Abstract
Purpose
Therapeutic regimens for ATL are limited with unsatisfactory results, thereby warranting development of novel therapies.
This study investigated antitumor activity and toxicity of alemtuzumab with regard to response, duration of response, progression free survival, and overall survival in patients with human T-cell lymphotropic virus-1 (HTLV-1)-associated adult T-cell leukemia/lymphoma (ATL).
Experimental Design
Twenty-nine patients with chronic, acute, and lymphomatous types of ATL were enrolled in a single institution, nonrandomized, open-label Phase II trial wherein patients received intravenous alemtuzumab 30 mg three times weekly for a maximum of 12 weeks.
Results
Twenty-nine patients were evaluable for response and toxicity. The overall objective response was 15 out of 29 patients (95% CI: 32.5 to 70.6%). The 15 patients that responded manifested a median time to response of 1.1 months. Median response duration was 1.4 months for the whole group and 14.5 months among responders. Median progression free survival was 2.0 months. Median overall survival was 5.9 months. The most common adverse events were 2 with vasovagal episodes (7%) and 3 with hypotensive episodes (10%), leukopenia (41%)grade 3 and (17%) grade 4, lymphocytopenia (59%) grade 3, neutropenia (31%) grade 3, anemia (24%) and thrombocytopenia 10%. All patients developed cytomegalovirus antigenemia (CMV). Three were symptomatic and all responded to antiviral therapy. Grade 3 or 4 infections were reported in 4 (14%) of patients.
Conclusions
Alemtuzumab induced responses in patients with acute HTLV-1-associated ATL with acceptable toxicity, but with short duration of responses. These studies support inclusion of alemtuzumab in novel multi-drug therapies for ATL.
Keywords: Alemtuzumab, HTLV-1, leukemia/ lymphoma, T-cell
Introduction
Adult T-cell leukemia/lymphoma (ATL) is an aggressive lymphoproliferative disorder occurring in individuals infected with the human T-cell leukemia virus type-1 (HTLV-1)1-3. ATL is characterized by the proliferation of malignant CD4+ CD25+ T-cells in the peripheral blood, lymph nodes and other tissues 3. The disease develops in 3-5% of HTLV-1 infected individuals after a latent period of 40-60 years 2, 4-6. ATL cells exhibit characteristic morphological features (flower-like cells) with deeply indented nuclei, derived from mature Tregs with the surface phenotype (CD3+ dim, CD4+, CD7−, CD8−, and CD25+) 1, 5. The aggressiveness of ATL varies and the disease has been grouped into four clinical subtypes : the “smoldering” subtype is characterized by at least 5% abnormal circulating T-lymphocytes with a normal peripheral blood lymphocyte count (4 × 109/L), lack of hypercalcemia, serum lactic dehydrogenase (LDH) values no greater than 1.5 × the normal upper limit, and no lymphadenopathy or organ infiltration other than skin and lung; the chronic form of ATL manifests an absolute lymphocytosis (4 × 109/L) and a T-cell lymphocytosis, elevated serum LDH values up to twice the upper limit of normal, without hypercalcemia or involvement of the central nervous system, bone or gastrointestinal tract; the lymphoma subtype lacks a lymphocytosis and has less than or equal to 1% abnormal T-cells in the circulation in conjunction with histologically proven malignant lymphadenopathy; and the acute type that includes patients with leukemic manifestations 7.
Despite significant advances in the understanding and treatment of many lymphoproliferative diseases, the prognosis for ATL remains poor with median survivals of 6.2, 10.2 and 24 months for acute, lymphomatous and chronic subtypes respectively 5, 8, 9. The majority of patients with the three more aggressive categories of ATL chronic, acute and lymphomatous type have routinely been treated with anthracycline-based combination therapy including CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone). The Japanese Clinical Oncology Group demonstrated superiority of a complex multidrug chemotherapy regimen compared to biweekly CHOP in ATL in which the one-year progression free survival was 28% 8,9. Early relapse in ATL remains problematic despite any improvement in response rates or progression free survival and the overall survival is poor. In the study by Gill partial (6) or complete (5) remissions were observed in 11 of 19 patients with ATL treated with the combination of interferon-alpha and zidovudine (AZT)10. However, the median survival of the whole group was only 3 months, and was 13 months for patients who obtained a complete or partial remission. A meta-analysis suggested that AZT therapy in combination with interferon may provide value 11. However, this combination has shown lack of efficacy in patients with prior chemotherapy who have p53 mutations or a high expression of IRF412.
To develop an alternative to CHOP we have turned to monoclonal antibodies directed toward receptors expressed on the surface of ATL cells. The development of therapeutic monoclonal antibodies has been a major advance in the treatment of B-cell lymphomas and other tumors. The introduction of rituximab (chimerized anti-CD-20) into the clinic has improved the response rate, progression-free survival and overall survival of many patients with non-Hodgkin’s lymphoma of the B-cell linages, especially when combined with chemotherapy. We have evaluated receptor-directed monoclonal antibodies in a xenograft murine model of human
ATL 13-15. Using the MET-1 in vivo model of ATL, we demonstrated efficacy with the CD2 directed monoclonal antibody, MEDI-507 (siplizumab), the anti-CD25 antibody, daclizumab, and the anti-CD52 monoclonal antibody, alemtuzumab 13-15. On the basis of these encouraging preclinical results siplizumab, daclizumab and now alemtuzumab (CAMPATH-1H) have been translated into clinical trials involving patients with T-cell malignancies with partial and complete responses observed 16.
Alemtuzumab is a humanized monoclonal antibody engineered by grafting the rodent hypervariable complementarity determining regions into a human immunoglobulin molecule 17. It is directed at CD52, a 12 amino acid peptide that is processed from a larger precursor, that is highly glycosylated and linked to the cell membrane by a phosphatidylinositolglycan linkage. CD52 is expressed on lymphocytes and monocytes, but monocytes and NK cells appear resistant to alemtuzumab-mediated lysis 18. It is estimated that there are 5 × 105 antibody sites per lymphocyte and that the antigen does not modulate from the cell surface.
