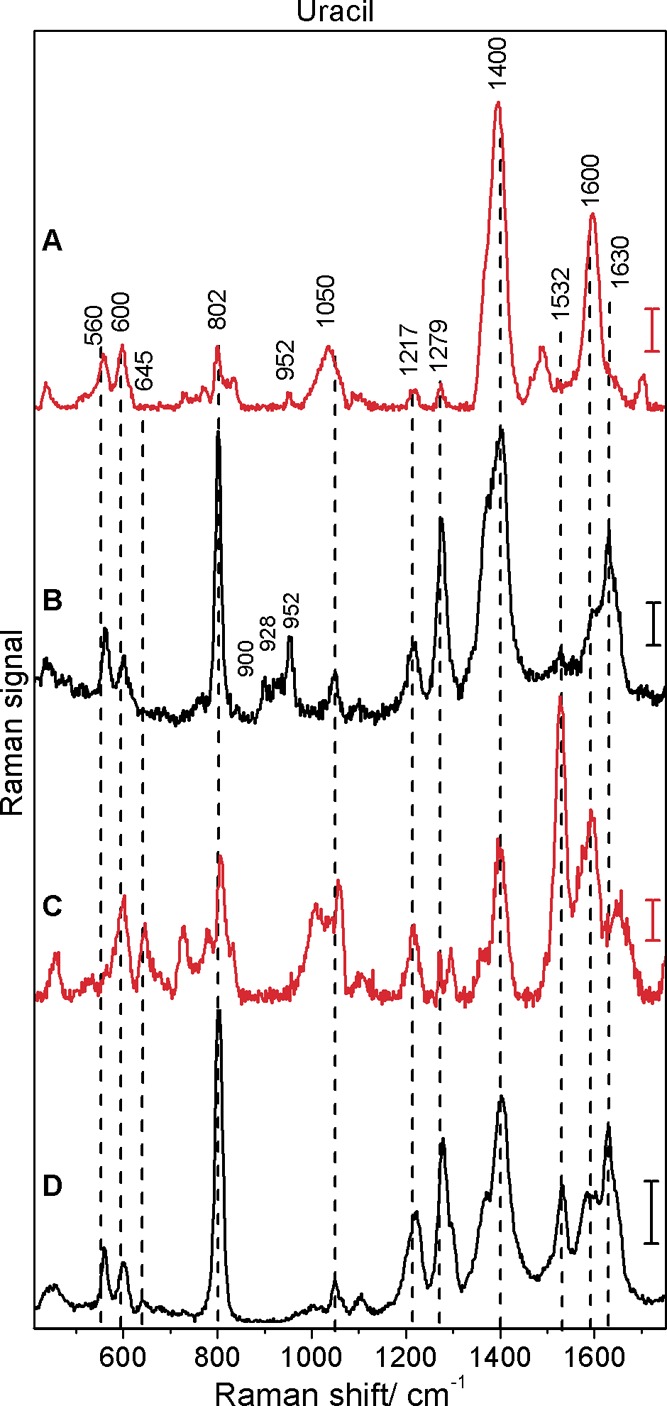
Surface-enhanced hyper-Raman (A, C) and surface-enhanced Raman (B, D) spectra of uracil obtained with citrate (A, B) and hydroxylamine (C, D) reduced silver nanoparticles: excitation, 1064 nm (A, C) and 532 nm (B, D); photon flux density, 1.7 × 1028 photons cm–2 s–1 (A, C) and 1.4 × 1027 photons cm–2 s–1 (B, D); acquisition time, 40 s (A), 100 s (C), and 1 s (B, D); scale bars, 5 cps (A), 300 cps (B), 1 cps (C), and 1500 cps (D); uracil concentration, 5 × 10–5 M. Spectra with hydroxylamine reduced silver nanoparticles were obtained at pH 10.
