Abstract
Background
Polycystic ovary syndrome (PCOS) is one of the most common hormonal disorders that can lead to irregular menstrual cycles and hyperandrogenism. Reduced levels of progesterone and increased estrogen in these women can perpetually stimulate the endometrial tissue of the uterus. In this study, we assess the effect of PCOS induction by estradiol valerate (EV) in a rat model.
Materials and Methods
In this experimental study, adult female Wistar rats that weighed approximately 200 g were divided into control, sham, and experimental groups (n=6 per group). The experimental group received subcutaneous injections of 2 mg EV for induction of PCOS. We confirmed the presence of PCOS in the experimental group rats. Rats from all groups were subsequently killed, after which their uteri were removed and fixed for histological and cytological analyses. The uterine tissue sections were stained with hematoxylin and eosin (H&E) and iron hematoxylin (iron-H). We examined epithelium height, thickness of the uterus wall, and frequency of the mitotic cells. The data were assessed at α=0.05.
Results
Uterine tissue findings from the experimental group showed significant increases in the height of the uterus luminal epithelium, the thickness of the uterus wall, and the frequency of eosinophils in the endometrial stroma. We observed an increased frequency of mitotic cells in the experimental group in both luminal and glandular epithelia of the uterus. An increased rate of the glandular epithelium region was noticeable and significant.
Conclusion
Induction of PCOS by EV could change the proliferation rate in the endo- metrial tissue of the uterus.
Keywords: Uterus, Estradiol Valerate, Polycystic Ovary Syndrome, Mitosis, Rat
Introduction
Polycystic ovary syndrome (PCOS) is a hormonal imbalance disorder (1, 2) that occurs in approximately 4-18% of reproductive-aged women (12 to 45 years) (3). PCOS is a metabolic and reproductive disorder with characteristic features that include hyperandrogenism, irregular menstrual cycles, insulin resistance, obesity, hirsutism, and acne (4). Anovulation that results from PCOS is the most common cause of infertility in women (5). Features of PCOS may manifest at any age and range from childhood (premature puberty), teenage (hirsutism, menstrual abnormalities), early adulthood and middle life (infertility, glucose intolerance), to later life (diabetes mellitus and cardiovascular diseases) (6).
Numerous evidences affirm the fact that endocrinologic and metabolic abnormalities in PCOS may have complex effects on endometrial tissue, thus contributing to infertility and endometrial disorders in women with this syndrome (7). Long-term PCOS increases the risk of hyperplasia, endometrial cancer (EC), and metabolic syndrome (8). Endometrial hyperplasia is a premalignant condition that usually heralds EC (9). It has been reported that women with PCOS and endometrial hyperplasia have a four times greater risk of developing EC than women without PCOS (10). Hyperplasia and uterine cancer have been observed in women with PCOS who received no treatment (11).
The two main types of EC are estrogen-dependent type Ι and estrogen-independent type ΙΙ (12). It is widely believed that PCOS is one of the most impressive risk factors that promote type I EC (10, 13, 14). Prolonged exposure of the endometrium to estrogen, as a consequence of anovulation, is suggested to be the prime cause of this increased risk (15). Therefore, the hormonal imbalance associated with PCOS can alter endometrial tissue homeostasis and promote cell proliferation (16). In humans, continuous exposure of the endometrium to estrogen can lead to endometrial hyperplasia (17). Progesterone acts as a protective factor against estrogen-driven uterine growth and proliferation (18).
Steroid hormone levels regulate the cycle of cellular proliferation and apoptosis in the endometrial tissue. Therefore, a firm balance between these two processes would secure the normal function of the endometrium (19). Endocrine-metabolic situations associated with abnormalities in plasma hormone concentrations, as seen with PCOS, can affect the processes that occur in the endometrium, which includes cell proliferation, differentiation and response to biological stimuli (20). Estrogen is a hormone that affects the uterus. Strong activation of proliferative activity is the most important physiological effect of estrogen hormones in the uterus (21). Significant consequences of (particularly long-term) endometrial exposure to estrogen are morphogenetic alterations that include modified type of luminal and glandular epithelia, glandular shape, and the glandular to stromal ratio (22, 23).
