Abstract
Objective
Socioeconomic hardship is common among children hospitalized for asthma but often not practically measurable. Information on where a child resides is universally available. We sought to determine the correlation between neighborhood-level socioeconomic data and family-reported hardships.
Methods
Caregivers of 774 children hospitalized with asthma answered questions regarding income, financial strain, and primary care access. Addresses were geocoded and linked to zip code-, census tract-, and block group-level (neighborhood) data from the U.S. Census. We then compared neighborhood median household income to family-reported household income; percentage of neighborhood residents living in poverty to family-reported financial strain; and percentage of neighborhood households without an available vehicle to family-reported access to primary care. We constructed heat maps and quantified correlations using Kendall’s rank correlation coefficient. Receiver operator characteristic curves were used to assess predictive abilities of neighborhood measures.
Results
The cohort was 57% African American and 73% publicly-insured; 63% reported income <$30,000, 32% endorsed ≥4 financial strain measures, and 38% reported less than adequate primary care access. Neighborhood median household income was significantly and moderately correlated with and predictive of reported household income; neighborhood poverty was similarly related to financial strain; neighborhood vehicle availability was weakly correlated with and predictive of primary care access. Correlations and predictions provided by zip code measures were similar to those of census tract and block group.
Conclusions
Universally available neighborhood information may help efficiently identify children and families with socioeconomic hardships. Systematic screening with area-level socioeconomic measures has the potential to inform resource allocation more efficiently.
Keywords: Asthma, Pediatrics, Social Determinants of Health, Geography
Social risk factors related to socioeconomic status (SES) and access to care affect health outcomes.1,2 Inequalities in pediatric asthma-related morbidity are largely driven by socioeconomic hardships and other social determinants of health (SDH),3–6 and children hospitalized with asthma are disproportionately from disadvantaged backgrounds and neighborhoods. The hardships faced by many of these children and their families may be difficult to identify though they may be amenable to interventions. However, many of the most successful interventions are multifaceted and resource intense.7–9
Universal implementation of such interventions may be cost prohibitive; therefore, targeting interventions to the most appropriate populations is essential. Determining the best ways to efficiently target and allocate resources can be a challenge. One approach to risk assessment, identification, and subsequent stratification, is an in-depth social history of factors that place children at risk for worse outcomes and may be amenable to intervention (e.g., financial hardships or strain, inadequate housing, barriers to accessing preventive care).10,11 However, universal comprehensive screening in a busy clinical setting, such as the inpatient asthma unit, may not be feasible. It may also be the case that parents or caregivers are not present at a time when screening can take place.
With such challenges in mind, the use of area-based socioeconomic measures, connected to a patient’s street address, has the potential to guide or tailor risk assessments. Such measures have shown promise as proxies for SES in understanding health risk for adult patients at the population level.12–14 Less is known regarding the utility of such measures in pediatrics; although, studies have demonstrated their potential utility in population-level assessment of health outcomes and in the characterization of the home environment.15,16 Still, there remains just limited evidence of what these area-based measures are proxies for and how they might be used clinically.
Thus, our first objective was to determine the correlation of neighborhood-level, area-based socioeconomic data to analogous family-reported hardships in a cohort of children hospitalized with asthma. Specifically, we sought to compare: 1) neighborhood median household income to family-reported household income; 2) neighborhood poverty rates to family-reported household financial strain; and 3) neighborhood vehicle availability to family-reported primary care access. Clearly, neighborhoods can be defined in multiple ways, and different neighborhood definitions may lead to varying correlations with family-level data. Consequently, our second objective was to investigate how correlations varied across different neighborhood definitions. Taking our cue from the Public Health Disparities Geocoding Project,13,17 we sought to compare zip code, census tract, and census block group with one another. We hypothesized that smaller, homogenous tracts and block groups would correlate better to patient and family data than zip codes.
METHODS
This was a cross-sectional analysis of data collected as part of the Greater Cincinnati Asthma Risks Study (GCARS). GCARS is a population-based, prospective, observational cohort which enrolled 774 children, 1–16 years of age, who were admitted between August 2010 and October 2011 to Cincinnati Children’s Hospital Medical Center (CCHMC). CCHMC is an urban, tertiary care referral center that also serves as the primary pediatric inpatient provider for Southwestern Ohio, Northern Kentucky, and Southeastern Indiana.
Details related to GCARS’ inclusion and exclusion criteria along with comprehensive demographic information have been previously described.6 Briefly, patients were identified by use of the evidence-based clinical pathway for acute asthma or bronchodilator-responsive wheezing. Children were excluded if they had significant respiratory or cardiovascular co-morbidity, if they lived outside of the CCHMC 8-county primary service area, or if they had a non-English speaking caregiver (2% of those otherwise eligible). Notably, ~60% of those who were eligible were enrolled. The CCHMC Institutional Review Board approved this study.