Examination of leukemic cells and fine-needle aspiration biopsies have demonstrated that CD52 is highly expressed on over 90% of ATL cells, and its expression is comparable to the level of CD25 expression observed on the tumor cells in these patients. Binding of alemtuzumab to CD52 causes cell death through complement activation and antibody-dependent cell-mediated cytotoxicity (ADCC) 19. As a result, alemtuzumab exhibits a powerful T-cell depleting effect and has been used to reduce the risk of graft-versus-host disease in patients undergoing allogeneic stem cell transplantation 20.
Alemtuzumab has demonstrated anticancer activity in patients with B-CLL21-23, non-Hodgkin’s lymphoma24, 25, T-cell prolymphocytic leukemia 26-28, mycosis fungoides, and Sézary syndrome29-31. In a large number of patients studied with alemtuzumab by Wellcome, Inc.32 they used an approach with 3 mg on day one, 10 mg on day two, 30 mg on day three, followed by 30 mg intravenously 3 × weekly for up to 12 weeks. The incidence of common adverse events seen in about half of the treated patients included chills/rigors, fever, nausea, vomiting and skin rash. Hypotension occurred in about a third of the patients. Dyspnea occurred in 25% and bronchospasm occurred in about 10% of patients. Thrombocytopenia occurred in association with the early infusions in the course of treatment. Tumor responses were observed in 16 of 174 patients. These responses occurred in patients with CLL or PLL leading to the approval of alemtuzumab for this indication. Alemtuzumab was granted initial approval by the U.S. Food and Drug Administration (U.S. FDA) for intravenous use indicated as a single agent for the treatment of B-cell chronic lymphocytic leukemia (B-CLL) in 2001. In 2007, the FDA granted approval for the use of alemtuzumab in previously treated B-CLL patients after evaluation in an open-label: (1) active-controlled trial in previously untreated patients with B-CLL and (2) Rai stage I-IV with evidence of progressive disease requiring therapy 33.
In preclinical studies, alemtuzumab prolonged the survival of mice with human MET-1 ATL comparable to that of tumor-free controls 14. These results were the basis of this single institution, nonrandomized, open-label Phase II clinical trial which was initiated to assess the antitumor activity and toxicity profile of alemtuzumab in patients with HTLV-1 associated ATL excluding those with the smoldering subtype.
Patients, materials and methods
Eligibility Criteria
To be included on the study, all patients had to have serum antibodies directed to HTLV-1 confirmed by Western blot. A histologically confirmed diagnosis of ATL with more than 10% of the malignant cells expressing CD52 and CD25 as determined by flow cytometry or on immunohistochemical staining was required. CD25 (IL-2R alpha, Tac) expressing chronic, acute and lymphomatous ATL subtypes were eligible. ATL patients were required to have measurable disease. Other requirements included: age greater than or equal to 18 years; absolute granulocyte count (AGC) greater than or equal to 1,000/μL; platelet count greater than or equal to 50,000/μL; serum creatinine no more than 3.0 mg/dL, serum hepatic transaminases less than or equal to 2.5 fold greater than the upper limit of normal (ULN), total bilirubin less than or equal to 3.0 mg/dL, and a life expectancy of at least 2 months. Patients were required to discontinue all cytotoxic chemotherapy, zidovudine, biological response modifier therapy, monoclonal antibodies, and other investigational antitumor agents at least 3 weeks prior to study entry.
Study Design and Treatment
This was a single institution, nonrandomized, open-label Phase II trial. Alemtuzumab was supplied through the Cancer Trials Evaluation Program (CTEP), NCI. Alemtuzumab was administered as follows: on day 1 patients received alemtuzumab 3 mg, followed by 10 mg on day 2 and 30 mg on day 3, followed by alemtuzumab 30 mg three times per week, separated by one day (usually Monday, Wednesday, Friday, or Tuesday, Thursday, Saturday). A maximum of 12 weeks of alemtuzumab were administered. Patients also received antimicrobial prophylaxis with oral trimethoprim/sulfamethoxazole 160/800 mg three times per week and daily famciclovir or valganciclovir and fluconazole. Patients were switched to i.v. ganciclovir for rising cytomegalovirus (CMV) antigen levels, or new onset antigenemia with signs of active infection.
CMV antigenemia in the first 7 patients was determined by quantitative immunofluorescence and in all subsequent patients by quantitative Real-Time polymerase chain reaction (qRT-PCR) performed on peripheral blood mononuclear cells (PMBCs). Polymerase chain reaction (PCR) for detection of HTLV-1 and T-cell receptor (TCR) rearrangement was performed on DNA extracted from PBMCs by TaqMan™ assay (Applied Biosystems, Foster City, CA).
Pathological Evaluation
Bone marrow aspirate and biopsy were performed prior to treatment, and every 4 weeks while on alemtuzumab in patients whose marrow was initially positive for tumor. Flow cytometry and lymphocyte subset analysis were performed on the peripheral blood at enrollment, and at weeks 4, 8, 12, 16, and all subsequent follow-up visits and included assessment of CD3, CD4, CD7, CD8, CD16, CD19, CD20, CD52, and CD25, the latter using the 7G7/B6 monoclonal antibody. After completion of the study, the patients were serially followed at least monthly if clinically indicated until the CD4 count recovered to greater than 200/mm3. In patients with leukemia the PBMCs were analyzed for HTLV-1 integration and for clonal T-cell receptor-gene rearrangements by PCR.