Estradiol valerate (EV) is used to create PCOS by inducing hormone abnormalities (24). EV, which is introduced as a prodrug, is an ester derived from 17β-estradiol. EV is normally cleared in blood plasma and the liver into 17β-estradiol by esterase activity (25). The 17β-estradiol metabolizing procedure includes an array of reversible and non-reversible enzyme-mediated reactions (26). The metabolites 17β-estradiol and estron may predict the risk of breast (27) and other hormone-related cancers (28). Studies show that hormonal abnormalities attributed to EV can create a phenotype similar to PCOS (29). In this study we focus on tissue changes and proliferation activity of the uterus in a rat model of PCOS induced by EV.
Materials and Methods
Animals
The present experimental study used 18 adult female Wistar rats that weighed 200 ± 20 g. Animals were obtained from the Pharmacology Department of Tehran University and maintained in special cages under standard conditions of 22ºC, a 12-hour dark/light cycle, and free access standard chow and water. In order to conduct a comparative evaluation, we divided the rats into three groups of 6 animals per group: control (normal rats), experimental group or PCOS (rats that received EV), and sham (rats that received EV solvent). Before the induction, we confirmed the rats’ normal estrous cycles through daily vaginal smears over two weeks. Animals that had at least two normal estrus cycles were selected for PCOS induction.
The Ethics Committee of the Biological Sciences Faculty at Kharazmi University, Tehran, Iran approved this study.
Induction of polycystic ovary syndrome
We used EV to induce the polycystic condition. Each experimental rat received 2 mg of EV, dissolved in 0.2 ml sesame oil, through a single subcutaneous injection at the inguinal region. Rats in the sham group received an equal volume of sesame oil. Subsequently, vaginal smears of these rats were monitored for 60 days, until the time when abnormal estrus cycles and persistent vaginal cornification (PVC) occurred as a sign of the presence of ovarian cysts and early confirmation of PCOS induction (30). Rats in the sham group that received sesame oil showed no evidence of abnormalities in estrus cycles or vaginal smears. Hence, further experiments were concentrated mainly on control and PCOS rats.
Histological and cytological studies
On the 60th day after the EV injection, rats from all groups were sacrificed and the uterine specimens were fixed in 10% formaldehyde. The tissue samples were dehydrated by graded series of ethanol, embedded in paraffin, then sectioned into 5-7 µm sections prior to microscopic analysis.
Histological evaluations of the uterus and determination of mitosis
As mentioned, the effect of estrogens on the uterus tissue is chiefly related to its strong invigorating impact on cell proliferation. Long-term exposure to estrogen leads to uterus endometrium overgrowth and hyperplasia. We used hematoxylin and eosin (H&E) in addition to iron hematoxylin (iron-H) staining to conduct in-depth assessments of histological changes, the occurrence of mitosis, and proliferating cells.
For histological evaluations, tissue sections were stained with H&E. We measured the height of the epithelial cells, uterus wall thickness, accumulation of uterine glands, and the number of eosinophil cells in the uterine stroma as visualized by a light microscope at ×100, ×400, and ×1000 magnifications. The longitudinal measurements were obtained by Microstructure Measurement software ver.1.04 (Scalor, Crop Toky, Japan).
For iron-H staining, we stained the tissue sections with Heidenhain’s iron hematoxylin color. In this hematoxylin solution, iron salts are used both as an oxidizer and a mordant. This staining method can be used to demonstrate numerous structures, such as nuclear chromatin, according to the degree of differentiation (31). This staining method shows the presence of cells during the mitotic cycle. In order to measure the percentage ratio of proliferating cells to the total number of epithelial cells, we separately counted both the total and mitotic cell numbers in the uterus luminal and glandular epithelia in 10 microscopic fields of view for each tissue specimen at ×1000 magnification with a light microscope. Overall, we assessed 4359 cells.
Statistical analysis
Comparative assessments of the aforementioned parameters are reported as mean ± SE. Assessment between PCOS and the control group was performed through one-way ANOVA (Tukey post hoctest) by SPSS Statistics software ver. 20.0 (IBM), at α=0.05. Charts were drawn with Excel software.
