Abstract
Objective To evaluate the effects of angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists (AIIRAs) on renal outcomes and all cause mortality in patients with diabetic nephropathy.
Data sources Medline, Embase, the Cochrane controlled trials register, conference proceedings, and contact with investigators.
Study selection Trials comparing ACE inhibitors or AIIRAs with placebo or with each other in patients with diabetic nephropathy.
Data extraction Mortality, renal outcomes (end stage renal disease, doubling of serum creatinine concentration, prevention of progression of microalbuminuria to macroalbuminuria, remission of microalbuminuria), and quality of trials.
Data synthesis 36 of 43 identified trials compared ACE inhibitors with placebo (4008 patients), four compared AIIRAs with placebo (3331 patients), and three compared ACE inhibitors with AIIRAs (206 patients). We obtained unpublished data for 11 trials. ACE inhibitors significantly reduced all cause mortality (relative risk 0.79, 95% confidence interval 0.63 to 0.99) compared with placebo but AIIRAs did not (0.99, 0.85 to 1.17), although baseline mortality was similar in the trials. Both agents had similar effects on renal outcomes. Reliable estimates of the unconfounded relative effects of ACE inhibitors compared with AIIRAs could not be obtained owing to small sample sizes.
Conclusion Although the survival benefits of ACE inhibitors for patients with diabetic nephropathy are known, the relative effects of ACE inhibitors and AIIRAs on survival are unknown owing to the lack of adequate head to head trials.
Introduction
Diabetic nephropathy occurs in 25-40% of patients with type 1 or type 2 diabetes within 20-25 years of the onset of disease.1 Both types of patients probably share the same pathogenetic and clinical stages of renal damage, including renal hypertrophy, incipient (microalbuminuric) nephropathy, overt (macroalbuminuric) nephropathy and, finally, end stage renal disease.2,3 About one third of patients with diabetic nephropathy progress to end stage renal disease.1
Agents used to delay the progression of diabetic nephropathy include β blockers, calcium channel blockers, diuretics, angiotensin converting enzyme (ACE) inhibitors, and angiotensin II receptor antagonists (AIIRAs). Large scale randomised controlled trials have shown that ACE inhibitors and AIIRAs slow the deterioration of renal function and reduce proteinuria, and for this reason they are the most widely used agents in diabetic patients.4-8
Mortality is reported to be 10-40% within 10 years of diabetes being diagnosed, depending on cardiovascular comorbidities. The primary cause of early death is cardiovascular. Nephropathy has been shown to be an independent risk factor for early death due to cardiovascular diseases in diabetic patients.9 Microalbuminuria is associated with a twofold to fourfold increase in the risk of death, and overt proteinuria and hypertension are associated with an even higher risk when present together.
The Joint National Committee on Prevention, Diagnosis and Management of Hypertension and the American Diabetes Association recommend that hypertensive and normotensive patients with diabetic nephropathy should receive ACE inhibitors or AIIRAs as first line treatment.10,11 We searched for evidence from randomised controlled trials of the effects of ACE inhibitors and AIIRAs on renal outcomes and mortality in patients with diabetic nephropathy.
Methods
We included randomised controlled trials of at least six months duration in which ACE inhibitors or AIIRAs were compared with placebo or no treatment or in which the relative effects of the agents were compared directly, in patients with diabetic nephropathy. Any stage of diabetic nephropathy was included: microalbuminuria (albumin excretion 30-300 mg/d) or macroalbuminuria (albumin excretion > 300 mg/d).
Search strategy
We searched Medline (1966-September 2003) and Embase (1988-September 2003) using optimally sensitive search strategies developed by the Cochrane Collaboration.12 We also searched the Cochrane Renal Group trial register and the Cochrane central registry of randomised controlled trials. Medical subject heading terms and text words used were angiotensin converting enzyme inhibitors, captopril, enalapril, cilazapril, enalaprilat, fosinopril, lisinopril, perindopril, ramipril, saralasin, teprotide, losartan, angiotensin receptor antagonist(s), angiotensin (II) receptor antagonist(s), combined with diabetes mellitus or diabetic nephropathy.
Trials were considered without language restriction. Two authors (GFMS, MC) analysed the titles and abstracts of identified trials according to the inclusion criteria, searched the reference lists, and sought information about unpublished or additional trials from the internet and experts in the subject.
Data extraction and quality assessment
GFMS and MC assessed each trial independently. They extracted data on the characteristics of the participants, interventions, comparisons, and outcomes (all cause mortality, end stage renal disease, doubling of serum creatinine concentration, progression from microalbuminuria to macroalbuminuria, regression from microalbuminuria to normoalbuminuria, cough, headache, hyperkalaemia, and impotence). Whenever these were not reported, the primary investigator (GFMS) and the Cochrane Renal Group editorial office contacted the authors at least twice for the data.
We used standard criteria to assess the quality of the trials (allocation concealment, intention to treat analysis, loss to follow up, blinding). Differences were resolved by discussion.
Statistical analysis
We summarised the treatment effects as relative risks, used the DerSimonian and Laird random effects model to pool the data, and examined heterogeneity of treatment effects between studies using the Cochran Q and I2 statistics.13-14 We used subgroup analysis and random effects metaregression to explore the influence of possible sources of heterogeneity on treatment effect. These were duration of follow up, type of diabetes, type of drug, presence or absence of hypertension at baseline, stage of diabetic nephropathy, and specific quality items.
For trials that did not compare ACE inhibitors with AIIRAs directly, we computed indirect comparisons of treatment effects on all outcomes by using the control group as a common comparator from trials that compared the agents with placebo or with no treatment. Analysis was performed as a metaregression using the trial intervention as the explanatory variable. All analyses were undertaken in STATA version 8.0 and RevMan 4.2.
