Abstract
Objective
Intense emotions are known triggers of sudden cardiac death. However, the effect of typical daily emotion on repolarization has not been examined. We examined whether QT interval changes as a function of typical daily emotion in patients at risk for cardiac events in the context of emotion.
Methods
We studied 161 patients (114 females; mean age 35 years) with the congenital form of the Long QT Syndrome during daily activities. Each day for three days a 12-hour Holter recording was completed. Patients were paged 10 times per day at random times and rated the intensity of 16 prespecified emotions during the preceding 5 minutes. Measurements of QT interval and interbeat intervals were synchronized with emotion ratings.
Results
Low Arousal Positive Affect was associated with significant increases in QT interval corrected for heart rate (using Fridericia's QTc) (p<.001) whereas higher arousal Activated Positive Affect (p<.001) and Activated Negative Affect (p<.01) were associated with significant decreases in QTc. Changes in QTc as a function of daily emotion ranged from 5 msec increases to 11 msec decreases. High frequency heart rate variability (vagal tone) was positively correlated with QTc (p<.001). The effects of each positive emotion variable on QTc were greater in LQT2 than LQT1 patients (p<.001).
Conclusion
Ventricular repolarization duration (QTc) changes dynamically as a function of daily emotion. These changes are relatively small and do not constitute a risk in themselves. In the context of other risk factors, however, they may contribute to ventricular arrhythmias in vulnerable populations.
Keywords: QT interval, Long QT Syndrome, heart rate, heart rate variability, ecological momentary assessments, emotion
Introduction
Emotion is an evolutionarily designed system that has profound effects on the operation of nearly all systems in the human body. Consistent with this premise, Lane (1) argued that emotion and emotion regulation are the cornerstone of psychosomatic medicine. Yet many important questions remain about how emotion contributes to disease and death. For example, sudden cardiac death is the leading cause of death in the western world, responsible for at least 300,000 individuals per year in the U.S. alone (2), and is therefore a leading problem in contemporary cardiology (3). This is the case despite decades of work focused on the myocardium and cardiac-specific mechanisms. Most existing research on sudden cardiac death related to emotion focuses on instances of intense stress and strong emotions (4). Compelling evidence indicates that intense stress and negative emotion are triggers of cardiac events in about 20% of cases (5). Relatively little research, however, has examined the physiological effects of everyday emotions -- the more common, everyday affective states people experience on an ongoing basis. The current research examines the impact of everyday emotion on cardiac function in patients at risk for cardiac events in the context of emotion. By doing so, this research addresses the possibility that the influence of emotion on vulnerability to life-threatening arrhythmias is even broader than has been previously appreciated.
Long QT Syndrome (LQTS) has been called the Rosetta Stone for ventricular tachyarrhythmias (6). Because the myocardium, coronary arteries and conduction system are normal, the genetically-based repolarization abnormality that characterizes LQTS constitutes a simple but homogeneous abnormality that may make it possible to more easily detect the influence of factors that affect vulnerability to sudden cardiac death. Alternative clinical models such as coronary artery disease (CAD) are more biologically heterogeneous and would likely require study of many more individuals. In this study we examine patients with LQTS and examine how daily emotion influences changes in the QT interval.
Alterations in the QT interval, a marker of ventricular repolarization, may be due to inherited disorders such as the long- or short-QT syndrome (7,8) or from acquired conditions including drugs (9), cerebro-vascular disorders (10), acute coronary disease (11), and autonomic factors involving the sympathetic and parasympathetic nervous systems (12). These QT-altering disorders and conditions have been associated with increased risk for ventricular tachyarrhythmias, syncope, and sudden cardiac death. Recently, a common genetic variant has been identified that influences the QT interval by a few milliseconds in normal subjects (13). It is generally appreciated that a concordance of several factors may come into play at any time to explain variation in the QT interval and the occurrence of life-threatening arrhythmias in vulnerable subjects. Although various emotional triggers have been associated with life-threatening cardiac events in the LQTS (14,15), including startling events such as the ringing of an alarm clock, no studies in either healthy volunteers or any clinical group have examined changes in QT interval in relation to emotion during routine daily activities.
Changes in QT interval in relation to emotion have been studied in the context of stress and depression. A study of healthy physicians revealed that in the context of heart rate increases associated with emergency phone calls while on-call, QT interval was prolonged relative to the expected shortening associated with heart rate change (16). Another study of healthy volunteers showed that performance of stressful mental arithmetic was associated with prolongation of QT interval corrected for heart rate (17). A third study in patients with eating disorders revealed a positive correlation between QT interval corrected for heart rate and self-reported depression (18).
Previous studies of emotional triggers of cardiac events have typically relied on recalled emotions (14,19). The biases inherent in retrospections about emotions and behavior are well-established (e.g., retrospective reinterpretation; selection of events to describe; difficulties summarizing across diverse events; motivated forgetting) (20). “Event-sampling” techniques such as ecological momentary assessments (EMA) (21) are a recent innovation that overcomes many of these limitations. While in their natural social-ecological context subjects are asked to rate the intensity of emotions experienced at a particular moment. By virtue of contemporaneity, the EMA procedure provides less biased emotion self-reports than has been typical in previous research relating emotions to the propensity for life-threatening arrhythmias. Moreover, unlike emotion ratings made shortly after a cardiac event (19) subjects have no knowledge of their momentary QT interval and thus the latter cannot influence their ratings.
In a study involving 161 subjects with LQTS, we assessed the influence of emotions on the QT interval during usual daily activities. We hypothesized that alterations in emotional states during the day would have a definable influence on ventricular repolarization.