Results
Histology of the uterus
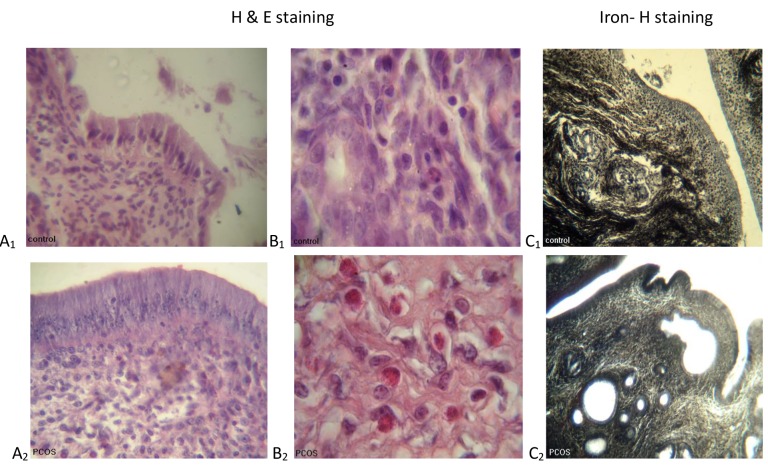
Microscopic study of the uterine tissue in the group treated by EV (PCOS) showed an increase in luminal epithelium height, accumulation of endometrial glands and their luminal diameter, and also the number of eosinophils in the endometrial stroma (Fig .1). Statistical comparison among the groups also revealed that luminal epithelium height and the thickness of the uterine wall in the PCOS group increased significantly compared to the control group (Fig .2A, B, Table 1). In addition, the percentage of eosinophils significantly increased in the experimental group (Fig .2C, Table 1).
Fig.1.
Histological sections of the uterus from control and estradiol valerate (EV)-treated polycystic ovary syndrome (PCOS) rats following hema- toxylin and eosin (H&E) and iron hematoxylin (iron-H) staining. A1 , A2. The uterine epithelium (×400), B1 , B2 . Eosinophil cells in the endometrial stroma (×1000), and C1 , C2 . The endometrial glands (×100).
Fig.2.
Statistical comparison between control and estradiol valerate (EV)-treated polycystic ovary syndrome (PCOS) rats. A. The height of the uter- ine epithelium (P<0.05), B. The thickness of the uterine wall (P<0.01), and C. The number of eosinophil cells in the endometrial stroma (P<0.05).
Table 1.
The height of the epithelial cells, uterine wall thicknesses, and the numbers of eosinophil cells in uterine stroma in control and estradiol valerate (EV)-treated polycystic ovary syndrome (PCOS) rats
| Group | Cell height (µm) | Wall thickness (µm) | Eosinophils (%) |
|---|---|---|---|
| Control | 31.81 ± 3.38 | 781.11 ± 53.59 | 5.49 ± 3.01 |
| PCOS | 48.57 ± 2.81* | 989.96 ± 22.07† | 21.06 ± 4.97* |
Values are mean ± SE. *; P<0.05 and †; P<0.01.
Researchers have reported that eosinophilic infiltration may be under the control of different hormones in rats. Eosinophilic infiltration is dependent upon the continued presence of elevated levels of estrogen in the blood and 17β-estradiol stimulates eosinophilic invasion (32). Therefore, in the present study, we have documented changes in the numbers of eosinophils after the injection of EV as a hormonal mechanism.