Results
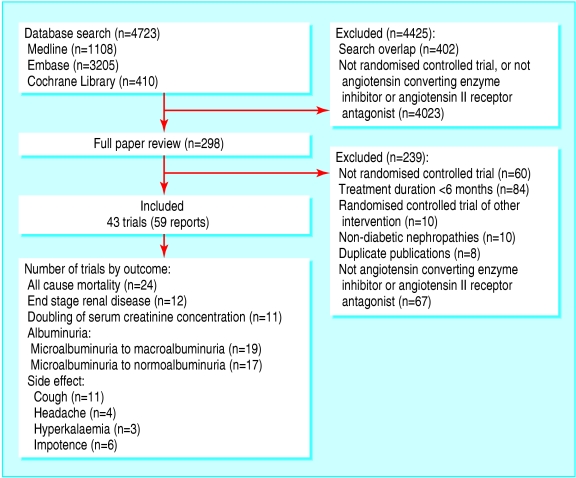
Of the 4723 articles we identified, 4425 were excluded after review of the title and abstract (fig 1). The major reasons for exclusion were a non-randomised design, non-antihypertensive interventions, study populations with non-diabetic nephropathy, and duplicate publications. After assessing the full text of 298 studies, we identified 43 eligible randomised controlled trials (59 publications), which enrolled 7545 patients.w1-w59
Fig 1.
Flow chart showing number of citations retrieved by individual searches and number of trials included in review
We obtained supplemental data on design features and outcomes from the authors of nine trials, or from publications relating to the primary trial.
Study characteristics
Of the 43 trials, 36 (4008 patients) compared ACE inhibitors with placebo, four (3331 patients) compared AIIRAs with placebo, and three (206 patients) compared ACE inhibitors with AIIRAs (table 1).
Table 1.
Characteristics of populations and interventions of included studies
| Trial | Level of albuminuria | Type of diabetes | Hypertension at baseline | Blood pressure equalised | Intervention* | No of patients | Follow up (months) |
|---|---|---|---|---|---|---|---|
| ACE inhibitors v placebo or no treatment:
|
|
|
|
|
|
|
|
| Estacio and Schrier (ABCD) 1998†w1 | Mixed | 2 | Yes | Yes | Enalapril 5-40 mg/d‡ | 246 | 63.6 |
| Ahmad et al 1997w3 | Microalbuminuria | 2 | — | Yes | Enalapril 10 mg/d‡ | 103 | 60 |
| Ahmad et al 2003w4 w5 | Microalbuminuria | 1 | — | Yes | Enalapril 10 mg/d‡ | 73 | 60 |
| O'Hare et al (ATLANTIS) 2000†w6 | Microalbuminuria | 1 | — | — | Ramipril 1.25 mg/d‡ | 134 | 24 |
| Bakris et al 1994w7 | Microalbuminuria | 1 | — | — | Lisinopril 78 (SD 8) mg/d | 15 | 18 |
| Bauer et al 1992w8 | Macroalbuminuria | Mixed | Yes | Yes | Enalapril 5-40 mg/d‡ | 33 | 18 |
| Bojestig et al 2001w9 | Microalbuminuria | 1 | — | — | Ramipril 1.25 mg/d v ramipril 5.0 mg/d | 55 | 24 |
| Capek et al 1994w10 | Microalbuminuria | 2 | Yes | — | Captopril 37.5 mg/d | 15 | 12 |
| Carella et al 1999w11 | Microalbuminuria | 1 | — | Yes | Fosinopril 10 mg/d‡ | 16 | 24 |
| Chase et al 1993w12 | Microalbuminuria | 1 | — | — | Captopril 50 mg twice daily | 16 | 24 |
| Cordonnier et al 1999w13 | Macroalbuminuria | 2 | Yes | Yes | Perindopril 4 mg/d† | 22 | 24 |
| Crepaldi et al 1998†w14 | Microalbuminuria | 1 | — | — | Lisinopril 2.5-20 mg/d | 96 | 36 |
| Baines (ESPRIT) 2001w15 | Mixed | 1 | — | Yes | Enalapril 10-20 mg/d | 33 | 36 |
| EUCLID Study Group 1997w16 | Mixed | 1 | — | — | Lisinopril 10-20 mg/d | 530 | 24 |
| Garg et al 1998†w18 | Microalbuminuria | 1 | — | — | Ramipril 5 mg/d | 11 | 12 |
| Hansen et al 1994w19 | Microalbuminuria | 1 | Yes | — | Captopril 50 mg twice daily | 22 | 24 |
| Jerums et al 2001†w21 | Microalbuminuria | 1 | — | — | Perindopril 2-8 mg/d | 23 | 36 |
| Katayama et al 2002†w22 | Microalbuminuria | 1 | Yes | Yes | Captopril 37.5 mg/d; imidapril 5 mg/d‡ | 79 | 18 |
| Laffel et al 1995w23 | Microalbuminuria | 1 | — | Yes | Captopril 50 mg twice daily‡ | 143 | 24 |
| Lebovitz et al 1994w24 | Mixed | 2 | Yes | Yes | Enalapril 5-40 mg/d† | 121 | 36 |
| Lewis et al 1993w25 | Macroalbuminuria | 1 | Yes | Yes | Captopril 25 mg three times daily‡ | 409 | 36 |
| Marre et al 1987w26 | Microalbuminuria | Mixed | Enalapril 20 mg/d | 20 | 6 | ||
| Maschio et al 1996w29 | Macroalbuminuria | 2 | Yes | Yes | Benazepril 10 mg/d‡ | 21 | 36 |
| Mathiesen et al 1999w30 | Macroalbuminuria | 1 | — | — | Captopril 100 mg/d | 45 | 48 |
| Micro-HOPE 2000w33 | Microalbuminuria | Mixed | Yes | — | Ramipril 10 mg/d‡ | 1140 | 54 |
| Nankervis et al 1998w35 | Microalbuminuria | Mixed | Yes | Yes | Perindopril 4 mg/d‡ | 31 | 36 |
| O'Donnell et al 1993w36 | Microalbuminuria | Mixed | — | Yes | Lisinopril 10 mg/d‡ | 32 | 12 |
| Parving et al 1989w37 | Macroalbuminuria | 1 | — | — | Captopril 25-100 mg/d | 33 | 12 |
| Parving et al 2001w38 w39 | Macroalbuminuria | 1 | — | — | Captopril 74 mg/d | 33 | 96 |
| Phillips et al 1993w40 | Mixed | 1 | Yes | — | Cilazapril 2.5-5 mg/d | 25 | 24 |
| Poulsen et al 2001w41 w42 | Microalbuminuria | 1 | Yes | — | Lisinopril 20 mg/d | 57 | 24 |
| Ravid et al 1993w43 w45 | Microalbuminuria | 2 | — | Yes | Enalapril 10 mg/d‡ | 94 | 60 |
| Romero et al 1993w47 | Microalbuminuria | 2 | — | — | Captopril 25 mg/d | 26 | 6 |
| Sano et al 1994w48 | Microalbuminuria | 2 | Yes | Yes | Enalapril 5 mg/d‡ | 52 | 48 |
| Stornello et al 1992w51 | Macroalbuminuria | 2 | Yes | — | Enalapril 5 mg/d | 24 | 6 |
| Trevisan and Tiengo 1995w52 | Microalbuminuria | 2 | Yes | — | Ramipril 1.25 mg/d | 122 | 6 |
| ACE inhibitors v AIIRAs: | |||||||
| Lacourciere et al 2000w53 | Microalbuminuria | 2 | Yes | Yes | Enalapril 5-10 mg/d v Iosartan 50 mg/d‡ | 92 | 12 |
| Tutuncu et al 2001w54 | Microalbuminuria | 2 | — | — | Enalapril 5 mg/d v Iosartan 50 mg/d | 24 | 12 |
| Muirhead et al 1989w55 | Microalbuminuria | 2 | Yes | — | Captopril 25 mg three times daily v valsartan 80 mg/d v valsartan 160 mg/d v placebo‡ | 90 | 13 |
| AIIRA v placebo or no treatment:
|
|
|
|
|
|
|
|
| Brenner et al 2001w56 | Macroalbuminuria | 2 | Yes | Yes | Losartan 50-100 mg/d‡ | 1513 | 40.8 |
| Lewis et al 2001w57 | Macroalbuminuria | 2 | Yes | Yes | Irbesartan 75-300 mg/d‡ | 1148 | 30 |
| Parving et al 2001w58 | Microalbuminuria | 2 | Yes | Yes | Irbesartan 150-300 mg/d‡ | 590 | 24 |
| Tan et al 2002w59 | Microalbuminuria | 2 | Yes | — | Losartan 50 mg/d‡ | 80 | 6 |
ACE=angiotensin converting enzyme; AIIRA=angiotensin II receptor anatagonist.
