Short abstract
Radiological and nuclear medicine examinations confer a definite (albeit low) long term risk of cancer, but patients undergoing such examinations often receive no or inaccurate information about these risks. Picano argues that this disregard of patient autonomy is no longer acceptable and suggests a practicable way of communicating risk
O voi ch'avete li `ntelletti sani, mirate la dottrina che s'asconde sotto `l velame de li versi strani [O You possessed of sturdy intellects, observe the teaching that is hidden here Beneath the veil of verses so obscure]
Dante, Inferno, Canto IX, 61-64
Shared decision making between patients and doctors is at the basis of modern medicine. One of the three fundamental principles of the “charter of medical professionalism” in the new millennium is the principle of patient autonomy: “Physicians must empower their patients to make informed decisions about their treatment.”1 The need to obtain free and informed consent is suggested by European and international texts.2,3 How are these principles translated into clinical practice involving radiological and nuclear medicine examinations?
Communicating radiological risk
Every radiological or nuclear medicine examination involves the administration of radiation, with its inherent risk. Life is a risky business, however, and any discussion of risk is complicated by people's tendency to underestimate large risks (such as the risk of dying from smoking tobacco), overestimate small risks (such as that of being struck by lightning), and be more willing to accept higher risks in situations where they think (usually wrongly) that they are in control (such as driving a car rather than being a passenger in an aeroplane).4 A risk of death of one in one million is generally ignored, since we face many risks of such magnitude every day, from travelling 100 miles by car or 1000 miles by aeroplane.4 It is less easy to ignore a risk of death of about one in 1000, which happens to be the long term risk of fatal cancer associated with a helical computed tomogram in a child5 or a thallium scintigraphy scan in an adult.6 Indeed, in 1983 the UK Royal Society stated that “a risk of one in one thousand is not totally unacceptable if: a), the individual knew the risk; b), received some commensurable benefit; and c), understood that everything reasonable had been done to reduce the risk.”7
In currently used informed consent forms there are three basic philosophies of risk communication—no mention of risk, understatement of risks, and specific detailing of risks.
Strategy 1: “Don't say a word”
One philosophy is not to mention radiological risk. Even for procedures with high radiation dose, such as interventions under fluoroscopic control, there is no explicit or implicit mention of long term risks. The risk exists and may be substantial, but it remains unheard (by the patient) and unspoken (by the doctor). The basic argument is that radiologists are too busy to lose time in obtaining informed consent and too wise to undertake inappropriate examinations.8 Patients' legal right to information is eclipsed by the two forces of efficiency and a paternalistic, “expert knows best” vision of individual autonomy. The long term nature of the risk, not its absolute amount, seems to be the excuse for overlooking the issue of informed consent.
Strategy 2: Understatement
In other aspects of radiological practice—such as in nuclear medicine—obtaining written informed consent is part of standard practice. In this case, the issue of efficiency bias is not raised: a patient must give informed consent before radionuclide is injected. But what is the quality of the information given to patients?
On the websites of scientific societies, in the information section for patients and in the informed consent forms to be signed by patients, we read statements such as “A nuclear medicine examination is safe, with an irradiation corresponding to a simple radiograph” or “almost always less than a common radiological examination”.9 Both patients and clinicians might believe that a “common radiological examination” or “a simple radiograph” would be a chest x ray, which is by far the simplest and commonest radiological examination, with 600 million examinations a year worldwide out of a total of two billion imaging examinations.10 In reality, however, the dose exposure ranges from 50 chest x rays for a thyroid scintigraphy to 4000 chest x rays for a cortical adrenal gland scintigraphy.
Such imprecise statements are probably intended to reassure patients, to avoid useless concern about an unavoidable risk. However, this attitude of “one consent fits all” for radiological examinations may mislead clinicians to underestimate the associated risks.