Proliferation
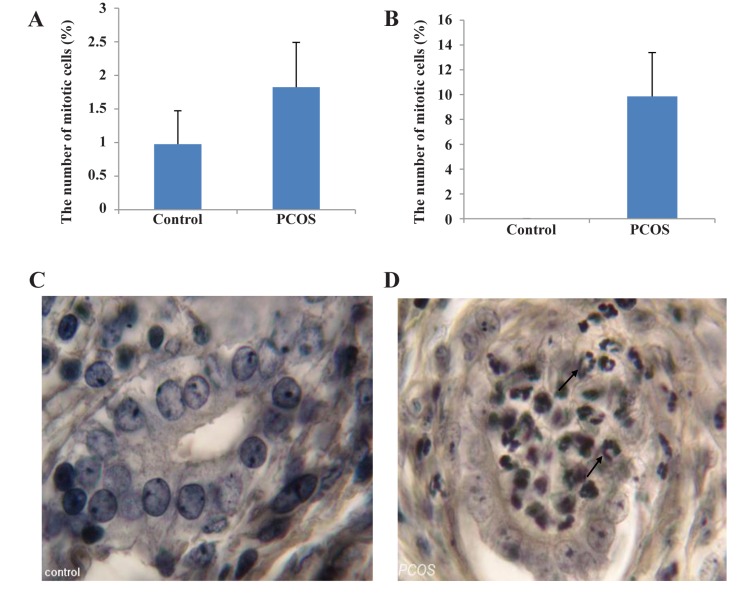
We examined and counted the epithelial cells in order to assess the frequency of mitotic cells in uterine luminal and glandular epithelia among the samples stained with iron-H. The numbers of mitotic cells were compared to the total numbers of epithelial cells. We assessed a total number of 2164 cells in the uterine luminal and 2195 cells in the glandular epithelia. A comparison between the groups revealed that the PCOS group had a nonsignificant increase in percentage of mitotic epithelial cells in the luminal region (Fig .3A, Table 2). On the other hand, the percentage of mitotic cells increased significantly in its glandular counterpart (Fig .3B, Table 2). Animals that received EV had remarkably more mitotic cells compared to animals in the control group (Fig .3C, D).
Fig.3.
Up-Statistical comparison between control and estradiol valerate (EV)-treated polycystic ovary syndrome (PCOS) rats. A. The number of mitotic cells in the luminal epithelium (P<0.05), B. The number of mitotic cells in the glandular epithelium (P<0.001). Down-Histological sections of the uterus from control and EV-treated PCOS rats following iron hematoxylin (iron-H) staining, C, and D. The glandular epithelium (×1000). ↗; Mitotic cells
Table 2.
Percentage of mitotic cells in luminal and glandular epithelia in control and estradiol valerate (EV)-treated polycystic ovary syndrome (PCOS) rats
| Group | Luminal epithelium (%) | Glandular epithelium (%) |
|---|---|---|
| Control | 0.97 ± 0.49 | 0.00 ± 0.00 |
| PCOS | 1.82 ± 0.67 | 9.86 ± 3.53* |
Values are mean ± SE. *; P<0.001.
It can be concluded that, as a sign of proliferation, the increase in numbers of mitotic cells leads to the development of a uterus with a thicker wall and dilated glands. This result can be considered as an overture for hyperplasia.
Discussion
The present study assessed the proliferative activity and histological changes in uterine tissue of an EV treated PCOS female rat model. Histological observations of uterine tissue sections showed a statistically reasonable increase in wall thickness of the uterus of EV treated (PCOS) rats in comparison with the control group. There was a significant rise in the average of the height of epithelial cells in PCOS rats compared to normal control rats. It has been shown that estrogen mediated stimulation of the uterus results in morphogenetic changes that include alterations in the type and morphology of luminal and glandular epithelia (24, 25). Similarly, the current study has proven that the stromal uterine glands of PCOS rats have larger luminal space and higher accumulation. In vitro studies of radiothymidine uptake by endometrium suggest that the maximal proliferation in uterine glands and stroma is chiefly associated with high concentrations of estradiol (33) and that ovarian steroids are among the most significant factors that affect both morphology and motility of the uterus (34). The results of this study have also supported the idea that noticeable changes in the epithelial surface, gland accumulation, and overall thickness of the uterus wall due to an abnormality at the level of ovarian steroids.
Based on the results, we observed a significantly higher eosinophil quantity in the endometrial stroma in the experimental group compared to the control rats. Experiments on the effect of hormonal perturbations on reproductive tissues suggested that the leukocyte invasion into these tissues have mainly occurred under hormone control. Eosinophil invasion is related to the continued presence of elevated blood estrogen levels as it is stimulated by estrogen (35). It has been reported that the immune system and inflammation are involved in the pathophysiological process of PCOS (36). Additionally, polymorphonuclear leukocyte infiltration may be relevant to an immunological process (37).
