Abstract
Aim
To determine whether diffusion tensor imaging (DTI) can be an independent assessment for identifying the corticospinal tract (CST) projecting from the more‐affected motor cortex in children with unilateral spastic cerebral palsy (CP).
Method
Twenty children with unilateral spastic CP participated in this study (16 males, four females; mean age 9y 2mo [standard deviation (SD) 3y 2mo], Manual Ability Classification System [MACS] level I–III). We used DTI tractography to reconstruct the CST projecting from the more‐affected motor cortex. We mapped the motor representation of the more‐affected hand by stimulating the more‐ and the less‐affected motor cortex measured with single‐pulse transcranial magnetic stimulation (TMS). We then verified the presence or absence of the contralateral CST by comparing the TMS map and DTI tractography. Fisher's exact test was used to determine the association between findings of TMS and DTI.
Results
DTI tractography successfully identified the CST controlling the more‐affected hand (sensitivity=82%, specificity=78%).
Interpretation
Contralateral CST projecting from the lesioned motor cortex assessed by DTI is consistent with findings of TMS mapping. Since CST connectivity may be predictive of response to certain upper extremity treatments, DTI‐identified CST connectivity may potentially be valuable for determining such connectivity where TMS is unavailable or inadvisable for children with seizures.
What this paper adds
Diffusion tensor imaging (DTI) can identify corticospinal tract (CST) projections from motor cortex.
DTI can safely examine CST projections in children with unilateral spastic cerebral palsy.
This article is commented on by Basu on pages 10–11 of this issue.
Abbreviations
- CST
Corticospinal tract
- DTI
Diffusion tensor imaging
- MACS
Manual Ability Classification System
- MEP
Motor evoked potential
- ROI
Region of interest
- TMS
Transcranial magnetic stimulation
Cerebral palsy (CP) is the most common cause of motor deficits in children. Unilateral spastic CP, the most common subtype of CP,1 is characterized by motor deficits lateralized to one side of the body. Studies using magnetic resonance imaging (MRI) showed that the etiology may include middle cerebral artery occlusion or hemorrhage, hypoxic–ischemic encephalopathy, brain malformation, and periventricular leukomalacia.2, 3 Unilateral spastic CP may also result from other causes of brain abnormality, such as prematurity4 or sinovenous thrombosis.5 Damage can affect the cerebral cortex, subcortical structures,3, 4 and the descending corticospinal tract (CST).6, 7 The CST is the primary motor pathway descending from the motor cortex innervating muscles controlling skilled voluntary movement.6, 7
In infants with typical development, the CST axons project bilaterally from the motor cortex to the spinal cord prenatally.8 Throughout the first 6 months of life, the ipsilateral CST is pruned, reinforcing the crossed contralateral CST sprouting into the spinal cord. A typical contralateral CST connection is established by 1 to 2 years of age.8, 9, 10 Perinatal brain injury can disrupt this typical course of activity‐dependent refinement. In children with unilateral spastic CP, damage to the more‐affected motor cortex weakens the contralateral/crossed projection.11 The ipsilateral CST projecting from the less‐affected motor cortex is hence strengthened.8, 9 Transcranial magnetic stimulation (TMS) can be used to assess this cortical control of the upper extremity.8, 9, 12 Approximately 50% of children with unilateral spastic CP have their more‐affected upper extremity controlled by the ipsilateral CST.13 Consequently, the less‐affected motor cortex controls bilateral movements whereas the more‐affected motor cortex controls no movement in these children. Stimulating the less‐affected motor cortex with TMS in this group of children with unilateral spastic CP often elicits muscle responses on both upper extremities, while no responses are observed when stimulating the more‐affected motor cortex.8
Constraint‐induced movement therapy has been shown to improve hand function and can produce long‐term improvements in children with unilateral spastic CP.14, 15 However, it is costly (thousands of dollars per child) and time‐consuming (60–90 h).16 It is therefore imperative to target constraint‐induced movement therapy to children who are most likely to benefit. CST connectivity or ‘rewiring’ (i.e. which motor cortex controls the more‐affected upper extremity) in children with unilateral spastic CP may be used as a biomarker to stratify patients before prescribing particular therapies.17 Kuhnke et al.13 demonstrated that children with an ipsilateral CST (absence of a contralateral CST) responded less than children with a preserved contralateral CST in the speed component of the Wolf Motor Function Test after constraint‐induced movement therapy. A recent review proposed using individual CST rewiring as a method to predict hand function and treatment outcome in children with unilateral spastic CP.17 These studies suggested CST connectivity should be carefully examined for targeting treatments based on individual pathology. TMS is a traditional neurophysiological method to examine CST connectivity and its function in children with unilateral spastic CP.8, 9, 12 TMS poses a risk in children with seizure disorder, a highly concomitant disorder (35%) in children with CP.1 DTI tractography is a neuroimaging method of reconstructing white matter tracts and allowing further investigation of pathway integrity.18, 19, 20 The benefit of using DTI to assess a preserved contralateral CST is that it typically takes a short period of time to administer (~10min) and does not pose a risk to children with seizure disorders. The aim of this study was to investigate whether DTI can be an assessment to identify the presence or absence of a contralateral CST in children with unilateral spastic CP. We hypothesized that DTI can be an independent assessment for identifying the contralateral CST projecting from the more‐affected motor cortex to the more‐affected upper extremity.