Methods
Overview
Due to the rarity of LQTS and the small number of patients in any one location, home visits were made to LQTS patients throughout the U.S. On each of three days a Holter recorder was attached to the patient for a 12-hour recording. Patients engaged in their usual daily activities and were paged (on vibration mode) 10 times per day at random times. Patients responded to the page by answering 59 questions using a Palm Personal Digital Assistant (PDA) pertaining to the 5 minutes preceding the page, including current activities (2 items), location (1 item), exertion intensity (1 item), social circumstances (24 items), and 22 emotion terms and 9 somatic symptoms rated on a 7-point intensity scale. Seventy-nine of the 3967 pages (1.7%) occurred while subjects were exercising. The intensity of exertion varied evenly across a 7-point exertion intensity scale. These data indicate that exertion had a negligible influence on our results. The current study focused on the emotion ratings. Clocks in the pager, Holter and PDA were synchronized.
Patients
Patients were recruited from the International Long QT Syndrome Registry located in Rochester, NY. Inclusion criteria limited enrollment to men and women ages 16 to 50 years who were genotype positive for LQT1 or LQT2, accounting for 90% of LQTS patients with genotypes (7). Exclusion criteria included diminished cognitive capacity interfering with informed consent or completion of the research procedures or lack of English fluency (needed for valid completion of self-report measures). Patients were not preselected for prior history of cardiac events, QTc duration, beta-blocker or ICD treatment. The study received approval from the appropriate IRB/ethics committees and all patients signed informed consent. Data were collected between January 2003 and July 2006.
Ecological Momentary Assessments (EMA)
Participants were paged 10 times per day for 3 days, using a modified random schedule. All signals were scheduled during a 12-hour window during usual waking hours, typically between 8 AM and 10 PM (only one subject had pages after midnight [25% for that subject]). Signals were constrained so that no two signals could occur within 60 minutes of each other. Participants were instructed to turn on their PDA as soon as possible after the page, to begin responding immediately, and to complete the 59-item protocol without interruption.
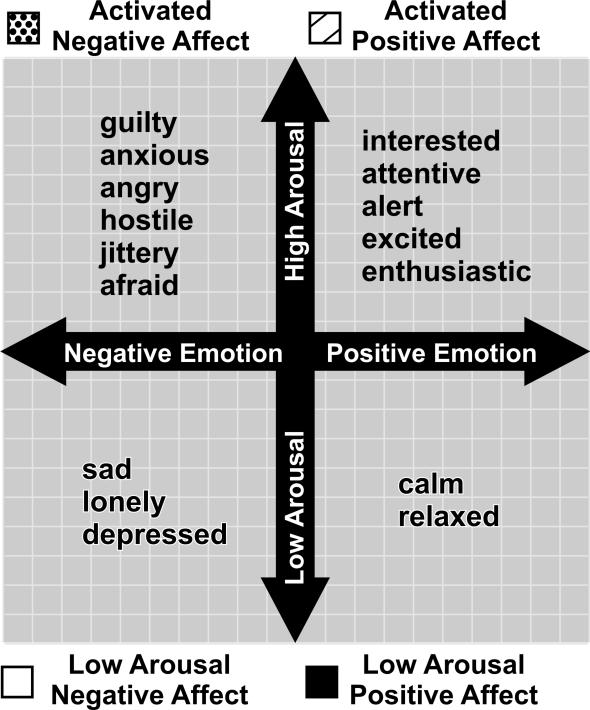
Based on previous demonstrations of the influence of intense emotions on ventricular arrhythmias and sudden death (5,22), we were particularly interested in activated (high arousal) forms of positive and negative affect. To minimize participant burden, we selected a briefer subset of 22 items for the EMA protocol from the 33-item Positive and Negative Affect Scale (23), a widely used and validated instrument that shows excellent discriminant validity between the positive and negative affect scales. Based on past psychometric studies and clinical relevance to this sample, we pre-selected a subset of 16 items for the current analysis: 1) Activated Positive Affect: interested, attentive, excited (in a positive way), enthusiastic, and alert (alpha = .82). 2) Activated Negative Affect: guilty, anxious, angry, hostile, jittery, and afraid (alpha = .70). We also included low-arousal affect terms representing constructs that have been linked to cardiovascular activity in the literature: 3) Low Arousal Negative Affect: sad (24), lonely (25), depressed (26) (alpha = .73), and 4) Low Arousal Positive Affect: calm, relaxed (27) (alpha = .81). Thus, the present pre-specified analysis included 16 of the 22 emotion terms rated. These four scales correspond to the four quadrants defined by two orthogonal dimensions of emotion self-reports, valence (positive-negative) and arousal (28) (see Figure 1).
Figure 1.
Location of Emotion Terms Used for EMA Ratings in Relation to Two Fundamental Dimensions of Emotion, Valence (Positive-Negative Emotion) and Arousal.
These adjectives were presented randomly with respect to the four affect scales but in the same order for all subjects in all trials. For each affect term, participants rated the extent to which they had experienced that emotion during the 5 minutes preceding the page, using a 0 (“not at all”) to 6 (“extreme”) scale (see Figure 2 caption for anchoring terms). To maximize variance among the emotion variables, and to maximize sensitivity to high intensity ratings, each of the four composite EMA variables was created by taking the maximum value among the individual items on that subscale for that particular page.
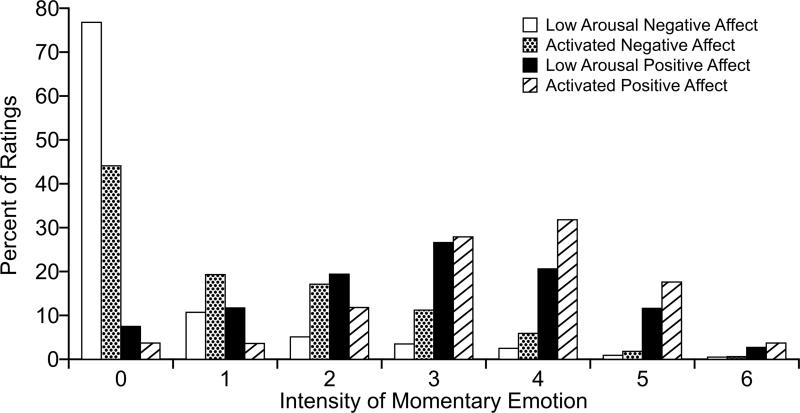
Figure 2. Percentage of Emotion Ratings At Each Intensity Level.