Results of the changes in proliferative activity in various regions of the uterus tissue showed a higher percentage of mitotic cells in luminal and glandular epithelia among rats of the experimental (PCOS) group compared to control rats. Estrogen has been well recognized as a strong factor which intensifies the proliferative activity of the uterus, with its major impact on uterine tissue (38, 39). The maximal proliferation in uterine glands and stroma occurs in the presence of high levels of estradiol (36). Studies have shown that the mitotic activity of estrogen in the endometrium of rodents is restricted to the luminal and gland neck epithelia (40, 41). In response to estrogen injection into ovariectomized mice, mitotic activity is first observed in the luminal, followed by the glandular region, while progesterone application can inhibit the mitotic response (42). Luminal epithelia have been suggested to undergo proliferation in the presence of 17β-estradiol (43, 44). In this study, we have shown that while mitotic activity was, to some extent, elevated in luminal epithelia in PCOS rats that received EV, this was not a statistically significant finding compared to the control group. We found that EV administration in PCOS rats had a surge in mitotic proliferation in the uterine glandular epithelia, which provided a probable explanation for the enlarged glands and thickened uteri wall.
Increased estrogenic environment may favor mitogenic activity in the breast and/or other reproductive tissues (45, 46). Estrogens lead to a reduction in the duration of all the stages of the cell cycle and drive cells from the G0 to the G1-phase; this is followed by an increase in the number of cells in passing the G1-and S- phases, as well as the quantity of dividing cells (47-49). Hyperplasia is an early response to an abnormal stimulation in the cell proliferation process which leads to an increase in the numbers of cells. Hyperplasia can cause the organ size to increase. It has been suggested that the development of estrogen related morphogenetic changes in the uterus can be considered as an early step towards endometrial hyperplasia and cancer (50). The persistent stimulation of endometrial tissue by estrogen (mainly estrone) in PCOS patients without the progesterone-induced inhibition leads to uterine hyperplasia as a preliminary step to carcinoma (1). Cellular proliferation and apoptosis in the human endometrial tissue take place in a cyclic procedure as they are regulated by steroid hormone levels (20). In the normal endometrium, pro-apoptotic and anti-apoptotic factors are under fine regulation that leads to tissue homeostasis which can be disturbed by hormonal alterations (51, 52). The uterus response to estrogen requires changes in the expression of genes whose products regulate successive and functionally interlinked cellular processes. Researchers suggest that the earliest changes after 17β-ethinyl estradiol treatment occur in the expression genes whose products are involved in transcriptional regulation and signal transduction, followed by those involved in mRNA and protein synthesis, cell cycle regulation, DNA replication, cell proliferation and differentiation, apoptosis, tissue remodeling, and immunological responses (53).
Conclusion
Administration of EV to induce an animal model of PCOS caused changes in epithelial height, uterus wall thickness, and the quantity of eosinophil cells. Additionally, PCOS rats showed considerably higher rates of proliferation in the glandular epithelium region of their uteri. Hence, it could be concluded that excessive estrogen content attributed to EV administration, caused an increase in the mitogenic activity of the uterus, which could be a prologue to endometrial hyperplasia and carcinoma.
Acknowledgments
This study was performed in the Histology Laboratory of Kharazmi University, Tehran (and Karaj), Iran. The authors would like to thank Dr. Mahnaz Azarnia for providing laboratory facilities. The au- thors declare that there is no conflict of interest in this study.