Table 3. Raman Shift Values in the SEHRS and SERS Spectra of Uracil with Hydroxylamine (AgHA) and Citrate (AgCit) Reduced Silver Nanoparticles and Assignment to Vibrations of the Uracil Molecule (Based on Reference (13)).
| Raman shift/cm–1a |
|||||
|---|---|---|---|---|---|
| SEHRS |
SERS |
||||
| AgHA | AgCit | AgHA | AgCit | plane | assignmentsb |
| 1654 s | in | str C4=O, C2=O | |||
| 1630 s | 1630 s | in | str C2=O, C4=O, bend N1–H, C5–H | ||
| 1590 s | 1595 s | 1590 m | 1600 m | in | str C4=O, C5–C6, C2=O, bend N1–H, C6–H |
| 1530 vs | 1532 m | 1530 vw | in | str C5–C6, C6–N1, bend C6–H | |
| 1489 br? | in | str C6–N1, C4–C5, C2=O | |||
| 1400 m | 1400 vs | 1402 s | 1402 vs | in | bend N1–H, C6–H, C5–H |
| 1374 m | 1372 m | in | bend N3–H, C5–H, C6–H | ||
| 1275 w | 1272 w | 1279 s | 1279 s | in | str N3–C4, C4–C5, C6–N1, bend N1–H, C5/6–H |
| 1215 m | 1217 w | 1219 m | 1217 m | in | bend N1–H, C6–H, C5–H, str C6–N1 |
| 1103 vw | 1098 vw | 1104 vw | 1098 vw | in | bend C5–H, str C5–C6, C6–N1 |
| 1056 br | 1040 br | 1050 br | 1050 br | in | ring def |
| 1011 br | out | wag C6–H | |||
| 808 w | 804 w | 803 vs | 802 vs | in | ring breath |
| 780 vw | 773 vw | 764 vw | out | ring def | |
| 643 m | 649 wv | 645 w | in | ring def | |
| 600 m | 600 m | 600 m | 600 m | in | ring def |
| 559 m | 561 m | 560 m | in | ring def | |
| 456 m | 440 m | 452 br | 448 br | in | bend C2=O, C4=O |
vs, very strong; s, strong; m, medium; w, weak; vw, very weak; br, broad.
Bend, bending; breath, breathing; def, deformation; str, stretching; wag, wagging.
Table 4. Raman Shift Values in the SEHRS and SERS Spectra of Thymine with Hydroxylamine (AgHA) and Citrate (AgCit) Reduced Silver Nanoparticles and Assignment to Vibrations of the Thymine Molecule (Based on References (35) and (12)).
| Raman shift/cm–1a |
|||||
|---|---|---|---|---|---|
| SEHRS |
SERS |
||||
| AgHA | AgCit | AgHA | AgCit | plane | assignmentsb |
| 1652 m | 1649 s | 1647 vs | 1649 s | in | str C2=O, C4=O12,35 |
| 1600 vs | 1601 vs | 1604 m | 1605 m | in | str C2=O, C4=O12 |
| 1522 m | 1521 m | 1520 vw | in | ring str12 | |
| 1456 vw | 1477 s | 1450 vw | in | bend CH312 | |
| 1401 m | 1397 vs | 1400 s | 1396 vs | in | bend N1–H, N3–H35 |
| 1347 m | 1352 s | 1350 vs | 1351 s | in | bend CH3, def C6–H12,35 |
| 1289 m | 1279 w | 1282 m | 1280 m | in | ring str12,35 |
| 1217 m | 1220 m | 1219 s | 1221 m | in | str C5–C935 |
| 1043 br | 1034 br | 1031 vw | 1034 vw | out | wag CH335 |
| 998 m | 1000 w | out | wag N1–H, N3–H35 | ||
| 821 w | 820 w | in | ring def12,35 | ||
| 776 w | 775 w | 785 vs | 786 s | in | ring breath12,35 |
| 650 br | 630 w | in | C2=O, C4=O def35 | ||
| 589 w | 590 w | 587 m | 584 m | in | ring def35 |
| 556 m | in | ring def12 | |||
| 502 m | 501 m | 502 m | 498 w | in | ring def12,35 |
| 452 m | 444 m | 446 w | 439 w | out | ring def35 |
vs, very strong; s, strong; m, medium; w, weak; vw, very weak; br, broad.
Bend, bending; breath, breathing; def, deformation; str, stretching; wag, wagging.
