Abstract
Purpose
Compare patient-centered outcomes in patients with proliferative diabetic retinopathy (PDR) treated with ranibizumab versus panretinal photocoagulation (PRP).
Design
Randomized clinical trial.
Methods
Setting: Multicenter (55 US sites). Patient Population: 216 adults with one study eye out of 305 adults (excluding participants with two study eyes since each eye received a different treatment) with PDR, visual acuity 20/320 or better, no history of PRP. Intervention: Ranibizumab (0.5-mg/0.05mL) versus PRP. Main Outcome Measures: Change from baseline to 2 years in composite and pre-specified subscale scores from the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25), University of Alabama Low Luminance Questionnaire (UAB-LLQ), Work Productivity and Activity Impairment Questionnaire (WPAIQ).
Results
For the NEI VFQ-25 and UAB-LLQ composite scores, ranibizumab-PRP treatment group differences (95% CI) were +4.0 (−0.2, +8.3, P=0.06) and +1.8 (−3.5, +7.1, P=0.51) at 1 year, and +2.9 (−1.5, +7.2, P=0.20) and +2.3 (−2.9, +7.5, P=0.37) at 2 years, respectively. Work productivity loss measured with the WPAIQ was 15.6% less with ranibizumab (−26.3%, −4.8%, P=0.005) at 1 year and 2.9% (−12.2%, +6.4%, P=0.54) at 2 years. Eighty-three ranibizumab participants (97%) were 20/40 or better in at least one eye (visual acuity requirement to qualify for an unrestricted driver license in many states) at 2 years compared with 82 PRP participants (87%, adjusted risk ratio=1.1, 95% CI: 1.0, 1.2 P=0.005).
Conclusions
While differences in some work productivity and driving-related outcomes favored ranibizumab over PRP, no differences between treatment regimens for PDR were identified for most of the other patient-centered outcomes considered.
INTRODUCTION
The Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol S, Prompt Panretinal Photocoagulation (PRP) versus Intravitreal Ranibizumab with Deferred Panretinal Photocoagulation for Proliferative Diabetic Retinopathy (PDR), determined that ranibizumab resulted in visual acuity not more than 5 letters worse than PRP treatment at 2 years.1 Secondary outcomes favoring ranibizumab included better average visual acuity over the course of 2 years, less visual field loss, fewer vitrectomies, and lower likelihood of developing diabetic macular edema (DME) with vision loss, supporting the consideration of ranibizumab as a treatment alternative to PRP for patients with PDR. However, ranibizumab does involve a greater number of treatments and visits than PRP. Over 2 years, eyes in the ranibizumab group had a median of 10 injections (for PDR and DME) and 22 visits compared with a median of only one injection (for DME) and 16 visits (over 2 years) in the PRP group. Of note, supplemental PRP was performed in 45% of the eyes in the PRP group.
The analysis of 2-year results1 did not identify statistically significant treatment group differences in the composite or subscale scores of the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25)2 or the University of Alabama at Birmingham Low Luminance Questionnaire (UAB-LLQ).3 In the current report, the results of additional analyses of these questionnaires as well as Work Productivity and Activity Impairment Questionnaire (WPAIQ) are provided. 4 These patient-centered outcomes could influence treatment decisions for using anti-vascular endothelial growth factor therapy in the management of PDR. Additional outcomes related to driving also are considered.
METHODS
Overview
The DRCR.net conducted a multicenter (55 US sites) randomized trial of 394 eyes among 305 adults enrolled between February and December 2012 (clinicaltrials.gov Identifier: NCT01489189). Participants were at least 18 years old and had type 1 or type 2 diabetes mellitus. The study eye had PDR, no history of PRP, and a best-corrected visual acuity letter score of 24 or better (approximate Snellen equivalent 20/320 or better). Eyes with or without central-involved DME were eligible. Eyes were randomly assigned to prompt PRP or ranibizumab injections (Lucentis®, Genentech Inc., South San Francisco, CA, USA). The use of ranibizumab for diabetic retinopathy only in the presence of DME is listed as an indication within its prescribing information in the U.S..5 Both groups received ranibizumab injections as needed for DME. Participants and investigators were unmasked to study treatment. The study adhered to the Declaration of Helsinki and was approved by multiple institutional review boards. The protocol and statistical methods have been reported previously,1 and more details can be found in eAppendix 1.