Bars depict the percentage of ratings at each intensity level for each of four scales of momentary emotion for 3967 events. Each scale was rated for each event. See Figure 1 for the terms comprising each scale. The 7-point rating scale was: 0-none, 1-mild, 2-somewhat, 3-moderate, 4-quite a bit, 5-very much, 6-extreme.
Compliance statistics for EMA ratings were computed by comparing the scheduled time of the page to the internal PDA record of when recording began. Subjects responded to 93.0% of the pages sent. Of these, 62.5%, 84.0%, 92.2%, 95.5%, and 96.9% were begun within 1, 5, 10, 15 and 20 minutes of the page, respectively. When we computed the percentage of reports begun within 15 minutes of the page for each participant, the median compliance rate was 98.3%. More than half the sample began all or all but one of their reports within 10 minutes, and only 19 participants began 4 or more reports more than 15 minutes after the page. On average it took 2.35 ±1.36 minutes to complete the EMA protocol.
These compliance statistics are very high for EMA research, based on comparable studies reported in the literature. To include as much data as possible, we decided to include all reports begun within 15 minutes of the page. This cutoff is well within the range typically recommended in the literature for EMA and similar protocols (20,29).
Electrocardiographic Measures
Holter ECGs were obtained during 24 hours on the first day and during 12 daytime hours on Day 2 and 3. Pre-specified 5-minute segments from 1-6 minutes prior to each page were used to calculate mean values of Fridericia's QTc (30) and heart rate. The summary Fridericia QTc value in Table 1 (mean =470 msec, SD = 33) was calculated from the Holter-derived values of QT interval and heart rate taken from the 3967 pages weighting each page equally. The Fridericia QTc correction for heart rate was chosen since this formula is preferred in studies evaluating changes in QTc over time (e.g., due to administration of drugs) in studied individuals (9,31). Although Bazett's formula is used most frequently clinically, this formula has several limitations by overestimating repolarization duration at fast heart rates and underestimating at low heart rates. Fridericia's correction is more reliable at low and high heart rates and therefore its use is preferred when there is a need for evaluating dynamic behavior of repolarization (9,31). QT was measured from the beginning of the QRS complex to the end of the T wave determined by the intersection of the T wave and the isoelectric line or to the nadir between T and U waves. U waves were not incorporated in the measurement of QT interval. The high frequency component of heart rate variability (HF-HRV) (0.15-0.40 Hz) was derived from a fast Fourier analysis of the RR interval spectrum over each 5-minute interval, reflecting mainly the influence of the parasympathetic system on the heart (32). ECG parameters were measured automatically using the Mortara H-Scribe System and the Super ECG program (Mortara Instruments, Milwaukee, WI). All electrocardiographic analyses were interpreted in a central core lab in a blinded manner regarding subject and timing of pages.
Table 1.
Demographic and Clinical Characteristics of the Study Sample.
| n = 161 | |
|---|---|
| Female | 117 (73%) |
| Age (years) | 35 (10) |
| QTc (msec, Fridericia correction for heart rate) | 470 (33) |
| Heart Rate (beats per minute) | 74 (14) |
| High Frequency Heart Rate Variability (msec2) | 39 (9) |
| Patients with previous arrhythmogenic cardiac event | |
| Yes | 80 (49.7%) |
| No | 80 (49.7%) |
| Unknown | 1 (.6%) |
| Patients taking beta blockers | 101 (63%) |
| Patients with Implanted Cardiac Defibrillator (ICD) | 18 (11%) |
| Genotype | |
| LQT1 | 103 (67%) |
| LQT2 | 58 (33%) |
Mean (SD) reported for age, QTc, heart rate, and HF-HRV. The QTc mean and SD values were derived from the 3967 events in this study.
Statistical Analysis
Descriptive statistics for time-independent participant-level variables were computed weighting each participant equally, while page-level time-dependent variables were summarized weighting each page equally. Each of the two 7-level positive affect measures were coarsened to 3 groups (0-1, 2-4, 5-6), yielding two parameters (2-4 vs. 0-1 and 5-6 vs. 0-1) to parsimoniously allow for potential nonlinearities. Negative affect measures were dichotomized as none (0) vs. any (1-6 coded as 1), given the high frequency of zeroes and the paucity of extreme levels of negative affect (see Figure 2). With all of the resulting predictors thus being indicator variables, multiple linear models are equivalent to multi-way analysis of variance (ANOVA) models, with no assumption of linearity for any given emotion.
We separately modeled each Holter outcome as a function of all measured emotions, using multivariable Conditional Linear Models (CLM) (33). Thus, the Holter outcome variables (e.g. Fridericia QTc) corresponding to the emotion ratings (e.g. 2-4 vs. 0-1) were compared. CLM conditions away the main effects of all participant-level time-independent variables (genotype, age, age2, genotype × sex, etc.) via the implicitly unconstrained participant-level intercepts, and thus controls for clustering by participant more completely than a mixed model with a random intercept. Inference was based on a robust sandwich estimator for the covariance matrix, using residuals from a more flexible mean function allowing separate coefficients for all 7 levels of each emotion, plus their interactions with genotype, as recommended with generalized estimating equations (34). Interactions with EMA variables were tested using robust 6-df F-tests for interactions with all 6 EMA parameters of our main effects model. Within-subject common correlations between Holter measures were computed after conditioning away participant-specific intercepts using Verbeke's orthonormal contrast matrix, and p-values were based on robust t-tests for the univariate CLM. Analyses were performed using Splus 7.0.0.
Results
Clinical Characteristics of Studied Patients (Table 1)
There were 161 patients (73% female) with a mean age of 35 years. The genotype distribution was 102 LQT1 (IKs), 58 LQT2 (IKr) and 1 LQT5 (the latter IKs mutation was grouped with LQT1 patients). As Table 1 indicates, 101 patients were taking beta blockers and 60 were not, half had prior arrhythmogenic cardiac events, and 11% had ICDs.
