Abstract
Background
Human herpesvirus 6 (HHV-6) has been associated with a wide spectrum of diseases. (r)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine (H2G) is an acyclic guanosine analogue that is structurally similar to acyclovir and is in clinical development for treatment of herpesvirus infections. H2G has been found to have activity against HSV type 1, HSV type 2, and HHV-6 in lymphoblast cell lines. A new antiviral duplex drug, 3′-azido-3′-deoxythymidylyl- (5′→2-O)-3-O-octadecyl-sn-glycerol (AZT-lipid-PFA), linking Zidovudine (AZT) and Foscarnet (PFA) via a lipophilic octadecylglycerol residue (lipid) also exhibits anti-viral activities against HIV, HSV type 1 and HCMV.
Objective
To assess the efficacy of H2G and AZT-lipid-PFA conjugate against HHV-6.
Study design
Drug associated toxicity and proliferative response were evaluated. We conducted in vitro experiments to determine the efficacy of H2G and an AZT-lipid-PFA conjugate in interfering with expression HHV-6 viral transcript in primary human peripheral blood mononuclear cells (PBMC).
Results
Both H2G and AZT-lipid-PFA were effective at inhibiting expression of HHV-6 gene transcript at comparable concentrations. Additionally, while AZT-lipid-PFA treatment was toxic to cells at concentrations above 5 μM, H2G treatment was associated with minimal cytotoxicity.
Conclusion
These data suggest the potential application of these anti-viral compounds in controlling HHV-6 infection.
INTRODUCTION
Human herpesvirus-6 (HHV-6) is a ubiquitous β-herpesvirus with a seroprevalence of approximately 90% in the adults. HHV-6 can establish latency following primary infection, typically before the age of 2, but can reactivate later in life 1. HHV-6A and HHV-6B are two variants that share high sequence homology, but differ in cellular tropism, antigenic specificity, and clinical manifestations2–5. HHV-6B is the etiological agent of exanthema subitum 6, while a specific disease association with HHV-6A remains elusive. HHV-6 is also implicated in other conditions such as encephalitis7, 8, rhomboencephalitis 9, multiple sclerosis 10–13, mesial temporal sclerosis 14, 15, post-bone marrow transplant (BMT) limbic encephalitis 16, and graft-versus-host disease following hematopoietic stem cell transplant17. HHV-6 may also play a role as a co-factor in HIV pathogenesis18.
The association of HHV-6 with a wide spectrum of clinical conditions makes it important to identify effective therapeutic agents with applications in clinical settings. There are a number of anti-herpetic drugs that have shown activity against herpes simplex virus 1, 2 (HSV-1, HSV-2), varicella-zoster virus (VZV) and cytomegalovirus (CMV)3. Some of these compounds, such as ganciclovir (GCV), foscarnet (PFA) and cidofovir (CDV), also exhibit anti-HHV-6 activity19. GCV is a guanosine analog that requires intracellular phosphorylation by a viral kinase for activation to the phosphorylataed product that competes with GTP for the viral DNA polymerase. PFA and CDV are non-guanosine derivates that do not require enzymatic activation. Another anti-herpetic compound, (r)-9-[4-hydroxy-2- (hydroxymethyl) butyl] guanine (H2G) that is structurally related to acyclovir demonstrated activity against HSV-1, HSV-2, VZV, and CMV in cell lines and primary cells. While efficacy of H2G towards HHV-6 infection in cell lines has been investigated, data based primary human cell infections are lacking20–22. Recently, a novel compound 3′-azido-3′-deoxythymidylyl- (5′→2-O)-3-O-octadecyl-sn-glycerol (AZT-lipid-PFA), was synthesized by covalently linking 3′-Azido-3′-deoxythymidine (AZT) and PFA via a lipophilic octadecylglycerol residue. This compound demonstrated antiviral activity not only against HIV, but also against drug-resistant strains and clinical isolates of HSV-1 and HCMV23. AZT is a reverse transcriptase inhibitor used for treating HIV infection that crosses the blood-brain-barrier due to its lipophilic nature 24. While previous studies indicated synergistic antiviral effects with PFA and AZT toward HIV and CMV25, 26, the activity of AZT-lipid-PFA towards HHV-6 has not been examined.