Patient variables
Upon GCARS enrollment, each caregiver participated in face-to-face survey with a trained research assistant, completing a 177-item questionnaire which included questions related to annual household income, markers of financial strain, and primary care access. These particular variables were chosen a priori for the analyses presented here given that lower income, heightened financial strain,6 and limited primary care access have all been associated with increased readmission risk in children with asthma.18
Caregivers reported annual household income within categories (<$15,000; 15,000–29,999; 30,000–44,999; 45,000–59,999; 60,000–89,999, ≥90,000). Financial strain was assessed using a series of seven previously described questions.19–21 These questions assessed, via self-report, a family’s ability to make ends meet, pay rent/mortgage, pay utilities, their need to move in with others due to finances, and ability to borrow money if needed, as well as home ownership and caregiver marital status.19–21 Strain questions were treated as dichotomous (yes/no, married/not married); the number of positive items was calculated for each patient. Access to primary care was assessed using the access subscale to the Parent’s Perception of Primary Care, a series of four questions, which assess a family’s ability to travel to their doctor, to see their doctor for routine or sick care, and to get help or advice on evenings or weekends. We scored the subscale as previously described; scores ranged from 0 (no access) to 100 (perfect access).18,22
Neighborhood variables
We identified area-based variables for each child based on where the patient lived. The reported street address was geocoded or mapped using ArcGIS software (ESRI, Redlands, California). Addresses were then linked to corresponding 2010 census geographies: zip code tabulation area (ZCTA), census tract, and census block group. ZCTAs are statistical entities constructed by the U.S. Census Bureau to enable demographic characterization of zip codes (areas defined by the U.S. Postal Service). Zip codes (and corresponding ZCTAs) can be socio-demographically heterogeneous and can vary in size. Census tracts, defined by the U.S. Census in collaboration with local municipalities, are more homogeneous areas of ~4,000 people that generally align with locally-recognized neighborhood boundaries. Census block groups are smaller still, areas of ~1,000 people located within census tracts. Each geographic level was then linked to geography-specific, neighborhood-level information available publically from the U.S. Census 2008–2012 American Community Survey.23
Analyses
We compared neighborhood measures to analogous patient (or family) measures, specifically assessing income, poverty/strain, and access (Table 1). Each correlation was assessed at three geographic levels: ZCTA or zip code, census tract, and census block group. The degree of correlation was assessed using Kendall’s tau B, a non-parametric rank correlation coefficient similar, in some ways, to the Spearman rank correlation coefficient. While both assess correlation, the Kendall’s tau B allows for more direct assessment of concordance between included variables. The resulting correlation coefficient ranges from −1, indicative of perfect discordance, to 0, indicative of neither concordance nor discordance, to 1, indicative of perfect concordance. To facilitate visualization, we also constructed heat maps for each of the tested relationships.
Table 1.
Primary comparisons assessed during correlation analyses
| Neighborhood Variables | ||||
|---|---|---|---|---|
| Zip Code | Census Tract | Block Group | ||
| Individual Patient Variables | Household Income | Individual Income* compared to Zip Code Tabulation Area Median Household Income* | Individual Income* compared to Census Tract Median Household Income* | Individual Income* compared to Block Group Median Household Income* |
| Financial Strain | Number of strain items compared to Zip Code Tabulation Area Poverty Decile** | Number of strain items compared to Census Tract Poverty Decile** | Number of strain items compared to Block Group Poverty Decile** | |
| Primary Care Access | Access score¶ compared to Zip Code Tabulation Area Vehicle Availability Decile** | Access score¶ compared to Census Tract Vehicle Availability Decile** | Access score¶ compared to Block Group Vehicle Availability Decile** | |
Income categories=<$15,000; 15,000–29,999; 30,000–44,999; 45,000–59,999; 60,000–89,999, ≥90,000
Deciles of the Greater Cincinnati Region; e.g. Highest Poverty Decile Neighborhood means neighborhood is amongst the highest 10% of impoverished neighborhoods in Cincinnati
Access score of <75, 75–99, or 100.
Given that family-reported income was collected in categories, we opted to categorize neighborhood median household income in analogous ways. That is, we determined whether a neighborhood’s median household income was <$15,000, 15,000–29,999, 30,000–44,999, 45,000–59,999, 60,000–89,999, or ≥90,000.
We then compared the neighborhood percentage of individuals living below the federal poverty level to the number of reported household strain items. Here, we used deciles of neighborhood poverty rates across CCHMC’s 8-county primary service area, GCARS’ inclusion area. For example, a child with a street address localized to a neighborhood with a poverty rate in the top 10% of our region’s neighborhoods would be designated as living in the highest (worst) poverty decile. This was then correlated to categorized measures of family-level financial strain. Categorization was done a priori after discussion with an inpatient social worker in an attempt to identify which patients might be most in need of assistance. Categories included those reporting 0–2, 3, 4, and ≥5 hardships.
Finally, we compared neighborhood-level vehicle availability, that is, the percentage of households within the defined geographic areas that own or have access to a vehicle, to a child’s reported primary care access. This was seen as relevant locally as our region has limited public transportation availability. Additionally, restricted primary care access has been shown to be associated with increased risk of asthma-related readmission.18 We used deciles of neighborhood vehicle availability for comparison, defined in the same way as for neighborhood poverty. Our family-level measure was categorized based on scores on the aforementioned scale, determined to be perfect access, almost always adequate access, and less than adequate access.