The spectra with citrate reduced silver nanoparticles (Figures 4A,B and 5A,B) were obtained by the addition of nucleobase solutions to the nanoparticle aggregates; the pH of the resulting mixtures was 7.5. It should be noted that it was not possible to obtain spectra of thymine and uracil under the same conditions (pH 7) with the hydroxylamine reduced silver nanoparticles. Increasing the pH to 10 by the addition of a sodium hydroxide–borax buffer allowed us to measure SEHRS and SERS spectra of uracil (Figure 4C,D) and thymine (Figure 5C,D) with these nanoparticles. Since the negatively charged forms of the molecules interact with the nanoparticle surface, we assume that the concentration of the anions at the surfaces at pH 7 is too small. These ions are only formed at very high solution pH values (the pKa values for uracil and thymine in water are 9.36 and 9.86, respectively41) or upon lowering of the pKa by the contact with the silver surface. This is supported by citrate reduced silver nanoparticles,13 but, as our results indicate, not by the hydroxylamine reduced silver nanoparticles.
As will be discussed in this and in the following paragraph, for SEHRS and SERS spectra of both molecules, there are differences between the data obtained at pH 7.5 with citrate reduced nanoparticles and at pH 10 with hydroxylamine reduced nanoparticles (Tables 3 and 4 and Figures 4 and 5). The slightly higher relative intensity of the ring breathing mode at 802 cm–1 of uracil and 780 cm–1 of thymine in the SERS spectra with the hydroxylamine reduced nanoparticles (compare panel B with panel D of Figure 4 and panel B with panel D of Figure 5) could result from the different orientation of the molecules.40,42 Under the basic conditions that were used to obtain the spectra with these nanoparticles (Figure 4D and Figure 5D), more nucleobase anions are present and a more upright orientation of the molecules would be less sterically demanding.
In addition to different orientation at different pH, the pH dependent differences in the spectra of uracil and thymine can also be caused by the presence of different tautomeric forms of the molecules: Thymine and uracil can be deprotonated at the N1 or N3 position, which leads to a tautomeric equilibrium between both deprotonated forms. The distribution of the two species depends on the temperature, the dielectric constant of the solvent, ionic strength, and pH of the solution13,43−45 and has been studied for uracil13 and thymine35 by means of SERS and DFT previously. According to the assignments proposed in ref (13), the N1–C6 stretching at 1532 cm–1 in the SERS spectrum of uracil is characteristic of the N1-deprotonated tautomer. In the SERS spectrum measured with the citrate reduced silver nanoparticles (Figure 4B), this band is only very weak. This indicates that, in the case of the citrate reduced nanoparticles, the N3-deprotonated tautomer contributes more to the SERS spectra, supporting earlier findings.13
Particularly, the SEHRS data can provide valuable additional information about the interaction of the molecules with the silver nanostructures at the two different pH values, due to the different selection rules that govern the two-photon excited Raman process. Similar to the SERS spectrum, the SEHRS spectrum of uracil at pH 7 (Figure 4A) does not show a contribution at 1532 cm–1. At alkaline pH (Figure 4C), a strongly enhanced band at 1532 cm–1 appears in the SEHRS spectrum, clearly indicating the presence of the N1-deprotonated species. The signal is the strongest in the SEHRS spectrum and is more pronounced than in the SERS spectrum of this tautomer (Figure 4D). Also the band at 645 cm–1 (Table 3) in the SEHRS spectrum at pH 10 (Figure 4C and Supporting Information Figure S1) can be related to a changed distribution of the uracil anion tautomers. In the SERS spectrum (Figure 4D), it is not visible as clearly. Figure S1, showing the same SEHRS spectra as Figure 4C, but for the citrate stabilized silver nanoparticles, illustrates that the differences observed in the spectra are indeed pH induced and are very similar for both types of silver nanoparticles. Analogous to this discussion of the contribution of the different tautomers to the uracil spectra at different pH, in the spectra of thymine at alkaline pH (Figure 5C,D) a new band due to the ring stretching vibration at 1521 cm–1 appears, which is more intense in the SEHRS spectrum (Figure 5C and Supporting Information Figure S2) and indicates the presence of N1-deprotonated thymine. Similarly, the band around 650 cm–1 becomes more intense than in the spectra at pH 7.5 (Figure 5A,B). These differences between the spectra at different pH can be associated with the shift of the tautomeric equilibrium between the N1- and N3-deprotonated thymine.35
It should be noted here that in the SERS spectra of uracil (Figure 4B) and thymine (Figure 5B) obtained with the citrate reduced nanoparticles, three additional bands can be observed at 900, 928, and 952 cm–1. These bands appear also in the SERS spectrum of citrate anions shown in ref (46). Since the same band pattern occurs in the SERS spectra of uracil and thymine, and only with citrate reduced nanoparticles (Figure 4B and Figure 5B), we conclude that they indicate coadsorption of citrate on the silver surface. In the SEHRS spectra (Figure 4A and Figure 5A, respectively), only a very weak band at 952 cm–1 can be observed. The weak citrate signals may present an additional advantage of SEHRS over SERS regarding the selective characterization of other analyte molecules as well.