Assessment of Toxicity and Treatment Modification
Drug safety was assessed by examining adverse events (AE), laboratory abnormalities, physical examination and Karnofsky performance score status change from baseline (Table 3). Adverse events, serious adverse events (SAE) and abnormal lab values were graded using the CTEP Common Toxicity Criteria v 2.0. For monitoring, toxicity was defined over the 28-day period after treatment began. Patients who experienced grade 3 or 4 infusion-related AEs or who developed a grade 2 rash during the initial dose escalation had the lower dose of alemtuzumab continued until these toxicities resolved to less than or equal to grade 1. Alemtuzumab was discontinued in the event of grade 4 granulocytopenia persisting for more than 14 days despite growth factor therapy or grade 4 thrombocytopenia persisting for more than 14 days, grade 3 allergic reaction, grade 4 non-hematological toxicity or grade 3 non-hematological toxicity that did not resolve to less than grade 2 within 3 days with the exception of grade 3 infection.
Table 3.
Summary of grade 3, 4 or 5 AEs possibly, probably or definitely related to alemtuzumab therapy in patients with ATL (N = 29)
| Adverse Event | Grade 3 No (%) | Grade 4 No (%) |
|---|---|---|
|
| ||
| Hematologic: | ||
| • Leukopenia | • 12 (41) | • 5 (17) |
| • Lymphocytopenia | • 17 (59) | • 0 |
| • Neutropenia | • 9 (31) | • 1 (3) |
| • Anemia | • 7 (24) | • 0 |
| • Thrombocytopenia | • 3 (10) | • 0 |
|
| ||
| Hepatic: | ||
| • Alkaline phosphatase | • 1 (3) | • 0 |
| • Gamma-glutamyl transpeptidase | • 1 (3) | • 0 |
| • Bilirubin | • 1 (3) | • 0 |
| • Hypoalbuminemia | • 2 (7) | • 0 |
|
| ||
| Metabolic: | ||
| • Creatinine phosphokinase | • 1 (3) | • 0 |
| • Hypercalcemia | • 1 (3) | • 0 |
| • Hypocalcemia | • 1 (3) | • 0 |
| • Hypokalemia | • 1 (3) | • 0 |
| • Hypophosphatemia | • 1 (3) | • 0 |
|
| ||
| Allergy Immunology: | ||
| • Allergic reaction | • 1 (3) | • 0 |
|
| ||
| Cardiovascular: | ||
| • Vasovagal episode | • 2 (7) | • 0 |
| Hypotension | • 3 (10) | • 0 |
|
| ||
| Constitutional symptoms: | ||
| • Fever in the absence of neutropenia | • 3 (10) | • 0 |
|
| ||
| Pulmonary: | ||
| • Pulmonary infiltrates | • 1 (3) | • 0 |
| • Pulmonary hypoxia | • 2 (7) | • 0 |
|
| ||
| Ocular/Visual: | ||
| • Blurred vision | • 1 (3) | • 0 |
| • Photophobia | • 1 (3) | • 0 |
| • (other) Uveitis | • 1 (3 | • 0 |
|
| ||
| Endocrine: | ||
| • Hyperthyroidism | • 1 (3) | • 0 |
|
| ||
| Dermatological: | ||
| • Rash, desquamation | • 1 (3) | • 0 |
|
| ||
| Infection: | ||
| • With Grade 3 or 4 neutropenia | • 2 (7) | • 0 |
| • Without Grade 3 or 4 neutropenia | • 2 (7) | • 0 |
Abbreviations
AE adverse events
Response
Tumor response was determined using the International Workshop Standardized Response Criteria for non-Hodgkin’s Lymphoma 34. Responses had to last for a minimum of 4 weeks to be determined as a response. Tumor status was evaluated at pretreatment, day 28, 56, and 72 on study and the date of study discontinuation. Patients achieving a complete response (CR) at their evaluation time point or who had progressive disease (PD) defined as a persistent (at least two determinations) doubling of the peripheral blood leukemic cell count, the development of new lesions or serum calcium elevations that were uncontrolled by conventional therapeutic procedures at any time were removed from the study. Patients with a partial response (PR) or stable disease (SD) received an additional 4 weeks of alemtuzumab therapy and were then similarly reevaluated. A maximum of 12 weeks of alemtuzumab were administered.
Statistical Methods
This was a single-arm, nonrandomized trial. An optimal 2-stage design (Simon 1989) to test for early evidence for futility based on complete and partial responses was used. The design was based on the following: (a) the response proportion (partial and complete response) will be less than 5% if the treatment is totally ineffective (Po = .05), (b) the treatment will be considered effective and worthy of future investigation if the true response proportion is consistent with 30% (P1 = .30), (c) a type I error rate of 5% (i.e., the probability of concluding that the treatment is effective if the true response rate is 5%, is 0.05), (d) a power of 95% i.e., the probability of concluding that the treatment is effective if the true response proportion is 30% , is 0.95.
In the first stage of the 2-stage design, nine patients were studied. An additional twenty patients (i.e., a total of twenty-nine patients in the trial) were studied in the second stage. The major objective of the study was to determine the antitumor activity of alemtuzumab with regard to response rate, time to progression and overall survival. Time to progression was measured from the date of registration until documentation of disease progression. Overall survival was determined from the date of registration to the event of death or for surviving patients, censored by the last day patients were known alive. The objective tumor response (CR + PR) rate and 95% confidence interval were calculated. For responders, the duration of response was measured from date of the response (CR + PR) to the documentation of disease progression or censored at the latest evaluation. Median duration of response was calculated using the Kaplan-Meier Method.
Results
Between February 2004 and November 2009, twenty-nine patients with HTLV-1 associated ATL were registered and treated with intravenously administered alemtuzumab on this trial at the National Institutes of Health Clinical Center in Bethesda, MD (Tables 1 and 2). There were nineteen women and ten men with a median age 48 years (age range 24-76 years). Twenty-eight patients were Afro-Caribbean or African-American and one was Japanese. Eleven patients had lymphoma type ATL, fifteen had acute ATL and three had chronic ATL. The median Karnofsky performance status was 89.4 (range 80-90).