At baseline, 1 year, and 2 years, participants completed the NEI VFQ-25 and UAB-LLQ. The WPAIQ was completed at baseline, four weeks and at each assessment visit (16, 32, 52, 68, 84, and 104 weeks). All subsequent analysis of DRCR.net data for this report included the 216 study participants (102 in the ranibizumab group and 114 in the PRP group) with only one study eye. The 89 participants with both eyes enrolled in the trial were not included because one eye was assigned to each treatment group. Since surveys were administered at the patient-level and treatment was administered at the eye-level, a treatment group comparison is not possible. Electronic-Early Treatment Diabetic Retinopathy Study (E-ETDRS) visual acuity testing following a protocol refraction was performed in each eye along with binocular E-ETDRS visual acuity testing with the participant's habitual correction (“everyday” glasses or contacts).
Questionnaires
The NEI VFQ-25 is a 25-item questionnaire measuring self-reported vision-targeted health status. It contains one question on general health and 11 vision-related scales: general vision, ocular pain, near activities, distance activities, social functioning, mental health, role difficulties, dependency, driving, color vision, and peripheral vision. A composite score averages each of the vision-related scales.
The UAB-LLQ is a 32-item questionnaire originally developed to assess vision under low luminance and at night in studies of age-related macular degeneration. It is comprised of the following 6 scales, which are averaged to form a composite score: driving, extreme lighting, mobility, emotional distress, general dim lighting, and peripheral vision.
The WPAIQ for specific health problems is a 6-item questionnaire yielding four scores related to employment and every-day activities: absenteeism (percent work time missed due to vision), presenteeism (percent impairment while working due to vision), work productivity loss (percent overall work impairment due to vision, a composite of the absenteeism and presenteeism scales), and activity impairment (percent activity impairment due to vision).
The NEI VFQ-25 and UAB-LLQ were interviewer-administered with all instructions read aloud, or self-administered in the clinic or at home up to 14 days prior to the study visit. The WPAIQ was always interviewer-administered. Spanish versions were available for the NEI VFQ-25 and WPAIQ but not the UAB-LLQ. Fifteen participants who completed the NEI-VFQ 25 and WPAIQ at randomization in Spanish did not complete the UAB-LLQ.
Outcomes
For each of the questionnaire subscales the following outcomes were pre-specified: (1) the mean change from baseline to 1 and 2 years, and (2) for the NEI VFQ-25 and UAB-LLQ, the proportion of participants gaining or losing at least 10 points (changes judged to be clinically relevant)3, 6. These same outcomes were evaluated post-hoc for the NEI VFQ-25 and UAB-LLQ composite scores. The following subscales were designated a priori as primary interest because investigators believed they were the ones most likely to show a difference: driving, peripheral vision, and color vision from the NEI VFQ-25, driving, peripheral vision, and general dim lighting from the UAB-LLQ, and work productivity loss from the WPAIQ.
Because patient-centered outcomes can differ substantially by whether the study eye is the better- or worse-seeing eye, a post-hoc subgroup analysis of the outcomes described above was performed using continuous outcome data where the study eye was the better-seeing eye as defined previously.7-10 A second post-hoc subgroup analysis was conducted to determine if the treatment effect differed by whether the study eye had DME at baseline for which ranibizumab was required by protocol in both groups (based on gender- and machine-specific thresholds of retinal thickness and visual acuity impairment of 20/32 or worse).
Because the driving subscales were of particular interest, two driving-related outcomes were pre-specified: (1) number (%) of participants driving at baseline who stopped driving wholly or partly because of vision and (2) number (%) of participants driving at night at baseline who stopped driving at night wholly or partly because of vision. In addition, the following driving-related outcomes were evaluated post-hoc: (1) the number (%) of participants driving among participants who, at baseline, were driving or had given up driving wholly or in part because of vision, (2) number (%) of participants with visual acuity letter score (approximate Snellen equivalent) of at least 69 (20/40 or better, the legal limit for driving in many US states) in (a) at least one eye, regardless of which eye was the study eye, (b) in the better-seeing eye, for participants whose study eye is the better-seeing eye, and (c) using both eyes (binocular visual acuity).
