Abstract
Serotonergic neurons of the raphe nucleus regulate sleep, mood, endocrine function, and other processes that mature during adolescence. Alcohol abuse and binge drinking are common during human adolescence. We tested the novel hypothesis that adolescent intermittent ethanol exposure would alter the serotonergic system that would persist into adulthood. Using a Wistar rat model of adolescent intermittent ethanol (AIE; 5.0 g/kg, i.g., 2-day on/2-day off from postnatal day [P]25 to P55), we found a loss of dorsal raphe nucleus (DRN) serotonin (5-HT)-immunoreactive (+IR) neurons that persisted from late adolescence (P56) into adulthood (P220). Hypothalamic and amygdalar DRN serotonergic projections were reduced following AIE. Tryptophan hydroxylase 2, the rate-limiting 5-HT synthesizing enzyme, and vesicular monoamine transporter 2, which packages 5-HT into synaptic vesicles, were also reduced in the young adult midbrain following AIE treatment. Adolescent intermittent ethanol treatment increased expression of phosphorylated (activated) NF-κB p65 as well as markers of microglial activation (i.e., Iba-1 and CD11b) in the adult DRN. Administration of lipopolysaccharide to mimic AIE-induced innate immune activation reduced 5-HT+IR and increased phosphorylated NF-κB p65+IR similar to AIE treatment. Voluntary exercise during adolescence through young adulthood blunted microglial marker and phosphorylated NF-κB p65+IR, and prevented the AIE-induced loss of 5-HT+IR neurons in the DRN. Together, these novel data reveal that AIE reduces 5-HT+IR neurons in the adult DRN, possibly through an innate immune mechanism, which might impact adult cognition, arousal, or reward sensitivity. Further, exercise prevents the deleterious effects of AIE on the serotonergic system.
Keywords: serotonin, binge drinking, adolescence, development, alcohol, microglia
1. Introduction
Adolescence is a highly conserved neurodevelopmental period that marks the transition from childhood to adulthood, and is characterized behaviorally by increased social interactions and risk-taking (i.e., novelty- and sensation-seeking [Spear, 2011]). In parallel, the brain undergoes significant maturation of neurocircuitry and refinement of several neurotransmitter systems (Coleman et al., 2011; Crews et al., In Press; Spear, 2000; Weir et al., 2012), including the serotonergic system (Lidov and Molliver, 1982; Shoval et al., 2014). Serotonin (5-hydroxytryptamine, 5-HT)-producing neurons are primarily located within the brainstem raphe nuclei and are generated prenatally in the brain (Lauder, 1990). Serotonergic neurons innervate multiple brain regions and these projections undergo significant refinement during adolescence (Dori et al., 1996; Xu et al., 2001). Serotonin is a neuromodulatory neurotransmitter involved in synaptic plasticity, learning and memory, mood regulation, sleep, and endocrine processes that mature during adolescence, and dysregulation of this system is linked to several psychiatric disorders, including depression, impulsivity, and alcohol dependence (Michelsen et al., 2007; Muller and Homberg, 2015; Nautiyal et al., 2015). It is currently unknown whether adolescent binge drinking alters populations of 5-HT-immunopositive neurons in the adult raphe nucleus.
In humans, adolescent risk-taking and sensation-seeking behaviors coincide with the onset of alcohol and drug experimentation (Windle et al., 2008). Studies reveal that by age 14, binge drinking is common among youth in the United States, with current statistics reporting heavy episodic binge drinking (i.e., >5 consecutive drinks per binge drinking episode) in approximately 5% of 13–14 year old 8th graders, 22% of 12th graders, and >40% of college students (Johnston et al., 2013; White et al., 2006). Since the adolescent rat brain has been found to be more sensitive to alcohol neurotoxicity (Crews et al., 2000), maturational processes occurring in the adolescent brain suggest adolescence may be a particularly vulnerable period of elevated risk for later development of addiction and other disorders (Crews and Boettiger, 2009). Employing the rodent adolescent intermittent ethanol (AIE) model of human adolescent binge drinking, our laboratory and others found evidence of long-term cognitive dysfunction, increased impulsivity and anxiety-like behaviors, increased alcohol preference and drinking, and increased expression of multiple innate immune genes in adulthood (Crews et al., In Press; Spear and Swartzwelder, 2014; Vetreno and Crews, 2012, 2015; Vetreno et al., 2013). However, it is unknown if adolescent binge ethanol exposure leads to long lasting changes in the adult serotonergic system.
Multiple studies have found that alcoholism is associated with innate immune gene induction in the brain (see e.g., Cui et al., 2015). There is increased expression of microglial (He and Crews, 2008) and innate immune markers (Crews et al., 2013; Vetreno et al., 2013) in postmortem human alcoholic brain samples. Adolescent intermittent ethanol treatment in rats also increases innate immune gene expression in the prefrontal cortex (Vetreno and Crews, 2012; Vetreno et al., 2013) and hippocampus (Vetreno and Crews, 2015). In ex vivo slice culture, innate immune signaling reduces 5-HT+IR neurons (Hochstrasser et al., 2011). Since adult alcohol use disorders and other drinking problems are associated with an earlier age of drinking onset (Sher and Gotham, 1999) and dysfunction of the serotonergic system is associated with increased alcohol consumption and dependence (LeMarquand et al., 1994a, b), it is imperative to determine the effect of adolescent binge ethanol exposure on the adult serotonergic system. In the current study, we tested the novel hypothesis that AIE treatment would alter serotonergic neurons that would persist into adulthood. To test this hypothesis, 5-HT+IR in the dorsal raphe nucleus was assessed following treatment with our model of adolescent intermittent ethanol (AIE). Lipopolysaccharide (LPS), which is known to increase brain innate immune gene expression, was used to determine if brain innate immune gene induction would mimic the AIE-induced loss of 5-HT+IR neurons in the adult raphe nucleus. Further, previous studies find that voluntary exercise prevents ethanol-induced neuropathology in adult mice (Crews et al., 2004). Thus, we sought to determine whether wheel running would prevent the AIE-induced innate immune response and 5-HT+IR neuronal loss in adulthood. Our findings suggest that voluntary exercise can prevent the loss of 5-HT expression and brain innate immune upregulation by AIE. The novel findings presented are consistent with adolescent binge drinking leading to long-lasting changes in innate immune signaling in the adult raphe nucleus that contribute to reductions in 5-HT+IR neurons.
2. Materials and Methods
2.1. Animals
Young time-mated pregnant female Wistar rats (embryonic day 17; Harlan Sprague-Dawley, Indianapolis, IN) were acclimated to our animal facility prior to birthing at the University of North Carolina at Chapel Hill. On postnatal day 1 (P1; 24 hr after birth), litters were culled to 10 pups (6 males and 4 females) and housed with their dam in standard clear plastic tubs with shavings until group housing with same-sex littermates at the time of weaning on P21. All animals were housed in a temperature- (20°C) and humidity-controlled vivarium on a 12 hr/12 hr light/dark cycle (light onset at 0700 hr), and provided ad libitum access to food and water. Experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill, and conducted in accordance with the National Institutes of Health regulations for the care and use of animals in research.
2.2. Adolescent intermittent ethanol (AIE) paradigm
On P21, male Wistar rats were randomly assigned to either: (i) AIE (n = 57) or (ii) water control (CON; n = 54). To minimize the impact of litter, no more than one subject from a given litter was assigned to any experimental condition. From P25 to P55, AIE animals received a single daily intragastric (i.g.) administration of ethanol (5.0 g/kg, 20% ethanol w/v) in the AM on a two-day on/two-day off schedule and CON subjects received comparable volumes of water. A separate naïve unmanipulated control group (NC) was included that was not handled for the duration of experimentation except for routine animal care. Tail blood was collected to assess blood ethanol concentrations (BECs) one hr after ethanol administration on P38 and P54, and BECs were assessed using a GM7 Analyzer (Analox, London, UK). On P38 and P54, mean BECs (±SEM) were 181 ± 5 mg/dL and 185 ± 7 mg/dL, respectively. Across experiments, body weights (g) were measured and all subjects evidenced dramatic increases across age (see Figure 1A).
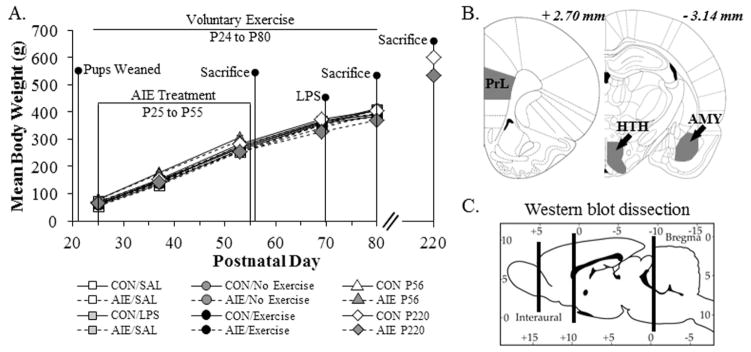
Figure 1. Graphical representation of the adolescent intermittent ethanol (AIE) exposure paradigm.
