Abstract
Sea urchins are broadly recognised as a delicacy and their quality as food for humans is highly influenced by their diet. Lipids in general and the long-chain polyunsaturated fatty acids (LC-PUFA) in particular, are essential nutrients that determine not only the nutritional value of sea urchins but also guarantee normal growth and reproduction in captivity. The contribution of endogenous production (biosynthesis) of LC-PUFA in sea urchins remained unknown. Using Paracentrotus lividus as our model species, we aimed to characterise both molecularly and functionally the repertoire of fatty acyl desaturases (Fads), key enzymes in the biosynthesis of LC-PUFA, in sea urchins. Three Fads, namely FadsA, FadsC1 and FadsC2, were characterised. The phylogenetic analyses suggested that the repertoire of Fads within the Echinodermata phylum varies among classes. On one hand, orthologues of the P. lividus FadsA were found in other echinoderm classes including starfishes, brittle stars and sea cucumbers, thus suggesting that this desaturase is virtually present in all echinoderms. Contrarily, the FadsC appears to be sea urchin-specific desaturase. Finally, a further desaturase termed as FadsB exists in starfishes, brittle stars and sea cucumbers, but appears to be missing in sea urchins. The functional characterisation of the P. lividus Fads confirmed that the FadsA was a Δ5 desaturase with activity towards saturated and polyunsaturated fatty acids (FA). Moreover, our experiments confirmed that FadsA plays a role in the biosynthesis of non-methylene interrupted FA, a group of compounds typically found in marine invertebrates. On the other hand, both FadsC desaturases from P. lividus showed Δ8 activity. The present results demonstrate that P. lividus possesses desaturases that account for all the desaturation reactions required to biosynthesis the physiological essential eicosapentaenoic and arachidonic acids through the so-called “Δ8 pathway”.
Introduction
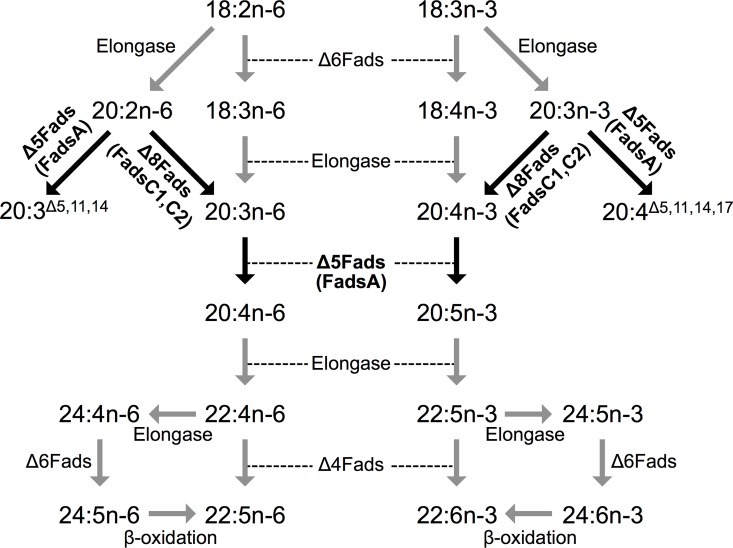
Long-chain (C20-22) polyunsaturated fatty acids (LC-PUFA) have been identified as essential components of biomembranes of all cells and tissues, and have important roles in growth and ontogenesis, particularly in development of the nervous system [1–2]. In addition, LC-PUFA have also key roles in inflammatory response and consequently in metabolic disorders, cardiovascular conditions and neurological diseases [3–5]. It is well known that vertebrates have some ability to biosynthesise LC-PUFA but are unable to endogenously produce their precursors, more specifically the C18 polyunsaturated fatty acids (PUFA) linoleic acid (18:2n-6, LA) and α-linolenic acid (18:3n-3, ALA). Consequently, diets for vertebrates must supply the C18 PUFA that are subsequently converted into the physiological important LC-PUFA, namely arachidonic acid (20:4n-6, ARA), eicosapentaenoic acid (20:5n-3, EPA) and docosahexaenoic acid (22:6n-3, DHA) [6]. The vertebrate LC-PUFA biosynthetic pathways consist of sequential reactions converting the dietary essential C18 PUFA into C20-22 LC-PUFA through the action of enzymes termed as fatty acyl desaturases (Fads) and elongation of very long-chain fatty acids proteins (Elovl) (Fig 1). Fads are key enzymes that mediate the introduction of an unsaturation (double bond) into a fatty acyl chain, while Elovl catalyse the condensation reaction within the elongation pathway resulting in the addition of two carbons into the fatty acid (FA) substrate [7–8].
Fig 1. Biosynthetic pathways of LC-PUFA from C18 PUFA precursors accepted in vertebrates.
Reactions catalysed by fatty acyl desaturases are designated as “Δx” (Δ6, Δ5, Δ4 and Δ8), whereas elongation reactions are indicated as “elongase”.
Unlike vertebrates, the biosynthetic pathways of LC-PUFA in marine invertebrates remain poorly understood [9]. Pioneer studies conducted in the common octopus Octopus vulgaris [10,11] and thereafter in the cephalopod Sepia officinalis [12], the gastropod Haliotis discus hannai [13] and the bivalve Chlamys nobilis [14–16] have confirmed that molluscs and potentially other marine invertebrates possess Fads and Elovl enzymes involved in the LC-PUFA biosynthesis. Additionally, the ability to synthesise non-methylene interrupted (NMI) FA, a group of PUFA with particular double bond distribution [17], was observed in several Fads enzymes from marine invertebrate species [10,12].
A recent study on Fads and Elovl repertoire among several classes of molluscs including cephalopods, gastropods and bivalves [18] highlighted that lineage-specific gene duplication events accounted for the presence of one or more Fads copies among the mollusc classes under investigation. These results further evidenced that the evolutionary history of genes involved in the LC-PUFA biosynthetic pathways of invertebrates, particularly the Fads-like desaturases, might differ markedly from those of vertebrates [6,12,19]. Clearly, marine invertebrates emerge as promising sources of LC-PUFA biosynthetic enzymes with potentially novel functionalities amenable for biotechnological production of n–3 oils [20].
Some invertebrate groups and specific species among them are becoming increasingly popular model species for comparative genomics and evolutionary developmental biology [21–23]. As a result, ever-increasing genomic data on certain species are becoming available. Among echinoderms, there exist genome projects developed for the sea urchin Strongylocentrotus purpuratus [24] and Lytechinus variegatus [25], and this offers us a unique opportunity to provide a comprehensive characterisation of key protein families involved in the biosynthesis of LC-PUFA in commercially interesting species such as Paracentrotus lividus (Lamark, 1816). P. lividus is a herbivorous sea urchin species that is commonly known as the Atlanto-Mediterranean sea urchin, due to its habitats comprising the sublittoral zone to 20 m in both the Mediterranean Sea [26] and throughout West coasts of continental Europe to Ireland and Scotland, UK [27]. Gonads from P. lividus are recognised as a high value seafood and delicacy [28], and thus P. lividus has been studied in different countries such as UK, Ireland and The Netherlands [29–31]. Recently comprehensive research has been conducted to optimise P. lividus culture, through assessing the nutritional requirements as a key aspect to fully develop a culture protocol for this species [32–35]. It has been reported that the growth and gonadal development are greatly affected by feed abundance and quality [36] and that adequate food supply during early development ensures optimal sexual maturation [31,33]. In addition, n-3 LC-PUFA such as EPA and DHA showed beneficial effect on early development of P. lividus [37]. Clearly, understanding the ability of P. lividus to endogenously produce essential LC-PUFA ensuring normal growth and reproduction is required to adequately formulate balanced diets. Therefore, it is important to elucidate the functions and substrate specificities of the endogenous enzymes that are responsible for the LC-PUFA biosynthesis. To the best of our knowledge, the specific Fads repertoire and functionalities of sea urchins remains to be investigated. Thus, the aim of this study was to identify the whole set of Fads-like desaturases found in P. lividus and characterise their function in yeast. The results will also provide valuable information on the evolution of the Fads family, and better understanding of LC-PUFA biosynthesis in sea urchin and, in extension, other echinoderms.