SEHRS Spectrum of Cytosine
Due to the very low stability of the hydroxylamine reduced silver nanoparticles in the presence of cytosine, we were not able to collect SEHRS spectra of the molecule using these nanoparticles without a strong background contribution. Figure 6 shows the SEHRS and SERS spectra obtained with the more stable citrate reduced nanoparticles; Table 5 contains the assignments of the bands. The most obvious differences between the SEHRS (Figure 6A) and the SERS spectra (Figure 6B) are the pronounced signals of the C–N stretching mode at 1482 cm–1 and a relatively strong NH2 bending mode around 1590 cm–1. In accord with the SEHRS data of the other molecules, the ring breathing mode at 798 cm–1 shows a much smaller contribution to the spectra than to the SERS spectrum. Both spectra display the contributions from citrate as discussed above for the spectra of uracil and thymine, with a very small signal at 952 cm–1 in the SEHRS spectrum.
Table 5. Raman Shift Values in the SEHRS and SERS Spectra of Cytosine with Citrate (AgCit) Reduced Silver Nanoparticles and Assignment to Vibrations of the Cytosine Molecule (Based on References (11, 47), and (36)).
| Raman shift/cm–1a |
||
|---|---|---|
| SEHRS | SERS | |
| AgCit | AgCit | assignmentsb |
| 1635 s | 1636 s | str C2=O11 |
| 1587 s | 1591 w | bend NH247 |
| 1547 w | 1544 vw | str N3–C4–C547 |
| 1482 s | 1482 w | str C4–N847 |
| 1424 s | 1422 s | bend N1–H, C5–H, C6–H47 |
| 1387 w | 1373 w | bend N1–H, C5–H, C6–H47 |
| 1307 vs | 1307 vs | ring str C–N11 |
| 1240 w | 1248 w | ring str C–N11 |
| 1196 w | 1196 w | ring str C–N11 |
| 1103 w | C=O11 | |
| 1036 m | 1038 w | |
| 798 w | 799 vs | ring breath11,47 |
| 703 w | 704 w | ring def36 |
| 632 vw | ring def36 | |
| 602 w | 600 w | bend C2=O11 |
| 562 w | 563 w | ring def47 |
| 433 w | 431 vw | |
vs, very strong; s, strong; m, medium; w, weak; vw, very weak; br, broad.
Bend, bending; breath, breathing; def, deformation; str, stretching.
Conclusions
We have discussed here nonresonant hyper-Raman spectra of the nucleic acid bases. In SEHRS experiments with silver nanostructures, at an excitation wavelength of 1064 nm, it is possible to obtain spectra of guanine, uracil, thymine, and cytosine, in addition to the SEHRS spectrum of adenine that has been reported before.23,28 In order to acquire more comprehensive vibrational information about the nanoparticle–nucleobase interaction and to interpret the SEHRS spectra, also one-photon excited SERS spectra of the same samples were acquired at a wavelength of 532 nm.