Table 1.
Demographics of patients with ATL
| New Patient | Age | Sex | Race | Number of Prior Therapies |
ATL Classification |
|---|---|---|---|---|---|
| 1 | 48 | F | AC | 1 | Lymphoma |
| 2 | 50 | F | AC | 2 | Lymphoma |
| 3 | 49 | M | AC | 2 | Lymphoma |
| 4 | 49 | F | AC | 3 | Lymphoma |
| 5 | 46 | F | AC | 4 | Lymphoma |
| 6 | 33 | M | AC | 1 | Lymphoma |
| 7 | 67 | F | AC | 0 | Lymphoma |
| 8 | 58 | M | AC | 2 | Lymphoma |
| 9 | 63 | F | AC | 2 | Lymphoma |
| 10 | 46 | F | AC | 1 | Lymphoma |
| 11 | 71 | M | AC | 0 | Lymphoma |
| 12 | 47 | M | AC | 1 | Acute Leukemia |
| 13 | 51 | F | AC | 3 | Acute Leukemia |
| 14 | 42 | M | AC | 2 | Acute Leukemia |
| 15 | 36 | F | AC | 0 | Acute Leukemia |
| 16 | 57 | F | AC | 1 | Acute Leukemia |
| 17 | 62 | F | Japanese | 0 | Acute Leukemia |
| 18 | 36 | F | AC | 1 | Acute Leukemia |
| 19 | 45 | M | AC | 0 | Acute Leukemia |
| 20 | 52 | M | AC | 0 | Acute Leukemia |
| 21 | 44 | M | AC | 1 | Acute Leukemia |
| 22 | 24 | M | AC | 1 | Acute Leukemia |
| 23 | 61 | F | AC | 0 | Acute Leukemia |
| 24 | 63 | F | AC | 2 | Acute Leukemia |
| 25 | 46 | F | AC | 0 | Acute Leukemia |
| 26 | 76 | F | AC | 2 | Acute Leukemia |
| 27 | 62 | F | AC | 2 | Chronic Leukemia |
| 28 | 57 | F | AC | 0 | Chronic Leukemia |
| 29 | 61 | F | AC | 1 | Chronic Leukemia |
| Median | 48 | 19F 10M |
1(0-4) |
AC Afro-Caribbean
Table 2.
Laboratory features of patients with ATL
| WBC | ALC | CD4+ CD25+ | IL-2R alpha | Hypercalcemia | |
|---|---|---|---|---|---|
| 1 | 3,300 | 1,016 | 302 | 9,434 | No |
| 2 | 2,840 | 826 | 257 | 1,528 | Yes |
| 3 | 5,080 | 1,158 | 595 | 1,567 | No |
| 4 | 2,790 | 625 | 90 | 6,329 | No |
| 5 | 6,000 | 1,157 | 203 | 1,039 | No |
| 6 | 11,000 | 101 | 772 | 4,880 | Yes |
| 7 | 6,100 | 793 | 1,061 | 29,780 | No |
| 8 | 5,450 | 763 | 162 | 3,940 | No |
| 9 | 10,800 | 194 | 367 | 20,630 | No |
| 10 | 3,200 | 1,024 | 246 | 5,872 | No |
| 11 | 3,760 | 1,339 | 581 | 1,461 | No |
| 12 | 5,470 | 1,597 | 1,076 | 18,178 | No |
| 13 | 49,300 | 38,701 | 40,640 | 1,627 | No |
| 14 | 19,200 | 1,536 | 6,994 | 16,324 | Yes |
| 15 | 111,000 | 98,790 | 13,768 | 30,500 | Yes |
| 16 | 48,000 | 31,959 | 36,201 | 61,137 | No |
| 17 | 63,000 | 40,128 | 4,834 | 23,580 | No |
| 18 | 6,000 | 26,818 | 1,486 | 1,240 | Yes |
| 19 | 271,000 | 252,030 | 213,339 | 2,935 | Yes |
| 20 | 35,700 | 23,562 | 26,595 | 18,000 | Yes |
| 21 | 225,000 | 204,750 | 193,571 | 41,230 | Yes |
| 22 | 2,830 | 1,007 | 519 | 3,633 | No |
| 23 | 26,400 | 13,200 | 12,457 | 44,530 | Yes |
| 24 | 64,500 | 41,280 | 53,261 | <1,125 | No |
| 25 | 339,000 | 332,050 | 296,913 | 8,015 | Yes |
| 26 | 99,650 | 20,930 | 167 | 3,633 | No |
| 27 | 4,660 | 559 | 224 | 3,975 | No |
| 28 | 6,270 | 3,950 | 2,099 | <1,135 | No |
| 29 | 3,540 | 1,841 | 27 | 855 | No |
| Mean | 49,684 | 39,487 | 31,338 | 6,329 | |
| Median | 6,270 | 1,597 | 1,061 | 13,650 | |
| Range | 2,790-339000 | 101-332050 | 27-296913 | 855-61,137* |
• Does not reflect that values below <1125 or <1135 are not known with certainty
Twenty patients (69%) received prior treatment for their ATL, receiving a median of one prior regimen (range 0-4). Prior chemotherapies included CHOP in fifteen patients, zidovudine and interferon-alpha in one patient, daclizumab (anti-CD25) monoclonal antibody in three and siplizumab (anti-CD2 monoclonal antibody) in four patients. One patient had received palliative radiation therapy. Nine patients were untreated prior to the trial. Six patients were receiving a stable dose of corticosteroids at the time of registration. Twenty-one patients (75%) completed treatment according to the protocol. The most common reason for early withdrawal was disease progression. The median time on the study was 6.7 weeks (range 6-143.6 weeks) and the patients received a median of 1.6 cycles of alemtuzumab (range 1-3 cycles). A cycle was defined as completion of 4 weeks of alemtuzumab at 30 mg three times weekly (total of 12 doses).