Control arm indicated if different from placebo or no treatment.
Included placebo or no treatment control arm along with additional treatment arm (non-ACE inhibitor, non-AIIRA).
Cointerventions: calcium channel blockers, β blockers, α blockers, or diuretics.
ACE inhibitors compared with placebo
Of the trials comparing ACE inhibitors with placebo, 20 enrolled patients with type 1 diabetes, 11 enrolled patients with type 2 diabetes, and five enrolled mixed populations. Sixteen trials included patients with hypertension at baseline. In 18 trials, other antihypertensive agents were given to equalise blood pressure in both groups and to minimise the confounding effect of blood pressure. Twenty three trials enrolled patients with microalbuminuria, eight enrolled patients with macroalbuminuria, and five enrolled mixed populations. Three trials also enrolled a few patients with normoalbuminuria.w2 w16 w53
AIIRAs compared with placebo
The four trials that compared AIIRAs with placebo enrolled hypertensive patients with type 2 diabetes. Antihypertensive cointerventions were given in all four trials. Two trials enrolled patients with microalbuminuria and the other two trials enrolled patients with macroalbuminuria.
ACE inhibitors compared with AIIRAs
The three trials that compared ACE inhibitors with AIIRAs enrolled microalbuminuric patients with type 2 diabetes. Two trials enrolled hypertensive patients and one trial enrolled normotensive participants. Antihypertensive cointerventions were given in two trials.
Study quality
Trial quality was variable. Allocation concealment was unclear in 36 (84%) trials, inadequate in one (2%) trial, and adequate in six (14%) trials. Participants were blinded in 33 (77%) trials, investigators in 29 (66%) trials, and outcome assessors in four (9%) trials. Thirteen (30%) trials used an intention to treat analysis. Between 0% and 20% of patients were lost to follow up in 40 (93%) trials and between 21% and 40% were lost to follow up in three (7%) trials.
All cause mortality and renal outcomes
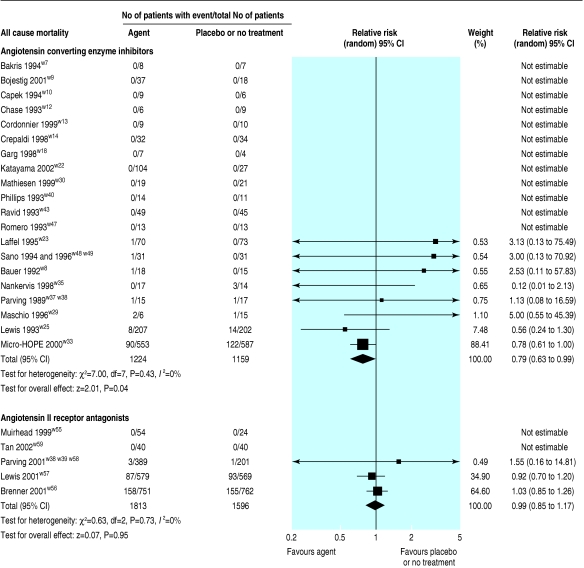
In 20 trials (2838 patients), all cause mortality was lower with ACE inhibitors than with placebo or no treatment (relative risk 0.79, 95% confidence interval 0.63 to 0.99; fig 2). This analysis was dominated by two trials, which contributed 88.4% and 7.5% of the weight to the summary estimate.w25 w33
Fig 2.