References
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352(12):1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 3.Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;7(2):Cd007506–Cd007506. doi: 10.1002/14651858.CD007506.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Albert-Puleo M. Fennel and anise as estrogenic agents. J Ethnopharmacol. 1980;2(4):337–344. doi: 10.1016/s0378-8741(80)81015-4. [DOI] [PubMed] [Google Scholar]
- 5.Engmann L, Maconochie N, Sladkevicius P, Bekir J, Campbell S, Tan SL. The outcome of in-vitro fertiliza- tion treatment in women with sonographic evidence of polycystic ovarian morphology. Hum Reprod. 1999;14(1):167–171. doi: 10.1093/humrep/14.1.167. [DOI] [PubMed] [Google Scholar]
- 6.Norman RJ, Wu R, Stankiewicz MT. 4: Polycystic ovary syndrome. Med J Aust. 2004;180(3):132–137. doi: 10.5694/j.1326-5377.2004.tb05838.x. [DOI] [PubMed] [Google Scholar]
- 7.Giudice LC. Endometrium in PCOS: implantation and pre- disposition to endocrine CA. Best Pract Res Clin Endo- crinol Metab. 2006;20(2):235–244. doi: 10.1016/j.beem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333(13):853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 9.Shafiee MN, Khan G, Ariffin R, Abu J, Chapman C, Deen S, et al. Preventing endometrial cancer risk in polycystic ovarian syndrome (PCOS) women: could metformin help? Gynecol Oncol. 2014;132(1):248–253. doi: 10.1016/j.ygyno.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM, Australian Ovarian Cancer Study Group and Aus¬tralian National Endometrial Cancer Study Group Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Australian case-control study. Cancer Causes Control. 2010;21(12):2303–2308. doi: 10.1007/s10552-010-9658-7. [DOI] [PubMed] [Google Scholar]
- 11.Prelevic GM. Insulin resistance in polycystic ovary syn- drome. Curr Opin Obstet Gynecol. 1997;9(3):193–201. [PubMed] [Google Scholar]
- 12.Di Cristofano A, Ellenson LH. Endometrial carcinoma. Annu Rev Pathol. 2007;2:57–85. doi: 10.1146/annurev.pathol.2.010506.091905. [DOI] [PubMed] [Google Scholar]
- 13.Lee WL, Lee FK, Su WH, Tsui KH, Kuo CD, Hsieh SL, et al. Hormone therapy for younger patients with endometrial cancer. Taiwan J Obstet Gynecol. 2012;51(4):495–505. doi: 10.1016/j.tjog.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Garg K, Soslow RA. Endometrial carcinoma in women aged 40 years and younger. Arch Pathol Lab Med. 2014;138(3):335–342. doi: 10.5858/arpa.2012-0654-RA. [DOI] [PubMed] [Google Scholar]
- 15.Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids. 2013;78(8):782–785. doi: 10.1016/j.steroids.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Plaza-Parrochia F, Bacallao K, Poblete C, Gabler F, Carvajal R, Romero C, et al. The role of androst-5-ene- 3beta,17beta-diol (androstenediol) in cell proliferation in endometrium of women with polycystic ovary syndrome. Steroids. 2014;89:11–19. doi: 10.1016/j.steroids.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Horn LC, Meinel A, Handzel R, Einenkel J. Histopathology of endometrial hyperplasia and endometrial carcinoma: an update. Ann Diagn Pathol. 2007;11(4):297–311. doi: 10.1016/j.anndiagpath.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011;22(4):145–152. doi: 10.1016/j.tem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada T, Kaponis A, Iwabe T, Taniguchi F, Makrydimas G, Sofikitis N, et al. Apoptosis in human endometrium and endometriosis. Hum Reprod Update. 2004;10(1):29–38. doi: 10.1093/humupd/dmh007. [DOI] [PubMed] [Google Scholar]