EMA Analyses
A total of 3967 pages, averaging 25 per patient, met inclusion criteria based on the presence and technical adequacy of both EMA and ECG data. The distribution of EMA ratings across the 7 intensity levels of each of the 4 EMA variables is depicted in Figure 2. The modal rating for the two negative affect variables (Low Arousal Negative Affect and Activated Negative Affect) was 0, whereas the modal rating for Low Arousal Positive Affect was “moderate” (3 on the 0-6 scale) and for Activated Positive Affect was “quite a bit” (4 on a 0-6 scale).
Association between EMA and ECG Parameters
Associations between EMA and ECG variables are shown in Table 2. The largest decreases in QTc occurred during Activated Positive Affect, with greater decreases in QTc when Activated Positive Affect was rated highest (5 “very much” or 6 “extreme”) and smaller decreases when Activated Positive Affect was rated as moderately intense (2 “somewhat,” 3 “moderate” or 4 “quite a bit”), relative to when Activated Positive Affect was rated 1 “mild” or 0 “none.” Activated Negative Affect when present (rated 1-6) was also associated with decreases in QTc relative to when Activated Negative Affect was rated 0, whereas when Low Arousal Negative Affect was present no significant changes occurred in QTc. Low Arousal Positive Affect at high levels (calm and/or relaxed rated 5 “very much” or 6 “extreme”) and moderate levels (calm and relaxed rated 2 “somewhat,” 3 “moderate” or 4 “quite a bit”) were associated with QTc increases. For QTc, beta of −7.9 msec on Activated Positive Affect rated 2, 3, or 4 means that compared to Activated Positive Affect values of 0 or 1, a value of 2, 3, or 4 is associated with a mean within-subject decrease of 7.9 msec in QTc. More generally, the QTc model states that as one emotion varies, while holding the subject and his/her other EMA variables constant, QTc changes on average by beta.
Table 2.
Multivariable Conditional Linear Regression Coefficients (Beta) for All Emotions, Separately Modeling Each of Four Outcomes: (1) QTc Based on our Genotype-Specific Correction for Heart Rate, (2) Fridericia's QTc, (3) Heart Rate, and (4) High Frequency Heart Rate Variability (HF-HRV).1
| QTc, adjusted for log(heart rate) and its interaction with genotype | QTc (Fridericia) | Heart Rate | HF-HRV | |
|---|---|---|---|---|
| Emotion Predictor | Beta (SE) p | Beta (SE) p | Beta (SE) p | Beta (SE) p |
| Activated Positive Affect 2-4 (vs. 0-1) | −5.1 (1.2) <.001 | −7.9 (1.5) <.001 | +4.8 (0.8) <.001 | −4.5 (0.7) <.001 |
| Activated Positive Affect 5-6 (vs. 0-1) | −8.1 (1.5) <.001 | −11.1 (2.0) <.001 | +4.8 (1.0) <.001 | −4.6 (0.7) <.001 |
| Low Arousal Positive Affect 2-4 (vs. 0-1) | +0.9 (1.0) .35 | +1.9 (1.0) .06 | −3.2 (0.6) <.001 | +1.5 (0.4) <.001 |
| Low Arousal Positive Affect 5-6 (vs. 0-1) | +3.1 (1.4) .03 | +4.8 (1.4) .001 | −5.2 (0.8) <.001 | +3.8 (0.6) <.001 |
| Activated Negative Affect 1-6 (any vs. none) | −2.1 (0.8) .01 | −2.2 (0.8) .01 | +0.8 (0.4) .03 | −0.9 (0.3) .002 |
| Low Arousal Negative Affect 1-6 (any vs. none) | −1.0 (1.0) .29 | −1.1 (1.0) .27 | −0.4 (0.5) .41 | +0.3 (0.4) .51 |
| R2 | 11.3% | 2.8% | 3.8% | 5.0% |
All effects of emotions above are adjusted for each other emotion; however, the results were similar when each emotion was modeled separately, unadjusted for the other three emotion predictors.
For each emotion predictor, the regression coefficient (Beta), its robust Standard Error (SE), and robust p-value are listed. From left to right, the four multivariable model outcomes are: (1) Fridericia's QTc adjusted for the natural log of heart rate plus its interaction with genotype, (2) Fridericia's QTc with no further adjustment for heart rate, (3) heart rate, and (4) HF-HRV. Regression coefficients for QTc are in msec, and heart rate in beats per minute. R2 refers to the percentage of variance in the dependent variable explained by the entire model, including log(heart rate) and its highly significant interaction with genotype when included in (1), conditional on the participant-specific intercepts (whose contribution is thus not counted in either the numerator nor the denominator).
Table 2 shows that the results were essentially the same, with slightly attenuated effect sizes, when additional variance due to heart rate was removed from QTc by further adjusting for log(heart rate) (p < .001) and its interaction with genotype (p < .001). Changes in HF-HRV and heart rate as a function of EMA variables showed similar patterns to those of QTc, except the signs of the significant effects were reversed for heart rate. The common within-subject correlations were: QTc and log(heart rate), −.21; QTc and HF-HRV, .26; log(heart rate) and HF-HRV, −.78; p < .001 for all.