Materials and Methods
Molecular cloning and phylogenetic analysis of novel fatty acyl desaturases from P. lividus
Fresh gonad, intestine and tube feet samples were collected from a P. lividus specimen from Ardtoe Marine Research Facility (Scotland, UK), and conserved in RNAlater (Thermo Fisher Scientific, Waltham, MA, USA) until further use. The total RNA was extracted from the selected tissues (~100 mg) using TRI Reagent (Sigma-Aldrich, Dorset, UK) following the manufacturer’s instructions. Complementary DNA (cDNA) were synthesised from 1 μg of total RNA using High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) following the manufacturer’s instructions.
In order to amplify the first fragment of P. lividus Fads cDNA, we retrieved several Fads-like sequences from expressed sequence tags (EST) information of P. lividus available in public databases. After alignment and phylogenetic analysis of obtained sequences, it was possible to distinguish two different Fads types. One of them closely related to several Fads genes characterised from other invertebrate species such as molluscs. Phylogenetic analysis performed by Surm and co-workers [18] indicated that this type of Fads formed a distinct cluster denoted as “clade A”, and consequently we termed the P. lividus Fads homologue as “FadsA”. On the other hand, we termed the other Fads-like sequence retrieved from the EST database search as “FadsC1”, to indicate it belongs to a cluster (“clade C”) different to Clades A or B reported in molluscs [18]. The first fragment of FadsA was amplified by PCR (Klear Taq polymerase, LGC, Teddington, UK) using the tube feet cDNA as template and the primers PLFAF and PLFAR, which were designed to anneal to a consensus sequence derived from EST (NCBI accession No. AM559332, AM220309, AM571147 and AM537908). The PCR amplification was performed with 35 cycles comprising denaturation for 20 s at 95°C, annealing for 20 s at 60°C and extension for 60 s at 72°C. In order to amplify the first fragment of P. lividus FadsC1, a forward primer PLFC1F (Table 1) was also designed to anneal to a consensus sequence derived from EST (NCBI accession No. AM569687, AM566789 and AM569285). Since the sequences obtained from EST databases were lacking most of the 3' region of putative coding sequence of FadsC1, we designed a reverse primer using consensus sequence derived from other sea urchin species in order to amplify a longer fragment. A reverse primer URCHFC1R (Table 1) was designed to anneal to a consensus sequence of FadsC1 homologues obtained from genomic and transcriptomic data of several sea urchin species (S. purpuratus NW_011995688, Evechinus chloroticus GAPB01052974, L. variegatus GAUR01019274, Sphaerechinus granularis GAVR01046553). The first fragment of FadsC1 was amplified from a mixture of the intestine and gonad cDNA by GoTaq® Colorless Master Mix (Promega, Madison, WI, USA). The PCR amplification was performed with 35 cycles comprising denaturation for 30 s at 95°C, annealing for 30 s at 55°C and extension for 60 s at 72°C. The amplified fragments were purified on agarose gels using IllustraTM GFXTM PCR DNA and Gel Band Purification Kit (GE Healthcare Life Sciences, Buckinghamshire, UK). The purified PCR products were then sequenced (GATC Biotech: DNA Sequencing and Bioinformatics, Konstanz, Germany).
Table 1. Sequences of the primer pairs used in the molecular cloning and functional characterisation in yeast of the Paracentrotus lividus fatty acyl desaturases (FadsC1, FadsA and FadsC2).
Restriction sites for HindIII and XbaI are underlined (AAGCTT and TCTAGA, respectively).
| Aim | Target | Primer | Primer sequence |
|---|---|---|---|
| First fragment | FadsA | PLFAF | 5'-TCACGCAGTGGGCCAAGAGACA-3' |
| PLFAR | 5'-ACAGAAGAGGGGGTCCAATGAGGA-3' | ||
| FadsC1 | PLFC1F | 5'-GCGTGAGTCATAACAAGCCA-3' | |
| URCHFC1R | 5'-TAGCAAGATTGTGTCTCGGCAT-3' | ||
| 5' RACE PCR | FadsA | PLFAR1 | 5'-GGAAGGTCCACCAACAGAATCCATA-3' |
| PLFAR2 | 5'-TCTTTGGCGATCTGCCCAATGTGGA-3' | ||
| FadsC1 | PLFC1R1 | 5'-GCGCGGTGTCATCAAGGTT-3' | |
| PLFC1R2 | 5'-TTGACTCACTGGAGCGATGAC-3' | ||
| 3' RACE PCR | FadsA | PLFAF1 | 5'-GCATGGCACACTACAGGCTCAGGT-3' |
| PLFAF2 | 5'-TCATTGGACCCCCTCTTCTGTTTC-3' | ||
| FadsC1 | PLFC1F1 | 5'-TCTTCAGATGCATGCCACGTGTA-3' | |
| PLFC1F2 | 5'-ACCTGGAGTCTTCTCTTTTCATTG-3' | ||
| ORF cloning | FadsA | PLFAVF | 5'-CCCAAGCTTACGATGGGTCTGGGAG-3' |
| PLFAVR | 5'-CCGTCTAGATTAGTCCGTTGAATACTGGT-3' | ||
| FadsC1 | PLFC1VF | 5'-CCCAAGCTTACAATGTGGACGATTAGAGA-3' | |
| PLFC1VR | 5'-CCGTCTAGACTACCCTACATAAGCTCCTA-3' | ||
| FadsC2 | PLFC2VF | 5'-CCCAAGCTTACGATGTGCAAGAAGGAAGATCTCT-3' | |
| PLFC2VR | 5'-CCGTCTAGATCAATGGTCGCCTGCAGGATCTA-3' |
The DNA sequences of the first fragments of FadsA and FadsC1 cDNAs were used to design primers for 5' and 3' Rapid Amplification of cDNA Ends (RACE) (Table 1). The RACE cDNAs were synthesised using the SMART RACE kit (Takara Bio USA, Inc., Mountain View, CA, USA) from the tube feet RNA and the FirstChoice® RLM-RACE Kit (Thermo Fisher Scientific) from the intestine and gonad RNA. Regarding the RACE PCR for FadsA, all reactions including first and second (nested) rounds were carried out with 30 cycles comprising denaturation for 20 s at 95°C, annealing for 20 s at 60°C and extension for 180 s at 72°C using adequate primers (Table 1) and the adapter primers supplied with the kit. Regarding FadsC1, both first and nested 5' RACE were carried out with 35 cycles comprising denaturation for 30 s at 95°C, annealing for 30 s at 55°C and extension for 120 s at 72°C using adequate primer sets (Table 1) and the adapter primers. Both first and second round 3' RACE PCRs were carried out with 35 cycles comprising denaturation for 30 s at 95°C, annealing for 30 s at 58°C and extension for 90 s at 72°C using adequate primer sets (Table 1) and the adapter primers. The nested PCR products were purified and then sequenced as described above.