- 20.Maliqueo M, Clementi M, Gabler F, Johnson MC, Palo- mino A, Sir-Petermann T, et al. Expression of steroid re- ceptors and proteins related to apoptosis in endometria of women with polycystic ovary syndrome. Fertil Steril. 2003;80(Suppl 2):812–819. doi: 10.1016/s0015-0282(03)00987-7. [DOI] [PubMed] [Google Scholar]
- 21.Nagaoka T, Takeuchi M, Onodera H, Mitsumori K, Lu J, Maekawa A. Experimental induction of uterine adenocar- cinoma in rats by estrogen and N-methyl-N-nitrosourea. In Vivo. 1993;7(6A):525–530. [PubMed] [Google Scholar]
- 22.Deligdisch L. Hormonal pathology of the endometrium. Mod Pathol. 2000;13(3):285–294. doi: 10.1038/modpathol.3880050. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg SG. Problems in the differential diagnosis of endometrial hyperplasia and carcinoma. Mod Pathol. 2000;13(3):309–327. doi: 10.1038/modpathol.3880053. [DOI] [PubMed] [Google Scholar]
- 24.Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod. 2012;86(5):149, 1-12. doi: 10.1095/biolreprod.111.097808. [DOI] [PubMed] [Google Scholar]
- 25.Dusterberg B, Nishino Y. Pharmacokinetic and pharma- cological features of oestradiol valerate. Maturitas. 1982;4(4):315–324. doi: 10.1016/0378-5122(82)90064-0. [DOI] [PubMed] [Google Scholar]
- 26.Yen SSC, RB J. Reproductive endocrinology. 3rd ed. Philadelphia: Saunders; 1991. [Google Scholar]
- 27.Bradlow HL, Michnovicz JJ. A new approach to the pre- vention of breast cancer. Edinburgh: Proceedings of the Royal Society of Edinburgh; 1989. pp. 77–86. [Google Scholar]
- 28.Sepkovic DW, Bradlow HL, Ho G, Hankinson SE, Gong L, Osborne MP, et al. Estrogen metabolite ratios and risk as- sessment of hormone-related cancers. Ann N Y Acad Sci. 1995;768(1):312–316. doi: 10.1111/j.1749-6632.1995.tb12149.x. [DOI] [PubMed] [Google Scholar]
- 29.Azarnia M, Kamyab SZ, Mirabolghasemi SG, Saeidnia S. Effect of hydroalcoholic extract of Melia azedarach L.seeds on serumconcentration of sex hormones in poly- cystic ovary syndrome induced in female wistar rats. Feyz. 2015;19(2):112–118. [Google Scholar]
- 30.Brawer JR, Munoz M, Farookhi R. Development of the polycystic ovarian condition (PCO) in the estradiol valer- ate-treated rat. Biol Reprod. 1986;35(3):647–655. doi: 10.1095/biolreprod35.3.647. [DOI] [PubMed] [Google Scholar]
- 31.Bancroft JD, Gamble M. Theory and practice of histologi- cal techniques. 6th ed. Edinburgh: Churchill Livingstone; 2008. pp. 131–132. [Google Scholar]
- 32.Luque EH, Munoz de Toro MM, Ramos JG, Rodriguez HA, Sherwood OD. Role of relaxin and estrogen in the control of eosinophilic invasion and collagen remodeling in rat cervical tissue at term. Biol Reprod. 1998;59(4):795–800. doi: 10.1095/biolreprod59.4.795. [DOI] [PubMed] [Google Scholar]
- 33.Ferenczy A, Bertrand G, Gelfand MM. Proliferation kinet- ics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol. 1979;133(8):859–867. doi: 10.1016/0002-9378(79)90302-8. [DOI] [PubMed] [Google Scholar]
- 34.Unsal F, Sonmez MF. The effects of ovariectomy on ghre- lin expression in the rat uterus. Adv Clin Exp Med. 2014;23(3):363–370. [PubMed] [Google Scholar]
- 35.Westerlind KC, Gibson KJ, Malone P, Evans GL, Turner RT. Differential effects of estrogen metabolites on bone and reproductive tissues of ovariectomized rats. J Bone Miner Res. 1998;13(6):1023–1031. doi: 10.1359/jbmr.1998.13.6.1023. [DOI] [PubMed] [Google Scholar]