Method
Participants
We recruited participants from our website (www.tc.edu/centers/cit/), ClinicalTrials.gov (NCT00305006), and online support groups. Participants were a convenience sample participating in our clinical trials.21 The inclusion criteria for the parent trial were established based on our prior trials14: (1) diagnosed with congenital unilateral spastic CP; (2) the ability to lift the more‐affected arm 15cm above a table surface and grasp light objects; (3) mainstreamed in school; (4) the ability to follow instructions during screening and complete the physical examination. Exclusion criteria of the parent study included: (1) health problems unassociated with CP; (2) current/untreated seizures; (3) visual problems; (4) severe spasticity at any joint (Modified Ashworth score >3.5); (5) orthopedic surgery on the more‐affected upper extremity within the previous year; and (6) botulinum toxin therapy in the upper extremity within the last 6 months. Children who met the following additional criteria were recruited for this study: (1) aged between 6 years and 17 years; (2) ability to comply with TMS and MRI procedures. Additional exclusion criteria were: (1) history of seizures after 2‐years‐old, (2) non‐removable metallic objects in the body, (3) claustrophobia, and (4) family history of epilepsy. Participants of this study were representative of the clinical population (Manual Ability Classification System [MACS] levels I–III, Table 1). Informed assent/consent was obtained from all participants and their caregivers. The study was approved by the Institutional Review Boards of Teachers College, Columbia University, the New York State Psychiatric Institute, and Burke Medical Research Institute.
Table 1.
Participant demographic and clinical characteristics (n=20)
| Mean age (SD) (y, mo) | 9.2 (3.2) |
| Sex | |
| Male | 16 |
| Female | 4 |
| Paretic hand | |
| Right | 10 |
| Left | 10 |
| Lesion type | |
| Right | 10 (0a, 5b, 5c) |
| Left | 10 (0a, 6b, 4c) |
| Bilateral | 0 |
| Race | |
| White | 11 |
| Hispanic | 4 |
| Mixed | 3 |
| African American | 1 |
| Asian | 1 |
| MACS level | |
| I | 3 |
| II | 14 |
| III | 3 |
| CST connectivity of the more‐affected UEd | |
| Ipsilateral | 9 |
| Contralateral | 2 |
| Bilateral | 9 |
aBrain malformation. bAbnormality of periventricular white matter. cCortical/subcortical lesion. dUpper extremity; identified by transcranial magnetic stimulation motor mapping. SD, standard deviation; MACS, Manual Ability Classification System; CST, corticospinal tract; UE, upper extremity.
Procedures
MRI protocols
Each child received structural MRI and DTI (in the same session). The structural scan was used in the TMS experiment to co‐register stimulation sites with brain landmarks, using a stereotaxic system (Brainsight, Rogue Research, Montreal, QC, Canada). The structural was also used for examining lesion location.22 The DTI was used to reconstruct the CST and obtain fiber characteristics.
T1‐weighted MRI was performed on a 3T scanner (Philips, Eindhoven, the Netherlands) at Columbia University Medical Center. Children were positioned head‐first‐supine. For the structural scan, 165 slices were taken (resolution 256×256 pixels). For the DTI, 75 slices were taken (resolution 112×112 pixels, 55 directions, b‐value=800s/mm2). An echo‐planar imaging sequence was used (TR=7638.99ms, TE=68.56ms).