Interactions with EMA Variables
Next we determined whether the QTc findings in Table 2 differed as a function of interactions of emotions with age, sex, genotype, beta blocker status, ICD treatment or previous cardiac events. For the purposes of testing these interactions, and for ease of interpretation, all potential modifiers were dichotomous, with age dichotomized at 35 years. There was insufficient evidence of interactions of EMA variables with age, sex, or ICD treatment (p > .10 for each), with or without adjusting QTc for heart rate and its interaction with genotype. There was borderline evidence of an interaction with beta-blocker status (p = .04), driven almost entirely by its interaction with Activated Negative Affect (p = .04), whereby the effect of Activated Negative Affect appeared to be solely among those on beta-blockers (Beta = −3.5 ms, compared with −2.2 ms in the main-effect model) and not those off beta-blockers (beta = .2 ms, p = .87). There was significant evidence of interactions with genotype (p<.001), which was driven by interactions with the positive emotion variables but not Activated Negative Affect (p = .15) nor Low Arousal Negative Affect (p = .97). Table 3 shows that the effects of each positive emotion variable on QTc were in the same direction for each genotype but significantly more pronounced for LQT2 compared to LQT1 patients. However, when testing these same interactions in models for QTc further adjusted for heart rate and its interaction with genotype (as in column 1 of Table 2), there was insufficient evidence of any such interactions, including those with genotype and beta-blockers. Thus, these interactions appear to be largely attributable to genotype-specific effects of heart rate on QTc that no global heart rate correction could eliminate. However, there was insufficient evidence of interactions of genotype with emotions when modeling heart rate (p=.22) or HF-HRV (p=.24) as the outcome; so the effects of emotions on heart rate and HF-HRV do not appear to significantly differ by genotype.
Table 3.
Genotype-Specific Effects (Beta) of Emotions on Fridericia's QTc, Estimated via a Single Conditional Linear Model for QTc, Simultaneously Including All Emotions and Their Interactions with Genotype.
| Emotion Predictor | LQT1 Beta (SE) p |
LQT2 Beta (SE) p |
Interaction p-value |
|---|---|---|---|
| Activated Positive Affect: 2-4 vs. 0-1 | −4.7 (1.2) <.001 | −12.9 (3.4) <.001 | .02 |
| Activated Positive Affect: 5-6 vs. 0-1 | −5.4 (1.3) <.001 | −20.6 (4.6) <.001 | .002 |
| Low Arousal Positive Affect: 2-4 vs. 0-1 | +0.3 (1.1) .75 | +4.6 (2.0) .02 | .06 |
| Low Arousal Positive Affect: 5-6 vs. 0-1 | +1.5 (1.4) .26 | +10.7 (3.0) <.001 | .005 |
| Activated Negative Affect: >0 vs. 0 | −1.2 (0.8) .16 | −4.1 (1.8) .03 | .15 |
| Low Arousal Negative Affect: >0 vs. 0 | −1.1 (0.9) .22 | −1.2 (2.4) .61 | .97 |
| Overall 6-df Robust F-test of Interaction | .001 |
Genotype-specific regression coefficients (Beta), SE, and p-values in the second and third columns refer to that genotype group alone, whereas the interaction p-values in the fourth column test equality of the genotype-specific effects for each emotion predictor (and overall, bottom row). R2 = 4.1% for this 12-parameter genotype interaction model, which does not include additional adjustments for heart rate and its interaction with genotype.
Discussion
The current study, to our knowledge, is the first of its kind in which people are paged at random times throughout the day and their momentary emotional experiences and ventricular repolarization values are simultaneously assessed. Our observation of statistically significant associations between emotion and QTc establishes for the first time that emotions during routine daily activities have a definable effect on ventricular repolarization duration in predisposed individuals.
The validity of the emotion ratings that we obtained were supported by the heart rate findings. Consistent with previous findings regarding the arousal dimension of emotion (35), heart rate increases were numerically greater during activated positive or negative emotion than during low arousal positive or negative emotion. Heart rate changes in relation to the valence (positive-negative) dimension were also consistent with previous findings showing greater effects for appetitive (positive) emotions (36), in that activated positive emotion was associated with greater heart increases than during activated negative emotion, and low arousal positive emotion was associated with greater heart rate decreases than low arousal negative emotion.
Previous research on dynamic changes in QT interval in relation to emotion has been quite limited. Prolonged QT intervals were observed in two previous studies involving acute stress (16,17), a condition that involves a predominance of sympathetic over parasympathetic mechanisms (37). In the current study the “activated” conditions, both positive and negative, were associated with QT interval decreases and the low arousal positive conditions were associated with QT interval increases. These findings appear paradoxical based on predictions derived from stress research, but make sense when considered from the perspective of routine daily activities. Under these circumstances parasympathetic control of cardiovascular regulation predominates (37). Consistent with this thesis, activated positive and activated negative affect were associated with vagal tone decreases and low arousal positive affect was associated with vagal tone increases. Moreover, heart rate was strongly negatively correlated with vagal tone and QTc was significantly positively correlated with vagal tone. Together these findings indicate that emotion influences repolarization even under routine circumstances. Given the strong association between daily emotions and vagal tone, however, the data may not be generalizable to emotional states accompanied by pronounced sympathetic nervous system activation.
In addition to the standard Fridericia correction of QT interval for heart rate (QTc) (30), we also more stringently controlled QTc for residual variance due to heart rate to eliminate chronotropic effects from our measure of repolarization. These analyses revealed that Fridericia's QTc in fact leaves significant residual variance due to heart rate embedded within it, at least for LQT2 subjects. However, removing this residual within-subject heart rate variance as in this study is not appropriate for routine clinical research that typically involves a comparison between individuals. For the latter purpose Fridericia's QTc is preferable (9,31). A second reason for more stringently controlling QTc for heart rate was to examine the association between emotion variables and repolarization without confounding by emotion-heart rate relationships. Our analyses showing strong inter-relationships between HF-HRV and both heart rate and QTc indicate that vagal tone influences the latter two parameters. Indeed, it is well-established that under conditions of relative safety, as in routine daily activities, emotion and emotion regulation are predominantly regulated by vagal tone mediated by the phylogenetically newer myelinated vagus under the control of the nucleus ambiguus in the brainstem (relative to the phylogentically older unmyelinated vagus under the control of the dorsal motor nucleus) (38). As such, given the strong covariation of heart rate and HF-HRV, the elimination of additional variance due to heart rate from QTc also removes vagal tone variance that is intrinsic to the physiology of emotion under everyday circumstances. Thus, Fridericia QTc provides the most accurate estimate of the magnitude of the association between daily emotion and ventricular repolarization duration because if all variance due to heart rate (and vagal tone) is eliminated the true association is underestimated.