Change in visual acuity, as well as visual acuity area under the curve (AUC), which were evaluated for the full cohort in the primary manuscript,1 were evaluated post-hoc for the subgroup of participants with one study eye. Area under the curve analyses were also conducted post-hoc for the NEI VFQ-25 and UAB-LLQ composite scores and the WPAIQ work productivity loss score.
Statistical analyses
Mean change in questionnaire scores from baseline was analyzed with analysis of covariance adjusting for baseline score and optical coherence tomography (OCT) central subfield thickness. Missing outcomes were imputed using last observation carried forward. Sensitivity analyses included only observed data (eTables 18-30).
For dichotomous outcomes, Poisson regression with a robust error variance was used to estimate the relative risk while adjusting for OCT central subfield thickness and baseline outcome values.11 Only participants who completed the 2-year visit were included in AUC analyses. AUC was calculated according to the trapezoidal rule and is analogous to taking a weighted average of the outcome over the course of the study.
To control the type I error rate, P<0.01 was pre-specified as statistically significant for analyses of the subscales of primary interest and suggestive of a difference for all other subscales, with 99% confidence intervals presented. For all other outcomes, P<0.05 was considered suggestive of a difference, rather than definitive, with 95% confidence intervals presented. All statistical analyses were conducted using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Relevant baseline characteristics and questionnaire scores appeared balanced by treatment group (eTables 1 and 2). The median age was 54, 95 (44%) were women, 119 (55%) were white, 40 (19%) had Type 1 diabetes, and the median HbA1c was 8.5%. Excluding deaths, overall completion rates for the NEI VFQ-25, UAB-LLQ and WPAIQ were 86%, 88% and 88% at 1-year and 85% at 2 years for all three questionnaires (eTable 3). The NEI VFQ-25 and UAB-LLQ were interviewer-administered 53% and 52% of the time.
The treatment group difference (ranibizumab group outcome minus PRP group outcome) in visual acuity, adjusted for baseline vision and central subfield thickness, was similar to the full cohort: +8.7 (95% CI: +5.3, +12.1, P<0.001) at 1 year, and +3.9 (95% CI: −0.0, +7.9, P=0.05) at 2 years.1 The change in visual acuity over the course of two years (AUC) was greater with ranibizumab by +6.2 letters (95% CI: +3.9, +8.4, P<0.001).
NEI VFQ-25
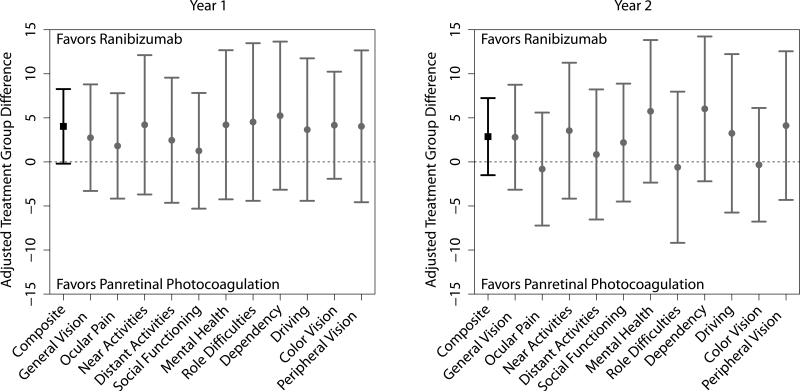
At 1 year, composite score change from baseline was greater with ranibizumab, although not statistically significant (+4.0, 95% CI: −0.2, 8.3, P=0.06), as was the case among all other subscales (Figure 1, eTable 4). At 2 years, the difference in composite score change from baseline was +2.9 (95% CI: −1.5, +7.2, P=0.20) and changes in pre-specified subscales of driving, color vision, and peripheral vision were not significantly different between treatment groups. Results were similar for the analysis of gain/loss of 10 points and the better-seeing eye subgroup (eTables 5 and 6) as well as for gain/loss of 5 points (data not shown).The difference in composite score change over the course of 2 years was greater with ranibizumab by +3.1 (95% CI: +0.0, +6.2, P=0.050).
Figure 1.
Treatment group differences, adjusted for baseline score and optical coherence tomography (OCT) central subfield thickness, between the ranibizumab and panretinal photocoagulation groups for the change from baseline in the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25) composite score and subscales at the 1-year (Left) and 2-year visits (Right). Whiskers represent the 95% confidence interval for the composite score (black square) or 99% confidence intervals for the subscales (gray circles). Values above the dashed line favor the ranibizumab group while values below the dashed line favor the PRP group.