After cloning of the full-length cDNA of FadsA and FadsC1, P. lividus transcriptome shotgun assembly was released in NCBI (http://www.ncbi.nlm.nih.gov) and this allowed us to screen other possible Fads-like transcripts in P. lividus. Thus, we obtained full-length open reading frame (ORF) sequence of another Fads-like sequence from the assembly (accession no. GCZS01077556). Since preliminary phylogenetic analysis suggested this Fads was closely related to FadsC1, we termed this desaturase as “FadsC2”.
The deduced amino acid (aa) sequences of P. lividus desaturases FadsA, FadsC1 and FadsC2 were aligned with homologous Fads sequences from various species following ClustalW algorithm. The phylogenetic tree of deduced aa sequences was constructed using the neighbour-joining method [38] with confidence in the resulting tree branch topology measured by bootstrapping through 1,000 iterations. All the sequencing analyses were carried out using CLC Main Workbench 7 (CLC bio, Aarhus, Denmark).
Functional characterisation of newly cloned Fads from P. lividus
The full-length ORF sequences of the P. lividus FadsA, FadsC1 and FadsC2 were amplified from the intestine cDNA using the high fidelity Pfu DNA polymerase (Promega). All the PCR amplifications were carried out with 35 cycles comprising denaturation for 30 s at 95°C, annealing for 30 s at 55°C and extension for 180 s at 72°C. The primer pairs contained restriction sites for HindIII (forward) and XbaI (reverse) for further cloning into the yeast expression vector pYES2 (Thermo Fisher Scientific). The PCR products were digested with HindIII and XbaI and then ligated into the similarly restricted pYES2 vector using T4 DNA ligase (Promega). Subsequently, the ligation reactions were transformed into competent E. coli JM109 cells (Promega). The plasmid constructs pYES2-FadsA, pYES2-FadsC1 and pYES2-FadsC2 were prepared (Gen EluteTM Plasmid Miniprep Kit, Sigma-Aldrich) and sequenced prior being transformed into competent yeast INvSc1 cells (Thermo Fisher Scientific) using S.c. EasyComp Transformation kit (Thermo Fisher Scientific). Selection of successful transformants and culture of transgenic yeast were performed as described in previous studies [10,11].
One single yeast colony transformed with either the P. lividus FadsA, FadsC1 or FadsC2 was used in each functional assay [10,11]. In order to investigate the ability of the P. lividus FadsA, FadsC1 and FadsC2 to desaturate PUFA, the transgenic yeast were grown in the presence of Δ6 (18:3n-3 and 18:2n-6), Δ8 (20:3n-3 and 20:2n-6), Δ5 (20:4n-3 and 20:3n-6) and Δ4 (22:5n-3 and 22:4n-6) substrates. Additionally, the transgenic yeast were also grown in the presence of 20:1n-9 to assess the ability of the P. lividus desaturases to biosynthesise NMI FAs. All FA substrates (>98–99% pure) used for the functional characterisation assays were obtained from Nu-Chek Prep, Inc. (Elysian, MN, USA), except 20:4n-3 that was purchased from Cayman Chemical Co. (Ann Arbor, USA). Since the newly cloned P. lividus Fads could potentially operate towards yeast endogenous saturated or monounsaturated FA, we further compared the FA profiles of yeast transformed with either pYES2-FadsA, pYES2-FadsC1 or pYES2-FadsC2 with that of yeast transformed with the empty pYES2 (control), and grown in all cases in the absence of exogenously added PUFA substrates. Each PUFA substrate was supplied to the yeast cultures in concentrations corresponding to 0.5 (C18), 0.75 (C20) and 1 mM (C22) as uptake efficiency decreases with increasing chain length [12].
Fatty acid analysis
Total lipids were extracted from yeast samples according to [39] and modifications as described in [10]. Fatty acid methyl esters (FAME) were prepared from total lipids as described previously [40]. FAME were quantified and identified using a Fisons GC-8160 (Thermo Fisher Scientific) gas chromatograph equipped with a 60 m x 0.32 mm i.d. x 0.25 μm ZB-wax column (Phenomenex, Cheshire, UK) and flame ionisation detector [41]. The desaturase conversions from exogenously added PUFA substrates were calculated from the fraction of FA substrate transformed to desaturase FA products as [product areas / (product areas + substrate area)] x 100. In order to determine the double bond positions of unusual FAs, the fatty acid 4,4-dimethyloxazoline (DMOX) derivatives were prepared from FAMEs. Briefly, the dried-up FAME samples were incubated over-night with 2-amino-2-methyl-1-propanol at 150°C. After cooling, the DMOX derivatives were extracted by adding 5 mL of distilled water and 5 mL diethyl ether-isohexane (1:1, v/v). The organic phase was transferred to fresh tubes and then evaporated under the stream of oxygen-free nitrogen. Subsequently, the dried-up DMOX samples were resuspended with isohexane. The DMOX derivatives were identified and quantified using a gas chromatograph (GC8035) equipped with a 30 m x 0.32 mm i.d. x 0.25 μm ZB-wax column (Phenomenex) and coupled to an MD800 mass spectrometer (ThermoFisher Scientific).