Strategy 3: Full disclosure
Some organisations, such as the US National Institutes of Health, describe radiological risk in more straightforward terms, at least when the test is performed within a research project and with a radiation dose greater than 15 millisieverts (corresponding to about two thirds of the dose from a thallium scintigraphy): “Your scan in Nuclear Medicine involves exposure to radiation. Although it can vary from person to person, your whole body radiation exposure during each scan will be about 15 millisieverts. This is about five times the average annual radiation exposure a person in the United States receives from natural background radiation. Although no harmful effects are expected, your long term risks of harm from this degree of radiation exposure might be as high as 1 in 1000. Harmful effects could include the development of cancer and genetic changes.”11
There is no doubt about the different ethical basis when participants are irradiated for purely research purposes, with no prospect of personal benefit, compared with diagnostic tests for patients (screening has a broadly intermediate ethical position). This is clearly a justification for a more explicit approach to obtaining informed consent. The standard of risk communication already adopted for irradiation in research might be fruitfully followed for irradiation in clinical practice.
Communicating risk
The language of radiation protection is not readily understood by non-specialists, and it is easy to get lost in a lexicon that expresses radiation doses in megabecquerels, millicuries, millirems, milliamperes, microsieverts, and “source-related dose constraints,” and risks as nominal probability coefficients for stochastic effects. The hapless prescribing (and practising) physician who wants to know about radiation risk enters a Tower of Babel, where essential information is “hidden beneath the veil of verses so obscure.”
As the best selling author Michael Crichton wrote when he was a young graduate from Harvard Medical School, “Medical writing is a highly skilled, calculated attempt to confuse the reader.”12 Unfortunately, a side effect of this confusion is that physicians become disoriented and eventually ignore the risks of what they are doing. The real issue is not that physicians do not communicate risks to patients, but rather that physicians do not communicate with other physicians, not even with themselves. They do not communicate radiological risks for the good reason that they are ignorant of risks.13 This may help to explain why 30% of tests involving ionising radiation are inappropriate—that is, patients take a long term risk without a commensurate acute benefit.5
How to wake up from this communication nightmare? One suggestion is that doctors should communicate risk through equivalents of ordinary life activities such as driving a car on the highway or smoking cigarettes.14 For example, a chest computed tomogram corresponds to about 400 chest x rays, implying a risk similar to that of having a car crash during 4000 km of highway driving or of smoking 700 cigarettes. Here, we have a paradox: in Europe, when you buy a cigarette pack you are faced with a large, bold, and funereal black notice stating that “Smoking severely damages your health” or “You can die from smoking”; then you have a thallium scan, and no one minds telling you that the risk corresponds to smoking 1400 cigarettes.14
Expressing a radiological dose as multiples of a chest x ray might be an even simpler means of communicating risk. This method has been suggested by the UK college of radiologists and has been endorsed in the European Commission's guidelines on imaging.15 The “dose unit” is familiar to both doctors and to patients, and it helps to express, in a straightforward fashion, the concept that the higher the radiation dose, the higher the long term risk of cancer.
A graphical presentation of radiological risk
The current lack of regulation for informed consent clashes with the accepted legal and ethical standards for giving patients information, shared decision making, and risk communication. Assessing radiological risk is certainly complicated, but some key information could (and probably should) be shared between patients and doctors.