- 36.Xiong YL, Liang XY, Yang X, Li Y, Wei LN. Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):148–150. doi: 10.1016/j.ejogrb.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Kelly RW. Pregnancy maintenance and parturition: the role of prostaglandin in manipulating the immune and in- flammatory response. Endocrine Reviews. 1994;15(5):684–706. doi: 10.1210/edrv-15-5-684. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Laping J, Glasser S, Day P, Mulholland J. Media- tors of estradiol-stimulated mitosis in the rat uterine lumi- nal epithelium. Endocrinology. 1998;139(3):961–966. doi: 10.1210/endo.139.3.5794. [DOI] [PubMed] [Google Scholar]
- 39.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 40.Marcus GJ. Mitosis in the rat uterus during the estrous cycle, early pregnancy, and early pseudopregnancy. Biol Reprod. 1974;10(4):447–452. doi: 10.1095/biolreprod10.4.447. [DOI] [PubMed] [Google Scholar]
- 41.Marcus GJ. Hormonal control of proliferation in the guin- ea-pig uterus. J Endocrinol. 1974;63(1):89–97. doi: 10.1677/joe.0.0630089. [DOI] [PubMed] [Google Scholar]
- 42.Weitlauf HM. Biology of implantation. In: Knobil EN, editor. The physiology of reproduction. 2nd ed. New York: Raven Press Ltd; 1994. pp. 391–429. [Google Scholar]
- 43.Quarmby VE, Korach KS. The influence of 17 beta-estra- diol on patterns of cell division in the uterus. Endocrinol- ogy. 1984;114(3):694–702. doi: 10.1210/endo-114-3-694. [DOI] [PubMed] [Google Scholar]
- 44.Nephew KP, Long X, Osborne E, Burke KA, Ahluwalia A, Bigsby RM. Effect of estradiol on estrogen receptor ex- pression in rat uterine cell types. Biol Reprod. 2000;62(1):168–177. doi: 10.1095/biolreprod62.1.168. [DOI] [PubMed] [Google Scholar]
- 45.Cauley JA, Lucas FL, Kuller LH, Vogt MT, Browner WS, Cummings SR. Bone mineral density and risk of breast cancer in older women: the study of osteoporotic fractures.Study of Osteoporotic Fractures Research Group. JAMA. 1996;276(17):1404–1408. [PubMed] [Google Scholar]
- 46.Zhang Y, Kiel DP, Kreger BE, Cupples LA, Ellison RC, Dorgan JF, et al. Bone mass and the risk of breast can- cer among postmenopausal women. N Engl J Med. 1997;336(9):611–617. doi: 10.1056/NEJM199702273360903. [DOI] [PubMed] [Google Scholar]
- 47.Evans GS, Gibson DF, Roberts SA, Hind TM, Potten CS. Proliferative changes in the genital tissue of female mice during the oestrous cycle. Cell Tissue Kinet. 1990;23(6):619–635. doi: 10.1111/j.1365-2184.1990.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 48.Galand P, de Maertelaer V. Models of oestrogen action: a cell kineticist's view. Epithelial Cell Biol. 1992;1(4):177–188. [PubMed] [Google Scholar]
- 49.Rumpel E, Michna H, Kuhnel W. PCNA-immunoreactivity in the uterus of rats after treatment with the antiestrogen tamoxifen. Ann Anat. 1995;177(2):133–138. doi: 10.1016/S0940-9602(11)80060-9. [DOI] [PubMed] [Google Scholar]
- 50.Gunin AG, Mashin IN, Zakharov DA. Proliferation, mitosis orientation and morphogenetic changes in the uterus of mice following chronic treatment with both estrogen and glucocorticoid hormones. J Endocrinol. 2001;169(1):23–31. doi: 10.1677/joe.0.1690023. [DOI] [PubMed] [Google Scholar]
- 51.Castro A, Johnson MC, Anido M, Cortinez A, Gabler F, Vega M. Role of nitric oxide and bcl-2 family genes in the regulation of human endometrial apoptosis. Fertil Steril. 2002;78(3):587–595. doi: 10.1016/s0015-0282(02)03304-6. [DOI] [PubMed] [Google Scholar]
- 52.Li A, Felix JC, Hao J, Minoo P, Jain JK. Menstrual-like breakdown and apoptosis in human endometrial explants. Hum Reprod. 2005;20(6):1709–1719. doi: 10.1093/humrep/deh824. [DOI] [PubMed] [Google Scholar]
- 53.Naciff JM, Overmann GJ, Torontali SM, Carr GJ, Kham- batta ZS, Tiesman JP, et al. Uterine temporal response to acute exposure to 17alpha-ethinyl estradiol in the im- mature rat. Toxicol Sci. 2007;97(2):467–490. doi: 10.1093/toxsci/kfm046. [DOI] [PubMed] [Google Scholar]