Given the potential role of HHV-6 in augmenting HIV pathogenesis and its associations with other diseases we examined the efficacy of AZT-lipid-PFA and H2G to against HHV-6B infection in human peripheral blood mononuclear cells (PBMC).
METHODS
Cell culture and HHV-6 infection of PBMC
SupT1 and PBMC were maintained at 37°C in complete RPMI media containing 10% FCS, 1% penicillin and streptomycin, and 1% L-glutamine (cRPMI). Prior to infection with HHV-6B (Z29), PBMC were activated with Phorbol 12-myristate 13-acetate (PMA,50 ng/mL) and ionomycin (0.5 μM) (Sigma) for 24 hours with IL-2 (30U/mL) (PeproTech) at 1.0 x 106 cells/mL. After 24 hours, three-quarters of the media was removed and HHV-6B viral supernatant was added at a multiplicity of infection of 100:1 for 3 days with AZT-lipid-PFA (laboratory of Dr. Herbert Schott) and H2G (Epiphany Biosciences). Infection was monitored by cytopathic effect and confirmed by TaqMan PCR.
Fluorescence-activated cell sorting (FACS) analysis
Annexin V and propidium iodine (PI) staining was performed using the Annexin-FITC apoptosis Detection Kit according to manufacture’s protocol (BD Pharmingen). Allophycocyanin (APC)-conjugated anti-CD4, Fluorescein isothiocyanate (FITC)-conjugated Ki-67, and Phycoerythrin (PE)-conjugated CD14 antibodies (BD Pharmingen). In a 96-well round bottom plate, 2μl of cell surface antibodies in 30 μl of FACS buffer were added and incubated at 4°C for 30 minutes. Intracellular staining, fixation and permeabilization were performed with Cytofix/CytoPerm (BD Pharmingen) according to manufacture’s instructions. Cells were suspended in FACS buffer and analyzed with a FACSCalibur. Isotype antibodies were used as staining controls
Proliferation assay
Proliferation assays were performed with SupT1 cells at 1x105 /well for 24 hours and 48 hours at 37°C. Cells were pulsed with 3[H]-labeled thymidine for 4 hours, harvested onto fiberglass filters, and measured with a β-scintillation counter (Wallac Instruments). Percent proliferation was calculated as followed: (CPM treated/CPM untreated) X100%.
HHV-6 U67 gene transcript detection
Total RNA was isolated from PBMC using the RNeasy RNA isolation kit (Qiagen) followed by treatment with amplification grade DNase I (Invitrogen). Complementary DNA was synthesized using random hexamer primers using TaqMan Reverese Transcription Reagents according to manufacturer’s instructions (Applied Biosystems). PCR amplification was performed as previously described27. Relative expression of U67 transcript was calculated using 2−ΔΔCT as followed: 2−(HHV-6B CT - β-actin CT) non-infected - (HHV-6B CT -β-actin CT) infected.
RESULTS
Determination of H2G and AZT-lipid-PFA cytotoxicity
We evaluated the drug-associated cytotoxicity of H2G or AZT-lipid-PFA by assessing levels of Annexin V and PI expression in SupT1 cell line at indicated concentrations for 2 days. Annexin V is a phospholipid binding protein that exhibits highly selective binding affinity to phosphatidylserine. The appearance of phosphatidylserine on the cell surface is often associated with early apoptotic events. PI is a DNA intercalating dye and its binding typically correlates with secondary necrosis of cells. As shown in Figure 1A, no appreciable cytotoxicity was associated with H2G treatment. However, a dose-dependent decrease in percent of viable cells was observed with AZT-lipid-PFA treatment starting at 5 μM. The individual contribution of apoptotic and necrotic processes to the cytotoxicity was investigated. H2G and AZT-lipid-PFA produced comparable levels of necrotic death (Figure 1B). However, a dose-dependent increase of apoptotic cells, as judged by Annexin V staining, occurred with AZT-lipid-PFA at concentrations above 5 μM (Figure 1C). These data suggest that short-term exposure to H2G did not significantly affect cell viability, while exposure to AZT-lipid-PFA was associated with cytotoxicity at higher drug concentrations.