Increased vagal tone is associated with increased QT interval duration, both in animal models with fixed heart rate (39,40) and in humans during fixed pacing and during sleep (41,42). The present observations therefore extend previous observations on the positive association between vagal tone and QT interval to the domain of emotion, which is important given the role of vagal tone in emotion and emotion regulation (38) and the role of emotion in sudden cardiac death (4,5).
Genotype proved to be an important explanatory variable, in that the effects of emotion on QTc were consistently stronger in LQT2 than LQT1 patients. These findings are consistent with a retrospective study of triggers of cardiac events in patients with LQTS, which showed that emotion was a more common trigger of cardiac events in LQT2 than LQT1 patients (14). The current findings raise the possibility that the vulnerability of LQT2 patients to emotions as electrically destabilizing influences may extend beyond sudden, short-lived events such as loud noises.
In a retrospective study of patients with LQTS we previously showed that lower levels of happiness during the prior day were a risk factor for arrhythmogenic cardiac events (43). We hypothesized that lower happiness might be associated with vagal withdrawal, which could increase the risk for cardiac events. That study did not attempt to disentangle high arousal from low arousal positive emotional states. In the current study, consistent with expectations based on arousal, as shown in Table 2, we observed that low arousal positive emotion (calm and relaxed) was associated with heighted vagal tone and that activated positive emotion was associated with reduced vagal tone and that low arousal positive emotion and activated positive emotion had opposite effects on QTc. Although longer QT intervals are associated with greater risk and shorter QT intervals are associated with lower risk in patients with LQTS (44), these associations are based on the entire QT interval derived from resting ECG data, not momentary changes in QT interval as a function of emotion. Whether momentary changes in QT interval due to emotion influence the timing of cardiac events has not been determined. Another fundamental reason why the data from the two studies may not be exactly comparable is that under everyday circumstances cardiac function and emotion are predominantly under vagal control (38), whereas emotional triggers of cardiac events typically involve negative states that are high arousal associated with sympathetic activation and/or vagal withdrawal (4,5). In this study high levels of activated negative emotional states were very uncommon.
The emotional states that we examined were ongoing, typically low-level emotional states that are part of daily living. Evidence shows that the majority of emotions experienced in life are of low intensity, and that low intensity emotions are more readily forgotten (45,46). Indeed, until the relatively recent advent of EMA and related experience sampling techniques, methods for reliably measuring such low intensity experiences did not exist. These considerations highlight the importance of our findings in the sense that they apply to the majority of one's waking experience and help to explain why such associations have not been previously observed.
As might be expected in the context of everyday emotion, the heart rate changes that we observed were of smaller magnitude than would typically be observed during exercise. Similarly, the changes observed in QT interval in this study were relatively small. The magnitude of the changes in QT interval, however, is comparable to those associated with certain specific gene variants (e.g., NOS1A associated with 2-5 msec changes [13]) and certain drugs (e.g. moxifloxacin causing a 4-7 msec increase in QT interval [47]). Their clinical significance is further supported when it is considered that sudden cardiac death is a multifactorial phenomenon (48). Many factors play a small role in affecting QTc, and when they coincide and act together, they provide our best current explanation as to why a cardiac event occurs at a give time and day of the week when it hasn't occurred on similar days and times in the past. The clinical importance of these findings is also evident when it is consider that our data reflect averages and thus any given individual may have larger effects in certain emotional contexts, particularly as a function of genotype, beta blocker status, or a combination thereof. Indeed, these results may provide a new lead in identifying that subgroup of patients with LQTS who will experience life-threatening ventricular arrhythmias in the future. Conversely, the present data also suggest that a reduction in the QTc duration with certain daily emotions could be associated with a decreased risk for ventricular arrhythmias. Should this be demonstrated in future research this association would have obvious clinical relevance. Our study did not involve a healthy control group. It is therefore not known whether the observed changes in QT interval as a function of daily emotion are specific for patients with LQTS or whether similar effects are present in other clinical groups or in healthy subjects. However, previous stress research has shown similar QT prolongation in healthy individuals (16,17) and in LQTS patients (49), and no previous study has examined dynamic changes in QT interval as a function of daily emotion in healthy individuals. Second, our subjects reported emotions in a limited range of intensity, particularly negative emotions. The latter were often of very low intensity (0 was the modal response for activated negative affect and low arousal negative affect), and thus extremely intense emotions that typically occur as precipitants of arrhythmias were generally not observed in this investigation. Future studies may benefit from studying the full range of positive and negative emotions. Third, we did not evaluate the effect of specific emotions such as anger, depression or happiness because of our desire to examine broad dimensions of emotion.
In conclusion, we observed that typical daily emotions have a definable effect on cardiac repolarization. As such, the current findings highlight the dynamicity of the QT interval in daily living in patients with an inherited cardiac repolarization disorder. It is likely that these emotion-related repolarization changes are among the multiple factors that contribute to arrhythmic cardiac events in patients with LQTS.
Acknowledgement
The authors thank Jennifer Robinson for assistance with identifying patients from the International Long QT Syndrome Registry, Sabrina Geoffrion, Marla Jirak and Gini Roberts for study coordination, Katherine Armstrong, Marcia Willis, Wendy Brittain, Paula Beerman and Martha Hoxley for home visit data collection, Rahul Raguram, Hemant Arora and Guruprasad Rajaraman for data processing, Cheryl Carmichael and Fen-Fang Tsai for EMA data management, Mark Andrews for data base management and Carolyn Fort for assistance with regulatory matters, all of whom received compensation.
Funding for this study was provided by NHLBI HL68764, HL51618, and HL33843, the Warmer Foundation and the University of Rochester. There was no funding from industry.