For the purposes of radiation protection, the dose-response curve for radiation induced cancer is assumed to be linear at low doses, with no minimum threshold. The attributable lifetime risk of radiation induced cancer for all members of the population can be calculated by multiplying the radiation dose from 50 chest x rays (1 millisievert) by the fatal cancer risk coefficient of 5×10-5 according to the best available estimates from the International Commission on Radiological Protection.16
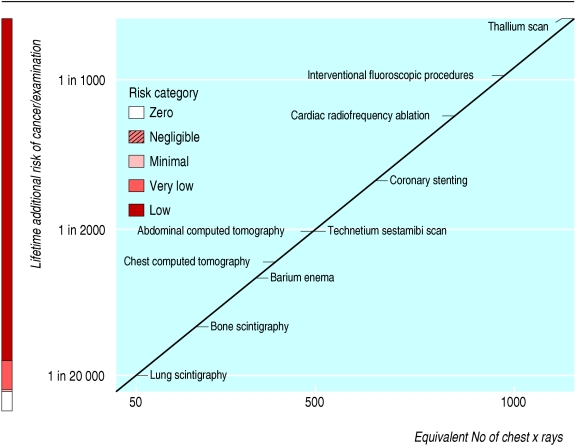
The figure on page 849 summarises the radiation dose and risk associated with some common radiological and nuclear medicine examinations. The dose of 50 chest x rays (for example, a lung scintigraphy) corresponds to an extra risk of cancer of about 1 in 20 000 exposed patients. The dose of 500 chest x rays (such as a technetium sestamibi scan) corresponds to an extra risk of about 1 in 2000 exposed patients. The dose of 1000 chest x rays (slightly less than that associated with a thallium scan) corresponds to an extra risk of about 1 in 1000 exposed patients. This graphic format might be useful for passing information from doctors to patients and between doctors. The basic information is the same as that suggested by the International Commission of Radiological Protection for communicating risk to patients,16 but our figure format has some potential advantages. Firstly, it shows risk as a continuum rather than in the traditional risk categories, which allows for greater differentiation of levels of risk: in radiology the “low risk” category encompasses both a chest computed tomogram (corresponding to 400 chest x rays) and an angiographic embolisation procedure (which may exceed the dose equivalent of 5000 chest x rays). Secondly, the figure format is more easily understood than the traditional table format: “images are like cannonballs, penetrate deeply into the mind of the reader and stay there,” in the words of communication guru Dale Carnegie. Thirdly, the colour coding helps readers to understand risk levels, as it does for total fatal cardiovascular risk in the SCORE risk prepared by the European Society of Cardiology, which offers a risk estimation system suitable to the constraints of clinical practice. Fourthly, use of the figure when obtaining patients' informed consent would allow use of a standard consent form but also provide a test-specific statement of dose and risk.
Figure 1.
Graphical presentation of cancer risk and radiation dose (in multiples of dose from a simple chest x ray) for some common radiological examinations
A proposed solution
The principle of patient autonomy in current radiological practice might be reinforced by making it mandatory to obtain informed consent for all “red code” examinations (that is, those with an associated risk of 1 in 10 000 or higher), with the consent form possibly incorporating a graphical portrayal of risk. The proposed graph underlines the linear relation between dose and risk. Obviously, this could be updated as new examination techniques become available and dose sparing techniques for current examinations are introduced.5 Regulatory bodies and radioprotection societies might take responsibility for disseminating the graph to prescribers, practitioners, and patients.
Hopefully, this simple, evidence based communication strategy, if used when obtaining informed consent, will increase the currently suboptimal level of radiological awareness among doctors and patients. Better knowledge of such risks will help us to avoid small individual risks translating into substantial population risks.17,18,19 Radiological awareness is essential to help doctors in the difficult task of balancing what is good for the individual patient against what is acceptable for society.20 Radiological protection should come to mean, not just another form to fill in, but a way of thinking, so that long term risk is familiar to doctors and patients and can be appropriately balanced against acute diagnostic benefits.
Summary points
Informed consent for radiological examinations is often not sought, and even when it is, patients are often not fully informed, even for considerable levels of radiation exposure and long term risk
Such practices disregard patients' rights and violate basic principles of modern medical practice
Risk might easily be communicated for each examination by reporting the dose in multiples of the dose from a chest x ray and the risk of cancer as number of extra cases in the exposed population
Communication of dose should be mandatory for the examinations with higher risk
Better knowledge of risks will help us to avoid small individual risks translating into substantial population risks
Contributors and sources: EP's views are based on 25 years of clinical and scientific work at the Institute of Clinical Physiology of the National Research Council in Pisa, mainly in cardiac ultrasonography. EP is also scientific director of cardiology in Clinica Cardiologica Montevergine, Mercogliano, Avellino, which has the greatest volume of invasive cardiology procedures in Italy.