(A) Rats were treated from postnatal day (P) 25 to P55 with either a single daily dose of ethanol (AIE; 5.0 g/kg 20% ethanol w/v, i.g.) or a comparable volume of water (CON) on a two-day on/two-day off administration schedule. Blood ethanol concentrations (BEC) were measured one hr after ethanol exposure on P38 and P54. Twenty-four hours (P56; CON = 8, AIE = 8), 25 days (P80; CON = 8, AIE = 8), and 165 days (P220; CON = 7, AIE = 7) following the conclusion of AIE, rats were sacrificed for immunohistochemistry and Western blot analysis (P80). A subset of CON- and AIE-treated animals was treated with lipopolysaccharide (LPS; 1.0 mg kg, i.p., CON = 8, AIE = 8) or saline (SAL; CON = 8, AIE = 8) on P70 and sacrificed on P80. An additional subset of CON- and AIE-treated animals was exposed to voluntary wheel running from P24 to P80 and sacrificed on P80 (No Exercise: CON = 8, AIE = 8; Exercise: CON = 9, AIE = 10). Body weights were assessed at the initiation of AIE (P25), the conclusion of AIE (P55), and the conclusion of each experiment, depending on the endpoint. Across experiments, we observed that all subjects evidence dramatic weight gains (main effect of Age: Exercise study, F[2,62] = 5152.9, p < 0.01; LPS study, F[2,54] = 4203.5, p < 0.01; Aging study: P56, F[1,14] = 3926.4, p < 0.01; P80, F[2,28] = 4434.8, p < 0.01; P220, F[2,24] = 1553.9, p < 0.01). Further, there were no differences in body weights across treatments and conditions (Exercise study: main effect of Treatment - F[1,31] = 0.9, p > 0.05; main effect of exercise - F[1,31] = 1.1, p > 0.05; LPS study: main effect of Treatment - F[1,28] = 1.8, p > 0.05; main effect of LPS - F[1,28] = 0.01, p > 0.05; Aging study: P56 - main effect of Treatment: F[1,14] = 1.4, p > 0.05; P80 - main effect of Treatment: F[1,14] = 0.03, p > 0.05) with the exception of the P220 study in which we observed that AIE-treated animals weighed approximately 10% less than CON subjects at P220 (CON = 622 ± 18 g, AIE = 553 ± 17 g [one-way ANOVA: F[1,12] = 7.9, p < 0.05]). (B) Representative photomicrographs based on the atlas of Paxinos and Watson (1998) defining the regions of interest assessed for serotonin terminal field immunoreactivity. PrL: prelimbic cortex; HTH: hypothalamus; AMY: amygdala. (C) Representative photomicrograph depicting the brain regions dissected for Western blot analysis. The frontal cortex and midbrain were dissected and used for Western blot analysis of serotonergic protein expression. (all p’s < 0.05) that did not differ as a function of treatment during AIE exposure (repeated measures ANOVAs: all p’s > 0.9; see Figure 1A). However, AIE-treated animals weighed approximately 10% less than CON subjects at P220 (CON = 622 ± 18 g, AIE = 553 ± 17 g [one-way ANOVA: p < 0.05]).
2.3. Voluntary wheel running exposure
On P24, a separate group of animals was randomly assigned to one of four groups and housed in pairs: CON- and AIE-treated animals housed with running wheels (Exercise; CON = 9, AIE = 10), and CON- and AIE-treated animals housed in normal cages (No exercise; CON = 8, AIE = 8). All groups were housed in pairs since social isolation can delay or counteract the beneficial effects of exercise (Leasure and Decker, 2009; Stranahan et al., 2006). Subjects in both exercising treatment groups were exposed to the running wheels 24 hr per day for the duration of the experiment. The exercise apparatus consisted of specifically designed cages with a running wheel attachment (Nalge designed for Minimitter Company, Sun River, OR). Dimensions of the fully assembled cage with the wheels were 50 cm × 27 cm × 36 cm. Although the running wheels did not contain a tachometer, all rats were observed daily running on the wheels and remained in their respective housing conditions until sacrifice on P80. Running wheel exposure did not affect BECs at either P38 (AIE/No exercise: 162 ± 16 mg/dL, AIE/Exercise: 169 ± 12 mg/dL; one-way ANOVA: F[1,16] = 0.14, p = 0.7) or P54 (AIE/No exercise: 160 ± 12 mg/dL, AIE/Exercise: 160 ± 22 mg/dL; one-way ANOVA: F[1,16] = 0.0, p = 0.99).
2.4. Lipopolysaccharide (LPS) treatment
On P70, separate groups of CON- and AIE-treated animals (LPS; CON = 8, AIE = 8) received a single intraperitoneal (i.p.) injection of 1.0 mg/kg LPS (E. Coli, serotype 0111:B4; Sigma-Aldrich, St. Louis, MO) or saline (SAL; CON = 8, AIE = 8). Subjects were monitored for sickness behavior 24 hr following LPS administration and sacrificed 10 days later on P80.
2.5. Perfusion, brain tissue preparation, and immunohistochemistry
For all immunohistochemistry experiments, subjects were anesthetized with a lethal dose of sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.4), followed by 4.0% paraformaldehyde in PBS. Brains were excised and post-fixed in 4.0% paraformaldehyde for 24 hr at 4ºC followed by 4 days of fixation in 30% sucrose solution.
In the initial experiment, intact brain samples from young adult P80 animals (16 subjects [CON (n = 8) and AIE (n = 8)]) were shipped to NeuroScience Associates (Knoxville, TN) to perform full brain 5-HT+IR immunohistochemistry. Upon receipt at NeuroScience Associates, brain samples were treated overnight with 20% glycerol and 2.0% dimethylsulfoxide to prevent freezing artifacts, and multiply embedded (16 brains per block [CON (n = 8) and AIE (n = 8)]) in a gelatin matrix using MultiBrain™ Technology. After curing, the block containing the brain samples was rapidly frozen by immersion in isopentane chilled to −70° C with crushed dry ice and mounted to the freezing stage of an AO 860 sliding microtome. The MultiBrain™ block was sectioned coronally at a thickness of 40 μm and collected sequentially into a 4 × 6 array of containers containing Antigen Preserve Solution (50% PBS [pH 7.0], 50% ethylene glycol, 1.0% polyvinyl pyrrolidone). Samples were stored at −20° C until immunohistochemistry. Free-floating MultiBrain™ sections were washed in Tris-buffered saline (TBS), incubated in H2O2 to inhibit endogenous peroxidase activity, and blocked with normal goat serum. Sections were incubated overnight at room temperature in the primary antibody rabbit anti-serotonin (5-HT; ImmunoStar, Hudson, WI, Cat. No. 20080) diluted in TBS containing 0.3% TritonX-100. Sections were then washed with TBS, incubated in a goat anti-rabbit secondary antibody solution, and incubated in an avidin-biotin-HRP complex solution (Vectastain ABC Kit; Vector Laboratories). After washing with TBS, the sections were treated with diaminobenzidine tetrahydrochloride (DAB) and H2O2 to visualize immunoreactivity. Sections were mounted on gelatinized (subbed) glass slides, air-dried, dehydrated in alcohol, cleared with xylene, and coverslipped. Stained samples were then shipped back to the University of North Carolina at Chapel Hill for immunohistological quantification.
For the remainder of immunohistochemical experiments, coronal sections were cut (40 μm) on a sliding microtome (MICROM HM450; ThermoScientific, Austin, TX), and sections were sequentially collected into well plates and stored at −20ºC in a cryoprotectant solution (30% glycol/30% ethylene glycol in PBS) for immunohistochemistry. Free-floating sections (1:6 series throughout the raphe nucleus, hypothalamus, basolateral amygdala, and prelimbic cortex) were washed in 0.1 M PBS, incubated in 0.3% H2O2 to inhibit endogenous peroxidases, and blocked with normal serum (MP Biomedicals, Solon, OH). Sections were incubated in either rabbit anti-5-HT (1:20,000; ImmunoStar), rabbit anti-ionized calcium-binding adapter molecule 1 (1:1000; Iba-1; Wako Chemicals, Richmond, VA, Cat. No. 019-19741), rabbit anti-phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells p65 (1:300; pNF-κB p65 [Ser 276]; Santa Cruz Biotechnologies, Dallas, TX, Cat. No. sc-101749), mouse anti-tryptophan hydroxylase 1 (1:200; TPH1; Millipore, Temecula, CA, Cat. No. AB15570-I), mouse anti-CD11b (1:1000; AbD Serotec, Raleigh, NC, Cat. No. MCA275R), or mouse anti-ED1 (1:1000; AbD Serotec, Cat. No. MCA341R) for 24 hr at 4ºC. Sections were washed with PBS, incubated for one hr in biotinylated secondary antibody (Vector Laboratories, Burlingame, CA), and incubated for one hr in avidin-biotin complex solution (Vectastain ABC Kit; Vector Laboratories). The chromogen, nickel-enhanced DAB (Sigma-Aldrich), was used to visualize immunoreactivity. Tissue was mounted onto slides, dehydrated, and coverslipped. Negative control for non-specific binding was conducted on separate sections employing the abovementioned procedures and omitting the primary antibody.
2.6. Microscopic quantification and image analysis
The BioQuant Nova Advanced Image Analysis system (R&M Biometric, Nashville, TN) was used for image capture and analysis. Images were captured using an Olympus BX50 microscope and Sony DXC-390 video camera linked to a computer. For each measure, the microscope, camera, and software were background corrected, and normalized to preset light levels to ensure fidelity of data acquisition.
Assessment of 5-HT+IR was performed in the dorsal and median raphe nuclei as well as serotonergic projection sites (i.e., hypothalamus, amygdala, and prelimbic cortex) according to the atlas of Paxinos and Watson (1998) (see Figure 1B). Pixel density was used to assess CD11b+IR in the DRN and 5-HT terminal fields in the prelimbic cortex, hypothalamus, and basolateral amygdala, and was rigorously thresholded to normalize pixel intensity (Vetreno et al., 2013). The threshold for pixel density was determined from the control subjects by calculating the average of the darkest and lightest values from each region of interest and sections were imaged under identical conditions to avoid non-systematic variations (Beynon and Walker, 2012). The outlined regions of interest were determined according to the atlas of Paxinos and Watson (1998) and staining density calculated by dividing the pixel count by the overall area (mm2). The total number of 5-HT+IR, pNF-κBp65+IR, Iba-1+IR, and ED-1+IR cells was quantified using a modified stereological approach (Crews et al., 2004), and data expressed as cells per mm2.