Results
P. lividus Fads sequences and phylogenetics
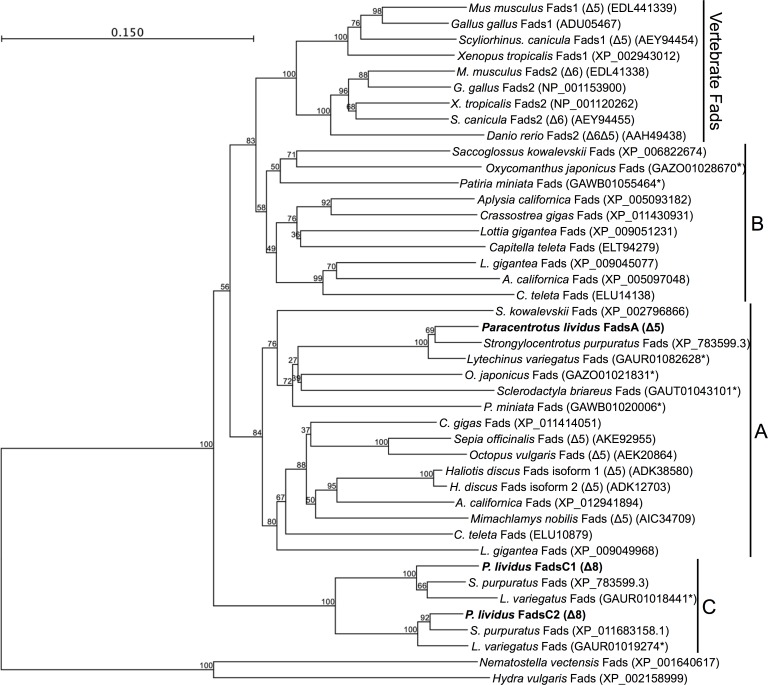
The ORF of the newly cloned P. lividus FadsA (GenBank accession number KY216020), FadsC1 (KY216021) and FadsC2 (KY216022) consisted of 1365 bp, 1374 bp and 1353 bp, respectively, encoding putative proteins of 454 aa, 457 aa and 450 aa, respectively. All three desaturases had representative domains of the “front-end” desaturases, including three histidine boxes (HXXXH, HXXHH and QXXHH), a putative cytochrome b5-like region and a heme-binding motif (HPGG) (Fig 2). The aa identity of P. lividus desaturases varied between FadsA and FadsC1 (45% identical) or FadsC2 (45%), and between FadsC1 and FadsC2 (64%). Phylogenetic analysis revealed that each P. lividus Fads protein was closely related to orthologues found in the sea urchin species S. purpuratus and L. variegatus (Fig 3). Compared with the three Fads-like desaturases found in the genome of S. purpuratus, the three P. lividus desaturases showed high aa sequence identity, namely 81% (FadsA vs XP_783599.3), 85% (FadsC1 vs XP_783599.3), and 85% (FadsC2 vs XP_011683158.1). Moreover, FadsA clustered with Fads from several molluscs that have all been functionally characterised as Δ5 desaturases (Fig 3, clade A) [10,12–14]. In contrast to FadsA, the desaturases FadsC1, FadsC2 and Fads-like sequences from other sea urchin species uniquely formed a distinct clade from other Fads (Fig 3, clade C). Interestingly, Fads-like sequences from some echinoderm species of starfish and sea lilies formed a distinct group denoted as “clade B” in Fig 3. Among all three types of Fads identified in echinoderms, Fads within clade B (FadsB) are the most closely related to vertebrate Fads, although this orthologue appears to be absent in the genomes of S. purpuratus and L. variegatus and was not found either in P. lividus transcriptomic databases. These results suggest that clade B desaturases do not exist in echinoids.
Fig 2. Comparison of the deduced amino acid sequence of the Paracentrotus lividus desaturases with those of mice (Mus musculus), zebrafish (Danio rerio) and common octopus (Octopus vulgaris).
Identical sequences are shaded black. All the Fads sequence from P. lividus contained typically conserved regions in members of the front-end desaturase family, including three histidine boxes (HXXXH, HXXHH and QXXHH), a putative cytochrome b5-like region (marked as a dotted line) and a heme-binding motif (HPGG).
Fig 3. Phylogenetic tree comparing the deduced amino acid (aa) sequence of the Paracentrotus lividus fatty acyl desaturases (Fads) with other Fads-like sequences from different organisms (vertebrates and invertebrates).
FadsA, FadsC1 and FadsC2 are highlighted in black. The tree was constructed using the Neighbour Joining method [38]. The horizontal branch length is proportional to aa substitution rate per site. The numbers represent the frequencies (%) with which the tree topology presented was replicated after 1,000 iterations. Asterisks denote deduced aa sequences derived from transcriptome shotgun assembly (TSA) from several echinoderm species.
Functional characterisation of the P. lividus Fads in yeast
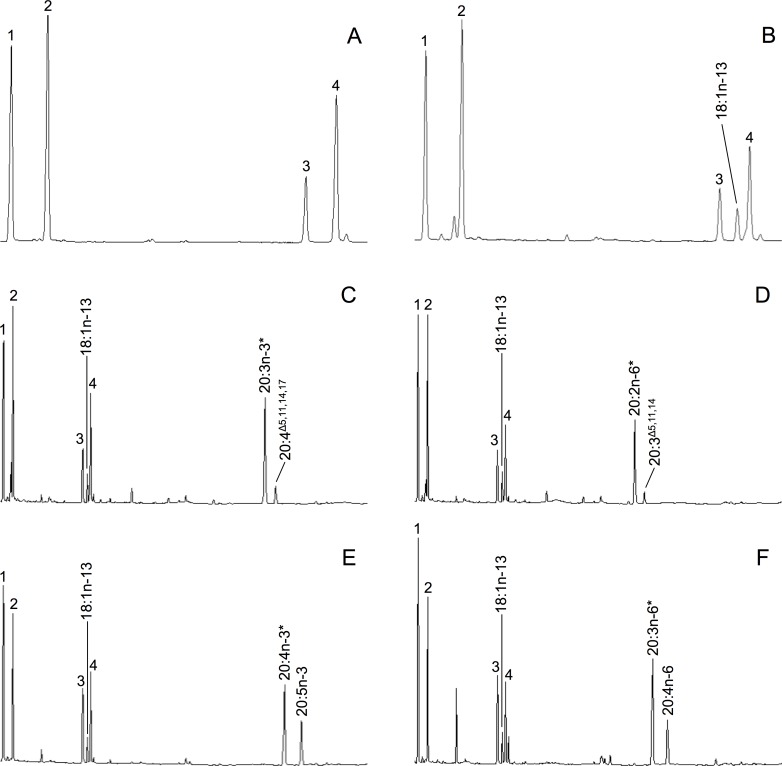
The activity of the P. lividus desaturases (FadsA, FadsC1 and FadsC2) was determined by expression of their ORF in yeast S. cerevisiae that were grown in presence of potential FA substrates. The FA profiles of yeast transformed with the empty vector (control), typically showed the endogenous S. cerevisiae FA, namely 16:0, 16:1 isomers (16:1n-9 and 16:1n-7), 18:0, 18:1 isomers (18:1n-9 and 18:1n-7) (Fig 4A). When additional PUFA substrates were exogenously supplied, these remained unmodified by control yeast confirming that the S. cerevisiae yeast strain INvSc1 lack Δ4, Δ5, Δ6 and Δ8 desaturase activities [42].
Fig 4. Functional characterisation of FadsA from Paracentrotus lividus in transformed yeast Saccharomyces cerevisiae.
Additional 18:1n-13 peak was observed in all the yeast transformed with pYES2-FadsA (B–F) compared with the yeast transformed with empty pYES2 vector (A). The yeast transformed with pYES2-FadsA were also grown in the presence of fatty acid (FA) substrates (indicated by asterisk), namely 20:3n-3 (C), 20:2n-6 (D), 20:4n-3 (E) and 20:3n-6 (F). Desaturation products are indicated accordingly in each panel. Peaks 1–4 in all panels are the main endogenous FA of S. cerevisiae, namely 16:0 (1), 16:1 isomers (2), 18:0 (3), 18:1n-9 (4). Vertical axis, FID response; horizontal axis, retention time.