Effect of angiotensin converting enzyme inhibitors or angiotensin II receptor antagonists compared with placebo or no treatment on overall mortality
No statistically significant reduction in all cause mortality was found in the four trials (3329 patients) of AIIRAs compared with placebo or no treatment (relative risk 0.99, 0.85 to 1.17; fig 2). This analysis was dominated by two trials, which contributed 64.6% and 34.9% of the weight to the summary estimate.w56 w57
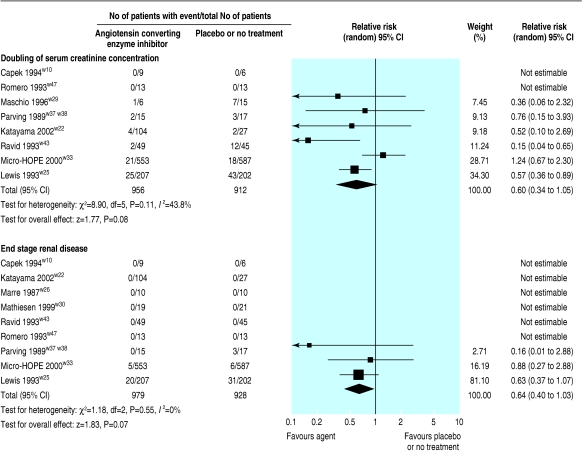
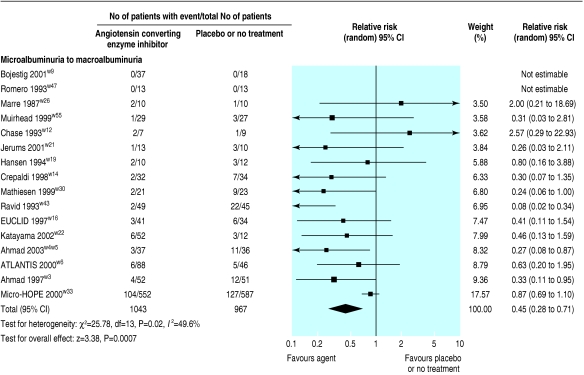
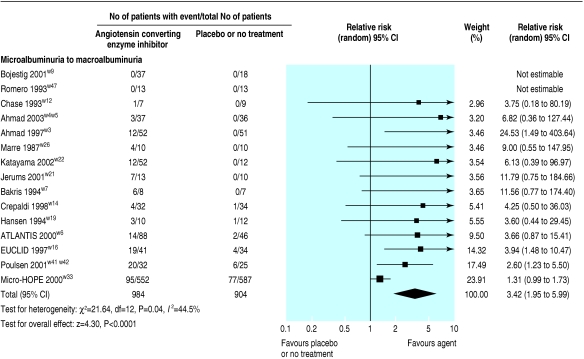
Nine of the trials (1907 patients) comparing ACE inhibitors with placebo showed weak evidence for a reduced risk of end stage renal disease (relative risk 0.64, 0.40 to 1.03) and eight of the trials (1868 patients) showed weak evidence for a doubling of serum creatinine concentration (0.60, 0.34 to 1.05; fig 3). In 16 trials (2010 patients), ACE inhibitors significantly reduced the risk of progression from microalbuminuria to macroalbuminuria (0.45, 0.28 to 0.71; fig 4), and in 15 trials (1888 patients) ACE inhibitors significantly increased the rate of regression from microalbuminuria to normoalbuminuria (3.42, 1.95 to .99; fig 5).
Fig 3.
Effect of angiotensin converting enzyme inhibitors compared with placebo or no treatment on renal function (doubling of serum creatinine concentration and end stage renal disease)
Fig 4.
Effect of angiotensin converting enzyme inhibitors compared with placebo or no treatment on risk of progression from microalbuminuria to macroalbuminuria
Fig 5.
Effect of angiotensin converting enzyme inhibitors compared with placebo or no treatment on rate of progression from microalbuminuria to normoalbuminuria
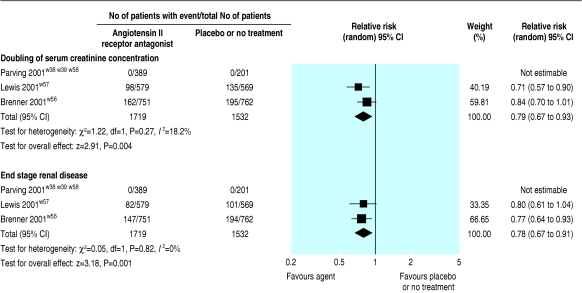
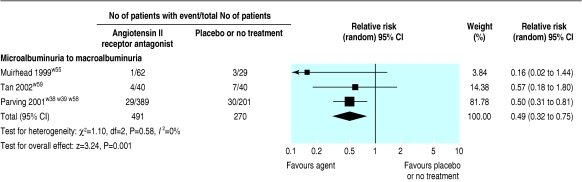
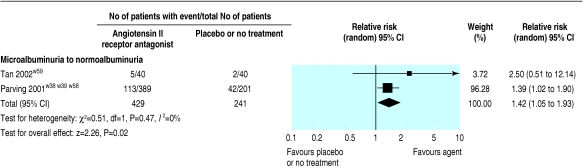
Three trials (3251 patients) comparing AIIRAs with placebo or no treatment showed a significantly reduced risk of end stage renal disease (relative risk 0.78, 0.67 to 0.91) and doubling of serum creatinine concentration (0.79, 0.67 to 0.93; fig 6). AIIRAs also significantly decreased the risk of progression from microalbuminuria to macroalbuminuria (three trials, 761 patients; 0.49, 0.32 to 0.75; fig 7) and increased the rate of regression from microalbuminuria to normoalbuminuria (two trials, 670 patients; 1.42, 1.05 to 1.93; fig 8).
Fig 6.
Effect of angiotensin II receptor antagonists compared with placebo or no treatment on renal function (doubling of serum creatinine concentration and end stage renal disease)
Fig 7.
Effect of angiotensin II receptor antagonists compared with placebo or no treatment on albuminuria, showing agent reduces risk of progression from microalbuminuria to macroalbuminuria
Fig 8.
Effect of angiotensin II receptor antagonists compared with placebo or no treatment on rate of regression from microalbuminuria to normoalbuminuria
ACE inhibitors compared with AIIRAs
The three trials that compared ACE inhibitors with AIIRAs did not report on all cause mortality, end stage renal disease, and doubling of serum creatinine concentration, and we were unable to obtain these data from the authors. Progression from microalbuminuria to macroalbuminuria was reported in one trial (92 patients) and there was no significant difference in risk, with the point estimate favouring ACE inhibitors (relative risk 0.16, 0.02 to 1.44).w53 Regression from microalbuminuria to normoalbuminuria in one trial showed a non-significant difference in the risk.w54
Indirect comparison of treatment effects
Regression analysis of treatment effects of ACE inhibitors compared with AIIRAs, using active treatment as the explanatory variable showed no significant difference between these two agents for the risk of any outcome (death: relative risk 0.79, 0.60 to 1.05; end stage renal disease: 0.82, 0.50 to 1.36; doubling of serum creatinine concentration: 0.83, 0.58 to 1.20; progression from microalbuminuria to macroalbuminuria: 1.14, 0.31 to 4.22; regression from microalbuminuria to normoalbuminuria: 0.76, 0.56 to 1.05).
The ACE inhibitor and AIIRA trials had potentially important differences in study design, particularly the two pairs of trials that dominate the summary estimates of effects (table 2).
Table 2.