The spectra obtained with silver nanostructures that are stabilized by different molecular species at their surfaces are very reproducible qualitatively, in spite of different SEHRS and SERS enhancement factors of the different nanoaggregates. They suggest that the interaction of the molecules with the silver nanoparticles, e.g., at different pH values, is independent of the type of nanoparticles that are used. The SEHRS spectra of the nucleobases differ greatly from the SERS spectra, due to the different selection rules of the one- and two-photon excited Raman process. Specifically, they show several characteristics of infrared-active vibrations. The very strong ring breathing mode in SERS, which is often used to estimate the adsorbate orientation with respect to the surface, is relatively weak in the SEHRS spectra of all five molecules. As seen for the spectra of uracil and thymine with the silver nanostructures obtained at alkaline pH, the SEHRS spectra can provide additional information about the interaction of the molecules with the nanoparticle surfaces. For example, the N1–C6 stretching characteristic for N1-deprotonated uracil is more enhanced in SEHRS compared to SERS and therefore allows more sensitive determination of the tautomer involved in the interaction. As a further advantage of SEHRS we have observed a greater sensitivity with respect to the nucleobase molecules and fewer contributions by the bands of citrate ions that are known to stabilize the citrate reduced nanoparticles. This can be seen by the presence of citrate bands in the SERS spectra of uracil, thymine, and cytosine, in contrast to almost no contribution of citrate in the corresponding SEHRS spectra.
In conclusion, it was shown that the combination of one- and two-photon excitation allows a comprehensive vibrational spectroscopic characterization of the nucleobase–nanoparticle interactions for a whole set of nucleobases. The possibility to obtain the nonresonant SEHRS spectra at relatively low excitation intensities opens new possibilities for future SEHRS applications, specifically the investigation of biological samples, which generally profits from near-infrared excitation. The SEHRS spectra of the nucleobases will help to interpret SEHRS data obtained from more complex systems, such as spectra from cells. The high sensitivity of the two-photon excited Raman scattering enhanced by plasmonic metal nanoparticles with respect to the orientation and contact with the silver nanoparticle surfaces is a very promising approach for the characterization of nanobiointeractions.
Acknowledgments
We thank Harald Kneipp for valuable discussions and support in setting up experiments. Funding by ERC Starting Grant No. 259432 (MULTIBIOPHOT) to all authors and by DFG (GSC 1013 SALSA) to Z.H. is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jpcc.6b02753.
SEHRS spectra of uracil and thymine obtained with citrate reduced silver nanoparticles at pH 10 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Erfurth S. C.; Peticolas W. L. Melting and Premelting Phenomenon in DNA by Laser Raman Scattering. Biopolymers 1975, 14, 247–264. 10.1002/bip.1975.360140202. [DOI] [PubMed] [Google Scholar]
- Fodor S. P. A.; Spiro T. G. Ultraviolet Resonance Raman Spectroscopy of DNA with 200–266-nm Laser Excitation. J. Am. Chem. Soc. 1986, 108, 3198–3205. 10.1021/ja00272a006. [DOI] [Google Scholar]