2.7. Brain tissue preparation and Western blot analysis
For Western blots, 14 subjects (CON [n = 7] and AIE [n = 7]) were anesthetized on P80 with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 0.1 M PBS. Immediately following perfusion, tissue samples were rapidly dissected, preserved in liquid nitrogen, and stored at −80° C until use. Since 5-HT expression was reduced in the DRN, hypothalamus, and basolateral amygdala, but increased in the prelimbic cortex, we dissected the frontal cortex and entire midbrain to determine whether other serotonergic markers were altered by AIE. Frozen frontal cortex and midbrain samples (see Figure 1C) were separately homogenized in RIPA lysis buffer (Sigma-Aldrich) containing protease inhibitor (1:100; Sigma-Aldrich) and centrifuged at 14,000 rpm at 4° C for 20 min. After centrifugation, protein content in the supernatant was assessed using the Pierce BCA Protein Assay Kit (ThermoScientific, Rockford, IL). A total of 20 ug of protein from each denatured sample was loaded into precast polyacrylamide mini-gel (4–15%; Bio-Rad, Hercules, CA) and transferred onto immunoblot PVDF membranes (Bio-Rad). Immunoblot membranes were blocked in Odyssey blocking buffer (LiCOR Biosciences, Lincoln, NE). Membranes were incubated overnight at 4°C in a primary antibody solution containing either rabbit anti-serotonin transporter (1:1000; SERT; Millipore), mouse anti-tryptophan hydroxylase 2 (1:1000; TPH2; Sigma-Aldrich), or rabbit anti-vesicular monoamine transporter 2 (1:1000; VMAT2; Abcam; Cambridge, MA), and one of the following housekeeping proteins: mouse anti-β-actin (Abcam) or rabbit anti-tubulin (Cell Signaling, Danvers, MA). After washing, membranes were incubated in appropriate fluorescent secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA), and bands were scanned with an Odyssey Infrared Imager (LiCOR Biosciences). Band intensity was quantified using Odyssey Imaging software and normalized to either β-actin or tubulin. All experiments were run in triplicate.
2.8. Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (Chicago, IL). Analysis of variance (ANOVA) was used to assess BECs, body weights, immunohistochemistry, and Western blot data. Post-hoc analyses were performed using Tukey’s HSD where appropriate. To determine outliers, the median, upper, and lower quartiles were calculated, and data points that exceeded the 1.5 × interquartile range were removed from further analysis. No more than one animal was removed from any group. All values are reported as mean ± SEM, and significance was defined as p ≤ 0.05.
3. Results
3.1. 5-Hydroxytryptamine-immunopositive serotonergic cell populations are persistently reduced in the DRN following AIE treatment
Immunohistochemistry was used to label and quantify serotonergic cell populations in the dorsal and median raphe nucleus of male Wistar rats across aging and following AIE treatment. Tissue samples were collected from subjects sacrificed on P56 (24 hr post-AIE treatment; CON = 8, AIE = 8), P80 (25 days post-AIE treatment; CON = 8, AIE = 8), and P220 (165 days post-AIE treatment; CON = 7, AIE = 7). Evaluation of 5-HT+IR neurons revealed well-defined heterogeneously distributed small and large darkly stained cell bodies and processes in dorsal and median raphe nucleus samples from CON- and AIE-treated subjects. Across aging from P56 to P220 in the CON subjects, we observed a 29% reduction of 5-HT+IR neurons in the DRN (one-way ANOVA: F[2,20] = 7.7, p < 0.01; see Figure 2). Importantly, 5-HT+IR cell counts did not differ between CON and NC animals (25 days post-AIE treatment; NC = 8, CON = 8) on P80 (one-way ANOVA: F[1,14] = 0.009, p = 0.93), suggesting that the gavage technique is not a contributing factor to the observed age-associated reduction. Thus, the number of 5-HT+IR cells shows an age-associated reduction in the DRN.
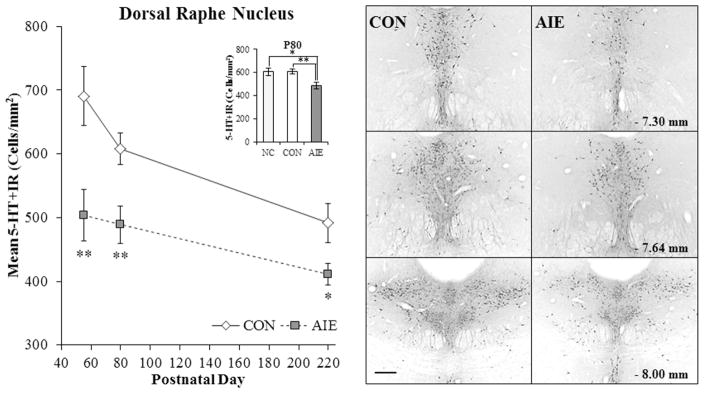
Figure 2. Adolescent intermittent ethanol (AIE) exposure leads to long-term reductions of serotonin-immunoreactive (5-HT+IR) cells in the dorsal raphe nucleus (DRN).
Left Modified unbiased stereological assessment revealed a 27% (±6%) (CON = 8, AIE = 8; one-way ANOVA: F[1,14] = 9.3, p < 0.01) decrease of 5-HT+IR in the adolescent (postnatal [P]56) DRN that persisted from young adulthood (P80 [20% ±5%]) (CON = 8, AIE = 8; one-way ANOVA: F[1,15] = 10.0, p < 0.01) into adulthood (P220 [16% ±1%]) (CON = 7, AIE = 7; one-way ANOVA: F[1,12] = 5.4, p < 0.05), relative to controls (CON). Further, across aging from P80 to P220 in CON subjects, we observed a 29% reduction in 5-HT+IR neurons in the DRN (one-way ANOVA: F[2,22] = 7.7, p < 0.01). (Inset) Modified unbiased stereological assessment revealed that 5-HT+IR did not differ between the naïve unmanipulated control group (NC; n = 8) and CON subjects (one-way ANOVA: F[1,14] = 0.009, p = 0.93). Post-hoc analysis revealed that 5-HT+IR was reduced by (19% [±5%]; p < 0.05) in AIE-treated animals relative to NC animals. Data are presented as mean ± SEM. * p < 0.05, ** p < 0.01, relative to CON rats. Right Photomicrograph images representing anterior to posterior sections within the DRN based on the atlas of Paxinos and Watson (1998). Scale bar = 50 microns. Note that 5-HT+IR neurons are clustered on the midline of the anterior DRN and extend laterally toward the posterior DRN.
Our adolescent binge ethanol paradigm involves an intermittent exposure schedule consistent with known patterns of heavy adolescent-typical weekend binge drinking, but not daily drinking behaviors associated with alcohol dependence. A 2 × 3 ANOVA (Treatment [CON vs. AIE] × Age [P56 vs. P80 vs. P220]) revealed that relative to CON subjects, AIE treatment significantly reduced 5-HT+IR in the DRN (main effect of Treatment: F[1,40] = 22.3, p < 0.01; see Figure 2). Follow-up analyses revealed that across each time point, AIE treatment reduced 5-HT+IR in the DRN by 27% (±6%) on P56 (one-way ANOVA: F[1,14] = 9.3, p < 0.01), 20% (±5%) on P80 (one-way ANOVA: F[1,14] = 10.0, p < 0.01), and 16% (±1%) on P220 (one-way ANOVA: F[1,12] = 5.4, p < 0.05). Further, while expression of 5-HT+IR did not differ between NC and CON animals (p = 0.93) on P80, post-hoc analysis revealed that 5-HT+IR was also reduced by (19% [±5%]; p < 0.05) in AIE-treated animals relative to NC animals (see Figure 2 inset). Interestingly, AIE-treated animals at P56 had similar levels of 5-HT+IR neurons as CON subjects on P220. Serotonergic process densities were similarly reduced by 29% (±11%) in the DRN of young adult (P80) AIE-treated animals, relative to CONs (one-way ANOVA: F[1,13] = 4.9, p < 0.05; CON = 8, AIE = 7). There was no effect of AIE treatment on 5-HT+IR in the median raphe nucleus (data not shown). Thus, AIE leads to long-term reductions of 5-HT+IR cell populations and process densities in the DRN.
3.2. 5-Hydroxytryptamine terminal field densities are altered in young adult serotonergic projection sites following adolescent binge ethanol exposure
Since serotonergic neurons project to multiple brain regions and AIE was found to reduce 5-HT+IR in the DRN, we next assessed 5-HT+IR terminal field densities in other brain regions. Across sampled regions, assessment of 5-HT+IR revealed darkly stained processes in both CON- and AIE-treated young adult (P80) (CON = 8, AIE = 8) animals. In the hypothalamus, AIE treatment reduced 5-HT+IR fiber densities by 38% (±7%), relative to CONs (one-way ANOVA: F[1,13] = 4.7, p = 0.05; CON = 8, AIE = 7; see Figure 3A). Similarly, serotonergic fiber densities were reduced by 20% (±7%) in the basolateral amygdala of AIE-treated animals, relative to CONs (one-way ANOVA: F[1,14] = 4.7, p = 0.05; see Figure 3B). On the contrary, serotonergic fiber densities were increased in the prelimbic cortex of AIE-treated animals (112% [±31%]) in comparison to CONs (one-way ANOVA: F[1,13] = 19.4, p < 0.01; CON = 8, AIE = 7; see Figure 3C), but further analysis of the frontal cortex did not reveal changes in other serotonin markers (see below) suggesting that prefrontal cortex changes are subtle. Further, we did not observe any TPH1 expression in the prelimbic cortex of CON- or AIE-treated animals (data not shown). Together, these data reveal that AIE treatment leads to long-lasting reductions of 5-HT+IR in the amygdala and hypothalamus, which are two key projection sites of the DRN.
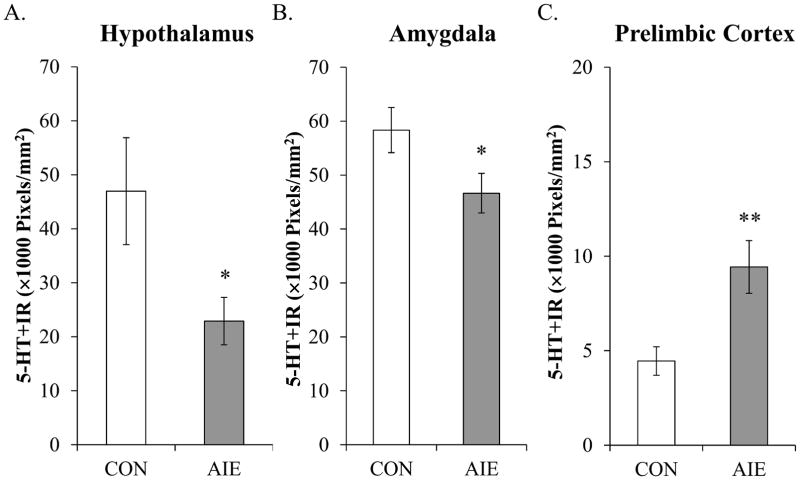
Figure 3. Adolescent binge ethanol (AIE) treatment reduces serotonin-immunoreactive (5-HT+IR) terminal field densities in the hypothalamus and amygdala of young adult rats.