Characteristics of study populations and trial designs of four influential trials of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists in patients with diabetic nephropathy. Values are means (standard deviations) unless stated otherwise
| Variable | Micro-HOPE 20004 | Lewis et al 19935 | Brenner et al 20017 | Lewis et al 20018 |
|---|---|---|---|---|
| Treatments | Ramipril v placebo | Captopril v placebo | Losartan v placebo | Irbesartan v placebo |
| Characteristics of patients at baseline*: | ||||
| Age (years) | 65.6 (6.6) | 35.0 (7.0) | 60.0 (7.0) | 58.3 (8.2) |
| No (%) with type 2 diabetes | 3 | 0 | 100 | 100 |
| Hypertension | Yes | Yes | Yes | Yes |
| Systolic blood pressure (mm Hg) | 142.3 (19.5) | 140.0 (20.0) | 153.0 (20.0) | 158.0 (20.0) |
| Diastolic blood pressure (mm Hg) | 79.3 (10.7) | 86.0 (12.0) | 82.0 (11.0) | 87.0 (11.0) |
| Arterial pressure (mm Hg) | NA | 104.0 (13.0) | 106.0 (11.6) | NA |
| Serum creatinine concentration (μmol/l) | 94.0 (27.6) | 114.4 (35.2) | 167.2 (44.0) | 148.7 (50.12) |
| Glomerular filtration rate (ml/min) | NA | 79.0 (35.0) | NA | NA |
| Urinary protein excretion (g/24 h) | NA | 3.0 (2.6) | NA | 2.9 (1.8-5.2)† |
| Stage of nephropathy | Microalbuminuria | Macroalbuminuria | Macroalbuminuria | Macroalbuminuria |
| Percentage of patients with cardiac disease (definition) | 80% (history of coronary artery disease, stroke, or peripheral vascular disease) | Excluded if scored >111 on criteria for New York Heart Association >III; no other data available | 10% (angina, myocardial infarction, stroke, or coronary revascularisation) | 27% (none) |
| Trial design and event rates (placebo group): | ||||
| Run-in phase‡ | Yes | No | No | No |
| Equalisation of blood pressure | No | Yes | No | Yes |
| Target blood pressure (systolic/diastolic, mm Hg) | NA | 140/90 | NA | <135/85 |
| Active v placebo group blood pressure (systolic/diastolic) at end of treatment (mm Hg) | 139.8/76.7 v 142.9/77.0 | 134.0/82.0 v 136.0/84.0 | 140.0/74.0 v 142.0/74.0 | 140.0/77.0 v 141.0/77.0 |
| Duration of study (months) | 54 | 48 | 48 | 52 |
| Mortality (%) | 20.7 | 6.9 | 20.3 | 16.3 |
| End stage renal disease (%) | 4.3 | 15.3 | 25.5 | 15.3 |
| Doubling of serum creatinine concentration (%) | 9.3 | 21.3 | 25.6 | 21.3 |
| Progression from microalbuminuria to macroalbuminuria (%) | 21.6 | NA | NA | NA |
| Regression from microalbuminuria to normoalbuminuria (%) | 13.1 | NA | NA | NA |
| Trial quality: | ||||
| Allocation concealment | Adequate | Unclear | Unclear | Adequate |
| Blinding of participants | Yes | Yes | Yes | Yes |
| Blinding of investigators | Yes | Yes | Yes | Yes |
| Blinding of outcome assessors | Yes | No | No | Yes |
| Intention to treat analysis | Yes | Yes | Yes | Yes |
| Lost to follow up (%) | 1.0 | 1.0 | 0.2 | 1.0 |
NA=not applicable.
Mean values provided for placebo group.
Data given as median (interquartile range).
To test compliance and toxicity.
Side effects and investigations for sources of heterogeneity
Reports of side effects were few in the smaller trials. Table 3 shows the summary estimates of the effects of ACE inhibitors and AIIRAs on cough, hyperkalaemia, headache, and impotence. We found no significant heterogeneity across all trials.
Table 3.
Comparative risk of developing drug related side effects with angiotensin converting enzyme inhibitors or angiotensin II receptor antagonists
|
Angiotensin converting enzyme inhibitor v placebo or no treatment (36 trials)
|
Angiotensin II receptor antagonist v placebo or no treatment (4 trials)
|
|||||
|---|---|---|---|---|---|---|
| Side effect | No of trials reporting outcome | No of patients | Relative risk (95% CI)* | No of trials reporting outcome | No of patients | Relative risk (95% CI)* |
| Cough | 10 | 2269 | 2.74 (1.74 to 4.30) | 1 | 91 | 1.87 (0.22 to 16.01) |
| Headache | 3 | 1326 | 0.97 (0.17 to 5.71) | 1 | 91 | 0.47 (0.03 to 7.22) |
| Hyperkalaemia | 2 | 1271 | 0.85 (0.35 to 2.08) | 1 | 1148 | 5.41 (1.20 to 24.28) |
| Impotence | 6 | 1569 | 1.26 (0.68 to 2.34) | — | — | — |
Values <1 favour intervention, values >1 favour placebo or no treatment.
Metaregression and subgroup analyses were possible only in trials comparing ACE inhibitors with placebo or no treatment, given the small number of trials evaluating AIIRAs (table 4). We found no evidence that the effect of ACE inhibitors on all cause mortality varied according to type of diabetes, presence or absence of hypertension, or microalbuminuria compared with macroalbuminuria at baseline. Differences in the risk of other outcomes according to type of diabetes, hypertension, and study design are all explained by the results of the micro-HOPE trial.4 When we excluded this trial from our analyses, results were homogeneous for all outcomes.
Table 4.
