ABSTRACT
Objectives
To assess the economic impact of introducing into clinical practice in the UK the soluble fms‐like tyrosine kinase (sFlt‐1) to placental growth factor (PlGF) ratio test for guiding the management of pre‐eclampsia.
Methods
We used an economic model estimating the incremental value of information, from a UK National Health Service payer's perspective, generated by the sFlt‐1/PlGF ratio test, compared with current diagnostic procedures, in guiding the management of women with suspected pre‐eclampsia. The economic model estimated costs associated with the diagnosis and management of pre‐eclampsia in pregnant women between 24 + 0 and 36 + 6 weeks' gestation, managed in either a ‘test’ scenario in which the sFlt‐1/PlGF test is used in addition to current diagnostic procedures, or a ‘no‐test’ scenario in which clinical decisions are based on current diagnostic procedures alone. Test characteristics and resource use were derived from PROGNOSIS, a non‐interventional study in women presenting with clinical suspicion of pre‐eclampsia. The main outcome measure from the economic model was the cost per patient per episode of care, from first suspicion of pre‐eclampsia to birth.
Results
Introduction of the sFlt‐1/PlGF ratio test into clinical practice is expected to result in cost savings of £344 per patient compared with a no‐test scenario. Savings are generated primarily through an improvement in diagnostic accuracy and subsequent reduction in unnecessary hospitalization.
Conclusions
Introducing the sFlt‐1/PlGF ratio test into clinical practice in the UK was shown to be cost‐saving by reducing unnecessary hospitalization of women at low risk of developing pre‐eclampsia. In addition, the test ensures that those women at higher risk are identified and managed appropriately. © 2016 Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: cost‐effectiveness, diagnosis, economic, hospitalization, model, prediction, pre‐eclampsia, sFlt‐1/PlGF
INTRODUCTION
Hypertensive disorders, including pre‐eclampsia, occur in approximately 10% of pregnancies in the UK1. Pre‐eclampsia, a condition characterized by hypertension and proteinuria, is reported in 3% of pregnancies, and is associated with substantial perinatal morbidity and mortality in mothers and infants1, 2. The management of pre‐eclampsia is also associated with significant healthcare costs3, 4.
The UK National Institute for Health and Care Excellence (NICE) guidelines recommend hospitalization for women diagnosed with pre‐eclampsia, but not for those with mild/moderate gestational hypertension only1. However, uncertainty in confirming the diagnosis leads to unnecessary admission of women with suspected but not proven pre‐eclampsia, leading to substantial healthcare costs.
In a UK study between 2006 and 2008, ‘substandard care’ was linked with 20/22 deaths related to pre‐eclampsia, of which 63% were described as ‘undoubtedly avoidable’5. Moreover, timely referral to a perinatal care center was reported to reduce perinatal morbidity and mortality by 20%6. Improved diagnostic testing could reduce costs and optimize management by triaging patients at low risk of pre‐eclampsia to an outpatient setting, while ensuring that patients at moderate/high risk are managed more intensively and receive interventions (e.g. antenatal corticosteroids for fetal lung maturation) to mitigate morbidity.
Quantification of the ratio between two angiogenic placental factors involved in the formation of new blood vessels – serum fms‐like tyrosine kinase‐1 (sFlt‐1) and placental growth factor (PlGF) – has provided valuable diagnostic information and forms the basis of the first automated biomarker test for pre‐eclampsia, the Elecsys® sFlt‐1/PlGF immunoassay ratio (Roche Diagnostics GmbH, Mannheim, Germany)7, 8, 9. PreOS, a multicenter, non‐interventional study evaluating the test for its aid in diagnosis and clinical decision‐making, found that changed decisions due to test results regarding hospitalization were in agreement with the maternal and neonatal outcomes10, 11, 12. Recently, PROGNOSIS, a global, multicenter, non‐interventional study derived and validated cut‐off values for the short‐term prediction of pre‐eclampsia. The study found that, in women presenting with clinical suspicion of pre‐eclampsia, a sFlt‐1/PlGF ratio of < 38 accurately ruled out the onset of pre‐eclampsia within 1 week13, 14, 15.
The objective of this study was to estimate the incremental value of the test information measured by a reduction in expected costs of patient management because of improved accuracy in the short‐term prediction of pre‐eclampsia when using the sFlt‐1/PlGF ratio test in addition to current practice.