DTI tractography
We used DTI Studio (Johns Hopkins, Baltimore, MD, USA) to reconstruct the contralateral CST of the more‐affected upper extremity. We first created an image to mask the background noise at the threshold of 30 dB, using standard linear‐regression for tensor calculation. We then excluded noisy images containing movement artifacts by visually inspecting the original images using the Apparent Diffusion Constant function.23 An average of 457 slices (standard deviation [SD]=192, 11.1% of images taken for each child) were excluded. We used the Fiber Assignment by Continuous Tracking method for fiber reconstruction. Fiber tracking started with the fractional anisotropy >0.3 and stopped with the fractional anisotropy <0.25 or if the tract turning angle was >70°.
We placed the first seed at pre‐ and post‐central gyri of the more‐affected hemisphere (a circular region of interest [ROI], 25cm2, centered at the central fissure) to reconstruct the contralateral CST on the DTI color map (same size/location for each child, Fig. 1a1, ‘OR’ function in DTI Studio).24 The size of this ROI was determined based on our recent findings.25 The seeded slice was always in the axial plane (average=12.4 slices, SD=2.1 slices below the first axial slice that showed visible cortex), examined in a cranial–caudal direction. Because central fissure was not always easily identified on a DTI color map of the lesioned motor cortex, we cross‐referenced with the fractional anisotropy map for precisely localizing the seed. An example of the obtained fiber after seeding the first ROI can be found in Figure 1b1. Then we placed a second seed at the pyramidal tract at the lower pons level on the more‐affected side (see Fig. 1a2, an anterior blue‐coded area where the CST typically passes through, ‘AND’ function in DTI Studio).6, 24, 26 An example of DTI tractography results after combining the two seeds can be found in Figure 1b2. Our criteria strictly excluded fibers that do not pertain to a ‘conventional’ CST. This first tractography approach was independent of TMS findings.
Figure 1.
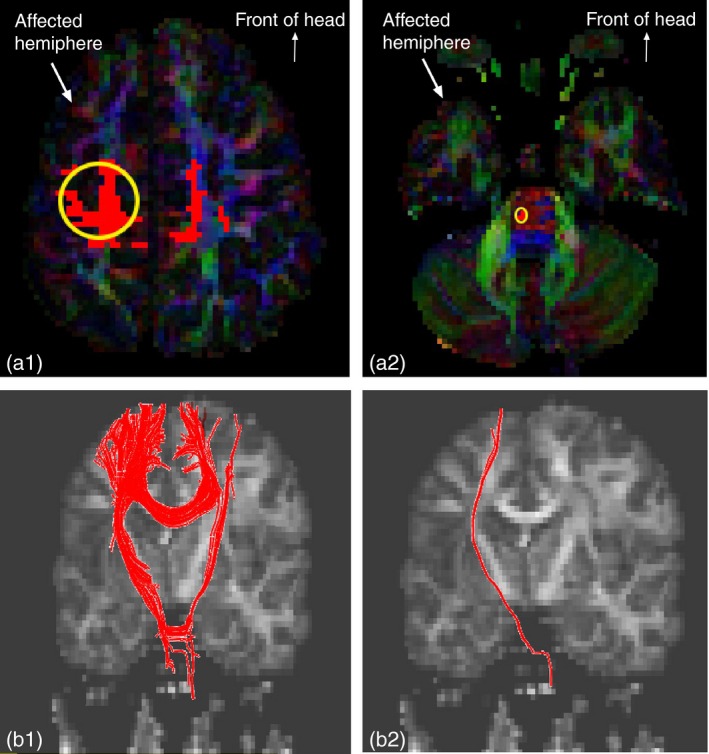
(a1 and a2) Yellow circled regions show the two seeds in diffusion tensor imaging (DTI) color map, axial slices. (a1) region of interest (ROI)1: 25cm2 circular region seeded at pre‐central and post‐central gyri of the more‐affected motor cortex. (a2) ROI2: seeded at pyramidal tract at the pons. (b1 and b2) Reconstructed tracts (red fibers) after seeding the ROIs. (b1) Reconstructed tracts after seeding ROI1. (b2) Reconstructed tracts after combining ROI1 and ROI2. [Colour figure can be viewed at wileyonlinelibrary.com].