Figure 1. Evaluation of H2G and AZT-lipid-PFA treatment associated cytotoxicity.
SupT1 cells incubated with various concentrations of H2G or AZT-lipid-PFA for 2 days as indicated. Annexin V and propidium iodine (PI) expression analyzed by FACS. Samples for each concentration were measured in duplicate. (A) Percent of live cells following drug treatments was calculated as [percent Annexin V and PI negative(treated)/percent Annexin V and PI negative (untreated)] x 100%. (B) Percent PI positive only. (C) Percent Annexin V positive only.
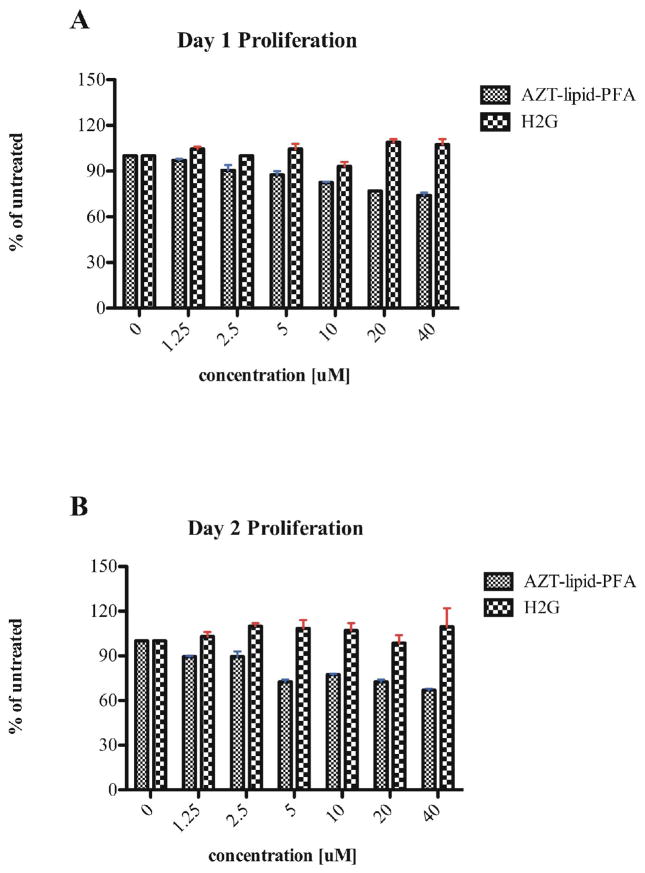
Effect of H2G and AZT-lipid-PFA on cell proliferation
The proliferative capability of SupT1 cells was examined after 24 and 48 hours of incubation with H2G and AZT-lipid-PFA by incorporation of 3[H]-labeled thymidine. In contrast to H2G-treated cells, SupT1 cells treated with AZT-lipid-PFA showed a decrease in proliferation in a dose-dependent manner (Figures 2A and B). This observed toxicity after exposure to AZT-lipid-PFA (Figure 1) was likely associated with cell death. Based on the results, subsequent experiments were performed using two concentrations, a non-toxic dose of 5 μM for H2G and AZT-lipid-PFA, and higher doses; namely, 40 μM of H2G or 10 μM of AZT-lipid-PFA.
Figure 2. Proliferative response of SupT1 cells after anti-viral treatments.
SupT1 cells treated with indicated concentration of H2G or AZT-lipid-PFA and proliferative response was measured by 3[H] incorporation for 4 hours at 37°C with 5% CO2. Triplicate wells were used for each treatment condition. (A) Proliferation of SupT1 cells after 1 day of anti-viral treatment. (B) Proliferation of SupT1 cells after 2 days of anti-viral treatment.