METHODS
PROGNOSIS study
PROGNOSIS was a prospective, non‐interventional study conducted across 30 sites globally, including the UK, in which serum sFlt‐1/PlGF ratios were measured in 1050 women with suspected pre‐eclampsia between 24 + 0 and 36 + 6 weeks' gestation. The aim of the study was to derive and validate a cut‐off of the ratio for the short‐term prediction of pre‐eclampsia. The serum ratio levels were measured after enrollment of the derivation cohort, and at the end of the study for the validation cohort and, as such, were not available to investigators, and patient management decisions were made in the absence of the test information. Data on fetal and maternal adverse events were collected. Resource use, including planned and unplanned hospital admissions and inpatient length of stay, were also recorded13.
The PROGNOSIS study data were used as a source of information for the proportion of women hospitalized on suspicion of pre‐eclampsia, in the absence of test information, in current practice (‘no‐test’ scenario), the correlation between hospitalization and the test ratio and the relationship between test ratio, hospitalization and a confirmed diagnosis of pre‐eclampsia. The study was also used to provide information on inpatient length of stay, before and after the onset of pre‐eclampsia, and for women who did not develop pre‐eclampsia.
In the PROGNOSIS study, each participating study site provided ethics committee/institutional review board approval of the study protocol and associated documents (participant informed consent, participant information) before the start of the clinical part of the study. All women provided written informed consent before enrollment13, 15. Being a health economic study, ethical approval was not required for the present study.
Model structure
An economic model was developed from a UK National Health Service (NHS) payer's perspective to estimate costs associated with the diagnosis and management of a cohort of women from first presentation with clinical suspicion of pre‐eclampsia to the point of delivery. The model simulates the progression of a woman through a treatment pathway that is determined by the assessed risk of her developing pre‐eclampsia and the consequent decision to hospitalize her or to manage the pregnancy in an outpatient setting.
The incremental value of the information generated by the test was evaluated by comparing expected management costs in two scenarios: a ‘test’ scenario (current diagnostic procedures plus the sFlt‐1/PlGF ratio) and a ‘no‐test’ scenario (current diagnostic procedures only) in a population of pregnant women presenting with a clinical suspicion of pre‐eclampsia, but in the absence of a definitive diagnosis. The incidence of pre‐eclampsia was assumed to be unaffected by the introduction of the test. Potential cost savings were expected to be driven by changes in clinical‐management strategy brought about by the test information. In particular, it was thought that the ability of the test to rule out the onset of pre‐eclampsia within 1 week may have reduced the number of women who were hospitalized unnecessarily. Treatment pathways for the test and no‐test scenarios are shown in Figure 1.
Figure 1.
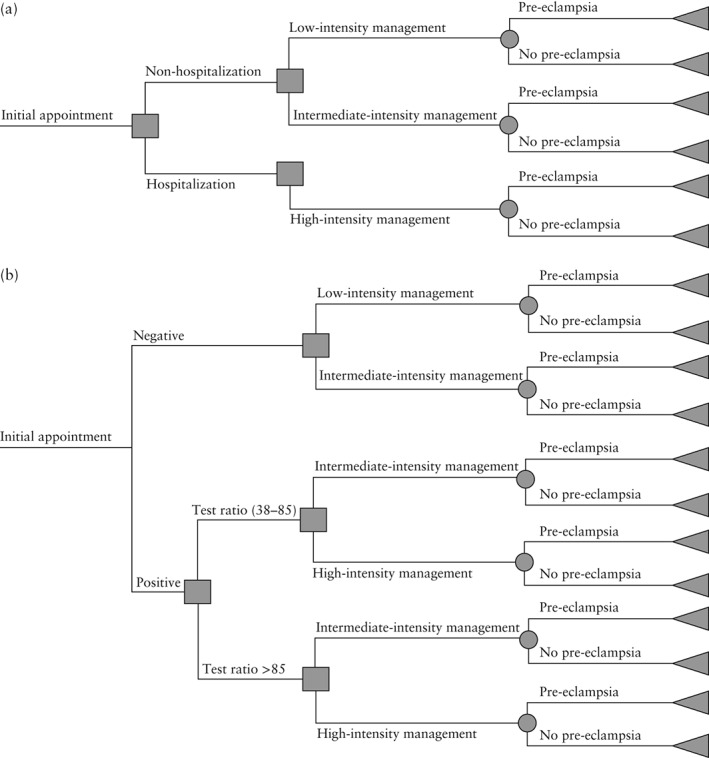
Decision tree: (a) in the ‘no‐test’ scenario and (b) in the ‘test’ scenario.