- Nishimura Y.; Tsuboi M.; Kubasek W. L.; Bajdor K.; Peticolas W. L. Ultraviolet Resonance Raman Bands of Guanosine and Adenosine Residues Useful for the Determination of Nucleic Acid Conformation. J. Raman Spectrosc. 1987, 18, 221–227. 10.1002/jrs.1250180314. [DOI] [Google Scholar]
- Benevides J. M.; Overman S. A.; Thomas G. J. Raman, Polarized Raman and Ultraviolet Resonance Raman Spectroscopy of Nucleic Acids and Their Complexes. J. Raman Spectrosc. 2005, 36, 279–299. 10.1002/jrs.1324. [DOI] [Google Scholar]
- Fleischmann M.; Hendra P. J.; McQuillan A. J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. 10.1016/0009-2614(74)85388-1. [DOI] [Google Scholar]
- Albrecht M. G.; Creighton J. A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. 10.1021/ja00457a071. [DOI] [Google Scholar]
- Jeanmaire D. L.; Van Duyne R. P. Surface Raman Spectroelectrochemistry. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. 10.1016/S0022-0728(77)80224-6. [DOI] [Google Scholar]
- Suh J. S.; Moskovits M. Surface-Enhanced Raman Spectroscopy of Amino Acids and Nucleotide Bases Adsorbed on Silver. J. Am. Chem. Soc. 1986, 108, 4711–4718. 10.1021/ja00276a005. [DOI] [Google Scholar]
- Sheng R.; Ni F.; Cotton T. M. Determination of Purine Bases by Reversed-Phase High-Performance Liquid Chromatography Using Real-Time Surface-Enhanced Raman Spectroscopy. Anal. Chem. 1991, 63, 437–442. 10.1021/ac00005a010. [DOI] [Google Scholar]
- Camafeita L. E.; Sánchez-Cortés S.; Garcia-Ramos J. V. SERS of Cytosine and its Methylated Derivatives on Gold Sols. J. Raman Spectrosc. 1995, 26, 149–154. 10.1002/jrs.1250260207. [DOI] [Google Scholar]
- Śanchez Cortés S.; Garcia-Ramos J. V. SERS of Cytosine and its Methylated Derivatives on Metal Colloids. J. Raman Spectrosc. 1992, 23, 61–66. 10.1002/jrs.1250230108. [DOI] [Google Scholar]
- Aroca R.; Bujalski R. Surface Enhanced Vibrational Spectra of Thymine. Vib. Spectrosc. 1999, 19, 11–21. 10.1016/S0924-2031(99)00003-X. [DOI] [Google Scholar]
- Giese B.; McNaughton D. Surface-Enhanced Raman Spectroscopic Study of Uracil. The Influence of the Surface Substrate, Surface Potential, and pH. J. Phys. Chem. B 2002, 106, 1461–1470. 10.1021/jp011986h. [DOI] [Google Scholar]
- Cyvin S. J.; Rauch J. E.; Decius J. C. Theory of Hyper-Raman Effects (Nonlinear Inelastic Light Scattering): Selection Rules and Depolarization Ratios for the Second-Order Polarizability. J. Chem. Phys. 1965, 43, 4083–4095. 10.1063/1.1696646. [DOI] [Google Scholar]
- Denisov V. N.; Mavrin B. N.; Podobedov V. B. Hyper-Raman Scattering by Vibrational Excitations in Crystals, Glasses and Liquids. Phys. Rep. 1987, 151, 1–92. 10.1016/0370-1573(87)90053-6. [DOI] [Google Scholar]
- Kelley A. M. Hyper-Raman Scattering by Molecular Vibrations. Annu. Rev. Phys. Chem. 2010, 61, 41–61. 10.1146/annurev.physchem.012809.103347. [DOI] [PubMed] [Google Scholar]
- Murphy D. V.; Vonraben K. U.; Chang R. K.; Dorain P. B. Surface-Enhanced Hyper-Raman Scattering from SO2–3 Adsorbed on Ag Powder. Chem. Phys. Lett. 1982, 85, 43–47. 10.1016/0009-2614(82)83457-X. [DOI] [Google Scholar]
- Baranov A. V.; Bobovich Y. S. Super-Enhanced Hyper-Raman Scattering from Dyes Adsorbed on Colloidal Silver Particles. JETP Lett. 1982, 36, 339–343. [Google Scholar]
- Chulhai D. V.; Hu Z.; Moore J. E.; Chen X.; Jensen L. Theory of Linear and Nonlinear Surface-Enhanced Vibrational Spectroscopy. Annu. Rev. Phys. Chem. 2016, 67, 541–564. 10.1146/annurev-physchem-040215-112347. [DOI] [PubMed] [Google Scholar]