The capability of P. lividus Fads to desaturate saturated FA was studied by comparing the FA profiles of yeast transformed with pYES2-FadsA, pYES2-FadsC1 and pYES2-FadsC2, with that of control yeast. The FA profiles from yeast transformed with either pYES2-FadsC1 or pYES2-FadsC2 and grown in the absence of exogenously added PUFA substrates, showed no additional peaks compared to controls. However, the FA profiles of yeast expressing the P. lividus FadsA had an additional peak corresponding to the Δ5-desaturated monoene 18:1n-13 (Fig 4B). The MS peak of a DMOX derivative of 18:1n-13 contained a diagnostic ion at m/z = 153, which shows a double bond at the Δ5 position (S1A Fig) and also matched the spectra presented in the AOCS lipid library website (http://lipidlibrary.aocs.org). Such Δ5 desaturation product from 18:0 occurred in all samples of yeast transformed with pYES2-FadsA, regardless the exogenously added PUFA substrate (data not shown). Conversion of 18:0 into 18:1n-13 was 32% (Table 2).
Table 2. Substrate conversions of yeast Saccharomyces cerevisiae transformed with pYES2 containing the open reading frame (ORF) of the Paracentrotus lividus desaturases (FadsC1, FadsA and FadsC2).
Results are expressed as a percentage of total fatty acid (FA) substrate converted to desaturated products, with the corresponding activity (Δx) detected also shown. FA are designated using the ‘n-‘ nomenclature, except for the non-methylene interrupted FA produced from 20:3n-3 and 20:2n-6 where the ‘Δ’ nomenclature is used.
| FA substrates | Product | Conversion rate (%) | Activity | ||
|---|---|---|---|---|---|
| FadsA | FadsC1 | FadsC2 | |||
| Saturate | |||||
| 18:0 | 18:1n-13 | 32 | nd | nd | Δ5 |
| Polyunsaturates | |||||
| 18:3n-3 | 18:4n-3 | nd | nd | nd | Δ6 |
| 18:2n-6 | 18:3n-6 | nd | nd | nd | Δ6 |
| 20:3n-3 | 20:4n-3 | nd | 4 | 42 | Δ8 |
| 20:4Δ5,11,14,17 | 13 | nd | nd | Δ5 | |
| 20:2n-6 | 20:3n-6 | nd | 2 | 38 | Δ8 |
| 20:3Δ5,11,14 | 12 | nd | nd | Δ5 | |
| 20:4n-3 | 20:5n-3 | 37 | nd | nd | Δ5 |
| 20:3n-6 | 20:4n-6 | 30 | nd | nd | Δ5 |
| 22:5n-3 | 22:6n-3 | nd | nd | nd | Δ4 |
| 22:4n-6 | 22:5n-6 | nd | nd | nd | Δ4 |
nd, not detected
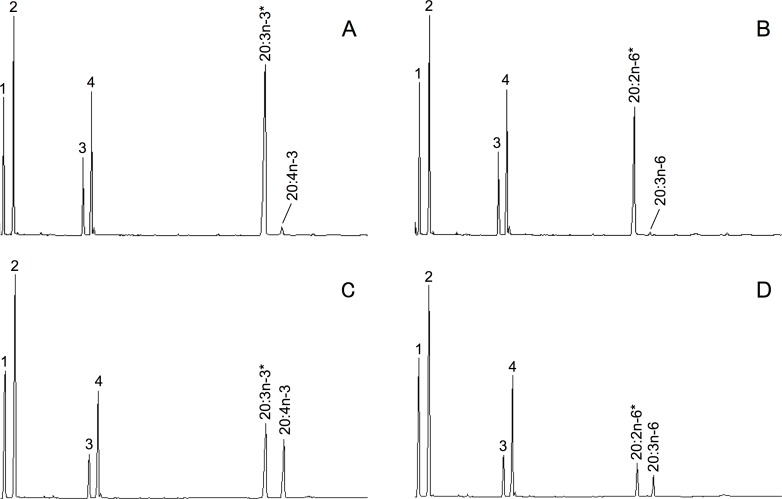
The function of the P. lividus FadsA, FadsC1 and FadsC2 in LC-PUFA biosynthetic pathways was investigated by growing transgenic yeast in the presence of PUFA precursors including 18:3n-3, 18:2n-6, 20:3n-3, 20:2n-6, 20:4n-3, 20:3n-6, 22:5n-3 and 22:4n-6. Transgenic yeast expressing the P. lividus FadsA were able to transform 20:4n-3 and 20:3n-6 into the corresponding Δ5 products, namely 20:5n-3 (EPA) and 20:4n-6 (ARA), respectively (Fig 4E and 4F). In addition, transgenic yeast carrying the P. lividus FadsA were able to desaturate 20:3n-3 and 20:2n-6 into the NMI FA 20:4Δ5,11,14,17 and 20:3Δ5,11,14, respectively (Fig 4C and 4D). The MS of the peaks identified as 20:4Δ5,11,14,17 and 20:3Δ5,11,14 contained a diagnostic ion for Δ5 desaturation at m/z = 153 (S1B and S1C Fig). Thus, 20:4Δ5,11,14,17-DMOX showed the characteristic gaps of 12 atomic mass units (amu) between m/z = 222 and 234, 262 and 274, and 302 and 314, which indicate the presence of the double bonds at Δ11, Δ14 and Δ17 position, respectively (S1B Fig). The MS profile of 20:3Δ5,11,14-DMOX also showed 12 amu between 222 and 234, and 262 and 274, which correspond to the double bonds at Δ11 and Δ14 position, respectively (S1C Fig). No activity towards 18:3n-3, 18:2n-6, 22:5n-3, 22:4n-6 was detected in the FA profiles (Table 2). These results confirmed that FadsA is a Δ5 desaturase. On the other hand, in yeast expressing the P. lividus FadsC1 and FadsC2, additional peaks identified as 20:4n-3 and 20:3n-6 were detected in yeast grown in the presence of 20:3n-3 and 20:2n-6, respectively (Fig 5A, 5B, 5C and 5D). These conversions clearly showed that both FadsC1 and FadsC2 are Δ8 desaturases. Neither FadsC1 nor FadsC2 showed any activity towards 18:3n-3, 18:2n-6, 20:4n-3, 20:3n-6, 22:5n-3 and 22:4n-6. In addition, no activity towards 20:1n–9 was observed in any of the three Fads cloned from P. lividus.
Fig 5. Functional characterisation of FadsC1 and FadsC2 from Paracentrotus lividus in transformed yeast S. cerevisiae.
The fatty acid (FA) profiles of yeast transformed with pYES2-FadsC1 (A, B) and pYES2-FadsC2 (C, D) were determined after they were grown in the presence of FA substrates (indicated by asterisk), namely 20:3n-3 (A, C) and 20:2n-6 (B, D). Moreover, desaturation products are indicated accordingly in each panel. Peaks 1–4 in all panels are the main endogenous FA of S. cerevisiae, namely 16:0 (1), 16:1 isomers (2), 18:0 (3), 18:1n-9 (4). Vertical axis, FID response; horizontal axis, retention time.
Discussion
Sea urchins are regarded as a delicacy in many countries; their gonads are commonly consumed as a raw product. As a result of their high economical value, the sea urchin culture industry aims to produce individuals with roe having the taste, appearance and the nutritional value as close as possible to those of wild specimens. Previous studies suggested that dietary lipids partly determined the lipid contents of P. lividus tissues [43] but it remained unclear whether endogenous biosynthesis of lipids and fatty acid can also contribute to the overall nutritional value. In the present study, we cloned and functionally characterised three distinct desaturases, namely FadsA, FadsC1 and FadsC2, with a role in the biosynthetic pathways of LC-PUFA in P. lividus.