Abbreviations
- CAD
coronary artery disease
- CLM
Conditional Linear Models
- ECG
electrocardiogram
- EMA
ecological momentary assessments
- HF-HRV
high frequency heart rate variability
- ICD
implanted cardioverter- defibrillator
- LQTS
Long QT Syndrome
- QTc
Fridericia correction of QT interval for heart rate
- PDA
Personal Digital Assistant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lane R. Neural substrates of implicit and explicit emotional processes: A unifying framework for psychosomatic medicine. Psychosom Med. 2008;70:213–230. doi: 10.1097/PSY.0b013e3181647e44. [DOI] [PubMed] [Google Scholar]
- 2.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 3.Richter S, Duray G, Gronefeld GW, Israel CH, Hohnloser S. Prevention of sudden cardiac death: lessons from recent controlled trials. Circulation Journal. 2005;69:625–9. doi: 10.1253/circj.69.625. [DOI] [PubMed] [Google Scholar]
- 4.Lampert R. Emotion and sudden cardiac death. Expert Review of Cardiovascular Therapy. 2009;2009;7:723–5. doi: 10.1586/erc.09.75. [DOI] [PubMed] [Google Scholar]
- 5.Ziegelstein RC. Acute emotional stress and cardiac arrhythmias. JAMA. 2007;298:324–329. doi: 10.1001/jama.298.3.324. [DOI] [PubMed] [Google Scholar]
- 6.Zipes DP. The long QT interval syndrome. A Rosetta stone for sympathetic related ventricular tachyarrhythmias. Circulation. 1991;84:1414–1419. doi: 10.1161/01.cir.84.3.1414. [DOI] [PubMed] [Google Scholar]
- 7.Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest. 2005;115(8):2018–2024. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zareba W, Cygankiewicz I. Long QT syndrome and short QT syndrome. Progr Cardiovasc Dis. 2008;51(3):264–278. doi: 10.1016/j.pcad.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Zareba W. Drug induced QT prolongation. Cardiol J. 2007;14(6):523–533. [PubMed] [Google Scholar]
- 10.Tatschl C, Stöllberger C, Matz K, Yilmaz N, Eckhardt R, Nowotny M, Dachenhausen A, Brainin M. Insular involvement is associated with QT prolongation: ECG abnormalities in patients with acute stroke. Cerebrovasc Dis. 2006;21(1-2):47–53. doi: 10.1159/000089594. [DOI] [PubMed] [Google Scholar]
- 11.Kenigsberg DN, Khanal S, Kowalski M, Krishnan SC. Prolongation of the QTc interval is seen uniformly during early transmural ischemia. J Am Coll Cardiol. 2007;49(12):1299–1305. doi: 10.1016/j.jacc.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Sundaram S, Carnethon M, Polito K, Kadish AH, Goldberger JJ. Autonomic effects on QTRR interval dynamics after exercise. Am J Physiol Heart Circ Physiol. 2008;294(1):H490–H497. doi: 10.1152/ajpheart.00046.2007. [DOI] [PubMed] [Google Scholar]
- 13.Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M, West K, Kashuk C, Akyol M, Perz S, Jalilzadeh S, Illig T, Gieger C, Guo CY, Larson MG, Wichmann HE, Marbán E, O'Donnell CJ, Hirschhorn JN, Kääb S, Spooner PM, Meitinger T, Chakravarti A. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38(6):644–651. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103(1):89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 15.Moss AJ, Robinson JL, Gessman L, Gillespie R, Zareba W, Schwartz PJ, Vincent GM, Benhorin J, Heilbron EL, Towbin JA, Priori SG, Napolitano C, Zhang L, Medina A, Andrews ML, Timothy K. Comparison of clinical and genetic variables of cardiac events associated with loud noise versus swimming among subjects with the long QT syndrome. Am J Cardiol. 1999;84(8):876–879. doi: 10.1016/s0002-9149(99)00458-0. [DOI] [PubMed] [Google Scholar]
- 16.Toivonen L, Helenius K, Viitasalo M. Electrocardiographic repolarization during stress from awakening on alarm call. J Am Coll Cardiol. 1997;30:774–779. doi: 10.1016/s0735-1097(97)00222-2. [DOI] [PubMed] [Google Scholar]
- 17.Andrássy G, Szabo A, Ferencz G, Trummer Z, Simon E, Tahy A. Mental stress may induce QT-interval prolongation and T-wave notching. Ann Noninvas Electro. 2007;12(3):251–259. doi: 10.1111/j.1542-474X.2007.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takimoto Y, Yoshiuchi K, Akabayashi A. Effect of mood states on QT interval and QT dispersion in eating disorder patients. Psychiatry & Clinical Neurosciences. 2008;62(2):185–9. doi: 10.1111/j.1440-1819.2008.01753.x. [DOI] [PubMed] [Google Scholar]
- 19.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. Triggering of acute myocardial infarction onset by episodes of anger. Circulation. 1995;92(7):1720–1725. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz N, Sudman S, editors. Answering Questions: Methodology for Determining Cognitive and Communicative Processes in Survey Research. Jossey-Bass; San Francisco, CA: 1996. [Google Scholar]
- 21.Reis HT, Gable SL. Event-sampling and other methods for assessing everyday experience. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Social Psychology. Cambridge University Press; New York, NY: 2000. pp. 190–222. [Google Scholar]
- 22.Verrier RL, Dickerson LW, Nearing BD. Behavioral states and sudden cardiac death. Pacing Clin Electophysiol. 1992;15:1387–93. [PubMed] [Google Scholar]
- 23.Watson D, Clark LA, Tellegan A. Development and validation of brief measures of positive and negative affect: the PANAS Scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 24.Rees WD, Lutkins SG. Mortality of bereavement. Br Med J. 1967;4(5570):13–16. doi: 10.1136/bmj.4.5570.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawkley LC, Burleson MH, Berntson GG, Cacioppo JT. Loneliness in everyday life: Cardiovascular activity, psychosocial context and health behaviors. J Pers Soc Psychol. 2003;85(1):105–120. doi: 10.1037/0022-3514.85.1.105. [DOI] [PubMed] [Google Scholar]