Patient‐level data from the PROGNOSIS study provided information for each woman on whether she was hospitalized before developing pre‐eclampsia, the test ratio at baseline and whether she ultimately developed pre‐eclampsia. In the economic model, patients not admitted to hospital were assumed to be managed in an outpatient setting. Outpatient resource use was modeled by distinguishing low‐intensity management (characterized by weekly midwife‐led outpatient appointments) and intermediate‐intensity management (twice‐weekly midwife‐led outpatient appointments with some specialist involvement). Table 1 shows the main differences in resource use between the three levels of patient management (low, intermediate and high intensity).
Table 1.
Modeled options for management of women with suspected pre‐eclampsia16
| Non‐hospitalized | Hospitalized | |
|---|---|---|
| Low‐intensity management | Intermediate‐intensity management | High‐intensity management |
| Midwife‐led hospital outpatient setting | Midwife‐led hospital outpatient setting | Inpatient management |
| Average weekly appointment | Average twice‐weekly appointment and specialist medical input | Not applicable |
| At each visit routine tests should be performed, including: | At each visit routine tests should be performed, including: | Tests including: |
| • Blood pressure | • Blood pressure | • Blood pressure (four times daily) |
| • Proteinuria | • Proteinuria | • Proteinuria (daily) |
| • Blood tests | • Blood tests (daily) | |
| • Kidney function | • Kidney function (twice daily) | |
| • Electrolytes | • Electrolytes (twice daily) | |
| • Transamines | • Transamines (twice daily) | |
| • Bilirubin | • Bilirubin (twice daily) | |
| No intervention | Oral antihypertensive therapy twice daily | Oral antihypertensive therapy twice daily |
Women were classified into one of three groups according to the sFlt‐1/PlGF ratio test results: < 38; 38–85; or > 85. The risk of pre‐eclampsia and probability of hospitalization were expected to be positively correlated with the value of the ratio8, 9. The lower cut‐off value of 38 to rule out pre‐eclampsia within 1 week, with a negative predictive value (NPV) of 99.3%, was derived from the PROGNOSIS study15. The higher cut‐off value of 85 for the diagnosis of pre‐eclampsia was derived from a multicenter case–control study9; according to a 2015 consensus statement, a ratio > 85 indicates that pre‐eclampsia was highly likely and administration of antenatal corticosteroids should be considered16. In the PROGNOSIS study, in which clinicians were blinded to the test ratio, 36% of women presenting with a clinical suspicion of pre‐eclampsia were hospitalized. In the absence of information on whether outpatient management was at low or intermediate intensity, the economic model assumed an equal split (32% low and 32% intermediate). Analysis of the test information from the PROGNOSIS study showed that, of the 13.2% of women with a ratio > 85, 65% were hospitalized. Of the 10.7% of women with a ratio in the range 38–85, 55% were hospitalized and of the 76.1% of women with a ratio of < 38, 28% were hospitalized (Table S1).
There is no direct information from the PROGNOSIS study on the management decisions that would have been made had the value of the ratio been known. For the purposes of modeling the test scenario, a clinical algorithm was developed to estimate the disposition of women according to the value of the ratio (Table S1), on the basis of a consensus statement on the management of pre‐eclampsia and current NICE guidelines1, 16. The conservative assumption was that, for a test ratio of > 38, the proportion of women hospitalized would be the same as was observed in the PROGNOSIS study (65% for a ratio > 85, and 55% for a ratio between 38 and 85). A ratio of < 38 denotes a low risk of pre‐eclampsia and, in principle, no woman in this group would need to be hospitalized to manage the risk. In practice, there may be other reasons for hospitalization, and the economic model is based on the assumption that a woman will be hospitalized with an sFlt‐1/PlGF ratio of < 38 and blood pressure higher than 160/110 mmHg, as recommended by current NICE guidelines. In the PROGNOSIS study, 1.7% of women met these joint criteria. All women with a ratio of > 38 were assumed to have received corticosteroids, irrespective of hospitalization, to form a conservative estimate in the economic model. The benefits of corticosteroid administration were not accounted for in the model.