Next, we constructed receiver operating characteristic (ROC) curves to assess how well neighborhood measures could discriminate between 1) children living in households reporting low income (focusing on those reporting incomes of <$15,000 and <$30,000); 2) those with high financial strain (≥4 and ≥5 positive strain items); and 3) those with suboptimal access to primary care (score <75). To plot each ROC curve, we calculated sensitivity and specificity for each patient measure as each neighborhood measure increased. For example, we calculated sensitivity and specificity for identifying a child with an annual household income of <$15,000 if they lived in a neighborhood with a median household income of <$1,000 then $2,000 then $3,000, etc. Predictive abilities (c-statistics) were then quantified using the area under the ROC curve (AUROC). AUROC values of 1 represent perfect prediction (corresponding to sensitivities and specificities of 1). AUROC values of 0.5 represent no predictive ability. Maximal total accuracy and the Youden Index were also used to provide further details on sensitivities and specificities. The total accuracy approach identifies the point on the ROC that is furthest from the diagonal line; the Youden Index identifies the point on the ROC that is closest to the 0,1 point.
RESULTS
A total of 774 children were enrolled in GCARS. Overall, 57% of included children were reported to be African American and 73% publicly-insured (Table 2). Compared to enrolled children, those who were eligible but not enrolled did not differ with respect to age, gender, or readmission rate at 12 months. Enrolled children were, however, more likely to be African American and publicly insured.6
Table 2.
Patient demographic characteristics for those enrolled in the Greater Cincinnati Asthma Risks Study cohort (n=774)
| Characteristic | n | % | |
|---|---|---|---|
| Age (years) | <4 | 294 | 38 |
| 4–11 | 396 | 51 | |
| ≥12 | 81 | 11 | |
| Male Sex | 503 | 65 | |
| Race/Ethnicity | African American | 441 | 57 |
| White | 254 | 33 | |
| Other, includes Hispanic | 76 | 10 | |
| Health Insurance | Public | 562 | 73 |
| Private | 171 | 22 | |
| Self-pay | 27 | 4 | |
| Annual household income ($) | <15,000 | 275 | 36 |
| 15–29,999 | 212 | 27 | |
| 30–44,999 | 108 | 14 | |
| 45,000–59,999 | 46 | 6 | |
| 60,000–89,999 | 78 | 10 | |
| ≥90,000 | 55 | 7 | |
| Number of Strain Items (7 possible) | 0–2 | 151 | 20 |
| 3 | 185 | 24 | |
| 4 | 252 | 32 | |
| 5+ | 182 | 24 | |
| Primary Care Access | Perfect (score=100) | 143 | 18 |
| Almost always adequate (75–99) | 337 | 44 | |
| Less than almost always adequate (<75) | 294 | 38 | |
Greater than 60% reported an annual household income <$30,000 while 32% endorsed 4 financial strain items and an additional 24% endorsed 5 or more. Additionally, 38% of participants had a primary care access score <75, indicative of sub-optimal access. All addresses were successfully geocoded and linked to U.S. Census information. A total of 29% of children lived in ZCTAs with median household incomes <$30,000; 42% lived in the highest poverty two deciles of regional ZCTAs, and 46% lived in the two deciles of ZCTAs with the lowest vehicle availability (Table 3).
Table 3.
Characteristics of neighborhoods where enrolled patients live
| Neighborhood characteristic | Zip Code Tabulation Area (% of children) | Census Tract (% of children) | Block Group (% of children) | |
|---|---|---|---|---|
| Median Household Income ($) | <15,000 | 5.6 | 9.8 | 12.3 |
| 15–29,999 | 23.1 | 27.7 | 26.2 | |
| 30–44,999 | 20.3 | 24.6 | 27.3 | |
| 45,000–59,999 | 33.1 | 19.9 | 15.1 | |
| 60,000–89,999 | 17.7 | 14.6 | 14.9 | |
| ≥90,000 | 0.3 | 3.5 | 4.3 | |
| Poverty* | Highest Decile | 25.1 | 22.9 | 27.5 |
| 2 | 16.8 | 19.9 | 16.8 | |
| 3 | 16.9 | 10.0 | 11.0 | |
| 4 | 13.7 | 9.7 | 10.1 | |
| 5 | 4.7 | 12.1 | 7.4 | |
| 6 | 7.8 | 5.3 | 7.5 | |
| 7 | 4.0 | 6.1 | 5.8 | |
| 8 | 4.4 | 4.4 | 5.0 | |
| 9 | 2.8 | 6.5 | 5.7 | |
| Lowest Decile | 3.9 | 3.2 | 3.2 | |
| Vehicle Availability** | Lowest Decile | 35.0 | 29.5 | 13.2 |
| 2 | 11.2 | 12.4 | 9.4 | |
| 3 | 16.9 | 11.4 | 13.6 | |
| 4 | 8.1 | 10.0 | 12.5 | |
| 5 | 6.6 | 8.5 | 9.7 | |
| 6 | 9.8 | 6.7 | 11.0 | |
| 7 | 3.8 | 5.9 | 9.2 | |
| 8 | 3.5 | 6.1 | 21.5 | |
| 9 | 3.1 | 6.1 | 0 | |
| Highest Decile | 1.9 | 3.5 | 0 |
Highest decile neighborhoods have poverty rates of >36%, 39%, and 40% at zip code, census tract and block group respectively. The lowest decile neighborhoods have poverty rates of <4%, 3%, and 1% respectively.