TMS motor mapping
TMS experiments were conducted at the New York State Psychiatric Institute. We used single‐pulse TMS (Magstim 200 stimulator, 70mm figure‐of‐eight coil) to assess the cortical control of the more‐affected upper extremity. Frameless stereotaxy (Brainsight) allowed for online tracking of the position of the TMS coil relative to a child's MRI. We used an electromyography (EMG) recording system (Brainvision, Morrisville, NC, USA) during TMS stimulation for simultaneously recording bilateral muscle responses using surface electrodes over the first dorsal interosseous and flexor carpi radialis muscles.7 We mapped the motor representation of the more‐affected first dorsal interosseous and flexor carpi radialis by probing the more‐ and the less‐affected motor cortex (as in Fig. 2). Details of TMS procedures are presented in Appendix S1 (online supporting information).
Figure 2.
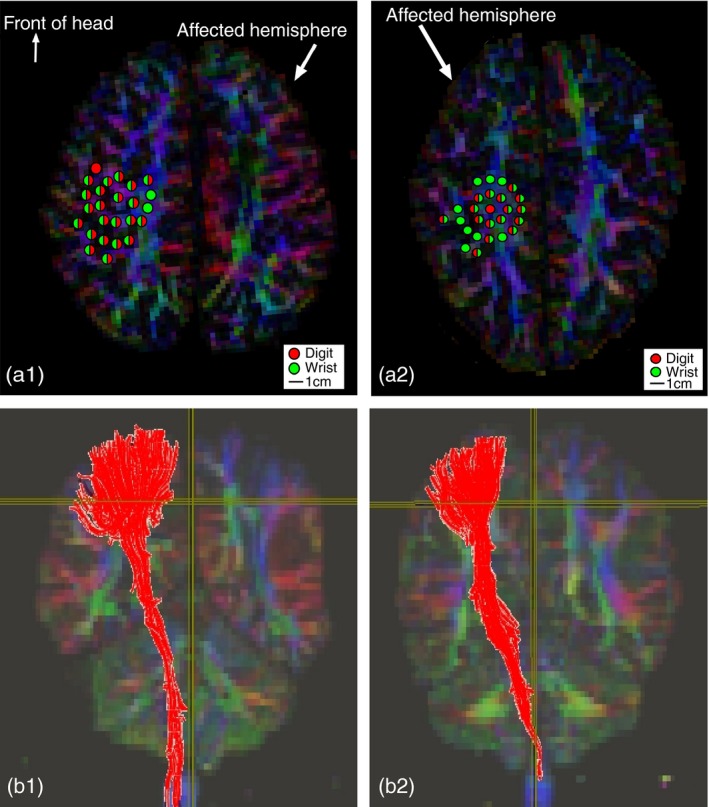
(a1 and a2) Color dots represent transcranial magnetic stimulation (TMS) motor map of the more‐affected hand (dark red, first dorsal interosseous; light green, flexor carpi radialis). (a1) A participant with TMS motor map (color dots) on the less‐affected motor cortex. (b1) An ipsilateral corticospinal tract (red fibers) after diffusion tensor imaging (DTI) tractography by seeding TMS motor map on the less‐affected motor cortex (region of interest [ROI] seeded based on the area of color dots in a1). (a2) A participant with TMS motor map on the more‐affected motor cortex. (b2) A contralateral CST (red fibers) after DTI tractography by seeding TMS motor map on the more‐affected motor cortex (ROI seeded based on the area of color dots in a2). [Colour figure can be viewed at wileyonlinelibrary.com].
TMS data analysis
TMS‐induced EMG data were imported into MATLAB (Mathworks, Natick, MA, USA). A MATLAB script was written to measure the motor evoked potential (MEP) amplitude for each muscle. Investigators identified the onset and offset of the MEP. For each grid point in the map, the average MEP strength was calculated. Each grid point was categorized as a digit (first dorsal interosseous), wrist (flexor carpi radialis), or a combination of the two muscles by the presence or absence of an MEP at that site.