- Hulteen J. C.; Young M. A.; Van Duyne R. P. Surface-Enhanced Hyper-Raman Scattering (SEHRS) on Ag Film over Nanosphere (FON) Electrodes: Surface Symmetry of Centrosymmetric Adsorbates. Langmuir 2006, 22, 10354–10364. 10.1021/la0612264. [DOI] [PubMed] [Google Scholar]
- Gühlke M.; Heiner Z.; Kneipp J. Combined Near-Infrared Excited SEHRS and SERS Spectra of pH Sensors Using Silver Nanostructures. Phys. Chem. Chem. Phys. 2015, 17, 26093–26100. 10.1039/C5CP03844H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valley N.; Jensen L.; Autschbach J.; Schatz G. C. Theoretical Studies of Surface Enhanced Hyper-Raman Spectroscopy: The Chemical Enhancement Mechanism. J. Chem. Phys. 2010, 133, 054103. 10.1063/1.3456544. [DOI] [PubMed] [Google Scholar]
- Kneipp J.; Kneipp H.; Kneipp K. Two-Photon Vibrational Spectroscopy for Biosciences Based on Surface-Enhanced Hyper-Raman Scattering. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 17149–17153. 10.1073/pnas.0608262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers Kelley A. Resonance Raman and Resonance Hyper-Raman Intensities: Structure and Dynamics of Molecular Excited States in Solution. J. Phys. Chem. A 2008, 112, 11975–11991. 10.1021/jp805530y. [DOI] [PubMed] [Google Scholar]
- Golab J. T.; Sprague J. R.; Carron K. T.; Schatz G. C.; Van Duyne R. P. A Surface Enhanced Hyper-Raman Scattering Study of Pyridine Adsorbed onto Silver: Experiment and Theory. J. Chem. Phys. 1988, 88, 7942–7951. 10.1063/1.454251. [DOI] [Google Scholar]
- Johnson C. K.; Soper S. A. Nonlinear Surface-Enhanced Spectroscopy of Silver Colloids and Pyridine: Hyper-Raman and Second-Harmonic Scattering. J. Phys. Chem. 1989, 93, 7281–7285. 10.1021/j100358a001. [DOI] [Google Scholar]
- Li W.-H.; Li X.-Y.; Yu N.-T. Surface-Enhanced Hyper-Raman Spectroscopy (SEHRS) and Surface-Enhanced Raman Spectroscopy (SERS) Studies of Pyrazine and Pyridine Adsorbed on Silver Electrodes. Chem. Phys. Lett. 1999, 305, 303–310. 10.1016/S0009-2614(99)00380-2. [DOI] [Google Scholar]
- Kneipp H.; Kneipp K. Surface-Enhanced Hyper Raman Scattering in Silver Colloidal Solutions. J. Raman Spectrosc. 2005, 36, 551–554. 10.1002/jrs.1333. [DOI] [Google Scholar]
- Kneipp J.; Kneipp H.; Wittig B.; Kneipp K. One- and Two-Photon Excited Optical pH Probing for Cells Using Surface-Enhanced Raman and Hyper-Raman Nanosensors. Nano Lett. 2007, 7, 2819–2823. 10.1021/nl071418z. [DOI] [PubMed] [Google Scholar]
- Lee P. C.; Meisel D. Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols. J. Phys. Chem. 1982, 86, 3391–3395. 10.1021/j100214a025. [DOI] [Google Scholar]
- Leopold N.; Lendl B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. 10.1021/jp027460u. [DOI] [Google Scholar]
- Zhang Z.-M.; Chen S.; Liang Y.-Z.; Liu Z.-X.; Zhang Q.-M.; Ding L.-X.; Ye F.; Zhou H. An Intelligent Background-Correction Algorithm for Highly Fluorescent Samples in Raman Spectroscopy. J. Raman Spectrosc. 2010, 41, 659–669. 10.1002/jrs.2500. [DOI] [Google Scholar]
- Giese B.; McNaughton D. Surface-Enhanced Raman Spectroscopic and Density Functional Theory Study of Adenine Adsorption to Silver Surfaces. J. Phys. Chem. B 2002, 106, 101–112. 10.1021/jp010789f. [DOI] [Google Scholar]