Lowest decile neighborhoods have vehicle availability rates of <83%, 75%, and 50% at the zip code, census tract and block group respectively. Highest decile neighborhoods have vehicle availability rates of ≥99% for all neighborhood groups.
Area-patient correlation
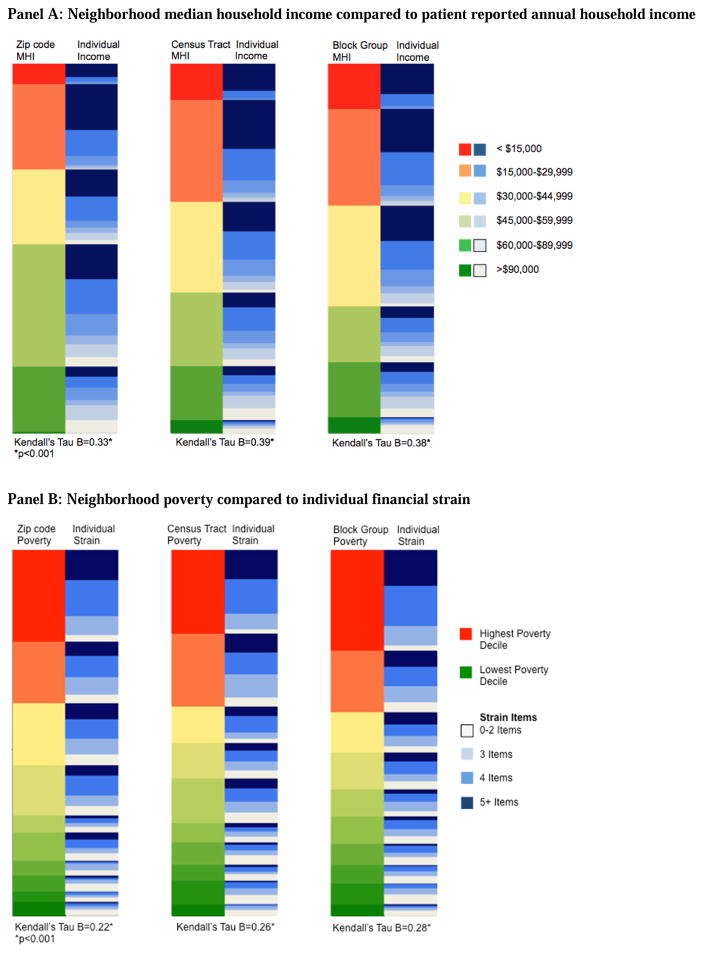
Heat maps display neighborhood next to patient attributes. The highest risk neighborhoods are depicted in red and lowest in green. Highest risk patient, or household, characteristics are depicted in dark blue, with lowest risk in white.
Income
Heat maps were used to visually compare neighborhood median household income to individual family-reported income. The red, orange, and yellow neighborhoods at the top of the figure include more darker shades of blue (corresponding to low individual income); the green neighborhoods at the bottom of graph include more lighter shades of blue (corresponding to higher individual income). For each of the studied geographic levels, area-based measures of low income (red/orange) captured a significant proportion, but far from all, of families with lower income (darker blue) (Figure 1A). Quantitatively, the Kendall’s tau B values at the ZCTA, census tract, and block group were moderate at 0.33, 0.39, and 0.38, respectively (all p<0.001).
Figure 1.
Heat maps* comparing neighborhood characteristics to patient/household characteristics at three geographies (zip code, census tract, census block group) for A) Income; B) Poverty and financial strain; and C) Vehicle availability (“car own”) and primary care access.
Abbreviations: MHI – median household income
*The left column of each pair represents neighborhood-level data; the right column is caregiver-reported, patient/household data. Highest risk neighborhoods are depicted in red and lowest risk neighborhoods in green. Highest risk patients are depicted in dark blue and lowest risk in white.
Children living in neighborhoods characterized by low median household incomes tended to have lower reported household incomes themselves. This positive predictive value was highest at the census tract geography. Indeed, 96% of children living in census tracts with median household incomes of <$15,000 had family-reported incomes of <$30,000 (compared to 88% for ZCTA and 94% for block group). Still, children living in neighborhoods with median household incomes of >$60,000 had a wide range of actual reported incomes. In these neighborhoods, approximately one-third of children had reported household incomes of <$30,000 (32% for ZCTA, 31% for census tract, and 34% for block group). This equates to negative predictive values of 70%, 69%, and 66% for ZCTA, tract, and block group, respectively.
Poverty and strain
Neighborhood poverty was also correlated with caregiver-reported financial strain. Kendall’s tau B values ranged from 0.22–0.28 for the three assessed geographies (all p<0.001) (Figure 1B). Of the children residing in the poorest two deciles of Cincinnati neighborhoods, 66–69% had ≥4 strain items (using ZCTA, census tract, or census block group to define the neighborhood). For the small percentage of hospitalized children living in the two deciles of neighborhoods with the lowest poverty rates for our region (i.e., the richest neighborhoods), a wide range of financial strain items was reported: 43–46% of these families reported <2 strain items, but 25–33% endorsed ≥4 items (across the three neighborhood definitions).