CST fiber characteristics assessed by DTI
We obtained CST fiber characteristics by using a second approach (independent of the first approach). We first seeded at TMS‐derived motor area on the DTI color map. We then excluded fibers that do not pertain to the CSTs (e.g. fibers passing through corpus callosum, cerebellum, and medial lemniscus at the pons). This second approach allowed us to obtain fiber characteristics more precisely by using individual motor maps. Figure 2 shows examples of using individual TMS‐derived motor maps to reconstruct CSTs for studying corticospinal fiber characteristics.
Statistical design
We used SPSS (version 19; IBM, Armonk, NY, USA) for statistical analysis. Two‐sided Fisher's Exact Test was used to determine the association between TMS and the first DTI tractography approach for determining the presence or absence of a contralateral CST. We calculated the sensitivity and specificity of using DTI as an assessment to identify the contralateral CST. We used paired t‐tests to determine if the means of DTI measures of the more‐affected CST were significantly different from those of the less‐affected CST. p‐values<0.05 were considered statistically significant for the study.
Results
Twenty children with unilateral spastic CP (age range 6y 1mo–17y 1mo; mean age 9y 2mo; SD 3y 2mo), who met inclusion criteria, participated in the study. Clinical characteristics of all participants are summarized in Table 1. In addition, we show the stratification of participants by TMS‐identified connectivity: nine children had CST projecting from the less‐affected motor cortex (ipsilateral connectivity), two children had CST projecting from the more‐affected motor cortex (contralateral connectivity), and nine children had CST projecting from both motor cortices (bilateral connectivity).
DTI is an assessment of contralateral CST connectivity
Table 2 summarizes the association between the two methods under study: TMS versus DTI. Each participant was categorized into one of the four categories by verifying whether there were TMS‐derived muscle responses by probing the more‐affected motor cortex, and by examining the presence or absence of the CST projecting from the more‐affected motor cortex using DTI tractography. Fisher's Exact Test showed that DTI is an independent assessment of CST connectivity (p=0.022). When using TMS as the standardized assessment, the sensitivity of using DTI for assessing CST connectivity was 81.8% (95% confidence interval [CI] 48–97%), and the specificity was 77.8% (95% CI 40–96%). Two participants with bilateral connectivity (examined by TMS) showed discrepancy between the two methods in their contralateral CST (Table 2, upper right cell, TMS ‘yes’, DTI ‘no’). Stimulating the more‐affected motor cortex using TMS‐induced MEPs in the more‐affected upper extremity in these two participants. However, their presumed CST passed through the medial lemniscus at the pons after we placed the first seed on the motor cortex (see Fig. S1, online supporting information).24, 26 Therefore, we considered these as tracts other than the CST for these two participants. Two other participants did not have a TMS‐evoked MEP from stimulating the more‐affected motor cortex, yet a visible CST projecting from the more‐affected motor cortex was visible (Table 2, TMS ‘no’, DTI ‘yes’).
Table 2.
2×2 Contingency table summarizing association between findings of transcranial magnetic stimulation (TMS) and diffusion tensor imaging (DTI)
| Contralateral corticospinal tracts (CST) detected by: DTI | ||||
|---|---|---|---|---|
| Yesa | Nob | |||
| TMS‐evoked muscle responses by probing the more‐affected motor cortex | Yesc | 9 | 2 | 11 |
| Nod | 2 | 7 | 9 | |
| 11 | 9 | 20 | ||
aDTI yes: presence of contralateral CST reconstructed by DTI tractography. bDTI no: absence of contralateral CST reconstructed by DTI tractography. cTMS yes: presence of TMS‐induced motor evoked potential (MEP) responses from electromyography (EMG) recordings on the more‐affected upper extremity (UE). dTMS no: absence of TMS‐induced MEP responses from EMG recordings on the more‐affected UE.
Integrity of the more‐affected CST is compromised
We investigated the differences in CST fiber integrity between the more‐ and less‐affected sides in children with bilateral connectivity using the second tractography approach. Significant differences between bilateral tracts could provide neuroanatomical evidence of a compromised CST descending from the more‐affected motor cortex in the mild to moderate form of children with unilateral spastic CP in this study (MACS level I–III). The DTI measures of nine children with bilateral connectivity were used to investigate this comparison. The DTI measures examined included fractional anisotropy, fiber volumes (mean number of fibers/voxel), radial diffusivity ((λ2+λ3)/2), and mean diffusivity ((λ1+λ2+λ3)/3) (Table 3). Two‐tailed paired t‐tests demonstrated that the means of DTI measures of the more‐affected CST were significantly different from those of the less‐affected CST. Specifically, the values of the ipsilateral fractional anisotropy and volume were higher (indicating better integrity) than those of the contralateral side (both p=0.003). In addition, the ipsilateral radial diffusivity (p=0.001) and mean diffusivity (p=0.007) were lower than those of the contralateral side.