The economic model includes an option for a retest 2 weeks after the initial test, if the initial test was negative (i.e. sFlt‐1/PlGF ratio < 38). Given that the NPV of the test was still very high after 2 weeks, with a value of 97.9% (95% CI, 96.0–99.0%)14, this period was chosen in the model for the retest, despite the rule‐out period for pre‐eclampsia being 1 week in the PROGNOSIS study (with an NPV of 99.3%)16. Additional criteria for a retest were continuing symptoms of pre‐eclampsia including epigastric pain, severe edema and headache; confirmed hypertension or proteinuria; one of the criteria for HELLP syndrome; intrauterine growth restriction; or abnormal uterine perfusion.
Costs
The analysis includes the cost of the ratio test (£65), treatment costs associated with hospitalization, outpatient appointments, antihypertensive medication, regular testing, the cost of preventing complications and the cost of treating complications. The level of resource use for each of the management intensities was informed by the NICE guidelines for the management of women with hypertension in pregnancy, and unit costs were taken from UK‐specific sources (Table S2). Hospitalization costs per episode were derived from a unit cost of £2639 for a 7‐day hospital stay (£377 per day)17, multiplied by the length of stay obtained from the PROGNOSIS study for each of the treatment arms in the model.
The costs of treating complications include the cost of unplanned re‐attendance of women at hospital and the cost of admission of neonates to the neonatal intensive care unit (NICU). In the absence of evidence, the model assumes that information from the test had no effect on unplanned readmissions of women, or on admission of a neonate to the NICU.
Scenario analysis
Three sets of scenario analysis were performed to test the robustness of the results:
Variations in inpatient length of stay. Two separate sensitivities were run: (a) the value of all length‐of‐stay parameters was reduced by 50%; and (b) length of stay was reduced to 1.6 days for women who were hospitalized but did not develop pre‐eclampsia (in both the test and the no‐test scenarios), in line with hospital statistics for women with gestational hypertension.
Variations in the proportion of women admitted to hospital, depending on the value of the test ratio: (a) the proportion of women admitted was increased by 10% and 20% for women with a ratio value of < 38 and ≥ 38 respectively; and (b) the proportion of women admitted was increased by 5% and 10% for women with a ratio of < 38.
No retest. Women were tested only once, at the time of the initial suspicion of pre‐eclampsia.
RESULTS
The additional information provided by the test may result in management decisions for women with suspected pre‐eclampsia that are better correlated with pre‐eclampsia outcomes than are current diagnostic procedures alone. Without the test information, 36% of women were hospitalized before a diagnosis of pre‐eclampsia, of whom 27% went on to develop pre‐eclampsia. If the additional information from the test had been available, the proportion of women hospitalized could have been reduced to around 16%, of whom 38% would have subsequently developed pre‐eclampsia. Among women who were not hospitalized, approximately the same proportion subsequently developed pre‐eclampsia. The introduction of the test is also expected to reduce the number of women hospitalized at first presentation, before developing pre‐eclampsia, from 36% to 16%. In the PROGNOSIS study population (n = 1050), this would equate to 213 fewer women hospitalized to manage the risk of pre‐eclampsia. This reduction in hospitalization would be expected to generate a cost saving of £344 per patient (8.3%) (Table 2). The additional costs of the test and retest are more than offset by savings in the cost of hospitalization. The expected annual cost savings for the UK NHS would be in the region of £24 million, based on a cohort of 68 900 women presenting annually with hypertensive disorders including suspected pre‐eclampsia18.
Table 2.
Cost analysis for introduction of serum fms‐like tyrosine kinase‐1/placental growth factor (sFlt‐1/PlGF) ratio test in addition to current diagnostic procedures (test scenario) compared with costs of current diagnostic procedures only (no‐test scenario), for guiding management of pre‐eclampsia (PE) in a cohort of 1050 women with suspected PE from the PROGNOSIS study
| Treatment | No‐test scenario cost (£) | Test scenario cost (£) | Difference (£) |
|---|---|---|---|
| Initial appointment | 445 673 | 445 673 | 0 |
| sFlt‐1/PlGF test | — | 68 250 | 68 250 |
| sFlt‐1/PlGF retest | — | 40 043 | 40 043 |
| Management costs prior to PE for patients who develop PE | 399 103 | 422 755 | 23 652 |
| Low risk | 25 629 | 25 506 | −123 |
| Intermediate risk | 77 169 | 126 907 | 49 738 |
| High risk | 296 306 | 270 343 | −25 963 |
| PE management | 616 337 | 609 049 | −7288 |
| Management costs for patients without PE | 2 811 942 | 2 326 603 | −485 340 |
| Low risk | 304 432 | 351 135 | 46 703 |
| Intermediate risk | 916 656 | 1 273 271 | 356 616 |
| High risk | 1 590 855 | 702 196 | −888 658 |
| Use of corticosteroids | 2737 | 2237 | −500 |
| Unplanned re‐attendance at hospital | 69 591 | 69 591 | — |
| Total per cohort | 4 345 382 | 3 984 200 | −361 182 |
| Total per patient | 4138 | 3794 | −344 |
Slight discrepancies between numbers and totals are due to rounding.