Vehicle availability and primary care access
Finally, primary care access was significantly correlated with neighborhood rates of vehicle availability; however, this relationship was the weakest of those assessed (Figure 1C). The Kendall’s tau B values ranged from 0.14–0.16 across the three geographies (all p<0.001). Of the children who lived in neighborhoods in the two deciles with the lowest rates of vehicle availability (i.e., least cars), 46–47% had primary care access scores of <75. Conversely, 14–15% of these children reported perfect primary care access (score=100). Of children who lived in neighborhoods in the two deciles with the highest rates of vehicle availability (i.e., most cars), 19–23% had access scores of <75 and 22–26% had perfect primary care access scores.
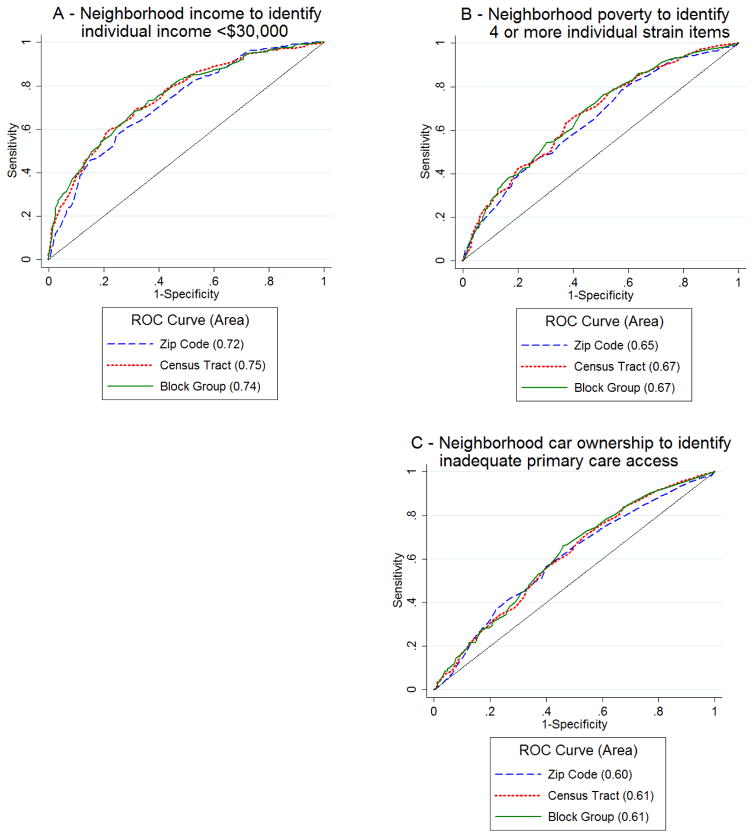
Area-patient prediction
We constructed ROC curves to determine the predictive ability of neighborhood variables on analogous family-reported variables. This was done to simulate the use of area-level measures to help stratify families for further services.
Neighborhood (census tract) median household income had the highest AUROC for identifying low-income families. The AUROC for the ability to predict a patient with a reported household income of <$30,000 income was 0.75 using census tract median household income (Figure 2A). The AUROC for predicting children with annual household incomes of <$15,000 ranged from 0.68–0.72 across geographies. These values represent moderate predictive ability. For the neighborhood poverty rate predicting children experiencing ≥4 financial strain items, the AUROCs ranged from 0.65–0.67 for the three geographies (Figure 2B). The AUROC for models predicting children with ≥5 strain items ranged from 0.60–0.66. Finally, the AUROC for predicting primary care access based on neighborhood rates of vehicle availability was 0.60 for ZCTA and 0.61 for both census tract and block group (Figure 2C). In general, the AUROC values for the census tract and block group level models were higher than the AUROC values for the ZCTAs models; however, these differences did not reach the level of statistically significant (income: p=0.09 for ZCTA versus tract and ZCTA versus block group; strain: p=0.1 for ZCTA versus tract and ZCTA versus block group; access: p=0.6 for ZCTA versus tract and p=0.4 for ZCTA versus block group).
Figure 2.
A: Receiver operator characteristics (ROC) curves at zip code, census tract, and block group level for predicting individuals with annual household income <$30,000. B: ROC curves for predicting individuals with 4 or more strain items. C: ROC curves for predicting individuals with inadequate primary care access (score < 75).
Finally, Table 4 illustrates an approach aimed at maximizing accuracy in the prediction of pertinent individual outcomes. The total accuracy approach suggests that a ZCTA cut-point of $41,948 would be 57% sensitive and 75% specific in the prediction of individual income <$30,000. The Youden Index identifies a cut-point of $60,276 to obtain a sensitivity of 95% and a specificity of 30%.
Table 4.