(A) Quantification of pixel density in the hypothalamus of AIE-treated animals revealed a 38% (±7%) (CON = 8, AIE = 7; one-way ANOVA: F[1,13] = 4.7, p = 0.05) reduction of 5-HT+IR terminal field densities, relative to controls (CON). (B) Quantification of pixel density in the AIE-treated animals revealed a 20% (±7%) (CON = 8, AIE = 8; one-way ANOVA: F[1,14] = 4.7, p = 0.05) reduction of 5-HT+IR terminal field densities in the amygdala, relative to CONs. (C) Quantification of pixel density in the AIE-treated animals revealed a 112% (±31%) (CON = 8, AIE = 7; one-way ANOVA: F[1,13] = 19.4, p < 0.01) increase of 5-HT+IR terminal field densities in the prelimbic, relative to CONs. Data are presented as mean ± SEM. * indicates a p < 0.05, ** p < 0.01, relative to CONs.
3.3. Adolescent binge ethanol treatment decreases serotonergic markers in the young adult midbrain but not frontal cortex
Since 5-HT+IR cell populations were reduced in the DRN, we next assessed the effect of AIE on protein expression of other serotonergic markers in the young adult brain (P80) (CON = 7, AIE = 7) using Western blot analysis. We assessed serotonergic marker expression in separately dissected frontal cortex and midbrain tissue samples due to the terminal field findings described in the previous section. Expression of TPH2, the rate-limiting enzyme responsible for synthesis of 5-HT in the brain, was unchanged in the frontal cortex samples (one-way ANOVA: F[1,12] = 1.5, p = 0.3) and reduced by 21% (±6%) in the midbrain of young adult AIE-treated animals, relative to CONs (one-way ANOVA: F[1,12] = 9.3, p < 0.01). Expression of SERT, which transports extracellular 5-HT into the presynaptic neuron, was unchanged in the frontal cortex samples (one-way ANOVA: p = 0.8) and insignificantly reduced in the young adulthood midbrain following AIE treatment (one-way ANOVA: p = 0.14). Further, while protein levels of VMAT2, which transports intracellular monoamines (e.g., 5-HT) into synaptic vesicles, were unchanged in the frontal cortex samples (one-way ANOVA: F[1,12] = 0.5, p = 0.5), protein expression was reduced by 24% (±5%) in the midbrain of young adult AIE-treated animals, relative to CONs (one-way ANOVA: F[1,12] = 6.5, p < 0.05; see Figure 4). Thus, AIE treatment leads to reductions in serotonergic protein markers in the young adult midbrain, but not frontal cortex, 25 days following the conclusion of AIE treatment.
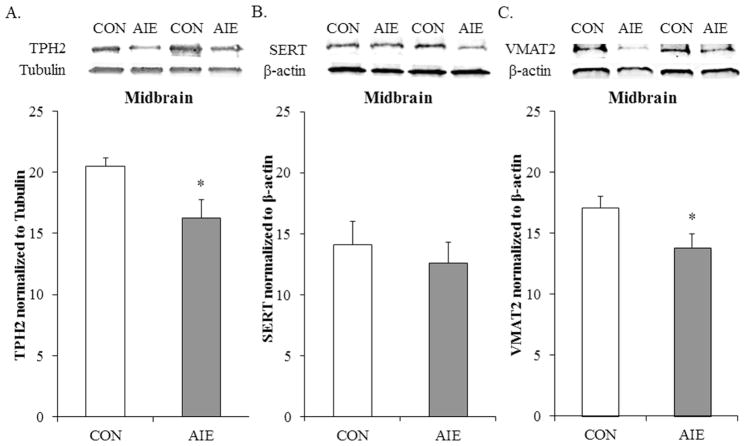
Figure 4. Adolescent intermittent ethanol (AIE) exposure reduces tryptophan hydroxylase 2 (TPH2) and vesicular monoamine transporter 2 (VMAT2) expression in the young adult (P80) midbrain.
(A) Western blot assessment of TPH2 protein expression revealed a significant 21% (±6%) (CON = 7, AIE = 7; one-way ANOVA: F[1,12] = 5.4, p < 0.05) reduction in AIE-treated animals, relative to controls (CON). (B) Western blot assessment of serotonin transporter (SERT) expression revealed an insignificant reduction following AIE treatment in young adulthood (CON = 7, AIE = 7; one-way ANOVA: F[1,12] = 5.4, p < 0.05). (C) Western blot assessment of VMAT2 protein expression revealed a significant 24% (±5%) (CON = 7, AIE = 7; one-way ANOVA: F[1,12] = 5.4, p < 0.05) reduction in AIE-treated animals, relative to CONs. Western blot analyses were run in triplicate and the mean was reported. The representative immunoblots are contiguous and obtained from the same gels. Data are presented as mean ± SEM. * indicates a p < 0.05, relative to CONs.
3.4. Adolescent binge ethanol treatment increases expression of microglia markers in the young adult DRN
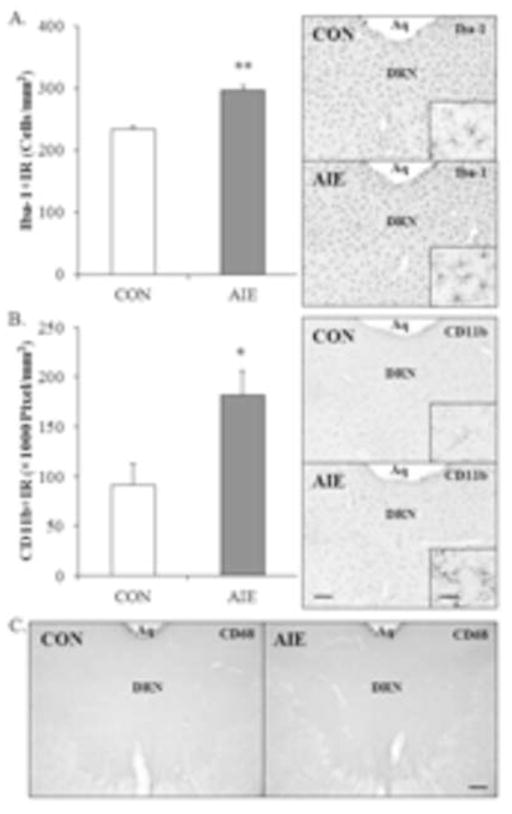
Adolescent intermittent ethanol and chronic ethanol exposure in adulthood result in microglial activation, and increased expression of cytokines and other innate immune signaling molecules in brain (Crews and Vetreno, 2014; Crews et al., In Press; Marshall et al., 2013; McClain et al., 2011). To determine if AIE leads to long-term alterations in microglia, Iba-1 (microglial marker), CD11b (marker of microglial activation), and ED-1 (marker of phagocytic amoeboid microglia) were assessed in the young adult (P80) (CON = 8, AIE = 8) DRN using immunohistochemistry. In CON- and AIE-treated animals, assessment of Iba-1+IR revealed darkly stained cell bodies and processes. Adolescent binge ethanol treatment led to a significant 27% (±3%) increase in the number of Iba-1+IR cells in the young adult DRN, relative to CONs (one-way ANOVA: F[1,14] = 54.1, p < 0.01; see Figure 5A). Analysis of CD11b+IR revealed darkly stained microglia in the AIE-treated animals and light staining in CONs. Adolescent binge ethanol treatment resulted in a significant 99% (±26%) increase in CD11b+IR pixel density in the young adult DRN, relative to CONs (one-way ANOVA: F[1,14] = 8.1, p < 0.05; see Figure 5B). ED-1 (CD68) immunohistochemistry did not show any staining in the raphe nucleus of either CON- or AIE-treated animals (see Figure 5C), but was evident in kainic acid positive control subjects. These findings are consisted with other studies reporting ethanol-induced increases in microglial markers and evidence of hyper-ramified activation, but not fully activated bushy or phagocytic morphology. Thus, AIE treatment leads to increased expression of microglial activation markers in the young adult DRN.
Figure 5. Adolescent intermittent ethanol (AIE) exposure increases the population and partial activation of microglia in the young adult (P80) dorsal raphe nucleus (DRN).
(A) Modified unbiased stereological assessment revealed that AIE treatment led to a 27% (±3%) (CON = 8, AIE = 8; one-way ANOVA: F[1,14] = 54.1, p < 0.01) increase in the population of Iba-1+IR microglia in the young adult (P80), relative to controls (CON). Representative photomicrographs of Iba-1+IR in the DRN from CON- and AIE-exposed animals. (B) Quantification of CD11b pixel density in the young adult following AIE revealed a 99% (±26%) (CON = 8, AIE = 8; one-way ANOVA: F[1,14] = 8.1, p < 0.05) increase in the partial activation of microglia, relative to CONs. Representative photomicrographs of CD11b+IR in the DRN from CON- and AIE-exposed animals. (C) Representative photomicrographs of ED1+IR in the DRN from CON- and AIE-exposed animals. Expression of ED1 was unaffected by AIE treatment in the young adult DRN. Aq; Cerebral aqueduct. Scale bar = 50 microns; scale bar inset = 10 microns. Data are presented as mean ± SEM. * p < 0.05, ** p < 0.01, relative to CON rats.
3.5. Lipopolysaccharide exposure mimics AIE-induced reductions of 5-HT+IR cell populations in the young adult DRN
Lipopolysaccharide (LPS) treatment of mice leads to a persistent increase in expression of proinflammatory cytokines and oxidases in the brain (Qin et al., 2007). To determine whether innate immune activation might contribute to the reduction of 5-HT+IR cells in the DRN, CON- and AIE-treated animals received a single dose of LPS (1.0 mg/kg, i.p.) on P70, and 5-HT+IR was assessed in young adulthood (P80) (CON/SAL = 8, CON/LPS = 8, AIE/SAL = 8, AIE/LPS = 8). A 2 × 2 ANOVA (Treatment × Drug [saline (SAL) vs. LPS]) replicated in an additional group revealed that AIE treatment significantly reduced 5-HT+IR in the DRN, relative to CONs (main effect of Treatment: F[1,28] = 4.2, p < 0.05). Furthermore, LPS treatment resulted in a significant reduction of 5-HT+IR, relative to SAL-treated subjects (main effect of Drug: F[1,28] = 12.7, p < 0.01). Post-hoc analysis found that 5-HT+IR was reduced by AIE/SAL (14% [±3%]; p < 0.05), CON/LPS (19% [±3%]; p < 0.01), and AIE/LPS (19% [±3%]; p < 0.01), relative to SAL-treated CONs (interaction of Treatment × Drug: F[1,28] = 5.2, p < 0.05; see Figure 6). Thus, administration of the innate immune activator LPS induced a similar decrease in 5-HT+IR cells in the CON subjects, but did not further reduce 5-HT+IR cells in the AIE-treated subjects.