At 1 year, the treatment effect for the driving subscale (interaction P=0.002) differed by whether the study eye had DME at baseline (eTable 7). Specifically, eyes with baseline DME (N=36) tended to have better outcomes with PRP, while eyes without baseline DME (N=146) tended to have better outcomes with ranibizumab. For example, the difference in driving subscale change from baseline was −16.9 (99% CI: −35.4, +1.7) less with ranibizumab for eyes with DME versus +8.2 (95% CI: −0.4, +16.7) more with ranibizumab for eyes without DME. This interaction was not observed at 2 years (interaction P=0.69 for driving subscale).
UAB-LLQ
Change from baseline to 1 year was numerically greater with ranibizumab among all subscales and the composite score (+1.8, 95% CI: −3.5, +7.1, P=0.51), although not statistically significant (Figure 2, eTable 8). At 2 years, there were also no significant differences identified in the composite score (+2.3, 95% CI: −2.9, +7.5, P=0.37), or any of the subscales. Results were similar for the analysis of gain/loss of at least 10 points and the better-seeing eye subgroup (eTables 9 and 10) as well as for gain/loss of at least 5 points (data not shown). The change in composite score AUC was +1.2 greater with ranibizumab although not statistically significant (95%CI: −2.5, +4.9, P=0.53).
Figure 2.
Treatment group differences, adjusted for baseline score and optical coherence tomography (OCT) central subfield thickness, between the ranibizumab and PRP groups for the change from baseline in the University of Alabama at Birmingham Low Luminance Questionnaire (UAB-LLQ) composite score and subscales at the 1-year (Left) and 2-year visits (Right). Whiskers represent the 95% confidence interval for the composite score (black square) or 99% confidence intervals for the subscales (gray circles). Values above the dashed line favor the ranibizumab group while values below the dashed line favor the PRP group.
The treatment effect on the change in composite score (interaction P=0.008) and mobility subscale (interaction P=0.002) at 1 year, but not 2 years (P=0.36, 0.05, respectively), differed by whether the study eye had baseline DME (eTable 11). Eyes with baseline DME (N=34) tended to have better outcomes with PRP, while eyes without baseline DME (N=139) tended to have better outcomes with ranibizumab.
WPAIQ
The change from baseline (Figure 3, eTable 12) in all four WPAIQ scores was numerically greater with ranibizumab at 1 year. This included a significant difference in the pre-specified scale of interest, the work productivity loss score (−15.6%, 99% CI: −29.8%, −1.3%, P=0.005), indicating less productivity loss with ranibizumab versus PRP. No significant differences were identified at 2 years, including the work productivity loss score (−2.9%, 99% CI: −15.2%, +9.5%, P=0.54). The AUC analysis of change in the work productivity loss scale demonstrated a difference of −10.3% (95% CI: −16.6%, −4.0%, P=0.002), indicating less productivity loss with ranibizumab compared to PRP over the course of two years.
Figure 3.
Treatment group differences, adjusted for baseline score and optical coherence tomography (OCT) central subfield thickness, between the ranibizumab and PRP groups for the change from baseline in Work Productivity and Activity Impairment Questionnaire (WPAIQ) scores at the 1-year (Left) and 2-year visits (Right). Whiskers represent 99% confidence intervals. Values above the dashed line favor the PRP group while values below the dashed line favor the ranibizumab group.
Results were similar when the study eye was the better-seeing eye (eTable 13). There was no indication of a treatment group by baseline DME interaction (eTable 14).
Driving-related Outcomes
The driving status of participants from the NEI VFQ-25 at annual visits is shown for all eyes in eTable 15 and stratified by baseline driving status in eTable 16. The percentage of participants still driving or who stopped driving (at night or completely) due wholly or partly to vision did not differ by group (Table 1). Visual acuity outcomes related to driving, however, were better in the ranibizumab group. At 2 years, 83 participants (97%) in the ranibizumab group had 20/40 or better vision in at least one eye compared with 82 (87%) in the PRP group (risk ratio=1.1, 95% CI: 1.0, 1.2, P=0.005). Furthermore, 73 participants (91%) in the ranibizumab group had 20/40 or better binocular vision versus 73 participants (81%) in the PRP group (risk ratio=1.1, 95% CI: 1.0, 1.3, P=0.020).