Toxicity
Grade 3 or higher infusion reactions were limited to 2 (7%) grade 3 vasovagal episodes and 3 (10%) hypotensive episodes (Table 3). All patients developed CMV antigenemia. Of these three were symptomatic but all responded to antiviral therapy. Grade 3 or 4 infections were reported in four (14%) of the patients. Grade 3 and 4 adverse events occurring in 10% or more of the patients, outlined in Table 3, included: hematologic (leukopenia 41% grade 3 and 17% grade 4, lymphocytopenia 59% grade 3, neutropenia 31% grade 3 and 3% grade 4, anemia 24% grade 3 and thrombocytopenia 10% grade 3). After completion of the study, the patients were serially followed at least monthly if clinically indicated until the CD4 count recovered to greater than 200/mm3. The median time to recovery of ALC to > 200 cells/μL was 1.8 months.
Response
The primary objective of this study was to determine the antitumor activity and toxicity profile of alemtuzumab in HTLV-1-associated ATL. The number of circulating cells expressing leukemic cell phenotype was monitored by direct and indirect immunocytofluoroscopy using a fluorescence-activated cell sorter (FACS). Fifteen (52%) of twenty-nine patients with ATL responded to alemtuzumab, including 12 of 15 with acute ATL and 2 of 3 with chronic ATL (95% confidence interval on 15/29: 32.5-70.6%; Table 4). There was only one response among the 11 patients with lymphoma type ATL. Six patients achieved a CR (21%) and nine a PR (31%). For those who responded, the median time to response was 1.1 months (range 1.0 to 4.2 months). Median duration of response was 1.4 months for the whole group and 14.5 months for responders. A Kaplan-Meier plot of overall survival is shown in Figure 1; median survival was 5.9 months. A plot of survival for responders is shown in Supplementary Figure 1. Median progression-free survival was 2.0 months, and is shown in Supplementary Figure 2.
Table 4.
Response of ATL Patients to Alemtuzumab
| ATL Classification | Response | Response Duration (Weeks) |
|---|---|---|
| Lymphoma | PD | 0.0 |
| Lymphoma | PD | 0.0 |
| Lymphoma | PD | 0.0 |
| Lymphoma | PD | 0.0 |
| Lymphoma | PD | 0.0 |
| Lymphoma | PD | 0.0 |
| Lymphoma | PR | NA |
| Lymphoma | PD | 0.0 |
| Lymphoma | PD | 0.0 |
| Lymphoma | PD | 0.0 |
| Lymphoma | PD | 0.0 |
| Acute Leukemia | PR | 12.1 |
| Acute Leukemia | CR | 132 |
| Acute Leukemia | PR | 2.9 |
| Acute Leukemia | PD | 0.0 |
| Acute Leukemia | PR | 4.4 |
| Acute Leukemia | CR | 28.4 |
| Acute Leukemia | CR | 27.9 |
| Acute Leukemia | CR | 11.7 |
| Acute Leukemia | PR | 7.9 |
| Acute Leukemia | PD | 0.0 |
| Acute Leukemia | CR | 119 |
| Acute Leukemia | PD | 0.0 |
| Acute Leukemia | PR | 33.9 |
| Acute Leukemia | PR | 5.6 |
| Acute Leukemia | PR | 12.0 |
| Chronic Leukemia | PD | 0.0 |
| Chronic Leukemia | CR | 33.3 |
| Chronic Leukemia | PR | 17.0 |
| Summaries | ||
| Mean | 6.9 | |
| Median | 1.4* | |
| Range | 0-132 | |
| 14.5 months for responders |
Figure Legend 1.
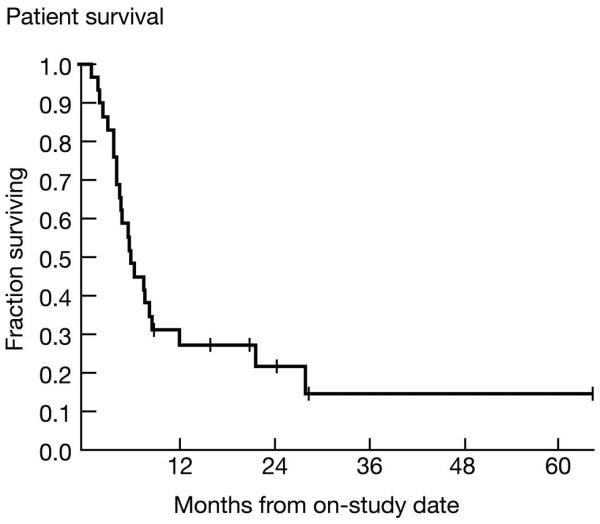
Kaplan-Meier Plot of Survival of patients with ATL (3 chronic, 15 acute and 11 lymphomatous) who were treated with alemtuzumab (CAMPATH-1, anti-CD52). The median survival was 5.9 months.