Metaregression and subgroup analysis of sources of variability for major outcomes analysed in review (in respect of categorical and continuous study factors) in studies of angiotensin converting enzyme inhibitors compared with placebo
|
Mortality
|
Doubling of serum creatinine concentration
|
End stage renal disease
|
Microalbuminuria to macroalbuminuria
|
Microalbuminuria to normoalbuminuria
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Relative risk (95% CI): No of trials | P value | Relative risk (95% CI) | P value | Relative risk 95% CI); No of trials | P value | Relative risk (95% CI); No of trials | P value | Relative risk (95% CI); No of trials | P value |
| Type of diabetes: | ||||||||||
| 1
|
0.71 (0.33 to 1.52); n=4
|
0.84* | 0.57 (0.38 to 0.88); n=3
|
0.04* | 0.60 (0.36 to 1.01); n=2
|
0.56* | 0.44 (0.27 to 0.70); n=9
|
0.12* | 3.62 (2.24 to 5.83); n=10
|
<0.001* |
| 2
|
4.23 (0.69 to 25.83); n=2
|
0.21 (0.06 to 0.66); n=2
|
Not estimable
|
0.20 (0.08 to 0.52); n=3
|
24.53 (1.49 to 403.6); n=1
|
|||||
| Mixed | 0.54 (0.12 to 2.36); n=2 | 1.23 (0.67 to 2.30); n=1 | 0.88 (0.27 to 2.88); n=1 | Not estimable | 2.04 (0.41 to 10.11); n=2 | |||||
| Hypertension: | ||||||||||
| Present
|
0.80 (0.48 to 1.32); n=6
|
0.45 | 0.72 (0.43 to 1.23); n=4
|
0.22 | 0.67 (0.41 to 1.08); n=1
|
0.34 | 0.84 (0.67 to 1.05); n=4
|
0.001 | 2.12 (1.12 to 4.02); n=5
|
0.01 |
| Absent | 1.73 (0.22 to 13.44); n=2 | 0.32 (0.07 to 1.58); n=2 | 0.66 (0.41 to 1.07); n=2 | 0.37 (0.21 to 0.65); n=9 | 4.99 (2.52 to 9.89); n=7 | |||||
| Stage of nephropathy:
|
|
|
|
|
|
|
|
|
|
|
| Microalbuminuria
|
0.79 (0.53 to 1.16); n=4
|
0.92 | 0.52 (0.14 to 1.98); n=3
|
0.88 | 0.88 (0.27 to 2.88); n=1
|
0.55 | 0.47 (0.28 to 0.79); n=12
|
0.86 | 3.35 (1.83 to 6.16); n=12
|
0.80 |
| Macroalbuminuria
|
1.07 (0.37 to 3.12); n=4
|
0.56 (0.37 to 0.86); n=3
|
0.60 (0.36 to 1.01); n=2
|
0.24 (0.06 to 1.00); n=1
|
Not estimable
|
|||||
| Mixed | Not estimable | Not estimable | Not estimable | 0.41 (0.11 to 1.54); n=1 | 3.94 (1.48 to 10.48); n=1 | |||||
| Allocation concealment:
|
|
|
|
|
|
|
|
|
|
|
| Adequate
|
0.78 (0.61 to 1.00); n=1
|
0.88 | 1.11 (0.62 to 1.98); n=2
|
0.03 | 0.88 (0.27 to 2.88); n=1
|
0.55 | 0.83 (0.67 to 1.04); n=5
|
<0.001 | 2.71 (1.20 to 1.07); n=5
|
0.21 |
| Unclear | 0.98 (0.41 to 2.33); n=7 | 0.48 (0.29 to 0.80); n=4 | 0.60 (0.36 to 1.01); n=2 | 0.31 (0.18 to 0.52); n=9 | 3.72 (2.06 to 6.73); n=8 | |||||
| Blinding of participants or investigators:
|
|
|
|
|
|
|
|
|
|
|
| Yes
|
1.70 (0.22 to 13.18); n=2
|
0.46 | 0.32 (0.66 to 1.58); n=2
|
0.22 | 0.16 (0.00 to 2.88); n=1
|
0.34 | 0.27 (0.12 to 0.62); n=4
|
0.06 | 6.24 (1.96 to 19.85); n=3
|
0.21 |
| No | 0.79 (0.48 to 1.32); n=6 | 0.72 (0.43 to 1.23); n=4 | 0.67 (0.41 to 1.08); n=2 | 0.64 (0.43 to 0.95); n=10 | 2.73 (1.59 to 4.68); n=10 | |||||
| Intention to treat analysis:
|
|
|
|
|
|
|
|
|
|
|
| Yes
|
0.77 (0.61 to 0.97); n=4
|
0.26 | 0.80 (0.44 to 1.44); n=3
|
0.08 | 0.64 (0.40 to 1.03); n=3
|
Not estimable | 0.83 (0.67 to 1.04); n=4
|
0.001 | 2.26 (1.21 to 4.22); n=4
|
<0.001 |
| No | 1.57 (0.27 to 9.07); n=4 | 0.28 (0.11 to 0.72); n=3 | Not estimable | 0.36 (0.21 to 0.62); n=10 | 6.91 (2.89 to 16.48); n=9 | |||||
| Lost to follow up (%):
|
|
|
|
|
|
|
|
|
|
|
| 0
|
5.0 (0.55 to 45.39; n=1
|
0.56† | 0.44 (0.13 to 1.52); n=2
|
0.98† | Not estimable
|
0.37† | 0.56 (0.28 to 1.13); n=5
|
0.05† | 3.50 (2.06 to 5.95); n=7
|
0.20† |
| 1-10
|
0.79 (0.62 to 1.01); n=2
|
0.48 (0.06 to 3.82); n=2
|
0.88 (0.27 to 2.88); n=1
|
0.43 (0.18 to 1.03); n=5
|
1.72 (0.88 to 3.35); n=3
|
|||||
| 11-20
|
3.13 (0.13 to 75.49); n=1
|
Not estimable
|
Not estimable
|
0.30 (0.13 to 0.66); n=2
|
13.30 (1.76 to 100.70); n=2
|
|||||
| >20 | 0.58 (0.27 to 1.23); n=4 | 0.58 (0.37 to 0.90); n=2 | 2 0.60 (0.36 to 1.01); n=2 | 0.28 (0.06 to 1.29); n=2 | 11.79 (0.75 to 184.66); n=1 | |||||
| Duration of follow up (months):
|
|
|
|
|
|
|
|
|
|
|
| 6-23
|
2.53 (0.11 to 57.83); n=1
|
0.95† | 0.52 (0.10 to 2.69); n=1
|
0.83† | Not estimable
|
0.63† | 0.56 (0.21 to 1.48); n=3
|
0.17† | 8.63 (1.76 to 42.42); n=3
|
0.001† |
| 24-47
|
0.92 (0.22 to 3.81); n=4
|
0.55 (0.36 to 0.86); n=2
|
0.63 (0.37 to 1.07); n=1
|
0.54 (0.29 to 1.01); n=6
|
3.36 (2.04 to 5.53); n=7
|
|||||
| ≥48 | 0.79 (0.62 to 1.01); n=3 | 0.58 (0.16 to 2.14); n=3 | 0.63 (0.16 to 2.44); n=2 | 0.31 (0.12 to 0.79); n=5 | 4.12 (0.56 to 30.28); n=3 | |||||
Some data are not estimable either because no trial with this variable reported outcome or because trials in this group reported 0 events in both treatment and control arms. P values calculated through random effects metaregression analysis.