Table 3.
Comparison of means of diffusion tensor imaging (DTI) measures of participants with bilateral connectivity (n=9)
| Ipsilateral CST (95% CI) | Contralateral CST (95% CI) | Paired mean difference (95% CI) | t | p a | Effect size (Cohen's d) | |
|---|---|---|---|---|---|---|
| Fractional anisotropy | 0.533 (0.509–0.557) | 0.495 (0.482–0.508) | 0.038 (0.017–0.059) | 4.121 | 0.003* | 1.52 |
| Volume (mean of fibers/voxel) | 12.193 (10.433–13.952) | 8.058 (6.144–9.972) | 4.135 (1.919–6.351) | 4.303 | 0.003* | 1.73 |
| Mean diffusivityb | 0.781 (0.772–0.789) | 0.838 (0.799–0.878) | −0.058 (−0.094 to −0.021) | −3.646 | 0.007* | −1.55 |
| Radial diffusivityb | 0.518 (0.500–0.536) | 0.583 (0.557–0.609) | −0.065 (−0.096 to 0.034) | −4.813 | 0.001* | −2.24 |
aTwo‐tailed paired t‐test. bUnit=10–3mm2/s. *Indicates p<0.05. CST, corticospinal tract; CI, confidence interval.
Discussion
DTI tractography can identify the contralateral CST in unilateral spastic CP
The primary aim of this study was to investigate whether DTI tractography can independently identify a preserved contralateral CST in children with unilateral spastic CP. Identifying the presence/absence of a contralateral CST controlling the more‐affected upper extremity may help in clinical decision‐making regarding treatment outcome and determining the location for brain stimulation treatments (e.g. stimulating the more‐affected motor cortex for children with a preserved contralateral CST; stimulating the less‐affected motor cortex for the absence of a contralateral CST). DTI tractography is sensitive (81.8%) and specific (77.8%) to identify the contralateral CST in children with unilateral spastic CP. Although two DTI tractography approaches were used, we compared findings of TMS mapping with the presence/absence of the contralateral CST derived from the first approach. This finding suggests that DTI can be used to assess a contralateral CST, especially for children who cannot receive TMS. We propose that DTI tractography can be used clinically as a tool for determining the CST connectivity in children with unilateral spastic CP.
Two participants (bilateral connectivity measured by TMS) showed discrepancy between DTI and TMS in the contralateral CST (Table 2, TMS ‘yes’, DTI ‘no’). DTI tractography showed that these fibers originating from the more‐affected motor cortex passed through the medial lemniscus, but not the anterior pyramidal tract at the lower pons.24 The clinical characteristics of these two cases cannot explain their disorganized CST (case 1: age 8y 11mo, MACS level III, lesion type=cortical/subcortical; case 2: age 17y 1mo, MACS level II, lesion type=cortical/subcortical). Nor did their TMS results show any discrepancy in MEP onset latency as compared with other children with bilateral connectivity (independent t‐test, t=0.05, p=0.966). The discrepancy between TMS and DTI in these two participants suggests that reorganization in the motor system may be variable and not always be measured by a single method. An example case showing incongruence of TMS and DTI outcomes is shown in Figure S1.
Two other participants (ipsilateral connectivity measured by TMS) did not have TMS‐evoked MEP responses from stimulating the more‐affected motor cortex, yet a visible CST projecting from the more‐affected motor cortex was reconstructed (Table 2, TMS ‘no’, DTI ‘yes’). These two cases both had brain lesion type categorized as periventricular leukolamacia with MACS level II (case 3: age 10y 1mo; case 4: age 14y 1mo), although this combination (periventricular leukolamacia and MACS level II) consisted of 35% of our participants. Children's tolerance for high intensity stimulation is sometimes low, and the motor threshold for the more‐affected motor cortex is typically high in children with unilateral spastic CP.9 It is possible that the stimulation intensity was not strong enough to activate their CSTs projecting from the more‐affected motor cortex (tested up to 85% TMS device output; stopped due to children's intolerance), despite the CSTs being anatomically present.