Discussion
There have been major advances in the therapy of aggressive B-cell lymphomas with the use of anthracycline-containing regimens such as CHOP and EPOCH with the anti-CD20 monoclonal antibody, rituximab. Therapeutic strategies with most forms of T-cell malignancies have been much less effective. By 2010 four agents (romidepsin, brentuximab vedotin, pralatrexate and panobinostat) have been approved by the FDA for the treatment of various T-cell malignancies 35. However, when used as single agents only about 30% of patients treated respond to therapy with a recurrence as the rule. A consensus has developed that multidrug combinations will be required for effective therapy of T-cell leukemia/lymphoma. Monoclonal antibodies have a number of features that support their inclusion in these multidrug combinations including excellent pharmacokinetics with a long survival duration in vivo, great specificity and usually lack of bone marrow toxicity that would complicate therapy in association with chemotherapeutic agents 36. Phenotypic analyses of ATL cells revealed a high (> 80%) incidence of expression of the chemokine receptor-4 (CCR4) as a hallmark of this disease 37, 38. Mogamulizumab (KW-0761) is a defucosylated humanized antibody with enhanced antibody dependent cellular cytotoxicity (ADCC) that binds to CCR4 and has yielded an overall response rate of 50% (in 13/26) in relapsed patients with CCR4 positive ATL 39, 40. Nevertheless, the anti-CCR4 monoclonal antibody is not curative and most of the patients relapse. As noted above, using the MET-I in vivo model of ATL we demonstrated efficacy in mice with the anti-CD2 monoclonal antibody, siplizumab, the anti-CD25 antibody, daclizumab and the anti-CD52 monoclonal antibody, alemtuzumab (CAMPATH-1)13-15. Furthermore, in our murine model additive/synergistic responses were observed with the combination of daclizumab with bortezomib, flavopiridol and romidepsin 41-43. In addition, in the MET-1 murine model a synergistic response was observed with the combination of alemtuzumab and the putative survivin inhibitor, YM155 44. Furthermore, we observed additivity/synergy when alemtuzumab was combined with 9AA, an agent that induces the activation of non-mutated but suppressed expression of p53 and activates NF-κB 45.
In the present study we evaluated monoclonal antibody mediated treatment in a phase II trial with patients receiving intravenous alemtuzumab 30 mg three times weekly for a maximum of 12 weeks. There was an overall objective response in 15 out of 29 patients. Twelve of 15 patients with acute, 2 of 3 patients with chronic, and only 1 of 11 patients with lymphomatous HTLV-1 associated ATL manifested a response. However, the responses were brief with a median response duration of only 1.4 months for the whole group, 14.5 months among the 15 responders and a median overall survival of only 5.9 months. These studies support further clinical trials in patients with chronic and acute forms of HTLV-1 associated ATL with alemtuzumab however in combination therapy.
One approach to be used with monoclonal antibodies such as alemtuzumab involves an effort to increase their efficacy by increasing their antibody dependent cellular cytotoxicity (ADCC). ADCC involves the interaction of the Fc element of the monoclonal antibody with Fc alpha III and IV activating receptors on effector cells46. In particular, in Fc receptor gamma−/− mice the efficacy of daclizumab and alemtuzumab in the MET-1 murine tumor model was lost 13, 14. A number of strategies have been used successfully to augment monoclonal antibody ADCC. These include defucosylation of the antibody or alteration of the Fc element to increase the binding of the monoclonal antibody to NK cells and monocytes that is required for its action 39. An additional approach involves the co-administration of an antibody to CD137 to activate NK cells-- the cellular partner of the monoclonal antibody in ADCC 47. We are investigating yet another alternative, the use of IL-15in conjunction with alemtuzumab. The administration of tolerable concentrations of IL-15 to mice, rhesus macaques, and to human patients has resulted in a 4 to 8-fold increase in the number of circulating activated NK cells 48. Furthermore, continuous intravenous infusion of IL-15 at 20 micrograms/kilogram to rhesus macaques yielded a 15-fold increase in the number of circulating monocytes and an 80 to 100-fold increase in the number of circulating effector memory CD8 T-cells 49. We have demonstrated a marked increase in the magnitude and duration of the antitumor response with the use of the anti-CD20 monoclonal antibody, rituximab through ADCC with IL-15 in a syngeneic model that involves EL4 cells transfected with human CD2050. Furthermore, in a syngeneic T-cell malignancy model using the cytokine independent 43Tb− ATL T-cell line in SCID/NOD mice we demonstrated a similar increase in the duration of the antitumor response when alemtuzumab was used 50. On the basis of these studies we have initiated a clinical trial that involves the evaluation of alemtuzumab in association with IL-15 for patients with severe chronic and acute ATL (Clinical Trial NCT02689453).
In conclusion, in the present study alemtuzumab induced responses in 12 of 15 patients with acute, and 2 of 3 patients with chronic HTLV-1-associated ATL with acceptable toxicity, but with a short duration of response. These studies support further clinical trials in patients with chronic and acute leukemic forms of HTLV-1 associated ATL with alemtuzumab given in conjunction with small- molecule agents that have shown efficacy in ATL or with IL-15 to augment the ADCC action of the monoclonal antibody.
Supplementary Material
Translational Relevance.
Therapeutic regimens including CHOP for human T-cell lymphotropic 1 (HTLV-1) associated adult T-cell leukemia (ATL) are limited by unsatisfactory outcomes thereby warranting development of novel therapies. Monoclonal antibody-mediated therapy with alemtuzumab induced responses in 15 of 29 patients including 12 of 15 patients with acute, 2 of 3 patients with chronic and one of 11 patients with lymphomatous HTLV-1 associated ATL with acceptable toxicity. These studies support further clinical trials in patients with chronic and acute leukemic forms of HTLV-1 associated ATL with alemtuzumab given in conjunction with small-molecule agents that have shown efficacy in ATL or with IL-15 to augment the ADCC action of the monoclonal antibody.
Footnotes
Presented in abstract form at the 50th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2008.