Figure 6. Exposure to lipopolysaccharide (LPS) mimics the loss of serotonin-immunoreactive (5-HT+IR) cells associated with adolescent intermittent ethanol (AIE) exposure in the dorsal raphe nucleus (DRN).
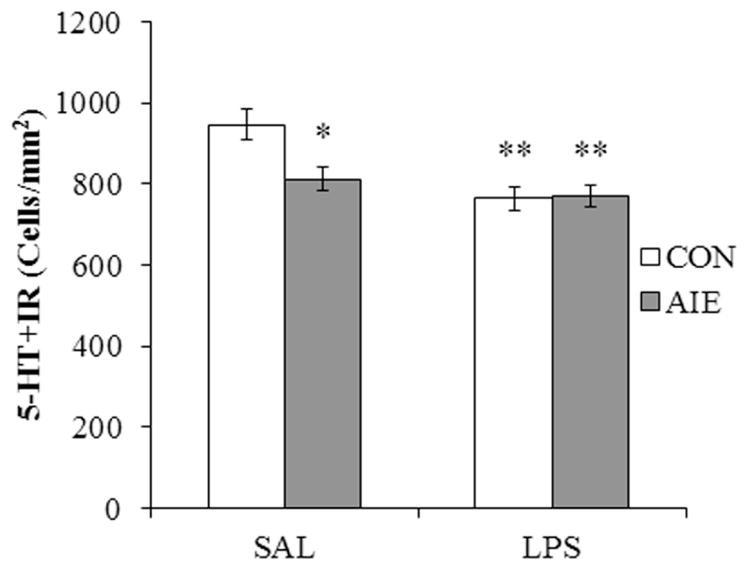
Modified unbiased stereological assessment of 5-HT+IR cells in the DRN of young adult rats revealed a significant reduction of 5-HT+IR neurons in AIE/saline (SAL)- (20% ±5%; n = 8), CON/LPS- (20% ±5%; n = 8), and AIE/LPS-exposed (20% ±5%; n = 8) rats, relative to CON/SAL (n = 8). Data are presented as mean ± SEM. * p < 0.05, ** p < 0.01, relative to CON/SAL rats.
3.6 Voluntary exercise prevents AIE-induced reductions of 5-HT+IR neurons and increased microglia marker expression in the young adult DRN
Voluntary exercise in the form of wheel running has previously been shown to reverse the deleterious effects of ethanol exposure in adult mice (Crews et al., 2004). To determine whether exercise prevents AIE-induced reductions of 5-HT+IR cells in the DRN, subjects were exposed to running wheels beginning on P24 (24 hr prior to the onset of AIE) that continued until sacrifice in young adulthood (P80) (CON/No exercise = 8, CON/Exercise = 9, AIE/No exercise = 8, AIE/Exercise = 10). A 2 × 2 ANOVA (Treatment × Exercise [No exercise vs. Exercise]) revealed that AIE treatment reduced 5-HT+IR in the DRN, relative to CONs (main effect of Treatment: F[1,31] = 4.1, p = 0.05). Furthermore, exercising subjects had significantly more 5-HT+IR cells in the DRN than non-exercising subjects (main effect of Exercise: F[1,31] = 6.0, p < 0.05). While a significant interaction of Treatment × Exercise was not observed, wheel running AIE-treated animals had significantly more 5-HT+IR cells than non-exercising AIE-treated animals (one-way ANOVA; F[1,16] = 10.8, p < 0.01; see Figure 7). Thus, these data reveal that voluntary exercise prevents the AIE-induced loss of 5-HT+IR cells in the DRN.
Figure 7. Voluntary exercise exposure prevents the adolescent intermittent ethanol (AIE)-induced loss of serotonin-immunoreactive (5-HT+IR) cells in the young adult (P80) dorsal raphe nucleus (DRN).
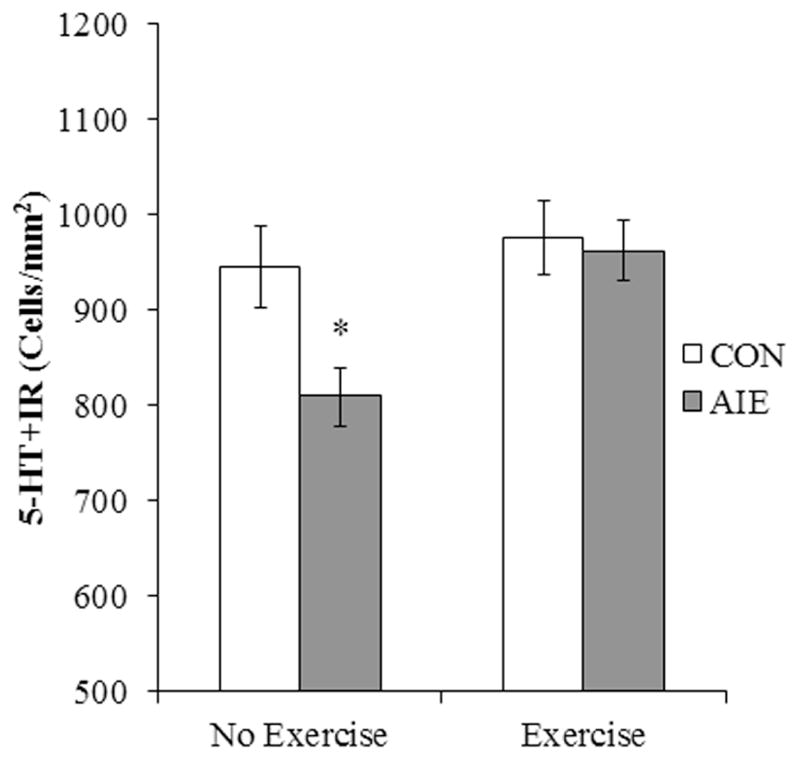
Modified unbiased stereological assessment of 5-HT+IR cells in the DRN of young adult rats in the no exercise condition revealed a significant (14% ± 3%) (CON/No exercise = 8, CON/Exercise = 9, AIE/No exercise = 8, AIE/Exercise = 10) reduction in the AIE-treated animals, relative to controls (CON). Interestingly, subjects in the AIE treatment group that were exposed to exercise in the form of voluntary wheel running from P24 to P80 did not evidence the observed loss of 5-HT+IR cells (one-way ANOVA; F[1,16] = 10.8, p < 0.01). Data are presented as mean ± SEM. * p < 0.05, relative to CON/No exercise rats.
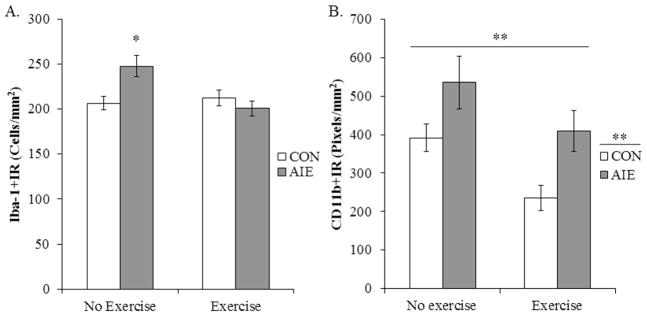
To determine if voluntary exercise exposure prevented the AIE-induced increase in microglia marker expression, Iba-1 and CD11b immunohistochemistry was assessed. A 2 × 2 ANOVA (Treatment × Exercise) revealed an exercise-induced reduction of Iba-1+IR microglia in the DRN, relative to non-exercising subjects (main effect of Exercise: F[1,31] = 5.1, p < 0.05). Further, post-hoc analysis of the significant interaction of Treatment × Exercise (F[1,31] = 8.2, p < 0.01) revealed that, relative to non-exercising CONs, Iba-1+IR was increased by 20% (±6%) in the non-exercising AIE-treated animals (p < 0.05) while there was no effect in the exercising AIE animals (see Figure 8A). A 2 × 2 ANOVA (Treatment × Exercise) revealed that AIE treatment increased CD11b+IR microglia in the DRN, relative to CON-treated subjects (main effect of Treatment: F[1,31] = 10.4, p < 0.01). Further, voluntary exercise was found to reduce CD11b immunoreactivity, relative to non-exercising subjects (main effect of Exercise: F[1,31] = 7.9, p < 0.01; see Figure 8B). Thus, these data reveal that voluntary exercise prevented the AIE-induced microglial activation in the DRN as well as the loss of serotonin marker expression.
Figure 8. Voluntary exercise exposure prevents adolescent intermittent ethanol (AIE)-induced increased population and partial activation of microglia in the young adult (P80) dorsal raphe nucleus (DRN).
A) Modified unbiased stereological assessment of Iba-1+IR cells in the DRN of young adult rats in the no exercise condition revealed a significant (20% ±6%) (CON/No exercise = 8, CON/Exercise = 9, AIE/No exercise = 8, AIE/Exercise = 10) increase in the AIE-treated animals, relative to controls (CON). Exercise-exposed AIE-treated subjects did not evidence an increase in Iba-1+IR microglia when compared to non-exercising CON-treated subjects. * p < 0.05, relative to CON/No exercise rats. (B) Quantification of CD11b pixel density in the DRN from young adult rats in the no exercise condition revealed a significant 38% (±4%) increase in the AIE-treated animals, relative to controls (CON). While the exercising AIE-treated animals did not differ from the non-exercising CON subjects, there was a 40% (±4%) reduction of CD11b+IR in the exercising CONs, relative to non-exercising CONs. Data are presented as mean ± SEM. ** p < 0.01, relative to CON/No exercise rats.