Calculated by analysing each category compared to first category.
Calculated with random effects metaregression for differences in risk across all trials considering variable on continuous scale, but relative risks and 95% confidence intervals provided for major categories.
Discussion
Trials have shown a survival benefit of angiotensin converting enzyme (ACE) inhibitors but not of angiotensin II receptor antagonists (AIIRAs) in patients with diabetic nephropathy. The relative survival advantage of one class of antihypertensives over the other in this population is, however, still unknown because only indirect comparisons based on small studies are available. ACE inhibitors significantly reduced the risk of all cause mortality (mainly cardiovascular) by about 20% and progression from microalbuminuria to macroalbuminuria by about 55%; they also increased the rate of regression from microalbuminuria to normoalbuminuria by about 3.4-fold. We found no evidence that these effects are related to baseline hypertension, type of diabetes, stage of diabetic nephropathy, and duration of treatment. In comparison, current trials of AIIRAs in patients with diabetic nephropathy have not shown a reduction in all cause mortality, with a relative risk of 0.99 and a narrow confidence interval (0.85 to 1.17). There is strong evidence that AIIRAs are beneficial for renal outcomes, with about a 22% reduction in risk of end stage renal disease and doubling of serum creatinine concentration, around a 51% reduction in progression rates from microalbuminuria to macroalbuminuria, and about a 42% increase in regression from microalbuminuria to normoalbuminuria.
Potential explanations for these apparent different effects between the two classes of antihypertensives are chance, confounding, and true differences. The usual 5% level for statistical significance was reached for all renal outcomes for AIIRAs, compared with ACE inhibitors, where this threshold was reached for the prevention of progression from microalbuminuria to macroalbuminuria, and regression of microalbuminuria to normoalbuminuria, but not end stage renal disease or doubling of serum creatinine concentration. The point estimates of effect for all renal outcomes favoured ACE inhibitors compared with AIIRAs, but there was considerable imprecision surrounding these summary point estimates for ACE inhibitors due to lower event rates and because of heterogeneity in trial results due to a large trial.4 For all cause mortality, the absence of benefit shown by AIIRAs is unlikely to be due to chance alone because the summary point estimate is close to unity (0.99) and the 95% confidence intervals are relatively narrow. Formal tests of differences in ACE inhibitors and AIIRAs did not show any differences in the risk of the outcomes beyond those expected by chance. The design and conduct of the ACEI and AIIRA trials have clear differences, which may explain apparent differences in results. In particular, micro-HOPE primarily included high cardiac risk patients with relatively low renal risk, and although end of treatment blood pressure was not different between the two groups (possibly due to survival bias), equalisation of blood pressure was not targeted and so may have confounded the observed benefit of ramipril. True differences in the relative effects of ACE inhibitors and AIIRAs can be established only by adequately powered trials that directly compare the two agents, which unfortunately are not available.
In trials that enrolled patients with diabetes without nephropathy (not been included in this review), intensive control of blood pressure with any agent reduced cardiovascular morbidity and mortality, independent of type of agent used.15 In addition, in one study comparing losartan with atenolol in hypertensive patients with diabetes, losartan significantly reduced the risk of all cause mortality.16 ACE inhibitors have been shown to reduce all cause mortality after myocardial infarction,17 whereas no relevant information with AIIRAs is available. Our findings are consistent with other large meta-analyses in patients with congestive heart failure, which showed a significant reduction in the risk of all cause mortality with ACE inhibitors compared with placebo, but not for AIIRAs.18,19 Previous studies have already analysed the role of various antihypertensive agents, including ACE inhibitors and AIIRAs, in patients affected by diabetic nephropathy. Particular focus was on the effect of ACE inhibitors in specific categories of patients (for example, only patients with type 1 diabetes). A recent meta-analysis of individual patient data from the ACEI in Diabetic Nephropathy Trialist Group concluded that in normotensive patients with type 1 diabetes and microalbuminuria, ACEI significantly reduced progression to macroalbuminuria and increased the chances of regression.20 An earlier metaregression analysis indicated that ACE inhibitors reduce proteinuria and preserve glomerular filtration rate in patients with diabetes, independent of changes in systemic blood pressure.6 The main difference with our study is that we included both ACEI and AIIRA trials, obtained additional data from the authors when possible, and evaluated all outcomes of interest, including all cause mortality, and not simply the traditional renal outcomes.
Limitations
The major limitation of our study is the indirect nature of the comparison between ACE inhibitors and AIIRAs, by using placebo as a common comparator. Trials directly comparing the two agents were few and small and did not report outcomes relevant to patients, therefore they were largely uninformative. The indirect comparison of treatment effects by regression analysis represents the best means available to compare relative treatment effects, but this evidence is inherently unreliable.21 Other limitations include the small number and suboptimal quality of included trials and the potential for publication bias. These issues are unlikely to be influential as the review is dominated by a few larger studies.
Possible mechanisms
A possible biological rationale for the benefit of ACE inhibitors but not of AIIRAs on all cause mortality could be that bradykinin antagonism occurs with ACE inhibitors but not with AIIRAs, and the selectivity of AIIRAs might not necessarily be an advantage. Although experimental renal models provide few data, information is available from cardiac models. Studies in rats with dilated cardiomyopathy showed that AIIRAs can affect blood pressure to a similar extent as high dose ACE inhibitors, but they do not confer sufficient protection against injury from the reninangiotensin system.22 ACE inhibitors were also more effective than AIIRAs in reducing the incidence of reperfusion induced arrhythmias and necrosis in rat models of ischaemia reperfusion.23 These aspects have recently been reviewed.24
The role of ACE inhibitors in the management of patients with diabetic nephropathy is well established. Recently, equivalence of the newer and more expensive class of antihypertensive agents, AIIRAs, has been widely advocated—the Joint National Committee on Prevention, Diagnosis and Management of Hypertension and the American Diabetic Association guidelines say that ACE inhibitors and AIIRAs can be used interchangeably—and are accepted in current practice. Our study shows that there is evidence from randomised controlled trials in patients with diabetic nephropathy that ACE inhibitors prevent early death but no such evidence for AIIRAs. Both agents prevent progression of nephropathy and promote regression to normoalbuminuria. The relative effects of ACE inhibitors and AIIRAs are unknown. Thus, outside of a comparative randomised controlled trial, the class of agent with proved survival benefit, ACE inhibitors, should be used as first line treatment.