The deduced aa sequences of the three desaturases isolated from P. lividus indicated they were all front-end desaturases, enzymes that introduce double bonds between a pre-existing unsaturation and the carboxyl end of the fatty acyl chain, in the position Δx from the carboxyl group [44]. The cytochrome b5 domain, heme–binding motif (HPGG) and three histidine boxes (HXXXH, HXXHH and QXXHH), which are typical features of front-end desaturases [6,8,44], were found in all desaturase sequences obtained from P. lividus. In spite of sharing common features though, distinctive sequence domains among Fads from sea urchins and other Echinodermata classes became obvious from our phylogenetic analysis. Clearly, our phylogenetic analysis confirms that the repertoire of Fads within the Echinodermata phylum varies among classes, similarly as reported for molluscs [18]. On one hand, the FadsA, confirmed be a Δ5 desaturase in P. lividus, is found in other echinoderm classes including starfish (Patiria miniata, Asteroidea), brittle stars (Ophiothrix spiculata, Ophiuroidea) and sea cucumbers (Parastichopus parvimensis, Holothuroidea), thus suggesting that this desaturase is virtually present in all echinoderms. On the other hand, the FadsC appears to be a Echinoidea-specific desaturase and, indeed, two distinct genes have been identified in P. lividus (FadsC1 and FadsC2) and genomes of S. purpuratus and L. variegatus. Interestingly, neither starfishes, brittle stars nor sea cucumbers appear to possess FadsC-like desaturases according to genomic information available from NCBI (accession no. GCA_000285935, GCA_000969725, GCA_000934455, respectively). Finally, the latter echinoderm classes but not sea urchins (Echinoidea), possess a desaturase herein denoted as FadsB. Importantly, our phylogenetic analysis suggested that the Echinodermata FadsB is the most closely related Fads-like desaturase to the vertebrate orthologues, and thus arises as an interesting protein to elucidate the evolutionary divergence of substrate specificity among metazoan Fads.
The differences in the amino acid sequences of the sea urchin Fads highlighted in the phylogenetic tree were further reflected in their regioselectivity. Thus the functional analyses in yeast confirmed that both FadsC1 and FadsC2 are Δ8 desaturases, a regioselectivity previously reported in a Fads from the scallop Chlamys nobilis [15]. Additionally, the P. lividus FadsA was confirmed to be a Δ5 desaturase, a desaturase ability reported in a variety of molluscs [10,12–14]. The present results demonstrate that P. lividus possesses desaturases that account for all the desaturation reactions required to convert the C18 PUFA precursors ALA (18:3n-3) and LA (18:2n-6) into the physiologically important EPA (20:5n-3) and ARA (20:4n-6), respectively, through the so-called “Δ8 pathway” [6] (Fig 1). These results are consistent with the fact that the two most abundant LC-PUFA in P. lividus lipids are usually EPA and ARA [45, 46], and can partly explain why high contents of ARA were found in the gonads of P. lividus individuals fed diets with low ARA content [45]. Similarly to the mollusc desaturases, the P. lividus FadsA also showed the capability to desaturate 18:0 into 18:1n-13 (18:1Δ5) but, in contrast to molluscs, the P. lividus FadsA was not able to desaturate 16:0 to 16:1n-11 (16:1Δ5) [10,12,14]. While dietary origin cannot be ruled out, the herein reported activity of the P. lividus FadsA is consistent with the presence of 18:1n-13 reported in several sea urchin species [47,48]. Overall, the functional analysis of the P. lividus FadsA clearly demonstrated that this enzyme is a Δ5 desaturase that can operate towards both saturated FA and PUFA substrates. Since the P. lividus FadsA clustered together with several mollusc Δ5 Fads in the phylogenetic tree (Clade A in Fig 3), it can be speculated that all Fads belonging to Clade A have Δ5 desaturase activity. Moreover, the FadsA appears to play a role in the biosynthesis of NMI FA in P. lividus.
The presence of NMI FA in sea urchins has been confirmed in previous studies [17,37,46–48,49–51], and biosynthetic pathways for certain NMI FA have even been postulated for Strongylocentrotus droebachiensis [52]. For instance, the biosynthesis of 20:2Δ5,11 and 20:2Δ5,13 has been hypothesised to proceed through Δ5 desaturation of 20:1n-9 (20:1Δ11) and 20:1n-7 (20:1Δ13), respectively [52]. In order to elucidate the molecular mechanisms underlying the biosynthesis of NMI FA in P. lividus, we incubated transgenic yeast expressing the newly cloned P. lividus Fads in the presence of 20:1n-9 (20:1Δ11). Our GC-MS analyses confirmed that no desaturation product was detected for any of the three Fads desaturases characterised from P. lividus. These results suggest that 20:2Δ5,11 and potentially other NMI FA reported in P. lividus tissues have a dietary origin rather than endogenous synthesis. Nevertheless, the P. lividus FadsA showed the ability to biosynthesise the NMI FAs 20:4Δ5,11,14,17 and 20:3Δ5,11,14 from the precursors 20:3n-3 (20:3Δ11,14,17) and 20:2n-6 (20:2Δ11,14), respectively. Similar functions have been also reported in Fads-like desaturases from common octopus, common cuttlefish and scallop [10,12,14]. Indeed these NMI FAs have been identified in several sea urchin species [47,48] and thus FadsA arises as the most likely candidate enzyme accounting for the biosynthesis of such NMI FA in vivo.
In conclusion, it has been demonstrated that P. lividus possess three fatty acyl desaturases with Δ5 desaturase (FadsA) and Δ8 desaturase (FadsC1 and FadsC2) activities. Moreover, we have provided evidence confirming that the P. lividus FadsA is involved in the biosynthesis of NMI FA, a role that could not be demonstrated for the FadsC desaturases. Our phylogenetic analysis revealed that the repertoire of Fads varies among echinoderm classes. FadsA desaturases can be found virtually in all Echinodermata classes, whereas other Fads-like desaturases have a more restricted distribution. Thus, the herein characterised FadsC appears to be a Echinoidea-specific enzyme, whereas other echinoderm classes such Asteroidea, Ophiuroidea and Holothuroidea possess an alternative desaturase herein denoted as FadsB.
Supporting Information
Mass spectra of DMOX derivatives prepared from desaturation products of the P. lividus FadsA (Δ5 desaturase), namely 18:1n-13 (A), 20:4Δ5,11,14,17 (B) and 20:3Δ5,11,14 (C). Mass of 153 in red is the diagnostic mass ion that shows Δ5 desaturation and other characteristic mass ions of each product fatty acid are indicated in blue. The corresponding fragmentation patterns are also shown in each panel.
(TIFF)
Acknowledgments
This work received funding from the MASTS pooling initiative (The Marine Alliance for Science and Technology for Scotland) and their support is gratefully acknowledged. MASTS is funded by the Scottish Funding Council (grant reference HR09011) and contributing institutions. This investigation also received support from the FP7 Project ENRICH (Ref: 222492).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work received funding from the MASTS pooling initiative (The Marine Alliance for Science and Technology for Scotland) and their support is gratefully acknowledged. MASTS is funded by the Scottish Funding Council (grant reference HR09011) and contributing institutions. This investigation also received support from the FP7 Project ENRICH (Ref: 222492).