Lymphocyte subsets were not altered by H2G and AZT-lipid-PFA treatments
Due to the cytopathic effect of HHV-6B (Z29), the number of lymphocytes was significantly reduced after 3 days of infection compared to the non-infected control (Figure 3A). Quantification of lymphocyte numbers as a percent of total PBMC is shown in Figure 3B. In addition, no significant changes in the proportion of CD4+ or CD4-lymphocytes was observed and addition of H2G or AZT-lipid-PFA did not reverse the cytopathic effect (Figure 3A, middle and bottom panels). Similarly, fewer numbers of CD14+ monocytes were detected after HHV-6B infection and anti-viral compounds did not change the number of monocytes (Figure 3C).
Figure 3. Analysis of PBMC subsets after HHV-6B infection.
Healthy donors PBMC were activated with PMA and ionomycin in the presence of recombinant human IL-2 for 24 hours. In the presence of H2G (5 μM and 40 μM) or AZT-lipid-PFA (5 μM and 10 μM), cell-free HHV-6B (Z29) inocula were used to infect the activated primary human PBMC on day 3. PBMC subsets were examined by staining for CD4+, CD14+, and Ki-67 expression followed by FACS analysis. (A) FACS profiles CD4+ and Ki-67+ staining gated on lymphocytes from 2 healthy donors. (B) Quantification of percent lymphocytes represented in Figure 3A. (C) Average of percent CD14+ cells in 2 healthy controls following mitogenic stimulation, HHV-6B infection, and anti-viral treatment.
Efficacy of H2G and AZT-lipid-PFA in inhibiting HHV-6B infection
The ability of H2G and AZT-lipid-PFA to interfere with de novo viral transcription following 3-day drug treatment was assessed by TaqMan PCR 27. At 5 μM of H2G treatment, the relative expression of U67 gene was reduced by approximately 1 log compared to HHV-6B infected PBMC without treatment (Figure 4). This inhibitory effect was further enhanced at a concentration of 40 μM of H2G. In contrast, dramatic reduction in U67 expression was observed at 5 μM and 10 μM concentrations of AZT-lipid-PFA.
Figure 4. Comparison of anti-viral treatments in inhibiting viral transcript expression.
Healthy donor PBMC infected with HHV-6B and treated with either H2G or AZT-lipid-PFA at the indicated drug concentrations were evaluated for the expression of viral U67 gene transcript. Significantly less U67 transcript was detected in HHV-6B infected PBMC after H2G (untreated vs. 5 μM, p = 0.0222; untreated vs. 40 μM, p = 0.0212; t test) or AZT-lipid-PFA treatment (untreated vs. 5 μM, p = 0.0215; untreated vs. 10μM, p = 0.0220; t test) for 3 days.
DISCUSSION
While recognized as the causative agent of exanthem subitum 6, HHV-6B has recently been implicated in various neurologic disorders 8, 14–16, and reactivation has been observed in patients with allogeneic post-BMT or HSCT procedures17, 28, 29. In non-human primates, HHV-6A infection accelerated HIV progression 18. While co-infection of HHV-6B and HIV has been described in human cord blood30, it is unclear whether HHV-6B exhibits similar ability to enhance HIV replication in vivo.
In spite of the associations of HHV-6 with various diseases10–17, there have been only a small number of studies of potential anti-HHV-6 compounds 19, 21, 31–34. Our study evaluated H2G and AZT-lipid-PFA and demonstrated that at the non-toxic concentration of 5 μM of H2G exhibited inhibitory effect on de novo gene transcription by HHV-6B, but the same concentration of AZT-lipid-PFA was less effective. Similar to other anti-herpetic compounds, such as acyclovir (ACV), and ganciclovir (GCV), H2G is a guanosine analog that requires the viral thymidine kinase for phosphorylation. However, since HHV-6 lacks a viral thymidine kinase, the observed efficacy suggests that H2G may be phosphorylated by cellular enzymes21. Alternatively, the HHV-6 U69 gene, which encodes a phosphotransferase and is the homolog of the CMV UL97 kinase, may activate H2G19.