3.7. Lipopolysaccharide exposure mimics and voluntary exercise prevents AIE-induced upregulation of pNF-κB p65 in the young adult DRN
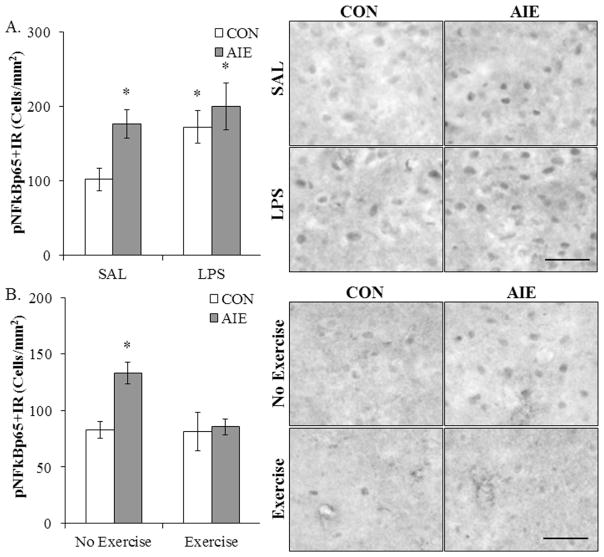
Our laboratory and others previously found that AIE upregulates innate immune signaling molecules and Toll-like receptor 4 expression in multiple brain regions that persist into young adulthood (Pascual et al., 2014; Vetreno and Crews, 2012, 2015). Phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells p65 is essential for NF-κB nuclear translocation and induction of proinflammatory cytokines. Since AIE is associated with long-term upregulation of proinflammatory cytokines in the brain, we assessed expression of pNF-κB p65 in the young adult (P80) (CON/SAL = 7, CON/LPS = 8, AIE/SAL = 8, AIE/LPS = 8) DRN following AIE and LPS treatment. A 2 × 2 ANOVA (Treatment × Drug) revealed that AIE treatment significantly increased expression of pNF-κB p65+IR in the DRN, relative to CONs (main effect of Treatment: F[1,27] = 4.9, p < 0.05). Further, LPS treatment increased pNF-κB p65+IR, relative to SAL-treated subjects (main effect of Drug: F[1,27] = 4.1, p = 0.05). While a significant interaction of Treatment × Drug was not observed, pNF-κB p65+IR was increased in AIE/SAL (73% [±19%]; p = 0.01), CON/LPS (69% [±21%]; p < 0.05), and AIE/LPS (96% [±31%]; p < 0.05), relative to SAL-treated CONs (see Figure 9A). Thus, AIE treatment increased expression of pNF-κB p65 and administration of LPS induced a similar increase in pNF-κB p65 in the CON subjects.
Figure 9. Lipopolysaccharide (LPS) mimics and voluntary exercise prevents adolescent intermittent ethanol (AIE)-induced increased expression of pNF-κBp65 in the young adult (P80) dorsal raphe nucleus (DRN).
(A) Modified unbiased stereological assessment of phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells p65 (pNF-κB p65)+IR cells in the DRN of young adult rats revealed a significant increase in AIE/saline (SAL)- (73% ±19%; n = 8), CON/LPS- (69% ±21%; n = 8), and AIE/LPS-exposed (96% ±31%; n = 8) rats, relative to SAL-treated controls (n = 7). (B) Modified unbiased stereological assessment in the DRN of young adult rats revealed a significant 61% (±12%) (CON+No exercise = 8, CON+Exercise = 8, AIE+No exercise = 8, AIE+Exercise = 8) increase in pNF-κB p65+IR cells in the non-exercise AIE-treated animals, relative to non-exercising CONs. Importantly, voluntary exercise exposure prevents the AIE-induced increase in pNF-κB p65+IR in the young adult DRN. Further, expression of pNF-κB p65+IR between control groups in Figure A and B did not differ statistically (one-way ANOVA: F[1,13] = 1.6, p > 0.05). Data are presented as mean ± SEM. * p < 0.05, relative to CON/SAL rats. Scale bar = 50 micron.
To determine if voluntary exercise exposure would prevent the AIE-induced increase in phosphorylated NF-κB expression, pNF-κB p65 immunohistochemistry was performed in the DRN in young adulthood (P80) (CON/No exercise = 8, CON/Exercise = 8, AIE/No exercise = 8, AIE/Exercise = 8). A 2 × 2 ANOVA (Treatment × Exercise) revealed an exercise-induced reduction of pNF-κB p65+IR in the DRN, relative to non-exercising subjects (main effect of Exercise: F[1,28] = 5.1, p < 0.05). Further, post-hoc analysis of the significant interaction of Treatment × Exercise (F[1,28] = 4.5, p < 0.05) revealed that, relative to non-exercising CONs, pNF-κB p65+IR was increased by 61% (±12%) in the non-exercising AIE-treated animals (p < 0.05) while there was no effect in the exercising AIE animals (see Figure 9B). Thus, these data reveal that voluntary exercise prevented the increase in pNF-κB p65 expression in the DRN following AIE treatment.
4. Discussion
We report here that adolescent binge ethanol exposure leads to long-term reductions of 5-HT+IR cells in the dorsal, but not median, raphe nucleus that persists from late adolescence (P56) into adulthood (P220). These findings are consistent with work from Evrard and colleagues (2006) that found late adolescent rats (i.e., P45) exposed to ethanol liquid diet for six weeks (6.6% ethanol with BECs of ~19.0 mg/dL) had an approximate 30% reduction of 5-HT+IR staining density in the adult DRN. Although the blood ethanol levels in Evrard’s study were 10% of the levels in our study, both find that ethanol decreases DRN, but not median raphe nucleus 5-HT+IR (Evrard et al., 2006). Notably, the literature indicates that the DRN might be more susceptive to degeneration in response to a neurotoxic insult than other 5-HT nuclei (Gartside et al., 1997; Kosofsky and Molliver, 1987; O’Hearn et al., 1988; Tagliaferro et al., 1997). Further, Evrard and colleagues (2006) found that 5-HT+IR staining density in DRN returned to control levels 10 weeks after ethanol treatment consistent with either depletion of 5-HT or neuronal shrinkage. Our AIE treatment paradigm, which models human levels of binge drinking, caused a loss of 5-HT+IR neurons that persisted from P56 to P220 (i.e., over 23 weeks). Adolescent intermittent ethanol treatment also decreased midbrain protein levels of TPH2 and VMAT2, markers of 5-HT neurons that regulate 5-HT synthesis and vesicular 5-HT packaging, respectively. Further, we found reduced 5-HT+IR in amygdala and hypothalamus, brain regions with known projections from serotonergic DRN neurons. Together, each of these findings supports a persistent loss of 5-HT+IR neurons following AIE treatment in the adult brain.
The reduction in DRN 5-HT+IR neurons was paralleled by increased Iba-1+IR and CD11b+IR microglia, and pNF-κB p65 expression in the adult DRN that is consistent with AIE treatment leading to increased hyper-ramified microglial activation and induction of innate immunity (see Figure 10; Crews and Vetreno, 2015). Previous studies found that AIE treatment increased expression of microglial markers and/or innate immune signaling genes in adult frontal cortex (Vetreno and Crews, 2012; Vetreno et al., 2013) and hippocampus (Vetreno and Crews, 2015) as well as the post-mortem human alcohol brain (Crews et al., 2013; He and Crews, 2008; Vetreno et al., 2013). Multiple studies suggest that microglia and proinflammatory cytokines contribute to alcoholic brain pathologies (Crews et al., 2006; Crews and Vetreno, 2014, 2015). We found that AIE increased markers for hyper-ramified, but not amoeboid microglia, in the DRN that are associated with increased secretion of proinflammatory cytokines and other signaling molecules (Beynon and Walker, 2012). This relationship is further supported by our finding that exercise (i.e., voluntary wheel running) during AIE prevents upregulation of microglial activation markers, increased pNF-κB p65+IR, and the loss of 5-HT+IR neurons in the adult DRN. Similarly, in an animal model of ischemia, treadmill running was found to reduce CD11b+IR microglia in adult male Wistar rats (Ang et al., 2004). We observed that voluntary exercise prevented the AIE-induced loss of 5-HT+IR neurons in the adult DRN; however, caution must be exerted when interpreting these data as it is possible that exposure to the wheel was beneficial as we did not include a control group with a locked wheel.
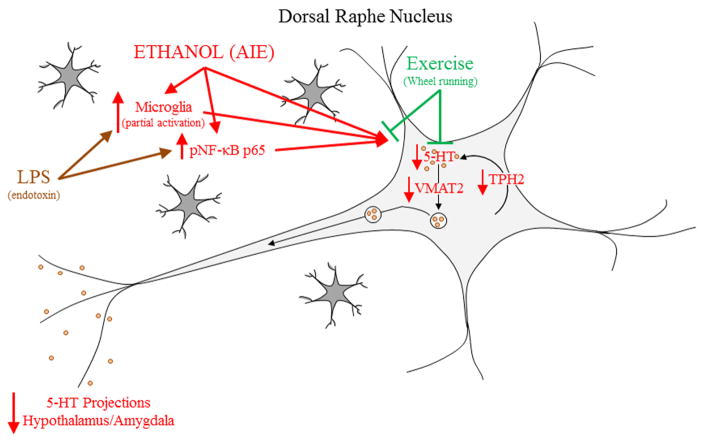
Figure 10. Proposed mechanism of adolescent intermittent ethanol (AIE)-induced serotonergic system deficits.
Adolescent intermittent ethanol led to a persistent reduction of serotonergic neurons in the dorsal raphe nucleus (DRN). This was accompanied by decreased levels of tryptophan hydroxylase 2 (TPH2), the rate-limiting enzyme in serotonin (5-HT) synthesis, decreased vesicular monoamine transporter 2 (VMAT2), a vesicular transporter of 5-HT, and decreases serotonergic axon terminal fields in the hypothalamus and amygdala. Lipopolysaccharide (LPS) mimicked the AIE-induced serotonergic neuron reduction and increase in phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells p65 (pNF-κB p65)+IR in the DRN, suggesting a possible innate immune mechanism of AIE-induced serotonergic neuron loss. Indeed, AIE increases microglial cell number and the CD11b marker of microglial activation in the DRN. Voluntary exercise prevented the loss of serotonergic neurons, and increases in microglial cell number and activation as well as expression of pNF-κB p65. These observations are consistent with AIE causing microglial proliferation/activation and innate immune activation that in turn contributes to the serotonergic neuron loss.