Fiber integrity in the more‐ versus less‐affected CST
Our results comparing the fiber integrity of the more‐ versus the less‐affected CSTs showed differential characteristics of the ipsilateral and the contralateral CSTs in children with bilateral connectivity (t‐tests, fractional anisotropy, p=0.003). Previous studies used MRI cross‐sectional areas of the cerebral peduncles to compare the integrity of two CSTs,6, 7 and one study used DTI tractography to directly compare the two CSTs in children with unilateral spastic CP.26 Our study added evidence that the two CSTs present differential fiber integrity projecting from bilateral motor cortices in children with unilateral spastic CP with MACS level I to III. This second approach allowed precisely seeding individualized ROIs since motor map location can be variable in children with unilateral spastic CP.
Limitations
The results of this study may not be generalizable in the clinical setting, because variability exists in DTI acquisition protocols. Our imaging protocols contained 55 diffusion directions. However, this level of precision may not be achievable in every MRI facility, particularly in the clinical setting. Second, we did not include the CSTs originating from the less‐affected motor cortex when comparing the two methods, given that it was challenging to determine to where those fibers descend (they could control either hand). This is a technical limitation of using brain DTI because it only captures images caudally to the junction of medulla and cervical spinal cord. Even if DTI of the cervical spine is available, tractography can be challenging when fibers are crossing.24 In addition, our sample encompassed children with mild to moderate levels of hand function impairments (MACS level I–III). Conceivably, children with more severe impairment (e.g. MACS level IV or V) may demonstrate a more disorganized CST, making DTI maps more difficult to define. Whereas the CST in severely affected children may be present, highly disorganized fibers would be more difficult to reconstruct. It would be ideal to compare our data with that from children with typical development or children whose lesions were postnatal. Last, due to the small number of the available contralateral CST in our participants, we were unable to perform correlation analysis between DTI‐derived fiber characteristics and hand function measures. We propose further studies to recruit a larger sample and age‐matched controls to study this relationship.
Supporting information
Appendix S1. Transcranial magnetic stimulation (TMS) motor mapping details.
Figure S1. A representative participant with discrepant findings between diffusion tensor imaging (DTI) tractography (A, B) and TMS motor mapping (C1).
Acknowledgements
We thank families and volunteers participating in this study. We also thank those individuals involved: Bruce Bassi, David Murphy, Bruce Luber, Marina Brandão, Jason Fuller, Stephen Dashnaw, Greg Westin, Charles Schroeder, Dan Javitt, Karen Chin. Grant support: KF (R01HD076436A1, K01NS062116, KL2 RR024157, UL1 RR024156, TL1 RR024158). JBC (K08 NS073796). The National Institutes of Health provided funding that paid for researchers' time and MRI fees. The funder was not involved in study design, data analysis, manuscript preparation, or publication decisions. The authors have stated that they had no interests which might be perceived as posing a conflict or bias.