The rate of hospitalization derived from data for UK subjects in the PROGNOSIS study showed that the hospitalization rate in the no‐test scenario was 58%, compared with 36% in the overall PROGNOSIS cohort, indicating that there may be further potential to reduce hospitalization in the UK. Of the 44 patients hospitalized in the UK cohort, nine (20.5%) developed pre‐eclampsia compared with 27% in the overall study cohort. This may indicate that the UK is more risk‐averse with regard to hospitalization than are other countries.
In the test scenario, all women with a sFlt‐1/PlGF ratio of > 38 (considered to be at intermediate or high risk of developing pre‐eclampsia) and an increased likelihood of clinical surveillance or hospitalization could be considered for antenatal corticosteroid administration in order to improve fetal lung maturation and neonatal outcome. The model conservatively accounts for the cost of corticosteroids, without quantifying the associated benefit. As such, in addition to the benefits that may be achieved by reducing unnecessary hospitalization, directed use of corticosteroids may also reduce the risk of neonatal morbidity.
Scenario analysis
The overall expectation of the positive value of the sFlt‐1/PlGF ratio test in terms of reducing costs is robust to plausible changes in the main parameters. The principal effect of the information derived from the test is to reduce hospitalization, and this is the driver of cost savings. Reducing mean length of stay has the effect of reducing the value of the test from £344 to between £265 and £281 (Table 3). Similarly, increasing the proportion of women admitted to hospital also has the effect of reducing expected cost savings. With the exception of the scenario in which admission rates are increased by 10% for women with a sFlt‐1/PlGF ratio of < 38, all the scenarios remain cost saving. This is the key assumption in the analysis. Removing the retest option increases the expected cost saving from £344 to £382.
Table 3.
Results of scenario analyses in which serum fms‐like tyrosine kinase‐1/placental growth factor (sFlt‐1/PlGF) ratio test was used in addition to current diagnostic procedures (test scenario) and in which current diagnostic procedures only were used (no‐test scenario) for guiding management of pre‐eclampsia in a cohort of 1050 women with suspected pre‐eclampsia from the PROGNOSIS study
| Cost (£) | ||||
|---|---|---|---|---|
| No‐test scenario | Test scenario | Cost difference (£) | Cost difference per patient (£) | |
| Variation in LOS | ||||
| Base–case | 4 345 382 | 3 984 200 | −361 182 | −344 |
| LOS scenario A (halved) | 4 024 584 | 3 729 431 | −295 153 | −281 |
| LOS scenario B (1.6 days) | 3 865 839 | 3 587 989 | −277 849 | −265 |
| Percentage admitted to hospital with: | ||||
| Variation in number of admissions | Positive test with sFlt‐1/PlGF ratio > 85 | Positive test with sFlt‐1/PlGF ratio of 38–85 | Negative test with sFlt‐1/PlGF ratio < 38 | Cost difference per patient (£) |
| Base–case | 64.75 | 55.36 | 1.71 | −344 |
| Increase admissions by 10% (proportionately) | 71.23 | 60.90 | 1.88 | −290 |
| Increase admissions by 20% (proportionately) | 77.70 | 66.43 | 2.05 | −235 |
| Increase admissions of patients with a ratio < 38 by 5 percentage points | 64.75 | 55.36 | 6.71 | −139 |
| Increase admissions of patients with a ratio < 38 by 10 percentage points | 64.75 | 55.36 | 11.71 | 56 |
| Cost (£) | ||||
| Variation in option of retest | No‐test scenario per patient | Test scenario per patient | Cost difference per patient (£) | |
| Base–case | £4138 | £3794 | −344 | |
| Exclude option of retest | £4138 | £3756 | −382 | |
LOS, length of stay.