References
- 1.Sargent JR, Tocher DR, Bell JG. The lipids In: Halver JE, Hardy RW, editors. Fish nutrition, third edition Academic Press; 2002. pp. 182–259. [Google Scholar]
- 2.van der Merwe LF, Moore SE, Fulford AJ, Halliday KE, Drammeh S, Young S, et al. Long-chain PUFA supplementation in rural African infants: a randomized controlled trial of effects on gut integrity, growth, and cognitive development. Am J Clin Nutr. 2013;97: 45–57. 10.3945/ajcn.112.042267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awada M, Meynier A, Soulage CO, Hadji L, Géloën A, Viau M, et al. n-3 PUFA added to high-fat diets affect differently adiposity and inflammation when carried by phospholipids or triacylglycerols in mice. Nutr Metabolism. 2013;10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montes R, Chisaguano AM, Castellote AI, Morales E, Sunyer J, López-Sabater MC. Fatty-acid composition of maternal and umbilical cord plasma and early childhood atopic eczema in a Spanish cohort. Eur J Clin Nutr. 2013;67: 658–663. 10.1038/ejcn.2013.68 [DOI] [PubMed] [Google Scholar]
- 5.Muhlhausler BS, Ailhaud GP. Omega-6 polyunsaturated fatty acids and the early origins of obesity. Curr Opin Endocrinol Diabetes Obes. 2013;20: 56–61. 10.1097/MED.0b013e32835c1ba7 [DOI] [PubMed] [Google Scholar]
- 6.Castro LFC, Tocher DR, Monroig Ó. Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of Fads and Elovl gene repertoire. Prog Lipid Res. 2016;62: 25–40. 10.1016/j.plipres.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 7.Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45: 237–249. 10.1016/j.plipres.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 8.Guillou H, Zadravec D, Martin PGP, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog Lipid Res. 2010;49: 186–199. 10.1016/j.plipres.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 9.Monroig Ó, Tocher DR, Navarro JC. Biosynthesis of polyunsaturated fatty acids in marine invertebrates: recent advances in molecular mechanisms. Mar Drugs. Multidisciplinary Digital Publishing Institute; 2013;11: 3998–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monroig Ó, Navarro JC, Dick JR, Alemany F, Tocher DR. Identification of a Δ5-like fatty acyl desaturase from the cephalopod Octopus vulgaris (Cuvier 1797) involved in the biosynthesis of essential fatty acids. Mar Biotechnol. 2012a;14: 411–422. [DOI] [PubMed] [Google Scholar]
- 11.Monroig Ó, Guinot D, Hontoria F, Tocher DR, Navarro JC. Biosynthesis of essential fatty acids in Octopus vulgaris (Cuvier, 1797): Molecular cloning, functional characterisation and tissue distribution of a fatty acyl elongase. Aquaculture. 2012b;360–361: 45–53. [Google Scholar]
- 12.Monroig Ó, Hontoria F, Varó I, Tocher DR, Navarro JC. Investigating the essential fatty acids in the common cuttlefish Sepia officinalis (Mollusca, Cephalopoda): Molecular cloning and functional characterisation of fatty acyl desaturase and elongase. Aquaculture. 2016;450: 38–47. [Google Scholar]
- 13.Li M, Mai K, He G, Ai Q, Zhang W, Xu W, et al. Characterization of two Δ5 fatty acyl desaturases in abalone (Haliotis discus hannai Ino). Aquaculture. 2013;416–417: 48–56. [Google Scholar]
- 14.Liu H, Guo Z, Zheng H, Wang S, Wang Y, Liu W, et al. Functional characterization of a Δ5-like fatty acyl desaturase and its expression during early embryogenesis in the noble scallop Chlamys nobilis Reeve. Mol Biol Rep. 2014a;41: 7437–7445. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Zhang H, Zheng H, Wang S, Guo Z, Zhang G. PUFA biosynthesis pathway in marine scallop Chlamys nobilis Reeve. J Agric Food Chem. 2014b;62: 12384–12391. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Zheng H, Wang S, Wang Y, Li S, Liu W, et al. Cloning and functional characterization of a polyunsaturated fatty acid elongase in a marine bivalve noble scallop Chlamys nobilis Reeve. Aquaculture. 2013;416–417: 146–151. [Google Scholar]
- 17.Barnathan G. Non-methylene-interrupted fatty acids from marine invertebrates: Occurrence, characterization and biological properties. Biochimie. 2009;91: 671–678. 10.1016/j.biochi.2009.03.020 [DOI] [PubMed] [Google Scholar]
- 18.Surm JM, Prentis PJ, Pavasovic A. Comparative Analysis and Distribution of Omega-3 lcPUFA Biosynthesis Genes in Marine Molluscs. PLoS ONE. 2015;10: e0136301 10.1371/journal.pone.0136301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro LFC, Monroig Ó, Leaver MJ, Wilson J, Cunha I, Tocher DR. Functional desaturase Fads1 (Δ5) and Fads2 (Δ6) orthologues evolved before the origin of jawed vertebrates. PLoS ONE. 2012;7: e31950 10.1371/journal.pone.0031950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JM, Lee H, Kang S, Park WJ. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients. 2016;8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raible F, Tessmar-Raible K, Osoegawa K, Wincker P, Jubin C, Balavoine G, et al. Vertebrate-type intron-rich genes in the marine annelid Platynereis dumerilii. Science. 2005;310: 1325–1326. 10.1126/science.1119089 [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, McDougall C, Troscianko J, Chen W-C, Jayaraman-Nagarajan A, Shimeld SM, et al. An EST screen from the annelid Pomatoceros lamarckii reveals patterns of gene loss and gain in animals. BMC Evol Biol. 2009;9: 240 10.1186/1471-2148-9-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui JHL, McDougall C, Monteiro AS, Holland PWH, Arendt D, Balavoine G, et al. Extensive chordate and annelid macrosynteny reveals ancestral homeobox gene organization. Mol Biol Evol. 2012;29: 157–165. 10.1093/molbev/msr175 [DOI] [PubMed] [Google Scholar]
- 24.Sea Urchin Genome Sequencing Consortium, Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314: 941–952. 10.1126/science.1133609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sergiev PV, Artemov AA, Prokhortchouk EB, Dontsova OA, Berezkin GV. Genomes of Strongylocentrotus franciscanus and Lytechinus variegatus: are there any genomic explanations for the two order of magnitude difference in the lifespan of sea urchins? Aging. 2016;8: 260–271. 10.18632/aging.100889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lozano J, Galera J, Lopez S, Turon X, Palacin C, Morera G. Biological cycles and recruitment of Paracentrotus lividus (Echinodermata: Echinoidea) in two contrasting habitats. Mar Ecol Prog Ser. 1995;122:179–192. [Google Scholar]