References
- 1.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50(3):481–492. [PubMed] [Google Scholar]
- 2.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada Y, Tomonaga M, Fukuda H, Hanada S, Utsunomiya A, Tara M, et al. A new G-CSF-supported combination therapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br J Haematol. 2001;113(2):375–382. doi: 10.1046/j.1365-2141.2001.02737.x. [DOI] [PubMed] [Google Scholar]
- 4.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, et al. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broder S, Bunn PA, Jr, Jaffe ES, Blattner W, Gallo RC, Wong-Staal F, et al. NIH conference T-cell lymphoproliferative syndrome associated with human T-cell leukemia/lymphoma virus. Ann Intern Med. 1984;100:543–557. doi: 10.7326/0003-4819-100-4-543. [DOI] [PubMed] [Google Scholar]
- 6.Jeang KT, Boros I, Brady J, Radonovich M, Khoury G. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J Virol. 1988;62(12):4499–4509. doi: 10.1128/jvi.62.12.4499-4509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87) Br J Haematol. 1991;79(3):428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 8.Shimoyama M, Ota K, Kikuchi M, Yunoki K, Konda S, Takatsuki K, et al. Chemotherapeutic results and prognostic factors of patients with advanced non-Hodgkin’s lymphoma treated with VEPA or VEPA-M. J Clin Oncol. 1988;6(1):128–141. doi: 10.1200/JCO.1988.6.1.128. [DOI] [PubMed] [Google Scholar]
- 9.Tsukasaki K, Utsunomiya A, Fukuda H, Shibata T, Fukushima T, Takatsuka Y, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group. Study JCOG9801. J Clin Oncol. 2007;25(34):5458–5464. doi: 10.1200/JCO.2007.11.9958. [DOI] [PubMed] [Google Scholar]
- 10.Gill PS, Harrington W, Jr, Kaplan MH, Ribeiro RC, Bennett JM, Liebman HA, et al. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med. 1995;332(26):1744–1748. doi: 10.1056/NEJM199506293322603. [DOI] [PubMed] [Google Scholar]
- 11.Bazarbachi A, Plumelle Y, Carlos Ramos J, Tortevoye P, Otrock Z, Taylor G, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28(27):4177–4183. doi: 10.1200/JCO.2010.28.0669. [DOI] [PubMed] [Google Scholar]
- 12.Datta A, Bellon M, Sinha-Datta U, Bazarbachi A, Lepelletier Y, Canioni D, et al. Persistent inhibition of telomerase reprograms adult T-cell leukemia to p53-dependent senescence. Blood. 2006;108(3):1021–1029. doi: 10.1182/blood-2006-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Zhang M, Ravetch JV, Goldman C, Waldmann TA. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD2 monoclonal antibody, MEDI-507. Blood. 2003;102(1):284–288. doi: 10.1182/blood-2002-11-3601. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Zhang M, Goldman CK, Ravetch JV, Waldmann TA. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD52 monoclonal antibody, Campath-1H. Cancer Res. 2003;63(19):6453–6457. [PubMed] [Google Scholar]
- 15.Zhang M, Zhang Z, Garmestani K, Goldman CK, Ravetch JV, Brechbiel MW, et al. Activating Fc receptors are required for antitumor efficacy of the antibodies directed toward CD25 in a murine model of adult T-cell leukemia. Cancer Res. 2004;64(16):5825–5829. doi: 10.1158/0008-5472.CAN-04-1088. [DOI] [PubMed] [Google Scholar]
- 16.Berkowitz JL, Janik JE, Stewart DM, Jaffe ES, Stetler-Stevenson M, Shih JH, et al. Safety, efficacy and pharmacokinetics/pharmacodynamics of daclizumab (anti-CD25) in patients with adult T-cell leukemia/lymphoma. Clin Immunol. 2014;155(2):176–187. doi: 10.1016/j.clim.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332(6162):323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- 18.Rao SP, Sancho J, Campos-Rivera J, Boutin PM, Severy PB, Weeden T, et al. Human peripheral blood mononuclear cells exhibit heterogeneous CD52 expression levels and show differential sensitivity to alemtuzumab mediated cytolysis. PLoS One. 2012;7(6):e39416. doi: 10.1371/journal.pone.0039416. doi:10:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golay J, Manganini M, Rambaldi A, Introna M. Effect of alemtuzumab on neoplastic B cells. Haematologica. 2004;89(12):1476–83. [PubMed] [Google Scholar]
- 20.Hale G, Jacobs P, Wood L, Fibbe WE, Barge R, Novitzky N, et al. CD52 antibodies for prevention of graft-versus-host disease and graft rejection following transplantation of allogeneic peripheral blood stem cells. Bone Marrow Transplant. 2000;26(1):69–76. doi: 10.1038/sj.bmt.1702477. [DOI] [PubMed] [Google Scholar]
- 21.Osterborg A, Dyer MJ, Bunjes D, Pangalis GA, Bastion Y, Catovsky D, et al. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukemia. J Clin Oncol. 1997;15(4):1567–1574. doi: 10.1200/JCO.1997.15.4.1567. [DOI] [PubMed] [Google Scholar]
- 22.Lundin J, Kimby E, Björkholm M, Broliden PA, Celsing F, Hjalmar V, et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2002;100(3):768–773. doi: 10.1182/blood-2002-01-0159. [DOI] [PubMed] [Google Scholar]
- 23.Rai KR, Freter CE, Mercier RJ, Cooper MR, Mitchell BS, Stadtmauer EA, et al. Alemtuzumab in previously treated chronic lymphocytic leukemia patients who also had received fludarabine. J Clin Oncol. 2002;20(18):3891–3897. doi: 10.1200/JCO.2002.06.119. [DOI] [PubMed] [Google Scholar]
- 24.Lundin J, Osterborg A, Brittinger G, Crowther D, Dombret H, Engert A, et al. CAMPATH-1H monoclonal antibody in therapy for previously treated low-grade non-Hodgkin’s lymphomas: a phase II multicenter study. European Study Group of CAMPATH-1H Treatment in Low-Grade Non-Hodgkin’s Lymphoma. J Clin Oncol. 1998;16(10):3257–3263. doi: 10.1200/JCO.1998.16.10.3257. [DOI] [PubMed] [Google Scholar]