DISCUSSION
Main findings
Measurement of the sFlt‐1/PlGF ratio provides new information that is likely to be valuable for the short‐term prediction of pre‐eclampsia. A ratio of < 38 has a high NPV (99.3%) in ruling out the onset of pre‐eclampsia within 1 week, and this would be expected to lead to a reduction in unnecessary hospitalization in women with a clinical suspicion of pre‐eclampsia, but with no definitive diagnosis15. Our analysis of the PROGNOSIS patient‐level data shows that more than one‐third (36%) of women presenting for assessment with suspected pre‐eclampsia were admitted to hospital; however, the majority of these women did not subsequently develop pre‐eclampsia. The economic analysis quantified the impact of implementing a step‐down care approach for suspected pre‐eclampsia, taking into account the high NPV for pre‐eclampsia developing within 1 week of the sFlt‐1/PlGF ratio test.
The economic analysis suggests that introduction of the test could reduce the number of women hospitalized by more than half (56%), from 36% to 16%. The exact size of the reduction in hospitalization would depend on a number of local factors, but the general conclusion is robust to changes in all the main parameters of the economic model. The reduction in hospitalization is associated with a net saving of £344 in the base–case analysis; the additional cost of the test is more than offset by a saving in inpatient resource use.
The base–case analysis includes an option to retest women who initially tested negative (sFlt‐1/PlGF ratio < 38) 2 weeks after the initial test. In the PROGNOSIS study, the proportion of women who tested negative at baseline and had not developed pre‐eclampsia but still exhibited signs and symptoms 2 weeks after the initial test was high (59%). Of those women whose retest ratio was > 38 2 weeks after the initial test, 35.5% subsequently developed pre‐eclampsia. The retest identified around 10 women (from a study cohort of 1050) at high risk of pre‐eclampsia who went on to develop the condition. Including the retest option resulted in cost savings of £344 per woman compared with £382 without retesting.
Strengths and limitations of the study
This analysis was based on data on management and resource use, collected from a large observational study (PROGNOSIS); as such, the findings of the analysis are likely to reflect real‐world clinical practice. Moreover, consistency with clinical consensus and NICE guidelines is a strength of the analysis. Scenario analyses to determine the robustness of base–case assumptions indicated that the results were sensitive to both length of stay and the percentage of women hospitalized with a negative test result (sFlt‐1/PlGF ratio < 38). However, the conclusions of the analysis remain robust to plausible changes in model parameters.
The main limitation of the analysis is the absence of a randomized interventional study on the actual impact of the test information to rule out pre‐eclampsia within 1 week in clinical practice. Although the model can simulate the effect of the most likely outcomes under a range of plausible assumptions, further research may be required to quantify the value of the sFlt‐1/PlGF ratio test information more accurately in routine practice.
A limitation from the perspective of the UK healthcare system is that PROGNOSIS includes data from countries other than the UK, whose protocols may differ from those of the UK. However, management is expected to be similar in the UK to that in other countries, and indeed, in PROGNOSIS, a higher overall hospitalization rate was observed in the UK study center. Overall, therefore, the limitations of the model are not expected to impact significantly on the findings of the study.
Interpretation in light of other evidence
The impact of the sFlt‐1/PlGF ratio test as an aid to diagnosis and clinical decision‐making has been investigated in Austria and Germany in the PreOS study, where the test is in routine clinical use in accordance with their local guidelines10, 11, 12, 19. The cut‐off ratio of 85 was considered in PreOS to confirm the diagnosis of pre‐eclampsia and inform management of women with suspected pre‐eclampsia. The change in clinical management observed in PreOS was consistent with the assumptions made in this analysis of the PROGNOSIS data, in which it was assumed that patients with sFlt‐1/PlGF ratios indicating low risk would be managed less intensively than patients who were indicated to be at moderate/high risk. Resource savings based on the use of the test as an aid to diagnosis were reported previously in a UK‐based analysis published in 2010, which reported savings of £945 per patient using the sFlt‐1/PlGF ratio test20.
A consensus publication from 2015 stated that, in women with a particularly high sFlt‐1/PlGF ratio, there was an association with a need to deliver the infant within 48 h, and thus close surveillance and prompt initiation of corticosteroids were strongly recommended16. These data emphasize the clinical need to identify accurately women at high risk of complications from pre‐eclampsia, as well as the economic need to reduce unnecessary hospitalization of women at low risk.