- 27.Boudouresque CF, Verlaque M. Ecology of Paracentrotus lividus In: Lawrence JM, editors. Edible sea urchins: biology and ecology, second edition Elsevier; 2001. pp. 243–286. [Google Scholar]
- 28.Furesi R, Madau FA, Palomba A, Pulina Pietro. Stated preferences for consumption of sea urchin: a choice experiment in Sardinia (Italy). Proceedings in Food System Dynamics 2014; 303–311. [Google Scholar]
- 29.Cellario C, Fenaux L. Paracentrotus lividus (Lamarck) in culture (larval and benthic phases): Parameters of growth observed during two years following metamorphosis. Aquaculture. Elsevier; 1990;84: 173–188. [Google Scholar]
- 30.Cook EJ, Kelly MS. Co-culture of the sea urchin Paracentrotus lividus and the edible mussel Mytilus edulis L. on the West Coast of Scotland, United Kingdom. J Shellfish Res. 2009;28: 553–559. [Google Scholar]
- 31.Kelly MS, Chamberlain J. Recent advances in sea-urchin aquaculture and enhancement in Scotland and Ireland. Bull Aquacult Assoc Can. 2010;108: 23–30. [Google Scholar]
- 32.Sartori D. Echinoculture: the rearing of Paracentrotus lividus in a recirculating aquaculture system—experiments of artificial diets for the maintenance of sexual maturation. Aquac Int. 2014;23: 111–125. [Google Scholar]
- 33.Carboni S, Hughes AD, Atack T, Tocher DR, Migaud H. Influence of broodstock diet on somatic growth, fecundity, gonad carotenoids and larval survival of sea urchin. Aquac Res. 2015;46: 969–976. [Google Scholar]
- 34.Sartori D, Gaion A. Can sea urchins benefit from an artificial diet? Physiological and histological assessment for echinoculture feasibility evaluation. Aquac Nutr. 2015. [Google Scholar]
- 35.Sartori D, Pellegrini D, Macchia S, Gaion A. Can echinoculture be a feasible and effective activity? Analysis of fast reliable breeding conditions to promote gonadal growth and sexual maturation in Paracentrotus lividus. Aquaculture. 2016;451: 39–46. [Google Scholar]
- 36.Agatsuma Y, Sakai Y, Tajima K. Recent advances in sea-urchin aquaculture in Japan. Bull Aquacult Assoc Can. 2010;108: 4–10. [Google Scholar]
- 37.Liu H, Kelly MS, Cook EJ, Black K, Orr H, Zhu JX, et al. The effect of diet type on growth and fatty-acid composition of sea urchin larvae, I. Paracentrotus lividus (Lamarck, 1816) (Echinodermata). Aquaculture. 2007;264: 247–262. [Google Scholar]
- 38.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4: 406–425. [DOI] [PubMed] [Google Scholar]
- 39.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226: 497–509. [PubMed] [Google Scholar]
- 40.Hastings N, Agaba MK, Tocher DR, Leaver MJ, Dick JR, Sargent JR, et al. A vertebrate fatty acid desaturase with Delta 5 and Delta 6 activities. Proc Natl Acad Sci USA. 2001;98: 14304–14309. 10.1073/pnas.251516598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sprague M, Walton J, Campbell PJ, Strachan F, Dick JR, Bell JG. Replacement of fish oil with a DHA-rich algal meal derived from Schizochytrium sp. on the fatty acid and persistent organic pollutant levels in diets and flesh of Atlantic salmon (Salmo salar, L.) post-smolts. Food Chem. 2015;185: 413–421. 10.1016/j.foodchem.2015.03.150 [DOI] [PubMed] [Google Scholar]
- 42.Fonseca-Madrigal J, Navarro JC, Hontoria F, Tocher DR, Martinez-Palacios CA, Monroig Ó. Diversification of substrate specificities in teleostei Fads2: characterization of Δ4 and Δ6Δ5 desaturases of Chirostoma estor. J Lipid Res. 2014;55: 1408–1419. 10.1194/jlr.M049791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez C, Boudouresque CF. Nutrition of the sea urchin Paracentrotus lividus (Echinodermata: Echinoidea) fed different artificial food. Mar Ecol-Prog Ser. 2000;204: 131–141. [Google Scholar]
- 44.Meesapyodsuk D, Qiu X. The front-end desaturase: structure, function, evolution and biotechnological use. Lipids. Springer-Verlag; 2012;47: 227–237. 10.1007/s11745-011-3617-2 [DOI] [PubMed] [Google Scholar]
- 45.Carboni S, Hughes AD, Atack T, Tocher DR, Migaud H. Fatty acid profiles during gametogenesis in sea urchin (Paracentrotus lividus): Effects of dietary inputs on gonad, egg and embryo profiles. Comp Biochem Physiol A. 2013;164: 376–382. [DOI] [PubMed] [Google Scholar]
- 46.Siliani S, Melis R, Loi B, Guala I, Baroli M, Sanna R, et al. Influence of seasonal and environmental patterns on the lipid content and fatty acid profiles in gonads of the edible sea urchin Paracentrotus lividus from Sardinia. Mar Environ Res. 2016;113: 124–133. 10.1016/j.marenvres.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 47.Takagi T, Kaneniwa M, Itabashi Y, Ackman RG. Fatty acids in echinoidea: Unusualcis-5-olefinic acids as distinctive lipid components in sea urchins. Lipids. Springer-Verlag; 1986;21: 558–565. [Google Scholar]
- 48.Liyana-Pathirana C, Shahidi F, Whittick A, Hooper R. Lipid and lipid soluble components of gonads of green sea urchin (Strongylocentrotus droebchiensis). J Food Lipids. 2002;9: 105–126. [DOI] [PubMed] [Google Scholar]
- 49.Cook EJ, Bell MV, Black KD, Kelly MS. Fatty acid compositions of gonadal material and diets of the sea urchin, Psammechinus miliaris: trophic and nutritional implications. J Exp Mar Biol Ecol. 2000;255: 261–274. [DOI] [PubMed] [Google Scholar]
- 50.Castell JD, Kennedy EJ, Robinson SMC, Parsons GJ, Blair TJ, González-Durán E. Effect of dietary lipids on fatty acid composition and metabolism in juvenile green sea urchins (Strongylocentrotus droebachiensis). Aquaculture. 2004;242: 417–435. [Google Scholar]
- 51.Kelly JR, Scheibling RE, Iverson SJ, Gagnon P. Fatty acid profiles in the gonads of the sea urchin Strongylocentrotus droebachiensis on natural algal diets. Mar Ecol-Prog Ser. 2008;373: 1–9. [Google Scholar]
- 52.González-Durán E, Castell JD, Robinson SMC, Blair TJ. Effects of dietary lipids on the fatty acid composition and lipid metabolism of the green sea urchin Strongylocentrotus droebachiensis. Aquaculture. 2008;276: 120–129. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mass spectra of DMOX derivatives prepared from desaturation products of the P. lividus FadsA (Δ5 desaturase), namely 18:1n-13 (A), 20:4Δ5,11,14,17 (B) and 20:3Δ5,11,14 (C). Mass of 153 in red is the diagnostic mass ion that shows Δ5 desaturation and other characteristic mass ions of each product fatty acid are indicated in blue. The corresponding fragmentation patterns are also shown in each panel.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.