- 26.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105(9):1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 27.Benson H, Rosner BA, Marzetta BR, Klemchuk HM. Decreased blood-pressure in pharmacologically treated hypertensive patients who regularly elicited relaxation response. Lancet. 1974;1(7852):289–291. doi: 10.1016/s0140-6736(74)92596-3. [DOI] [PubMed] [Google Scholar]
- 28.Feldman Barrett L, Russell J. Independence and bipolarity in the structure of current affect. J Pers Soc Psychol. 1998;74(4):967–984. [Google Scholar]
- 29.Schwartz JE, Stone AA. The analysis of real-time momentary data: A practical guide. In: Stone AS, Shiffman S, Atienza AA, Nebeling L, editors. The Science of Real Time Data Capture. Oxford University Press; New York: 2007. pp. 76–113. [Google Scholar]
- 30.Indik JH, Pearson EC, Fried K, Woosley RL. Bazett and Fridericia QT correlation formulas interfere with measurement of drug-induced changes in QT interval. Heart Rhythm. 2006;3(9):1003–1007. doi: 10.1016/j.hrthm.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 31.J Morganroth J, Shah RR, Scott JW. Evaluation and management of cardiac safety using the electrocardiogram in oncology clinical trials: Focus on cardiac repolarization (QTc Interval). Clin Pharmacol Ther. 2010;87(2):166–175. doi: 10.1038/clpt.2009.214. [DOI] [PubMed] [Google Scholar]
- 32.Bigger JT., Jr. Heart rate variability: frequency domain. In Noninvasive Electrocardiology: Clinical Aspects of Holter Monitoring. In: Moss AJ, Stern S, editors. W.B.Saunders; London: 1996. pp. 175–198. [Google Scholar]
- 33.Verbeke G, Spiessens B, Lesaffre E. Conditional Linear Mixed Models. Am Stat. 2001;55:25–34. [Google Scholar]
- 34.Diggle PJ, Heagerty P, Liang K-Y, Zeger S. Analysis of Longitudinal Data. 2nd ed. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- 35.Brosschot JF, Thayer JF. Heart rate response is longer after negative emotions than after positive emotions. Int J Psychophysiol. 2003;50(3):181–187. doi: 10.1016/s0167-8760(03)00146-6. [DOI] [PubMed] [Google Scholar]
- 36.Fowles DC. Motivational effects on heart rate and electrodermal activity: Implications for research on personality and psychopathology. Journal of Research in Personality. 1983;17(1):48–71. [Google Scholar]
- 37.Pagani M, Mazzuero G, Ferrari A, Liberati D, Cerutti S, Vaitl D, Tavazzi L, Malliani A. Sympathovagal interaction during mental stress. A study using spectral analysis of heart rate variability in healthy control subjects and patients with a prior myocardial infarction. Circulation. 1991;83(4 Suppl):II43–51. [PubMed] [Google Scholar]
- 38.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martins JB, Zipes DP, Lund DD. Distribution of local repolarization changes produced by efferent vagal stimulation in the canine ventricles. J Am Coll Cardiol. 1983;2(6):1191–9. doi: 10.1016/s0735-1097(83)80350-7. [DOI] [PubMed] [Google Scholar]
- 40.Harada T, Abe J, Shiotani M, Hamada Y, Horii I. Effect of autonomic nervous function on QT interval in dogs. J Toxicol Sci. 2005;30(3):229–37. doi: 10.2131/jts.30.229. [DOI] [PubMed] [Google Scholar]
- 41.Browne KF, Prystowsky E, Heger JJ, Chilson DA, Zipes DP. Prolongation of the Q-T interval in man during sleep. Am J Cardiol. 1983;52(1):55–9. doi: 10.1016/0002-9149(83)90068-1. [DOI] [PubMed] [Google Scholar]
- 42.Bexton RS, Vallin HO, Camm AJ. Diurnal variation of the QT interval--influence of the autonomic nervous system. Br Heart J. 1986;55(3):253–8. doi: 10.1136/hrt.55.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lane RD, Reis H, Peterson D, Zareba W, Moss A. Happiness and stress alter susceptibility to cardiac events in Long QT Syndrome. Ann Noninvas Electro. 2009;14:191–198. doi: 10.1111/j.1542-474X.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, Folli R, Cappelletti D. Risk stratification in the Long-QT Syndrome. NEJM. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 45.Diener E, Larsen RJ, Levine S, Emmons RA. Intensity and frequency: dimensions underlying positive and negative affect. J Pers Soc Psychol. 1985;48:1253–1265. doi: 10.1037//0022-3514.48.5.1253. [DOI] [PubMed] [Google Scholar]
- 46.Redelmeier DA, Kahneman D. Patients' memories of painful medical treatments: real-time and retrospective evaluations of two minimally invasive procedures. Pain. 1996;66:3–8. doi: 10.1016/0304-3959(96)02994-6. [DOI] [PubMed] [Google Scholar]
- 47.Van Bambeke F, Tulkens PM. Safety profile of the respiratory fluoroquinolone moxifloxacin: comparison with other fluoroquinolones and other antibacterial classes. Drug Safety. 2009;32(5):359–78. doi: 10.2165/00002018-200932050-00001. [DOI] [PubMed] [Google Scholar]
- 48.Spooner PM, Albert C, Benjamin EJ, Boineau R, Elston RC, George AL, Jr, Jouven X, Kuller LH, MacCluer JW, Marbán E, Muller JE, Schwartz PJ, Siscovick DS, Tracy RP, Zareba W, Zipes DP. Sudden cardiac death, genes, arrhythmogenesis: consideration of new population and mechanistic approaches from a National Heart, Lung, and Blood Institute Workshop, Part I. Circulation. 2001;103(19):2361–2364. doi: 10.1161/01.cir.103.19.2361. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu W, Antzelevitch C. Cellular basis for long QT, transmural dispersion of repolarization, and torsade de pointes in the long QT syndrome. J Electrocardiol. 1999;32(Suppl):177–184. doi: 10.1016/s0022-0736(99)90077-8. [DOI] [PubMed] [Google Scholar]