NICE assessed PlGF‐based testing to help diagnose suspected pre‐eclampsia21. The economic model showed cost reductions per patient compared with standard clinical assessment of £2488 for the Elecsys immunoassay sFlt‐1/PlGF ratio for women presenting with suspected pre‐eclampsia before 35 weeks' gestation, and NICE diagnostic guidance recommends the Elecsys immunoassay sFlt‐1/PlGF ratio to help rule out pre‐eclampsia and avoid unnecessary hospital admissions21. The NICE assessment focused on women with suspected pre‐eclampsia before 35 weeks' gestation (the PROGNOSIS study and the present analysis included women with suspected pre‐eclampsia between 24 and 36 + 6 weeks' gestation) and also included different sources for clinical inputs and costs of NICU stay, resulting in greater cost reductions than those found in the current analysis. The NICE assessment confirms in a separately developed model the cost‐saving potential of the sFlt‐1/PlGF ratio test in women with suspected pre‐eclampsia.
Conclusion
This study demonstrates that use of the sFlt‐1/PlGF ratio test in the UK may lead to a reduction in unnecessary hospitalization for women with suspected pre‐eclampsia, resulting in substantial cost savings.
Supporting information
Table S1 Clinical algorithm used in the model
Table S2 Treatment costs to prevent complications in women with pre‐eclampsia, stratified by management intensity
ACKNOWLEDGMENTS
We thank John Posnett of PAREXEL for advice and input into the economic model and Fiona Sheppard, Michael Tang and John Posnett (all with PAREXEL) for writing support. PAREXEL provided consultancy services funded by Roche Diagnostics. Elecsys and Cobas are trademarks of Roche Diagnostics. C.W., T.S.M., M.H. and D.A. are employees of Roche Diagnostics.
REFERENCES
- 1. National Institute for Health and Care Excellence (NICE) . Hypertension in pregnancy (NICE clinical guideline 107). NICE, 2011. [Google Scholar]
- 2. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre‐eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25: 391–403. [DOI] [PubMed] [Google Scholar]
- 3. Liu A, Wen SW, Bottomley J, Walker MC, Smith G. Utilization of health care services of pregnant women complicated by preeclampsia in Ontario. Hypertens Pregnancy 2009; 28: 76–84. [DOI] [PubMed] [Google Scholar]
- 4. Delahaije DH, Smits LJ, van Kuijk SM, Peeters LL, Duvekot JJ, Ganzevoort W, Oudjik MA, van Pampus MG, Scheepers HC, Spaanderman ME, Dirksen CD. Care‐as‐usual provided to formerly preeclamptic women in the Netherlands in the next pregnancy: health care consumption, costs and maternal and child outcome. Eur J Obstet Gynecol Reprod Biol 2014; 179: 240–245. [DOI] [PubMed] [Google Scholar]
- 5. Shennan AH, Redman C, Cooper C, Milne F. Are most maternal deaths from pre‐eclampsia avoidable? Lancet 2012; 379: 1686–1687. [DOI] [PubMed] [Google Scholar]
- 6. Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, Calda P, Holzgreve W, Galindo A, Engels T, Denk B, Stepan H. The sFlt‐1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol 2012; 206: 58.e1–8. [DOI] [PubMed] [Google Scholar]
- 7. Schiettecatte J, Russcher H, Anckaert E, Mees M, Leeser B, Tirelli AS, Fiedler GM, Luthe H, Denk B, Smitz J. Multicenter evaluation of the first automated Elecsys sFlt‐1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem 2010; 43: 768–770. [DOI] [PubMed] [Google Scholar]
- 8. Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, Pape J, Dudenhausen JW, Denk B, Stepan H. An automated method for the determination of the sFlt‐1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol 2010; 202: 161.e1–161.e11. [DOI] [PubMed] [Google Scholar]
- 9. Verlohren S, Herraiz I, Lapaire O, Schlembach D, Zeisler H, Calda P, Sabria J, Markfeld‐Erol F, Galindo A, Schoofs K, Denk B, Stepan H. New gestational phase‐specific cutoff values for the use of the soluble fms‐like tyrosine kinase‐1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014; 63: 346–352. [DOI] [PubMed] [Google Scholar]