References
- 1. Himmelmann K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth‐year period 1995–1998. Acta Paediatr 2005; 94: 287–94. [DOI] [PubMed] [Google Scholar]
- 2. Himmelmann K, Beckung E, Hagberg G, Uvebrant P. Gross and fine motor function and accompanying impairments in cerebral palsy. Dev Med Child Neurol 2006; 48: 417–23. [DOI] [PubMed] [Google Scholar]
- 3. Krägeloh‐Mann I, Horber V. The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: a systematic review. Dev Med Child Neurol 2007; 49: 144–51. [DOI] [PubMed] [Google Scholar]
- 4. Kuban KC, Leviton A. Cerebral palsy. N Engl J Med 1994; 330: 188–95. [DOI] [PubMed] [Google Scholar]
- 5. deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol 2000; 15: 316–24. [DOI] [PubMed] [Google Scholar]
- 6. Bleyenheuft Y, Grandin CB, Cosnard G, Olivier E, Thonnard JL. Corticospinal dysgenesis and upper‐limb deficits in congenital hemiplegia: a diffusion tensor imaging study. Pediatrics 2007; 120: e1502–11. [DOI] [PubMed] [Google Scholar]
- 7. Duque J, Thonnard JL, Vandermeeren Y, Sébire G, Cosnard G, Olivier E. Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain 2003; 126: 732–47. [DOI] [PubMed] [Google Scholar]
- 8. Eyre JA, Tyalor JP, Villagra F, Smith M, Miller S. Evidence of activity‐dependent withdrawal of corticospinal projections during human development. Neurology 2001; 57: 1543–54. [DOI] [PubMed] [Google Scholar]
- 9. Eyre JA, Smith M, Dabydeen L, et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol 2007; 62: 493–503. [DOI] [PubMed] [Google Scholar]
- 10. Friel KM, Chakrabarty S, Martin JH. Pathophysiological mechanisms of impaired limb use and repair strategies for motor systems after unilateral injury of the developing brain. Dev Med Child Neurol 2013; 55(Suppl 4): 27–31. [DOI] [PubMed] [Google Scholar]
- 11. Martin JH, Friel KM, Salimi I, Chakrabarty S. Activity‐ and use‐dependent plasticity of the developing corticospinal system. Neurosci Biobehav Rev 2007; 31: 1125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krägeloh‐Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol 2004; 56: 854–63. [DOI] [PubMed] [Google Scholar]
- 13. Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint‐induced movement therapy? Dev Med Child Neurol 2008; 50: 898–903. [DOI] [PubMed] [Google Scholar]
- 14. Gordon AM, Hung YC, Brandão M, et al. Bimanual training and constraint‐induced movement therapy in children with hemiplegic cerebral palsy: a randomized trial. Neurorehabil Neural Repair 2011; 25: 692–702. [DOI] [PubMed] [Google Scholar]
- 15. Sakzewski L, Ziviani J, Abbott DF, Macdonell RA, Jackson GD, Boyd RN. Participation outcomes in a randomized trial of 2 models of upper‐limb rehabilitation for children with congenital hemiplegia. Arch Phys Med Rehabil 2011; 92: 531–39. [DOI] [PubMed] [Google Scholar]
- 16. Wallen M, Ziviani J, Herbert R, Evans R, Novak I. Modified constraint‐induced therapy for children with hemiplegic cerebral palsy: a feasibility study. Dev Neurorehabil 2008; 11: 124–33. [DOI] [PubMed] [Google Scholar]
- 17. Jaspers E, Byblow WD, Feys H, Wenderoth N. The corticospinal tract: a biomarker to categorize upper limb functional potential in unilateral cerebral palsy. Front Pediatr 2015; 3: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshida S, Hayakawa K, Yamamoto A, et al. Quantitative diffusion tensor tractography of the motor and sensory tract in children with cerebral palsy. Dev Med Child Neurol 2010; 52: 935–40. [DOI] [PubMed] [Google Scholar]
- 19. Lee SK, Kim DI, Kim J, et al. Diffusion‐tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics 2005; 25: 53–65. [DOI] [PubMed] [Google Scholar]
- 20. Hoon AH, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol 2009; 51: 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brandão MB, Ferre C, Kuo H‐C, et al. Comparison of structured skill and unstructured practice during intensive bimanual training in children with unilateral spastic cerebral palsy. Neurorehabil Neural Repair 2013; 28: 452–61. [DOI] [PubMed] [Google Scholar]
- 22. Staudt M. Brain plasticity following early life brain injury: insights from neuroimaging. Semin Perinatol 2010; 34: 87–92. [DOI] [PubMed] [Google Scholar]
- 23. Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 2006; 81: 106–16. [DOI] [PubMed] [Google Scholar]
- 24. Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PC, Mori S. Fiber tract‐based atlas of human white matter anatomy. Radiology 2004; 230: 77–87. [DOI] [PubMed] [Google Scholar]
- 25. Friel KM, Kuo HC, Fuller J, et al. Skilled bimanual training drives motor cortex plasticity in children with unilateral cerebral palsy. Neurorehabil Neural Repair 2016; 30: 834–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas B, Eyssen M, Peeters R, et al. Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain 2005; 128: 2562–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Transcranial magnetic stimulation (TMS) motor mapping details.
Figure S1. A representative participant with discrepant findings between diffusion tensor imaging (DTI) tractography (A, B) and TMS motor mapping (C1).