In contrast to the dose-dependent cytotoxicity observed with AZT-lipid-PFA-treated SupT1 cells (Figure 1), the same trend was not observed with HHV-6B infected PBMC. This difference could be related to culture conditions where PBMC were activated with PMA and ionomycin in the presence of IL-2, but mitogenic simulations were not used for SupT1 cells. In addition, H2G and AZT-lipid-PFA also inhibited viral replication in SupT1 cells (data not shown); however, the efficiency was less robust compared to HHV-6B infection in PBMC. Although the 1 log reduction in viral transcript observed with H2G in PBMC from 2 independent donors suggest its potential for controlling HHV-6B replication, the overall reduction was rather modest. In this study, only short term effects relating to anti-viral treatments were examined. Experiments that investigate the effects over time will further shed light on the pharmacokinetics of these compounds. The strong inhibition of HHV-6B viral expression by AZT-lipid-PFA may have potential implications as a therapeutic for HIV and HHV-6 co-infections.
A major challenge with many anti-viral compounds is drug-associated cytotoxicity. We showed that H2G treatment produced minimal effects on cell viability or proliferation. AZT-lipid-PFA exhibited significant cytotoxicity at concentration as low as 5 μM. However, the overall toxicity as determined by Annexin and PI staining was low and may be attributed to the short duration of the experiment. While PFA was found to be less toxic in vitro compared to other viral compounds such as ganciclovir and cidofovir36,while cell toxicity associated with AZT is well documented24. However, since only low concentration of AZT-lipid-PFA was required for effective inhibition of HHV-6 viral replication, the drug-associated toxicity should be minimal. As previously described, the half maximal inhibitory doses (IC50) of AZT-lipid-PFA against highly acyclovir (ACV)-resistant HSV isolates was determined by a plaque reduction assay ranged between 1.87 and 4.59 μM23. H2G, while less effective at decreasing viral gene expression, was associated with minimal toxicity even at high concentrations. The broad-spectrum of anti-viral activity20–22 and the relative non-toxic nature of H2G make it suitable for long-term administration.
Because HHV-6 infection and reactivation are linked to a variety of neurologic diseases12, the ability of anti-HHV-6 compounds to cross the blood brain barrier is important. In a normal rodent model, H2G concentrations detectable in the CNS were equivalent to approximately 10–20% of concentrations achieved in blood and muscle37. The achievable concentration may therefore be higher in a diseased model where the blood brain barrier is compromised. AZT is used to treat HIV-associated neurologic complications and it crosses the blood brain barrier at a modest level38, but the ability of AZT-lipid-PFA to penetrate the brain barrier in vivo remains to be determined.
Our results indicate that H2G and AZT-lipid-PFA inhibit HHV-6B in vitro. Experiments that evaluate the bioavailability of these compounds in vivo using animal models, or in clinical trials, and measure their ability to interfere with viral infection or reactivation,, are required to determine the benefit of these anti-viral compounds for use in clinical settings.
Acknowledgments
We thank Dr. Herbert Schott for critical reading of the manuscript and Epiphany Bioscience for providing the (R)-9-[4-hydroxy-2- (hydroxymethyl) butyl] guanine (H2G) compound. This research was supported in part by the Intramural Research Program of the NIH: National Institute of Neurological Disorders and Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prober C. Sixth disease and the ubiquity of human herpesviruses. N Engl J Med. 2005;352:753–755. doi: 10.1056/NEJMp048302. [DOI] [PubMed] [Google Scholar]
- 2.Dominguez G, Dambaugh TR, Stamey FR, et al. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J Virol. 1999;73:8040–8052. doi: 10.1128/jvi.73.10.8040-8052.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217–245. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Bolle L, Van Loon J, De Clercq E, Naesens L. Quantitative analysis of human herpesvirus 6 cell tropism. J Med Virol. 2005;75:76–85. doi: 10.1002/jmv.20240. [DOI] [PubMed] [Google Scholar]