- 10. Hund M, Verhagen‐Kamerbeek W, Reim M, Messinger D, van der Does R, Stepan H. Influence of the sFlt‐1/PlGF ratio on clinical decision‐making in women with suspected preeclampsia – the PreOS study protocol. Hypertens Pregnancy 2015; 34: 102–115. [DOI] [PubMed] [Google Scholar]
- 11. Klein E, Schlembach D, Ramoni A, Langer E, Bahlmann F, Grill S, Schaffenrath H, van der Does R, Messinger D, Verhagen‐Kamberbeek WD, Reim M, Hund M, Stepan H. PreOS (Preeclampsia Open Study), a multicenter, prospective, non‐interventional study evaluating the influence of the sFlt‐1/PlGF ratio on physician decision‐making in pregnant women with suspicion of preeclampsia. 20th World Congress on Controversies in Obstetrics, Gynecology and Infertility; 2014, Paris. Abstract P54. [Google Scholar]
- 12. Klein E, Schlembach D, Ramoni A, Langer E, Bahlmann F, Grill S, Schaffenrath H, van der Does R, Messinger D, Verhagen‐Kamerbeek WD, Reim M, Hund M, Stepan H. Influence of the sFlt‐1/PlGF ratio on clinical decision‐making in women with suspected preeclampsia. PLoS One 2016; 11: e0156013. doi: 10.1371/journal.pone.0156013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hund M, Allegranza D, Schoedl M, Dilba P, Verhagen‐Kamerbeek W, Stepan H. Multicenter prospective clinical study to evaluate the prediction of short‐term outcome in pregnant women with suspected preeclampsia (PROGNOSIS): study protocol. BMC Pregnancy Childbirth 2014; 14: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verlohren S, Llurba E, Chantraine F, Vatish M, Staff A, Sennström M, Olovsson M, Brennecke S, Stepan H, Allegranza D, Schoedl M, Dilba P, Hund M, Zeisler H. The sFlt‐1/PlGF ratio can rule out preeclampsia up to four weeks in women with suspected preeclampsia. ISSHP XX World Congress; 2016, Sao Paulo, Brazil. Submitted abstract. [Google Scholar]
- 15. Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Dilba P, Schoedl M, Hund M, Verlohren S. Predictive value of the sFlt‐1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 2016; 374: 13–22. [DOI] [PubMed] [Google Scholar]
- 16. Stepan H, Herraiz I, Schlembach D, Verlohren S, Brennecke S, Chantraine F, Klein E, Lapaire O, Llurba E, Ramoni A, Vatish M, Wertaschnigg D, Galindo A. Implementation of the sFlt‐1/PlGF ratio for prediction and diagnosis of pre‐eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound Obstet Gynecol 2015; 45: 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. NHS. PbR tariff information spreadsheet for 2013–2014. https://www.gov.uk/government/publications/payment‐by‐results‐pbr‐operational‐guidance‐and‐tariffs [Accessed July 2016].
- 18. Office for National Statistics. Births in England and Wales , 2014. http://www.ons.gov.uk/ons/dcp171778_410897.pdf [Accessed October 2015].
- 19. DGGG . Diagnostik und Therapie hypertensiver Schwangerschaftserkrankungen. http://www.awmf.org/uploads/tx_szleitlinien/015‐018l_S1_Diagnostik_Therapie_hypertensiver_Schwangerschaftserkrankungen_2014‐verlaengert.pdf [Accessed July 2016].
- 20. Hadker N, Garg S, Costanzo C, Miller JD, Foster T, van der Helm W, Creeden J. Financial impact of a novel pre‐eclampsia diagnostic test versus standard practice: a decision‐analytic modeling analysis from a UK healthcare payer perspective. J Med Econ 2010; 13: 728–737. [DOI] [PubMed] [Google Scholar]
- 21. National Institute for Health and Care Excellence (NICE) . PlGF‐based testing to help diagnose suspected pre‐eclampsia (Triage PlGF test, Elecsys immunoassay sFlt‐1/PlGF ratio, DELFIA Xpress PlGF 1‐2‐3 test, and BRAHMS sFlt‐1 Kryptor/BRAHMS PlGF plus Kryptor PE ratio): NICE diagnostics guidance [DG23]. 2016. https://www.nice.org.uk/guidance/dg23/chapter/1‐Recommendations [Accessed May 2016].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Clinical algorithm used in the model
Table S2 Treatment costs to prevent complications in women with pre‐eclampsia, stratified by management intensity