Table 1.
Panretinal Photocoagulation versus Ranibizumab for Proliferative Diabetic Retinopathy—Analysis of Driving Outcomes
| Outcome | Ranibizumab N (%) |
PRP N (%) |
Adjusted Risk Ratio (95% CI)a | P-value |
|---|---|---|---|---|
| Year 1 | ||||
| Stopped driving due to visionb | 1 (1%) | 2 (3%) | 0.5 (0.1, 4.7) | 0.55 |
| Stopped driving at night due to visionc | 3 (4%) | 4 (6%) | 0.6 (0.2, 2.4) | 0.49 |
| Currently drivingd | 79 (92%) | 77 (86%) | 1.1 (1.0, 1.2) | 0.15 |
| Binocular visual acuity ≥ 20/40 | 80 (94%) | 76 (85%) | 1.1 (1.0, 1.2) | 0.016 |
| Better-seeing eye visual acuity ≥ 20/40e | 42 (93%) | 30 (77%) | 1.2 (1.0, 1.4) | 0.021 |
| Visual acuity ≥ 20/40 in at least one eye | 87 (96%) | 87 (89%) | 1.1 (1.0, 1.2) | 0.021 |
| Year 2 | ||||
| Stopped driving due to visionb | 5 (7%) | 1 (1%) | 5.2 (0.6, 46.0) | 0.09 |
| Stopped driving at night due to visionc | 2 (3%) | 3 (4%) | 1.0 (0.2, 4.2) | 0.98 |
| Currently drivingd | 67 (84%) | 74 (89%) | 1.0 (0.9, 1.1) | 0.84 |
| Binocular visual acuity ≥ 20/40 | 73 (91%) | 73 (81%) | 1.1 (1.0, 1.3) | 0.020 |
| Better-seeing eye visual acuity ≥ 20/40e | 36 (90%) | 29 (76%) | 1.2 (1.0, 1.4) | 0.07 |
| Visual acuity ≥ 20/40 in at least one eye | 83 (97%) | 82 (87%) | 1.1 (1.0, 1.2) | 0.005 |
PRP=panretinal photocoagulation, CI=confidence interval.
Adjusted risk ratios, confidence intervals, and P-values from Poisson regression adjusting for baseline binocular visual acuity and OCT central subfield thickness
Includes participants who were driving at baseline
Includes participants who were driving at night at baseline
Includes participants who were driving or gave up driving wholly or partly due to eyesight at baseline
Includes participants whose study eye was the better-seeing eye at baseline. The eye with the higher (better) visual acuity letter score (approximate Snellen equivalent) at baseline was considered the better-seeing eye if the difference in visual acuity between eyes was at least 5 letters and the study eye visual acuity letter score was at least 54 (20/80 or better) or the difference was at least 10 letters and the study eye visual acuity letter score was <54 (20/100 or worse). Risk ratio adjusted for baseline visual acuity in the better-seeing eye rather than binocular visual acuity.
DISCUSSION
In comparing the treatment of PDR with ranibizumab or PRP, while differences in some work productivity and driving-related outcomes favored ranibizumab, significant differences at 2 years were not identified in a majority of the patient-centered outcomes evaluated. These results differ from studies of anti-vascular endothelial growth factor treatment for DME, which have reported better patient-centered outcomes than no treatment or macular laser treatment.7, 8
The UAB-LLQ outcomes were similar to the NEI VFQ-25 outcomes, which might be expected since the composite score and certain subscales evaluate similar domains of visual function, even though the UAB-LLQ was designed for studying patients with age-related macular degeneration.3
No meaningful differences in the peripheral vision subscales from the NEI VFQ-25 or UAB-LLQ were identified, even though Humphrey Visual Field testing, an objective measure of peripheral vision, identified substantial differences in visual fields at the 1-year visit, which persisted through the 2-year visit.1 It is possible that this discordance represents patient adaptation to the permanent visual field loss of PRP or that the peripheral vision subscales from the NEI VFQ-25 (1 question) and UAB-LLQ (3 questions) are not sensitive enough to detect differences in this aspect of visual function. Another possibility is that the difference found in Humphrey visual field testing is not a substantial impediment in a patient's perception of their function due to their peripheral vision. Although peripheral visual field loss in a non-study eye at baseline due to PRP could impact patient-reported outcomes, particularly in the PRP group where both eyes would have peripheral visual field loss following placement of PRP in the study eye, there were no treatment group differences identified in the peripheral vision subscales from the NEI VFQ-25 or UAB-LLQ for the subgroup of participants that had a history of PRP in their fellow eye at baseline (eTable 17). Recent work suggests that the detrimental effects of PRP on peripheral visual fields may not impact driving eligibility (by UK standards),12 although this research did not evaluate driving safety and performance, which could be affected by visual field loss following PRP for PDR.13 While the data from this trial clearly show a loss of visual field due to PRP, we are not able to comment on how this may affect driving eligibility.