- 5.Dewhurst S, Skrincosky D, van Loon N. Human herpesvirus 6. Expert Rev Mol Med. 1997;1997:1–17. doi: 10.1017/S146239949700001X. [DOI] [PubMed] [Google Scholar]
- 6.Yamanishi K, Okuno T, Shiraki K, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;1:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 7.Birnbaum T, Padovan CS, Sporer B, et al. Severe meningoencephalitis caused by human herpesvirus 6 type B in an immunocompetent woman treated with ganciclovir. Clin Infect Dis. 2005;40:887–889. doi: 10.1086/427943. [DOI] [PubMed] [Google Scholar]
- 8.Tavakoli NP, Nattanmai S, Hull R, et al. Detection and typing of human herpes virus 6 by molecular methods, in specimens from patients diagnosed with encephalitis/meningitis. J Clin Microbiol. 2007 doi: 10.1128/JCM.01692-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford JR, Kadom N, Santi MR, et al. Human herpesvirus 6 rhombencephalitis in immunocompetent children. J Child Neurol. 2007;22:1260–1268. doi: 10.1177/0883073807307086. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Lafuente R, De Las Heras V, Bartolome M, et al. Human herpesvirus 6 and multiple sclerosis: a one-year follow-up study. Brain Pathol. 2006;16:20–27. doi: 10.1111/j.1750-3639.2006.tb00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cermelli C, Berti R, Soldan SS, et al. High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J Infect Dis. 2003;187:1377–1387. doi: 10.1086/368166. [DOI] [PubMed] [Google Scholar]
- 12.Opsahl ML, Kennedy PG. Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain. 2005;128:516–527. doi: 10.1093/brain/awh390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soldan SS, Berti R, Salem N, et al. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997;3:1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- 14.Fotheringham J, Akhyani N, Vortmeyer A, et al. Detection of active human herpesvirus-6 infection in the brain: correlation with polymerase chain reaction detection in cerebrospinal fluid. J Infect Dis. 2007;195:450–454. doi: 10.1086/510757. [DOI] [PubMed] [Google Scholar]
- 15.Fotheringham J, Donati D, Akhyani N, et al. Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med. 2007;4:e180. doi: 10.1371/journal.pmed.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seeley WW, Marty FM, Holmes TM, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2007;69:156–165. doi: 10.1212/01.wnl.0000265591.10200.d7. [DOI] [PubMed] [Google Scholar]
- 17.Zerr DM, Corey L, Kim HW, et al. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:932–940. doi: 10.1086/428060. [DOI] [PubMed] [Google Scholar]
- 18.Lusso P, Crowley RW, Malnati MS, et al. Human herpesvirus 6A accelerates AIDS progression in macaques. Proc Natl Acad Sci U S A. 2007;104:5067–5072. doi: 10.1073/pnas.0700929104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Clercq E, Naesens L, De Bolle L, et al. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev Med Virol. 2001;11:381–395. doi: 10.1002/rmv.336. [DOI] [PubMed] [Google Scholar]
- 20.Abele G, Karlstrom A, Harmenberg J, et al. Inhibiting effect of (RS)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine on varicella-zoster virus replication in cell culture. Antimicrob Agents Chemother. 1987;31:76–80. doi: 10.1128/aac.31.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akesson-Johansson A, Harmenberg J, Wahren B, Linde A. Inhibition of human herpesvirus 6 replication by 9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine (2HM-HBG) and other antiviral compounds. Antimicrob Agents Chemother. 1990;34:2417–2419. doi: 10.1128/aac.34.12.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowe DM, Alderton WK, Ellis MR, et al. Mode of action of (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine against herpesviruses. Antimicrob Agents Chemother. 1995;39:1802–1808. doi: 10.1128/aac.39.8.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schott H, Hamprecht K, Schott S, et al. Synthesis and in vitro activities of a new antiviral duplex drug linking Zidovudine (AZT) and Foscarnet (PFA) via an octadecylglycerol residue. Bioorg Med Chem. 2008 doi: 10.1016/j.bmc.2008.10.081. [DOI] [PubMed] [Google Scholar]