- 25.Khorana A, Bunn P, McLaughlin P, Vose J, Stewart C, Czuczman MS. A phase II multicenter study of CAMPATH-1H antibody in previously treated patients with nonbulky non-Hodgkin’s lymphoma. Leuk Lymphoma. 2001;41(1-2):77–87. doi: 10.3109/10428190109057956. [DOI] [PubMed] [Google Scholar]
- 26.Pawson R, Dyer MJ, Barge R, Matutes E, Thornton PD, Emmett E, et al. Treatment of T-cell prolymphocytic leukemia with human CD52 antibody. J Clin Oncol. 1997;15(7):2667–2672. doi: 10.1200/JCO.1997.15.7.2667. [DOI] [PubMed] [Google Scholar]
- 27.Dearden CE, Matutes E, Cazin B, Tjonnfjord GE, Parreira A, Nomdedeu B, et al. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood. 2001;98(6):1721–1726. doi: 10.1182/blood.v98.6.1721. [DOI] [PubMed] [Google Scholar]
- 28.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99(10):3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy GA, Seymour JF, Wolf M, Januszewicz H, Davison J, McCormack C, et al. Treatment of patients with advanced mycosis fungoides and Sézary syndrome with alemtuzumab. Eur J Haematol. 2003;71(4):250–256. doi: 10.1034/j.1600-0609.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 30.Lundin J, Hagberg H, Repp R, Cavallin-Stahl E, Fredén S, Juliusson G, et al. A. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced fungoides/Sézary syndrome. Blood. 2003;101(11):4267–4272. doi: 10.1182/blood-2002-09-2802. [DOI] [PubMed] [Google Scholar]
- 31.Gautschi O, Blumenthal N, Streit M, Solenthaler M, Hunziker T, Zenhäusern R. Successful treatment of chemotherapy-refractory Sezary syndrome with alemtuzumab (Campath-1H) Eur J Haematol. 2004;72(1):61–63. doi: 10.1046/j.0902-4441.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 32.Waldmann H, Hale G. CAMPATH: from concept to clinic. Trans R Soc Lond Biol Sci. 2005;360(1461):1707–1711. doi: 10.1098/rstb.2005.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demko S, Summers J, Keegan P, Kazdur R. FDA drug approval summary: alemtuzumab as single-agent treatment for B-cell chronic lymphocytic leukemia. Oncologist. 2008;13(2):167–174. doi: 10.1634/theoncologist.2007-0218. [DOI] [PubMed] [Google Scholar]
- 34.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workup to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 35.Foss F. Evolving Therapy of Peripheral T-Cell Lymphoma: 2010 and beyond. Ther Adv Hematol. 2011;2(3):161–173. doi: 10.1177/2040620711408491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldmann TA, Morris JC. Development of antibodies and chimeric molecules for cancer immunotherapy. Adv Immunol. 2006;90:83–131. doi: 10.1016/S0065-2776(06)90003-0. [DOI] [PubMed] [Google Scholar]
- 37.Yoshie O, Fujisawa R, Nakayama T, Harasawa H, Tago H, Izawa D, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transfromed T cells. Blood. 2002;99(5):1505–1511. doi: 10.1182/blood.v99.5.1505. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa M, Schmitz R, Xiao W, Goldman CK, Xu W, Yang Y, et al. Gain-of-function CCR4 mutations in adult T cell leukemia/lymphoma. J Exp Med. 2014;211(13):2497–2505. doi: 10.1084/jem.20140987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010;28(9):1591–1598. doi: 10.1200/JCO.2009.25.3575. [DOI] [PubMed] [Google Scholar]
- 40.Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837–842. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, Zhang Z, Goldman CK, Janik J, Waldmann TA. Combination therapy for adult T-cell leukemia-xenografted mice: flavopiridol and anti-CD25 monoclonal antibody. Blood. 2005;105(3):1231–1236. doi: 10.1182/blood-2004-05-1709. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Zhang M, Ju W, Waldmann TA. Effective treatment of a murine model of adult T-cell leukemia using depsipeptide and its combination with unmodified daclizumab directed toward CD25. Blood. 2009;113(6):1287–1293. doi: 10.1182/blood-2008-04-149658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan C, Waldmann TA. Proteasome inhibitor PS-341, a potential therapeutic agent for adult T-cell leukemia. Cancer Res. 2002;62(4):1083–1086. [PubMed] [Google Scholar]
- 44.Chen J, Pise-Masison CA, Shih JH, Morris JC, Janik JE, Conlon KC, Keating A, Waldmann TA. Markedly additive antitumor activity with the combination of a selective survivin suppressant YM155 and alemtuzumab in adult T-cell leukemia. Blood. 2013;121(11):2029–2037. doi: 10.1182/blood-2012-05-427773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ju W, Zhang ML, Petrus M, Maeda M, Pise-Masison CA, Waldmann TA. Combination of 9-aminoacridine with Campath-1H provides effective therapy for a murine model of adult T-cell leukemia. Retrovirology. 2014;11:43. doi: 10.1186/1742-4690-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 47.Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117(8):2423–2432. doi: 10.1182/blood-2010-08-301945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117(18):4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sneller MC, Kopp WC, Engelke KJ, Yovandich JL, Creekmore SP, Waldmann TA, et al. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood. 2011;118(26):6845–6848. doi: 10.1182/blood-2011-09-377804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wen B, Zhang M, Dilillo D, Ravetch JV, Waldmann TA. Interleukin-15 enhances rituximab-dependent cytotoxicity ex vivo and in vivo against mouse lymphoma expressing human CD20. Cancer Res. 2015;75(supplement 15) doi: 10.1158/1538-7445. meeting abstract 1332. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