Sensitivities and specificities for neighborhood prediction of individual variable using a maximal total accuracy and maximal Youden Index approach
| Neighborhood value used to maximize total accuracy | Maximal total accuracy (Sensitivity, Specificity) | Neighborhood value used to maximize Youden Index | Maximal Youden Index (Sensitivity, Specificity) | |
|---|---|---|---|---|
| Prediction of individual income <$30,000 | ||||
| Zip code | 41948 | (0.57, 0.75) | 60276 | (0.95, 0.30) |
| Census tract | 34271 | (0.60, 0.78) | 34271 | (0.60, 0.78) |
| Block group | 50602 | (0.84, 0.51) | 38036 | (0.69, 0.69) |
| Prediction of individuals with ≥4 strain items | ||||
| Zip code | 8.5 | (0.93, 0.25) | 13.0 | (0.78, 0.43) |
| Census tract | 57.1 | (0.91, 0.13) | 19.7 | (0.53, 0.52) |
| Block group | 92.8 | (1.00, 0.00) | 9.9 | (0.28, 0.76) |
| Prediction of inadequate primary care access | ||||
| Zip code | 25.3 | (0.36, 0.78) | 11.7 | (0.56, 0.60) |
| Census tract | 61.8 | (0.03, 0.99) | 9.6 | (0.70, 0.48) |
| Block group | 66.6 | (0.09, 0.96) | 12.1 | (0.66, 0.54) |
DISCUSSION
Universally available neighborhood information may help identify risks related to asthma morbidity. To this end, we found significant though moderate correlations between neighborhood median household income and family-reported income and between neighborhood poverty and family-reported financial strain. Neighborhood measures of vehicle availability were just weakly correlated with a patient’s primary care access. We also found that these neighborhood characteristics were moderately predictive of patient or family attributes (AUROCs up to 0.75). Notably, children living in poor neighborhoods were found to be almost universally disadvantaged. Conversely, children in middle class neighborhoods had wider range of individual or family-level attributes. Such knowledge has the potential to inform risk assessment, identification, and resource allocation in clinical settings using data that can be efficiently linked to a patient’s street address.
Allocation of clinical resources (e.g., social work, financial counseling) often relies on reactive processes – a care team member must assess for risks, identify a need, and then refer. Use of neighborhood data may enhance more proactive allocation of resources and may provide context for social and environmental histories. For relatively ubiquitous resources, such as hospital-based financial counseling, the goal may be to maximize sensitivity (minimizing false negatives). Our data suggest that these services could be targeted to patients who live in neighborhoods with low to moderate incomes in order to identify as many at-risk patients and families as possible. Conversely, a highly restricted resource such as case management could be offered to patients living neighborhoods with the lowest median household incomes as almost all of the children (88–96%) living in very low-income neighborhoods have low incomes themselves. It should be noted, however, that this approach, in isolation, would miss low-income children and families not living in low-income neighborhoods. As a result, different settings might explore different cut-points in ways to maximize sensitivity or specificity as they consider the potential needs of their patient panel as well as the resources they have available.
Despite our findings relating to correlation and prediction, defining who is “at risk” is not straightforward. We are not able to tell, from these analyses, whether children who have discordancy in their area- compared to their individual-level risk fare better, worse, or the same as those children with concordance. Data from the moving-to-opportunity studies suggest that, for some health outcomes that include asthma, low-income children living in higher-income neighborhoods may actually be at higher risk than their peers in low-income neighborhoods.24,25 Should this be the case, misidentification (or misclassification) may be particularly problematic. Yet, the ability of area-level data to provide context may then allow for more efficient, tailored discussions at the bedside to augment decisions with individual information.26
Understanding the correlation between individual attributes and neighborhood attributes has implications for health policy. Given the well-established link between health outcomes and SDH,2,4,12,13,27,28 there has been a call for adjusting hospital-level quality metrics for the SDH.29 As hospital quality metrics rely on administrative data, neighborhood characteristics are frequency utilized.30,31 In pediatrics, there is a paucity of information about how a patient’s neighborhood correlates with patient or family attributes. Here, we further such an understanding, providing analyses that informs the use of neighborhood-level data in patient-level risk assessment and risk identification.16,32
Smaller, more homogeneous geographies like census tracts and block groups correlate with and predict patient-level attributes better than zip codes in large multi-state analyses.15,17,33 We found similar patterns; however, in our analyses, ZCTA data were statistically equivalent to that of census tract and block group. This may reflect the smaller sample size (of neighborhoods and patients) at our single institution. It also should be noted that in clinical practice, identification of the census tract or block group requires the additional step of geocoding a patient’s street address and associating that address with the geography of choice. Zip code information is easier to access as it is readily available and known by most individuals as a component of the street address. Regardless, the neighborhood must be matched to easily accessible, available, and pertinent information. Further testing may be warranted that assesses how the additional step of geocoding affects usage of such information. As electronic health records advance, such a step may be possible in increasingly efficient, automated ways.34,35
Our results should be considered in the context of several limitations. We examined children admitted for asthma to a single center in a single region. As with many pediatric asthma studies,8 the majority of our patients were poor (just 7% reported annual incomes ≥$90,000). We also excluded caregivers who were unable to participate in written or oral English. While this may significantly limit generalizability in some populations, locally, this only resulted in the exclusion of 2% of those otherwise eligible. Second, regions with different attributes such as more mixed income neighborhoods, robust public transportation, or neighborhoods with rapidly evolving demographics may have different findings. More heterogeneous neighborhoods may be more affected the “ecological fallacy” – falsely ascribing area-level characteristics to individuals. Third, we chose several cut-points for predictive modeling. These cut-points, while based on pre-existing literature when available, may not be the correct cut-points for optimal use of neighborhood information and may differ across different settings. Fourth, caregiver report of income and financial strain is subject to social desirability bias; however, the same bias would likely exist in questioning on these topics during routine clinical practice. Finally, the widespread use of neighborhood attributes to assess patient or family risk in clinical practice may require electronic health record enhancements to allow for geocoding and data linkages before such processes can be put in place more broadly.