- 24.D’Andrea G, Brisdelli F, Bozzi A. AZT: an old drug with new perspectives. Curr Clin Pharmacol. 2008;3:20–37. doi: 10.2174/157488408783329913. [DOI] [PubMed] [Google Scholar]
- 25.Eriksson BF, Schinazi RF. Combinations of 3′-azido-3′-deoxythymidine (zidovudine) and phosphonoformate (foscarnet) against human immunodeficiency virus type 1 and cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1989;33:663–669. doi: 10.1128/aac.33.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snoeck R, Andrei G, Schols D, et al. Activity of different antiviral drug combinations against human cytomegalovirus replication in vitro. Eur J Clin Microbiol Infect Dis. 1992;11:1144–1155. doi: 10.1007/BF01961133. [DOI] [PubMed] [Google Scholar]
- 27.Nitsche A, Muller CW, Radonic A, et al. Human herpesvirus 6A DNA Is detected frequently in plasma but rarely in peripheral blood leukocytes of patients after bone marrow transplantation. J Infect Dis. 2001;183:130–133. doi: 10.1086/317651. [DOI] [PubMed] [Google Scholar]
- 28.Wilborn F, Schmidt CA, Zimmermann R, et al. Detection of herpesvirus type 6 by polymerase chain reaction in blood donors: random tests and prospective longitudinal studies. Br J Haematol. 1994;88:187–192. doi: 10.1111/j.1365-2141.1994.tb04995.x. [DOI] [PubMed] [Google Scholar]
- 29.Zerr DM. Human herpesvirus 6 and central nervous system disease in hematopoietic cell transplantation. J Clin Virol. 2006;37(Suppl 1):S52–56. doi: 10.1016/S1386-6532(06)70012-9. [DOI] [PubMed] [Google Scholar]
- 30.D’Agaro P, Burgnich P, Comar M, et al. HHV-6 is frequently detected in dried cord blood spots from babies born to HIV-positive mothers. Curr HIV Res. 2008;6:441–446. doi: 10.2174/157016208785861122. [DOI] [PubMed] [Google Scholar]
- 31.Naesens L, Bonnafous P, Agut H, De Clercq E. Antiviral activity of diverse classes of broad-acting agents and natural compounds in HHV-6-infected lymphoblasts. J Clin Virol. 2006;37(Suppl 1):S69–75. doi: 10.1016/S1386-6532(06)70015-4. [DOI] [PubMed] [Google Scholar]
- 32.Naesens L, Stephens CE, Andrei G, et al. Antiviral properties of new arylsulfone derivatives with activity against human betaherpesviruses. Antiviral Res. 2006;72:60–67. doi: 10.1016/j.antiviral.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 33.De Bolle L, Andrei G, Snoeck R, et al. Potent, selective and cell-mediated inhibition of human herpesvirus 6 at an early stage of viral replication by the non-nucleoside compound CMV423. Biochem Pharmacol. 2004;67:325–336. doi: 10.1016/j.bcp.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 34.De Bolle L, Manichanh C, Agut H, et al. Human herpesvirus 6 DNA polymerase: enzymatic parameters, sensitivity to ganciclovir and determination of the role of the A961V mutation in HHV-6 ganciclovir resistance. Antiviral Res. 2004;64:17–25. doi: 10.1016/j.antiviral.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Torres-Madriz G, Boucher HW. Immunocompromised hosts: perspectives in the treatment and prophylaxis of cytomegalovirus disease in solid-organ transplant recipients. Clin Infect Dis. 2008;47:702–711. doi: 10.1086/590934. [DOI] [PubMed] [Google Scholar]
- 36.Akhyani N, Fotheringham J, Yao K, et al. Efficacy of antiviral compounds in human herpesvirus-6-infected glial cells. J Neurovirol. 2006;12:284–293. doi: 10.1080/13550280600880772. [DOI] [PubMed] [Google Scholar]
- 37.Stahle L, Oberg B. Pharmacokinetics and distribution over the blood brain barrier of two acyclic guanosine analogs in rats, studied by microdialysis. Antimicrob Agents Chemother. 1992;36:339–342. doi: 10.1128/aac.36.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meier C, Aubertin AM, de Monte M, et al. Synthesis and antiviral evaluation of SATE-foscarnet prodrugs and new foscarnet-AZT conjugates. Antivir Chem Chemother. 1998;9:41–52. doi: 10.1177/095632029800900105. [DOI] [PubMed] [Google Scholar]