Among select scales of the NEI VFQ-25 and UAB-LLQ at 1 year, there was a treatment by baseline DME interaction in which outcomes were better in the ranibizumab group when there was no baseline DME but better in the PRP group when baseline DME was present (eTables 7 and 11). This result requires confirmation in future studies, especially since such an interaction was not seen for visual acuity outcomes.1
Driving-related outcomes favored the ranibizumab group over the laser group (Table 1). A similar result with the same driving-related outcomes was found in a study of ranibizumab versus macular laser treatment for DME.14
We were unable to identify another study comparing patient-centered outcomes using anti-vascular endothelial growth factor therapy versus PRP for PDR. Worth noting is the fact that NEI VFQ-25, although not the UAB-LLQ and WPAIQ, has been used previously to study the effects of diabetic retinopathy on vision-related quality of life.15-17 However, as these data were generated from 55 community- and university-affiliated sites across the United States, we believe the patient-centered outcome responses are likely generalizable throughout the United States. Patients with the same clinical features from other countries may respond differently because of cultural influences on responses to these questionnaires.
There are some limitations to this study. The responsiveness of these outcome tools has not been evaluated previously in eyes with PDR, 17% of participants with one study eye were lost to follow-up by the 2-year visit, and there were numerous analyses done, thus increasing the possibility that differences identified could be due to chance. In addition, 48% of participants with one study eye randomly assigned to PRP received ranibizumab for DME, which could make patient-centered outcomes more similar. Finally, because patient-centered outcomes can only be evaluated among participants with one study eye, only 216 participants were eligible for these analyses versus 394 eyes (from 305 participants) for the primary outcome, a 45% reduction in sample size that limits statistical power. When designing this study, the value of patient-centered outcomes was outweighed by the advantages of enrolling two study eyes, such as faster recruitment, reduced costs, and the ability to compare the two treatments within an individual, where systemic and environmental are identical.18
In summary, while some differences were identified favoring ranibizumab over PRP, no differences between treatment regimens for PDR were identified for most of the other patient-centered outcomes considered. In discussing treatment options with a patient, these results should be considered alongside the primary outcome from this trial which demonstrated that ranibizumab was no worse than (non-inferior to) PRP with respect to visual acuity, while multiple secondary outcomes favored ranibizumab over PRP, including reduced rates of vitrectomy, development of central-involved DME, and visual field loss.1 These outcomes also should take into consideration the increased number of treatments and visits with ranibizumab over PRP for patients without DME.
Supplementary Material
ACKNOWLEDGEMENTS
a. Funding/Support: This study was supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U. S. Department of Health and Human Services (grants EY14231, EY14229, EY18817). Genentech (South San Francisco, CA, USA) provided ranibizumab for the study and funds to the DRCR.net to defray the study's clinical site costs.
The National Institutes of Health participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study nor in the collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Per the DRCR.net Industry Collaboration Guidelines (available at http://www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, and all editorial content of presentations and publications related to the protocol.