CONCLUSION
Information about a child’s neighborhood may help to efficiently identify low-income children and families who are experiencing socioeconomic hardships and barriers related to the SDH. This could guide risk assessment and identification starting the moment a family arrives and registers in the clinical setting. Bringing this information into the clinical realm could be beneficial as a means by which systematic risk screening is more efficiently pursued, resources are targeted and allocated more effectively, and policy decisions around payment reform are made more appropriately.
WHAT’S NEW.
Socioeconomic hardship is common in children hospitalized with asthma. Universal socioeconomic risk screening is challenging to implement in clinical practice. Patient address may be an efficient way to identify at-risk children and allocate social support resources in the inpatient setting.
Acknowledgments
This work was (partially) supported through a grant from the National Institutes of Health (1R01AI88116 to Robert Kahn). Dr. Beck was also supported through the National Institutes of Health (NIH 1K23AI112916).
All authors had full access to the data and have approved of this work as submitted. This work was presented in abstract form at the Society of Hospital Medicine national conference between March 29 and April 1, 2015 (National Harbor, Maryland) and at the Pediatric Academic Societies on April 26, 2015 (San Diego, California).
Funding Source: The GCARS study was funded by a grant from the National Institutes of Health (1R01AI88116). Dr. Beck also received funding from the NIH through a career development award (1K23AI112916).
Abbreviations
- AUROC
area under receiver operating characteristic
- CCHMC
Cincinnati Children’s Hospital Medical Center
- GCARS
Greater Cincinnati Asthma Risks Study
- ROC
receiver operating characteristic
- SES
socioeconomic status
- SDH
social determinants of health
- ZCTA
Zip Code Tabulation Area
Footnotes
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose
Funding Disclosure: The authors have no financial relationships relevant to this article to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gornick . Disparities in Health Care- Methods for Studying the Effects of Race, Ethnicity, and SES on Access Use, and Quality of health care IOM. 2002. [Google Scholar]
- 2.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–398. doi: 10.1146/annurev-publhealth-031210-101218. [DOI] [PubMed] [Google Scholar]
- 3.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. National health statistics reports. 2011;(32):1–14. [PubMed] [Google Scholar]
- 4.Williams DR, Sternthal M, Wright RJ. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009;123(Suppl 3):S174–184. doi: 10.1542/peds.2008-2233H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck AF, Moncrief T, Huang B, et al. Inequalities in neighborhood child asthma admission rates and underlying community characteristics in one US county. The Journal of pediatrics. 2013;163(2):574–580. doi: 10.1016/j.jpeds.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck AF, Huang B, Simmons JM, et al. Role of financial and social hardships in asthma racial disparities. Pediatrics. 2014;133(3):431–439. doi: 10.1542/peds.2013-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegel J, Love AS, Wood PR, et al. The Inner-City Asthma Intervention: description of a community-based implementation of an evidence-based approach to asthma management. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2006;97(1 Suppl 1):S6–10. doi: 10.1016/s1081-1206(10)60778-8. [DOI] [PubMed] [Google Scholar]
- 8.Evans R, 3rd, Gergen PJ, Mitchell H, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. The Journal of pediatrics. 1999;135(3):332–338. doi: 10.1016/s0022-3476(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 9.Portnoy JM, Jennings D. Utilization patterns in an asthma intervention. Annals of Allergy, Asthma & Immunology. 2006;97(1):S25–S30. doi: 10.1016/s1081-1206(10)60782-x. [DOI] [PubMed] [Google Scholar]
- 10.Kenyon C, Sandel M, Silverstein M, Shakir A, Zuckerman B. Revisiting the social history for child health. Pediatrics. 2007;120(3):e734–738. doi: 10.1542/peds.2006-2495. [DOI] [PubMed] [Google Scholar]
- 11.Beck AF, Sauers HS, Kahn RS, Yau C, Weiser J, Simmons JM. Improved documentation and care planning with an asthma-specific history and physical. Hospital Pediatrics. 2012;2(4):194–201. doi: 10.1542/hpeds.2012-0016. [DOI] [PubMed] [Google Scholar]