Across maturation from P56 to P220, we observed a 29% decrease of 5-HT+IR neurons in the DRN of control subjects. While extensive studies have defined the embryonic origins and early postnatal development of the rat serotonergic system (Lauder, 1990; Lauder and Bloom, 1974), few studies have addressed its maturation from adolescence into adulthood. Lauder and colleagues (1982) observed an approximate 30% increase in synapses from P30 to P60 in the rat DRN. Levels of 5-HT in the cortex and basal ganglia, which are projection sites of the DRN, increase between P35 and adulthood (Loizou, 1972). Further, consistent with our findings of larger populations of 5-HT+IR neurons in the adolescent DRN, Nakamura and colleagues (2006) reported a maturational decline in 5-HT+IR in the mouse DRN from P21 to P65. Thus, our findings of an age-associated decline of 5-HT+IR from P56 to P220 suggest an age-associated decline of dorsal raphe serotonergic neurons.
Lipopolysaccharide is known to induce a systemic innate immune response that activates brain microglia leading to induction of cytokines and other innate immune signaling molecules (Qin et al., 2007). Studies in mice find that LPS administration 24 hrs post-ethanol treatment potentiates the innate immune response (Qin et al., 2008). Adolescent intermittent ethanol exposure has been shown in this study and others (e.g., Vetreno and Crews, 2012; Vetreno and Crews, 2015; Vetreno et al., 2013) to subtly increase innate immune expression in the adult brain. To model AIE neuroimmune induction in the adult brain, we used LPS to increase brain innate immune gene expression which we assessed using pNF-κB p65+IR, the active form of a key proinflammatory transcription factor. We found that a single dose of LPS assessed 10 days later increased pNF-κB p65+IR and reduced 5-HT+IR DRN neurons. These findings suggest that innate immune gene induction can reduce expression of 5-HT in the adult DRN. Further, we observed a trend toward increased pNF-κB p65+IR in the LPS-treated AIE subjects, but additional studies will be needed to understand the LPS-induced AIE responses and determine if AIE is desensitizing the LPS response in adulthood. Our studies find that AIE has a persistent effect of decreasing 5-HT+IR and increasing pNF-κB p65 consistent with long-lasting changes in the adult brain following adolescent alcohol exposure.
Application of LPS to DRN organotypic slice culture increase expression of proinflammatory cytokines (i.e., IL-1β, MCP-1, and TNFα) and reduced serotonergic neurons (Hochstrasser et al., 2011). Further, LPS-induced systemic inflammation in pregnant female rats causes a 31% decrease in TPH+IR DRN neurons in adult (P120) offspring (Wang et al., 2009), consistent with a developmental sensitivity of 5-HT neurons to LPS. Induction of brain innate immune signals is associated with most neurodegenerative diseases (Heneka et al., 2014), pain and depression (Walker et al., 2014), and alcoholism (Crews et al., 2011; Kelley and Dantzer, 2011), and is extensively modeled using LPS-induced innate immune activation. Previous studies in rats have found that exercise can reduce the induction of TLR2, TLR4, MyD88, and NF-κB (Ma et al., 2013) as well as microglial activation and proinflammatory cytokine induction (Ang et al., 2004) in a rat model of cerebral ischemia. However, other studies in mice have found that systemic LPS induction of depression-like behavior and proinflammatory cytokines in liver and spleen (Martin et al., 2013) or in brain (Martin et al., 2014) are not reduced by voluntary exercise. These findings are consistent with differences between rats and mice as well as exercise having a greater impact on brain insult-induced innate immune responses than peripheral LPS-induced systemic innate immune responses. Alternatively, brain regional changes may be specific and not resolved in whole brain studies. For example, a recent study in rats found that exercise reduced the induction of hippocampal IL-1β and IL-6 mRNA and protein during sleep deprivation, but the cerebral cortex was not altered by either sleep deprivation or exercise (Chennaoui et al., 2015). In our study, pNF-κB p65+IR and microglial markers in the DRN were used to assess innate immune responses. We found that LPS increased DRN pNF-κB p65+IR similar to AIE, and that running wheel exposure prevented the AIE-induced increases in DRN pNF-κB p65+IR and loss of DRN 5-HT+IR. Thus, our findings are consistent with AIE increased DRN pNF-κB p65+IR and microglial markers contributing to the long-lasting reduction in 5-HT+IR neurons following AIE treatment.
Our finding that AIE treatment leads to long-term alterations in the serotonergic neurotransmitter system are of significant import as it could contribute to the development of psychopathologies as well as alcohol dependence later in life (Wrase et al., 2006). Adolescent intermittent ethanol increases adult anxiety-like behaviors (Sakharkar et al., 2014; Vetreno et al., 2015), induces depressive-like behavior in adulthood (Ehlers et al., 2011; Slawecki et al., 2004), disrupts sleep (Criado et al., 2008), and increases impulsivity in adulthood (Ehlers et al., 2011; Gass et al., 2014) that might be secondary to reduced 5-HT+IR neurons in the DRN. Serotoninergic system dysfunction is associated with depression and alcoholism, which are co-morbid in humans (Kelley and Dantzer, 2011). Previous studies have linked innate immune induction to the development of depression (e.g., Dantzer et al., 2008) and depression with reduced levels of serotonin (Coppen and Doogan, 1988; Michelsen et al., 2007). Low brain 5-HT levels are linked to alcoholism and alleles of serotonergic genes associated with alcoholism, with strong evidence for decreased 5-HT turnover in Type 2 alcoholics with impaired impulse control and a history of suicide attempts (Linnoila et al., 1994; Virkkunen et al., 1996). Reductions in brain levels of 5-HT are associated with increased alcohol consumption (Barr et al., 2004; LeMarquand et al., 1994a, b) and AIE leads to increased ethanol self-administration in adulthood (Alaux-Cantin et al., 2013; Gass et al., 2014; Pandey et al., 2015; Pascual et al., 2009). Adolescent alcohol-preferring rats self-administering ethanol have reduced TPH2 and the vesicular transporter VMAT2 consistent with genetic and/or developmental raphe neuron sensitivity to alcohol (McClintick et al., 2015). Human studies using TPH+IR find reduced dorsal raphe neuron staining intensity in alcoholics, with no significant differences in numbers of neurons although variability was high limiting resolution of differences (Baker et al., 1996). More recent studies on post-mortem human alcoholics find increased dorsal raphe TPH2+IR expression (Bach et al., 2014; Underwood et al., 2007) as well as decreased serotonin transporter density as assessed by [3H]citalopram binding (Karkkainen et al., 2015) with both associated with variations in specific serotonin gene alleles. These findings confound clear conclusions while multiple serotonergic medications are being tested for treatment of alcoholism with some effect in certain populations with specific serotonergic genotypes (Kenna, 2010).
In summary, AIE treatment persistently reduced serotonergic neuron populations in the DRN and altered terminal field projections. While the mechanism underlying this reduction remains to be fully elucidated, the LPS effects, coupled with the increased populations of partially activated hyper-ramified microglia and increased expression of pNF-κB p65 in the DRN, implicate a role for the innate immune system in the AIE-induced persistent loss of serotonergic neurons. Interestingly, voluntary exercise prevented both the increase in hyper-ramified microglia and pNF-κB p65 as well as loss of serotonergic neurons in the DRN. The serotonergic system plays a neuromodulatory role in alcohol drinking and these novel findings might explain why adolescent onset of alcohol drinking drastically increases the likelihood of developing an alcohol use disorder later in adulthood.
Highlights.