A complete list of the Diabetic Retinopathy Clinical Research Network investigators who participated in this trial is available in JAMA. 2015;314(20):2137-2146.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Material available at AJO.com
DISCLOSURES
b. Financial Disclosures: A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net
Wesley T. Beaulieu: National Eye Institute, Regeneron, Genentech (Grant)
Neil M. Bressler : National Eye Institute, Bayer, Genentech/Roche, Novartis, Regeneron (Grant)
Michele Melia: National Eye Institute, Regeneron, Genentech (Grant)
Cynthia Owsley: Abbott Medical Optics (Consultancy), Genentech (Grant)
Calvin E. Mein: Genentech (Rebates)
Jeffrey G. Gross: non-financial support from Genentech and Regeneron and grants from Regeneron, Acucela (Grants)
Lee M. Jampol: National Eye Institute, Regeneron, Genentech (Grant)
Adam R. Glassman: National Eye Institute, Regeneron, Genentech (Grant)
REFERENCES
- 1.Writing Committee for the Diabetic Retinopathy Clinical Research Network Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA. 2015;314(20):2137–46. doi: 10.1001/jama.2015.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 3.Owsley C, McGwin G, Jr., Scilley K, Kallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47(2):528–35. doi: 10.1167/iovs.05-1222. [DOI] [PubMed] [Google Scholar]
- 4.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 5.Lucentis(R) [package insert] Genentech I; South San Francisco, CA: Feb, 2015. [July 1, 2016]. http://www.gene.com/download/pdf/lucentis_prescribing.pdf. [Google Scholar]
- 6.Suñer IJ, Kokame GT, Yu E, Ward J, Dolan C, Bressler NM. Responsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trials. Invest Ophthalmol Vis Sci. 2009;50(8):3629–35. doi: 10.1167/iovs.08-3225. [DOI] [PubMed] [Google Scholar]
- 7.Bressler NM, Varma R, Suner IJ, et al. Vision-related function after ranibizumab treatment for diabetic macular edema: results from RIDE and RISE. Ophthalmology. 2014;121(12):2461–72. doi: 10.1016/j.ophtha.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell P, Bressler N, Tolley K, et al. Patient-reported visual function outcomes improve after ranibizumab treatment in patients with vision impairment due to diabetic macular edema: randomized clinical trial. JAMA Ophthalmol. 2013;131(10):1339–47. doi: 10.1001/jamaophthalmol.2013.4592. [DOI] [PubMed] [Google Scholar]
- 9.Bressler NM, Chang TS, Suner IJ, et al. Vision-related function after ranibizumab treatment by better- or worse-seeing eye: clinical trial results from MARINA and ANCHOR. Ophthalmology. 2010;117(4):747–56.e4. doi: 10.1016/j.ophtha.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J, Klesert TR. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 2007;125(11):1460–9. doi: 10.1001/archopht.125.11.1460. [DOI] [PubMed] [Google Scholar]
- 11.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 12.Subash M, Comyn O, Samy A, et al. The effect of multispot laser panretinal photocoagulation on retinal sensitivity and driving eligibility in patients with diabetic retinopathy. JAMA Ophthalmol. 2016;134(6):666–72. doi: 10.1001/jamaophthalmol.2016.0629. [DOI] [PubMed] [Google Scholar]
- 13.Owsley C, McGwin G., Jr. Driving eligibility in proliferative diabetic retinopathy. JAMA Ophthalmology. 2016;134(6):672–3. doi: 10.1001/jamaophthalmol.2016.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bressler NM, Varma R, Mitchell P, et al. Effect of ranibizumab on the decision to drive and vision function relevant to driving in patients with diabetic macular edema: report From RESTORE, RIDE, and RISE trials. JAMA Ophthalmol. 2015:1–7. doi: 10.1001/jamaophthalmol.2015.4636. [DOI] [PubMed] [Google Scholar]
- 15.Hariprasad SM, Mieler WF, Grassi M, Green JL, Jager RD, Miller L. Vision-related quality of life in patients with diabetic macular oedema. Br J Ophthalmol. 2008;92(1):89–92. doi: 10.1136/bjo.2007.122416. [DOI] [PubMed] [Google Scholar]
- 16.Warrian KJ, Lorenzana LL, Lankaranian D, Dugar J, Wizov SS, Spaeth GL. The assessment of disability related to vision performance-based measure in diabetic retinopathy. Am J Ophthalmol. 2010;149(5):852–60.e1. doi: 10.1016/j.ajo.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Gabrielian A, Hariprasad SM, Jager RD, Green JL, Mieler WF. The utility of visual function questionnaire in the assessment of the impact of diabetic retinopathy on vision-related quality of life. Eye (Lond) 2010;24(1):29–35. doi: 10.1038/eye.2009.56. [DOI] [PubMed] [Google Scholar]
- 18.Glassman AR, Melia M. Randomizing 1 eye or 2 eyes: a missed opportunity. JAMA Ophthalmol. 2015;133(1):9–10. doi: 10.1001/jamaophthalmol.2014.3600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.