Competing interests: None declared.
References
- 1.Medical professionalism in the new millennium: a physician charter. JAMA 2002;136: 243-6. [DOI] [PubMed] [Google Scholar]
- 2.European Parliament. The charter of fundamental rights of the European Union. www.europarl.eu.int/charter/default_en.htm (accessed 21 May 2004).
- 3.World Medical Association. Declaration of Helsinki. www.wma.net/e/policy/b3.htm (accessed 21 May 2004).
- 4.Meara J. Getting the message across: is communicating risk to the public worth it? J Radiol Prot 2002;22: 79-85. [DOI] [PubMed] [Google Scholar]
- 5.Hall EJ. Lessons we have learned from our children: cancer risks from diagnostic radiology. Pediatr Radiol 2002;32: 700-6. [DOI] [PubMed] [Google Scholar]
- 6.Overbeek FJ, Pauwels EK, Bloem JL, Camps JA, Geleijns J, Broerse JJ. Somatic effects in nuclear medicine and radiology. Appl Radiat Isot 1999;50: 63-72. [DOI] [PubMed] [Google Scholar]
- 7.Lawton MP. Legal aspects of iatrogenic disorders: discussion paper. J R Soc Med 1983;76: 289-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewars M. Sustainability of medical imaging. To obtain informed consent from everyone is impossible [letter]. BMJ 2004;328: 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SFBMN. Societé Française de Biophysique et Médecine Nucléaire. Informations du public. www.sfbmn.org/pub/index.htm (accessed 4 Sep 2004).
- 10.United Nations Scientific Committee on the Sources and Effects of Ionising Radiation. Report on the effects of atomic radiation to the General Assembly, 2000. Medical radiation exposures. New York, NY: United Nations, 2001.
- 11.Office for Protection from Research Risks, Department of Health and Human Services. Code of federal regulations. Title 45: public welfare. Part 46—protection of human subjects. Bethesda, MD: National Institutes of Health, 1991. www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm (accessed 30 Sep 2004).
- 12.Crichton M. Sounding board: Medical obfuscation: structure and function. N Engl J Med 1975;293: 1257-9. [DOI] [PubMed] [Google Scholar]
- 13.Shiralkar S, Rennie A, Snow M, Galland RB, Lewis MH, Gower-Thomas K. Doctors' knowledge of radiation exposure: questionnaire study. BMJ 2003;327: 371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamenhof R. Risk list. JPNM Physics, 1997. www.med.harvard.edu/JPNM/physics/safety/risks/risklist.html (accessed 1 Sep 2004).
- 15.European Commission. Radiation protection 118: referral guidelines for imaging. Luxembourg: Office for Official Publications of the European Communities, 2001. http://europa.eu.int/comm/environment/radprot/118/rp-118-en.pdf (acessed 30 Sep 2004).
- 16.International Commission on Radiological Protection. Radiation and your patient: a guide for medical practitioners. 2001. www.icrp.org/docs/Rad_for_GP_for_web.pdf (accessed 4 Sep 2004).
- 17.Gofman JW. Radiation-induced cancer from low-dose exposure: an independent analysis. San Francisco, CA: Committee for Nuclear Responsibility, 1990.
- 18.Berrington de Gonzalez A, Darby S. Risk of cancer from diagnostic x-rays: estimates for the UK and 14 other countries. Lancet 2004;363: 345-51. [DOI] [PubMed] [Google Scholar]
- 19.Picano E. Risk of cancer from diagnostic x-rays [letter]. Lancet 2004;363: 1909-10. [DOI] [PubMed] [Google Scholar]
- 20.Picano E. Sustainability of medical imaging. BMJ 2004;328: 578-80. [DOI] [PMC free article] [PubMed] [Google Scholar]