AIE reduces adult raphe TPH2, VMAT2, and 5-HT+IR neurons
AIE increases innate immune marker expression in the adult raphe nucleus
LPS increases immune marker expression and mimics AIE loss of 5-HT neurons
Exercise prevents AIE immune expression and0loss of 5-HT neurons
Acknowledgments
This work was supported in part by the National Institutes of Health, National Institute on Alcoholism and Alcohol Abuse (AA019767, AA011605, AA007573, and AA021040), the Neurobiology of Adolescent Drinking in Adulthood (NADIA [AA020023, AA020024, and AA020022]), and the Bowles Center for Alcohol Studies. The authors thank Diana Lotito for help with preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Ang ET, Wong PT, Moochhala S, Ng YK. Cytokine changes in the horizontal diagonal band of Broca in the septum after running and stroke: a correlation to glial activation. Neuroscience. 2004;129:337–347. doi: 10.1016/j.neuroscience.2004.06.087. [DOI] [PubMed] [Google Scholar]
- Bach H, Arango V, Kassir SA, Tsaava T, Dwork AJ, Mann JJ, Underwood MD. Alcoholics have more tryptophan hydroxylase 2 mRNA and protein in the dorsal and median raphe nuclei. Alcohol Clin Exp Res. 2014;38:1894–1901. doi: 10.1111/acer.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Kril JJ, Harper CG. Chronic alcoholics without Wernicke-Korsakoff syndrome or cirrhosis do not lose serotonergic neurons in the dorsal raphe nucleus. Alcohol Clin Exp Res. 1996;20:61–66. doi: 10.1111/j.1530-0277.1996.tb01045.x. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Newman TK, Higley JD. The use of adolescent nonhuman primates to model human alcohol intake: neurobiological, genetic, and psychological variables. Ann N Y Acad Sci. 2004;1021:221–233. doi: 10.1196/annals.1308.027. [DOI] [PubMed] [Google Scholar]
- Beynon SB, Walker FR. Microglial activation in the injured and healthy brain: what are we really talking about? Practical and theoretical issues associated with the measurement of changes in microglial morphology. Neuroscience. 2012;225:162–171. doi: 10.1016/j.neuroscience.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Chennaoui M, Gomez-Merino D, Drogou C, Geoffroy H, Dispersyn G, Langrume C, Ciret S, Gallopin T, Sauvet F. Effects of exercise on brain and peripheral inflammatory biomarkers induced by total sleep deprivation in rats. J Inflamm (Lond) 2015;12:56. doi: 10.1186/s12950-015-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Jr, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcoholism, clinical and experimental research. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppen AJ, Doogan DP. Serotonin and its place in the pathogenesis of depression. J Clin Psychiatry. 1988;49(Suppl):4–11. [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology, biochemistry, and behavior. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism, clinical and experimental research. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2013;73:602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP. Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol. 2014;118:315–357. doi: 10.1016/B978-0-12-801284-0.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP. Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, Robinson DL. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacological Reviews. doi: 10.1124/pr.115.012138. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol. 2008;42:631–639. doi: 10.1016/j.alcohol.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Noronha A, Warren KR, Koob GF, Sinha R, Thakkar M, Matochik J, Crews FT, Chandler LJ, Pfefferbaum A, Becker HC, Lovinger D, Everitt BJ, Egli M, Mandyam CD, Fein G, Potenza MN, Harris RA, Grant KA, Roberto M, Meyerhoff DJ, Sullivan EV. Brain pathways to recovery from alcohol dependence. Alcohol. 2015;49:435–452. doi: 10.1016/j.alcohol.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dori I, Dinopoulos A, Blue ME, Parnavelas JG. Regional differences in the ontogeny of the serotonergic projection to the cerebral cortex. Exp Neurol. 1996;138:1–14. doi: 10.1006/exnr.1996.0041. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–345. doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard SG, Duhalde-Vega M, Tagliaferro P, Mirochnic S, Caltana LR, Brusco A. A low chronic ethanol exposure induces morphological changes in the adolescent rat brain that are not fully recovered even after a long abstinence: an immunohistochemical study. Exp Neurol. 2006;200:438–459. doi: 10.1016/j.expneurol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Gartside SE, McQuade R, Sharp T. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on 5-HT cell firing and release: comparison between dorsal and median raphe 5-HT systems. Neuropharmacology. 1997;36:1697–1703. doi: 10.1016/s0028-3908(97)00171-8. [DOI] [PubMed] [Google Scholar]
- Gass JT, Glen WB, Jr, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ. Adolescent Alcohol Exposure Reduces Behavioral Flexibility, Promotes Disinhibition, and Increases Resistance to Extinction of Ethanol Self-Administration in Adulthood. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2570–2583. doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental neurology. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014;14:463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- Hochstrasser T, Ullrich C, Sperner-Unterweger B, Humpel C. Inflammatory stimuli reduce survival of serotonergic neurons and induce neuronal expression of indoleamine 2,3-dioxygenase in rat dorsal raphe nucleus organotypic brain slices. Neuroscience. 2011;184:128–138. doi: 10.1016/j.neuroscience.2011.03.070. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on drug use: 2012 Overview, Key Findings on Adolescent Drug Use. Institute for Social Research, The University of Michigan; Ann Arbor: 2013. [Google Scholar]
- Karkkainen O, Laukkanen V, Haukijarvi T, Kautiainen H, Tiihonen J, Storvik M. Lower [3H]Citalopram binding in brain areas related to social cognition in alcoholics. Alcohol Alcohol. 2015;50:46–50. doi: 10.1093/alcalc/agu074. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Dantzer R. Alcoholism and inflammation: neuroimmunology of behavioral and mood disorders. Brain, behavior, and immunity. 2011;25(Suppl 1):S13–20. doi: 10.1016/j.bbi.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA. Medications acting on the serotonergic system for the treatment of alcohol dependent patients. Curr Pharm Des. 2010;16:2126–2135. doi: 10.2174/138161210791516396. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Molliver ME. The serotoninergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. discussion 314. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, Raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol. 1974;155:469–481. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Petrusz P, Wallace JA, Dinome A, Wilkie MB, McCarthy K. Combined serotonin immunocytochemistry and 3H-thymidine autoradiography: in vivo and in vitro methods. J Histochem Cytochem. 1982;30:788–793. doi: 10.1177/30.8.6749972. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Decker L. Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus. 2009;19:907–912. doi: 10.1002/hipo.20563. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry. 1994a;36:326–337. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol Psychiatry. 1994b;36:395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- Lidov HG, Molliver ME. An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain Res Bull. 1982;8:389–430. doi: 10.1016/0361-9230(82)90077-6. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, George T, Eckardt M, Higley JD, Nielsen D, Goldman D. Serotonin, violent behavior and alcohol. EXS. 1994;71:155–163. doi: 10.1007/978-3-0348-7330-7_16. [DOI] [PubMed] [Google Scholar]
- Loizou LA. The postnatal ontogeny of monoamine-containing neurones in the central nervous system of the albino rat. Brain Res. 1972;40:395–418. doi: 10.1016/0006-8993(72)90142-4. [DOI] [PubMed] [Google Scholar]
- Ma Y, He M, Qiang L. Exercise Therapy Downregulates the Overexpression of TLR4, TLR2, MyD88 and NF-kappaB after Cerebral Ischemia in Rats. Int J Mol Sci. 2013;14:3718–3733. doi: 10.3390/ijms14023718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiology of disease. 2013;54:239–251. doi: 10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SA, Dantzer R, Kelley KW, Woods JA. Voluntary wheel running does not affect lipopolysaccharide-induced depressive-like behavior in young adult and aged mice. Neuroimmunomodulation. 2014;21:52–63. doi: 10.1159/000356144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SA, Pence BD, Greene RM, Johnson SJ, Dantzer R, Kelley KW, Woods JA. Effects of voluntary wheel running on LPS-induced sickness behavior in aged mice. Brain Behav Immun. 2013;29:113–123. doi: 10.1016/j.bbi.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain, behavior, and immunity. 2011;25(Suppl 1):S120–128. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick JN, McBride WJ, Bell RL, Ding ZM, Liu Y, Xuei X, Edenberg HJ. Gene expression changes in serotonin, GABA-A receptors, neuropeptides and ion channels in the dorsal raphe nucleus of adolescent alcohol-preferring (P) rats following binge-like alcohol drinking. Pharmacol Biochem Behav. 2015;129:87–96. doi: 10.1016/j.pbb.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphe nucleus--from silver stainings to a role in depression. Brain Res Rev. 2007;55:329–342. doi: 10.1016/j.brainresrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Muller CP, Homberg JR. The role of serotonin in drug use and addiction. Behav Brain Res. 2015;277:146–192. doi: 10.1016/j.bbr.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sugawara Y, Sawabe K, Ohashi A, Tsurui H, Xiu Y, Ohtsuji M, Lin QS, Nishimura H, Hasegawa H, Hirose S. Late developmental stage-specific role of tryptophan hydroxylase 1 in brain serotonin levels. J Neurosci. 2006;26:530–534. doi: 10.1523/JNEUROSCI.1835-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Tanaka KF, Barr MM, Tritschler L, Le Dantec Y, David DJ, Gardier AM, Blanco C, Hen R, Ahmari SE. Distinct Circuits Underlie the Effects of 5-HT1B Receptors on Aggression and Impulsivity. Neuron. 2015;86:813–826. doi: 10.1016/j.neuron.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hearn E, Battaglia G, De Souza EB, Kuhar MJ, Molliver ME. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci. 1988;8:2788–2803. doi: 10.1523/JNEUROSCI.08-08-02788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, Zhang H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis. 2015;82:607– 619. doi: 10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Pla A, Minarro J, Guerri C. Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: a review with reference to human adolescent drinking. Alcohol and alcoholism. 2014;49:187–192. doi: 10.1093/alcalc/agt164. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Tang L, Zhang H, Chen Y, Grayson DR, Pandey SC. Effects of acute ethanol exposure on anxiety measures and epigenetic modifiers in the extended amygdala of adolescent rats. Int J Neuropsychopharmacol. 2014;17:2057–2067. doi: 10.1017/S1461145714001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Gotham HJ. Pathological alcohol involvement: a developmental disorder of young adulthood. Development and psychopathology. 1999;11:933–956. doi: 10.1017/s0954579499002394. [DOI] [PubMed] [Google Scholar]
- Shoval G, Bar-Shira O, Zalsman G, John Mann J, Chechik G. Transitions in the transcriptome of the serotonergic and dopaminergic systems in the human brain during adolescence. Eur Neuropsychopharmacol. 2014;24:1123–1132. doi: 10.1016/j.euroneuro.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Ann N Y Acad Sci. 2004;1021:448–452. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Developmental cognitive neuroscience. 2011;1:392–400. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro P, Ramos AJ, Lopez EM, Pecci Saavedra J, Brusco A. Neural and astroglial effects of a chronic parachlorophenylalanine-induced serotonin synthesis inhibition. Mol Chem Neuropathol. 1997;32:195–211. doi: 10.1007/BF02815176. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Mann JJ, Arango V. Morphometry of dorsal raphe nucleus serotonergic neurons in alcoholism. Alcohol Clin Exp Res. 2007;31:837–845. doi: 10.1111/j.1530-0277.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–488. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front Neurosci. 2015;9:35. doi: 10.3389/fnins.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiol Dis. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Yaxley R, Paniagua B, Crews FT. Diffusion tensor imaging reveals adolescent binge ethanol-induced brain structural integrity alterations in adult rats that correlate with behavioral dysfunction. Addict Biol. 2015:1–15. doi: 10.1111/adb.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkkunen M, Goldman D, Linnoila M. Serotonin in alcoholic violent offenders. Ciba Found Symp. 1996;194:168–177. doi: 10.1002/9780470514825.ch10. discussion 177–182. [DOI] [PubMed] [Google Scholar]
- Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev. 2014;66:80–101. doi: 10.1124/pr.113.008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yan JY, Lo YK, Carvey PM, Ling Z. Dopaminergic and serotoninergic deficiencies in young adult rats prenatally exposed to the bacterial lipopolysaccharide. Brain Res. 2009;1265:196–204. doi: 10.1016/j.brainres.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Weir JM, Zakama A, Rao U. Developmental risk I: depression and the developing brain. Child Adolesc Psychiatr Clin N Am. 2012;21:237–259. vii. doi: 10.1016/j.chc.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Kraus CL, Swartzwelder H. Many college freshmen drink at levels far beyond the binge threshold. Alcoholism, clinical and experimental research. 2006;30:1006–1010. doi: 10.1111/j.1530-0277.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, Smith GT, Giedd J, Dahl RE. Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121(Suppl 4):S273–289. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Reimold M, Puls I, Kienast T, Heinz A. Serotonergic dysfunction: brain imaging and behavioral correlates. Cogn Affect Behav Neurosci. 2006;6:53–61. doi: 10.3758/cabn.6.1.53. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Ali SF, Slikker W, Jr, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]