Our findings highlight the need for an adequately powered comparative trial of ACE inhibitors compared with AIIRAs, with renal and all cause mortality as primary outcomes. In general, trials of the newer pharmacological agents (AIIRAs) have been placebo controlled rather than direct comparisons with existing agents (ACE inhibitors). This makes it easier to prove a benefit with the new agent, but harder to prove a differential advantage compared with existing drugs, as this may be only done by indirect comparison. Future trials should compare these agents directly. Given the recent promising results achieved with combination therapy, a factorial trial may be the preferred design.25 Meanwhile, undertaking a meta-analysis using individual patient data may allow the effects of baseline cardiac and renal disease to be better understood and accounted for through subgroup analysis.
What is already known on this topic
Diabetic nephropathy is managed by angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists (AIIRAs)
ACE inhibitors and AIIRAs may be used interchangeably
What this study adds
Evidence exists that ACE inhibitors prevent early death in patients with diabetic nephropathy but no such evidence for AIIRAs
The two classes of drugs have equivalent effects on renal outcomes
The relative effects of ACE inhibitors and AIIRAs on survival are unknown owing to the lack of adequate head to head trials
Supplementary Material
 References w1-w59 are on bmj.com
References w1-w59 are on bmj.com
We thank Denise Campbell (medical editor) for editing the manuscript; Narelle Willis, Sharn Gökalp, and Sandra Puckeridge for editorial and administrative support; Ruth Mitchell, Linda Heslop, Gail Higgins (trial search coordinators with the Cochrane Renal Group) for search strategies for this review; and Janice Pogue and the HOPE trialists, M Ravid, PJ Phillips, HH Parving, R Romero, S Katayama, ER Mathiesen, BM Brenner, and KC Tan who provided data on the trials on request.
Contributors: GFMS was responsible for the conception and design of the study; extraction, analysis, and interpretation of data; and writing the article. He is guarantor. MC was responsible for the conception and design of the study and data extraction. JJD was responsible for analysis and interpretation of the data and critical revision of the manuscript for intellectual content. FPS was responsible for clinical revision for intellectual content. JCC was responsible for the conception and design of the study; analysis and interpretation of the data; and writing the article. He approved the final manuscript.
Funding: Australia-Europe Scholarship 2003 (Department of Education, Science and Training of Australia), an NHMRC Centre for Clinical Research Excellence Grant (2003), and the Italian Society of Nephrology (Young Investigator Scholarship 2003).
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 1999;341: 1127-33. [DOI] [PubMed] [Google Scholar]
- 2.Mogensen CE. Drug treatment for hypertensive patients in special situations: diabetes and hypertension. Clin Exp Hypertens 1999;21: 895-906. [DOI] [PubMed] [Google Scholar]
- 3.Mogensen CE. Microalbuminuria in prediction and prevention of diabetic nephropathy in insulin-dependent diabetes mellitus patients. J Diabetes Complications 1995;9: 337-49. [DOI] [PubMed] [Google Scholar]
- 4.Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355: 253-9. [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effects of angiotension converting enzyme inhibition to diabetic nephropathy. N Engl J Med 1993;329: 1456-62. [DOI] [PubMed] [Google Scholar]
- 6.Kasiske BL, Kalil RS, Ma JZ, Liao M, Keane WF. Effect of antihypertensive therapy on the kidney in patients with diabetes: a meta-regression analysis. Ann Intern Med 1993;118: 129-38. [DOI] [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345: 861-9. [DOI] [PubMed] [Google Scholar]
- 8.Lewis E, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345: 851-60. [DOI] [PubMed] [Google Scholar]
- 9.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med 1997;157: 1413-8. [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC-7 report. JAMA 2003;289: 2560-72. [DOI] [PubMed] [Google Scholar]
- 11.Arauz-Pacheo C, Parrott MA, Raskin P. Treatment of hypertension in adults with diabetes. Diabetes Care 2003;26: S80-2. [DOI] [PubMed] [Google Scholar]
- 12.Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309: 1286-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Contr Clin Trials 1986;7: 177-88. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327: 557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman E, Messerli FH, Goldbourt U. Intensive blood pressure control and drugs reduce morbidity and mortality in hypertension and diabetes mellitus. Evidence Based Med 2001;6: 44. [Google Scholar]
- 16.Wachtell K, Ibsen H, Olsen MH, Borch-Johnsen K, Lindholm LH, Mogensen CE, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med 2003;139: 901-6. [DOI] [PubMed] [Google Scholar]
- 17.Zuanetti G. Prognosis of diabetic patients post-MI: the role of ACE inhibitor treatment. GISSI-3 Investigators. J Diabetes Complications 1996;10: 139-40. [DOI] [PubMed] [Google Scholar]
- 18.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA 1995;273: 1450-6. [PubMed] [Google Scholar]
- 19.Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345: 1667-75. [DOI] [PubMed] [Google Scholar]
- 20.ACE-Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting-enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001;134: 370-9. [DOI] [PubMed] [Google Scholar]
- 21.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003;326: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe K, Juan W, Narasimman G, Ma M, Inoue M, Saito Y, et al. Comparative effects of angiotensin II receptor blockade (candesartan) with angiotensin-converting-enzyme inhibitor (quinapril) in rats with dilated cardiomyopathy. J Cardiovasc Pharmacol 2003;41: S93-7. [PubMed] [Google Scholar]
- 23.Ozer MK, Sahna E, Birincioglu M, Acet A. Effects of captopril and losartan on myocardial ischemia-reperfusion induced arrhythmias and necrosis in rats. Pharmacol Res 2002;45: 257-63. [DOI] [PubMed] [Google Scholar]
- 24.Klahr S, Morrissey J. Comparative effects of ACE inhibition and angiotensin II receptor blockade in the prevention of renal damage. Kidney Int 2002;62(Suppl 82): S23-6. [DOI] [PubMed] [Google Scholar]
- 25.Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet 2003;361: 117-24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.