- Giese B.; McNaughton D. Density Functional Theoretical (DFT) and Surface-Enhanced Raman Spectroscopic Study of Guanine and its Alkylated Derivatives Part 2: Surface-Enhanced Raman Scattering on Silver Surfaces. Phys. Chem. Chem. Phys. 2002, 4, 5171–5182. 10.1039/b203830g. [DOI] [Google Scholar]
- Cho K.-H.; Choo J.; Joo S.-W. Tautomerism of Thymine on Gold and Silver Nanoparticle Surfaces: Surface-Enhanced Raman Scattering and Density Functional Theory Calculation Study. J. Mol. Struct. 2005, 738, 9–14. 10.1016/j.molstruc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Frankel D. J.; Chen Q.; Richardson N. V. Formation of Hydrogen-Bridged Cytosine Dimers on Cu (110). J. Chem. Phys. 2006, 124, 204704. 10.1063/1.2190225. [DOI] [PubMed] [Google Scholar]
- Schrader B.Raman/Infrared Atlas of Organic Compounds; 2nd ed.; VCH: Weinheim, Germany, and New York, NY, USA, 1989. (Translation of Raman/IR Atlas organischer Verbindungen). [Google Scholar]
- Oh W. S.; Suh S. W.; Kim M. S. Surface-Enhanced Raman Scattering of Nucleic Acid Components in Silver Sol: Uracil and its Derivatives. J. Raman Spectrosc. 1988, 19, 261–265. 10.1002/jrs.1250190408. [DOI] [Google Scholar]
- Rivas L.; Sanchez-Cortes S.; Garcia-Ramos J. V. Raman Structural Study of Thymine and its 2′-Deoxy-ribosyl Derivatives in Solid State, Aqueous Aolution and When Adsorbed on Silver Nanoparticles. Phys. Chem. Chem. Phys. 2002, 4, 1943–1948. 10.1039/b110564g. [DOI] [Google Scholar]
- Cho K.-H.; Choo J.; Joo S.-W. Surface-Enhanced Raman Scattering and Density Functional Theory Calculation of Uracil on Gold and Silver Nanoparticle Surfaces. Spectrochim. Acta, Part A 2005, 61, 1141–1145. 10.1016/j.saa.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Ganguly S.; Kundu K. K. Protonation/Deprotonation Energetics of Uracil, Thymine, and Cytosine in Water from E.M.F./Spectrophotometric Measurements. Can. J. Chem. 1994, 72, 1120–1126. 10.1139/v94-143. [DOI] [Google Scholar]
- Creighton J. A. Surface Raman Electromagnetic Enhancement Factors for Molecules at the Surface of Small Isolated Metal Spheres: The Determination of Adsorbate Orientation from SERS Relative Intensities. Surf. Sci. 1983, 124, 209–219. 10.1016/0039-6028(83)90345-X. [DOI] [Google Scholar]
- Wierzchowski K. L.; Litońska E.; Shugar D. Infrared and Ultraviolet Studies on the Tautomeric Equilibria in Aqueous Medium between Monoanionic Species of Uracil, Thymine, 5-Fluorouracil, and Other 2,4-Diketopyrimidines. J. Am. Chem. Soc. 1965, 87, 4621–4629. 10.1021/ja00948a039. [DOI] [PubMed] [Google Scholar]
- Psoda A.; Kazimierczuk Z.; Shugar D. Structure and Tautomerism of the Neutral and Monoanionic Forms of 4-Thiouracil Derivatives. J. Am. Chem. Soc. 1974, 96, 6832–6839. 10.1021/ja00829a003. [DOI] [PubMed] [Google Scholar]
- Lippert B. Uracil and Thymine Monoanions in Solution: Differentiation of Tautomers by Laser Raman Spectroscopy. J. Raman Spectrosc. 1979, 8, 274–278. 10.1002/jrs.1250080511. [DOI] [Google Scholar]
- Bell S. E. J.; Sirimuthu N. M. S. Surface-Enhanced Raman Spectroscopy as a Probe of Competitive Binding by Anions to Citrate-Reduced Silver Colloids. J. Phys. Chem. A 2005, 109, 7405–7410. 10.1021/jp052184f. [DOI] [PubMed] [Google Scholar]
- Liu S.; Zheng G.; Li J. Raman Spectral Study of Metal-Cytosine Complexes: A Density Functional Theoretical (DFT) Approach. Spectrochim. Acta, Part A 2011, 79, 1739–1746. 10.1016/j.saa.2011.05.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.