- 12.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures--the public health disparities geocoding project. American journal of public health. 2003;93(10):1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. American journal of public health. 2005;95(2):312–323. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krieger N, Waterman PD, Chen JT, Soobader MJ, Subramanian SV. Monitoring socioeconomic inequalities in sexually transmitted infections, tuberculosis, and violence: geocoding and choice of area-based socioeconomic measures--the public health disparities geocoding project (US) Public health reports. 2003;118(3):240–260. doi: 10.1093/phr/118.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) Journal of epidemiology and community health. 2003;57(3):186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AF, Huang B, Chundur R, Kahn RS. Housing code violation density associated with emergency department and hospital use by children with asthma. Health affairs. 2014;33(11):1993–2002. doi: 10.1377/hlthaff.2014.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger N, Waterman P, Chen JT, Soobader MJ, Subramanian SV, Carson R. Zip code caveat: bias due to spatiotemporal mismatches between zip codes and US census-defined geographic areas--the Public Health Disparities Geocoding Project. American journal of public health. 2002;92(7):1100–1102. doi: 10.2105/ajph.92.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auger KA, Kahn RS, Davis MM, Beck AF, Simmons JM. Medical home quality and readmission risk for children hospitalized with asthma exacerbations. Pediatrics. 2013;131(1):64–70. doi: 10.1542/peds.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jencks C, Mayer S. Poverty and the distribution of material hardship. J Hum Resources. 1989;24:88–114. [Google Scholar]
- 20.Danziger S, Corcoran M, Danziger S, Heflin C. Work, income, and material hardship after welfare reform. J Consumer Affairs. 2000;34:6–30. [Google Scholar]
- 21.Ouellette T, Burstein N, Long D, Beecroft E. Measures of Material Hardship: Final Report. U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation; 2004. http://aspe.hhs.gov/hsp/material-hardship04/index.htm. [Google Scholar]
- 22.Seid M, Varni JW, Bermudez LO, et al. Parents’ Perceptions of Primary Care: measuring parents’ experiences of pediatric primary care quality. Pediatrics. 2001;108(2):264–270. doi: 10.1542/peds.108.2.264. [DOI] [PubMed] [Google Scholar]
- 23.United States Census Bureau. [Accessed June 9, 2015];Geography Terms and Concepts. https://www.census.gov/geo/reference/terms.html.
- 24.Schmidt NM, Lincoln AK, Nguyen QC, Acevedo-Garcia D, Osypuk TL. Examining mediators of housing mobility on adolescent asthma: results from a housing voucher experiment. Soc Sci Med. 2014;107:136–144. doi: 10.1016/j.socscimed.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leventhal T, Dupere V. Moving to Opportunity: does long-term exposure to ‘low-poverty’ neighborhoods make a difference for adolescents? Soc Sci Med. 2011;73(5):737–743. doi: 10.1016/j.socscimed.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 26.Bazemore AW, Cottrell EK, Gold R, et al. “Community Vital Signs”: Incorporating geocoded social determinants into electronic records to promote patient and population health. J Am Med Inform Assoc. 2015 doi: 10.1093/jamia/ocv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck AF, Simmons JM, Huang B, Kahn RS. Geomedicine: Area-Based Socioeconomic Measures for Assessing Risk of Hospital Reutilization Among Children Admitted for Asthma. American journal of public health. 2012 doi: 10.2105/AJPH.2012.300806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public health reports. 2014;129(Suppl 2):19–31. doi: 10.1177/00333549141291S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiscella K, Burstin HR, Nerenz DR. Quality measures and sociodemographic risk factors: to adjust or not to adjust. Jama. 2014;312(24):2615–2616. doi: 10.1001/jama.2014.15372. [DOI] [PubMed] [Google Scholar]
- 30.Sills MR, Hall M, Colvin JD, et al. Association of Social Determinants With Children’s Hospitals’ Preventable Readmissions Performance. JAMA pediatrics. 2016;170(4):350–358. doi: 10.1001/jamapediatrics.2015.4440. [DOI] [PubMed] [Google Scholar]
- 31.Colvin JD, Zaniletti I, Fieldston ES, et al. Socioeconomic status and in-hospital pediatric mortality. Pediatrics. 2013;131(1):e182–190. doi: 10.1542/peds.2012-1215. [DOI] [PubMed] [Google Scholar]
- 32.Beck AF, Klein MD, Schaffzin JK, Tallent V, Gillam M, Kahn RS. Identifying and treating a substandard housing cluster using a medical-legal partnership. Pediatrics. 2012;130(5):831–838. doi: 10.1542/peds.2012-0769. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. Comparing individual- and area-based socioeconomic measures for the surveillance of health disparities: A multilevel analysis of Massachusetts births, 1989–1991. Am J Epidemiol. 2006;164(9):823–834. doi: 10.1093/aje/kwj313. [DOI] [PubMed] [Google Scholar]
- 34.Miranda ML, Ferranti J, Strauss B, Neelon B, Califf RM. Geographic Health Information Systems: A Platform To Support The ‘Triple Aim’. Health affairs. 2013;32(9):1608–1615. doi: 10.1377/hlthaff.2012.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comer KF, Grannis S, Dixon BE, Bodenhamer DJ, Wiehe SE. Incorporating geospatial capacity within clinical data systems to address social determinants of health. Public health reports. 2011;126(Suppl 3):54–61. doi: 10.1177/00333549111260S310. [DOI] [PMC free article] [PubMed] [Google Scholar]