Abstract
This study systematically reviews the literature on the occurrence, incidence and case fatality rate (CFR) of invasive nontyphoidal Salmonella (iNTS) disease in Africa from 1966 to 2014. Data on the burden of iNTS disease in Africa are sparse and generally have not been aggregated, making it difficult to describe the epidemiology that is needed to inform the development and implementation of effective prevention and control policies. This study involved a comprehensive search of PubMed and Embase databases. It documents the geographical spread of iNTS disease over time in Africa, and describes its reported incidence, risk factors and CFR. We found that Nontyphoidal Salmonella (NTS) have been reported as a cause of bacteraemia in 33 out of 54 African countries, spanning the five geographical regions of Africa, and especially in sub-Saharan Africa since 1966. Our review indicates that NTS have been responsible for up to 39% of community acquired blood stream infections in sub-Saharan Africa with an average CFR of 19%. Salmonella Typhimurium and Enteritidis are the major serovars implicated and together have been responsible for 91%% of the cases of iNTS disease, (where serotype was determined), reported in Africa. The study confirms that iNTS disease is more prevalent amongst Human Immunodeficiency Virus (HIV)-infected individuals, infants, and young children with malaria, anaemia and malnutrition. In conclusion, iNTS disease is a substantial cause of community-acquired bacteraemia in Africa. Given the high morbidity and mortality of iNTS disease in Africa, it is important to develop effective prevention and control strategies including vaccination.
Author Summary
Although Salmonella are a common global cause of mild gastro-intestinal illness that usually presents with self-limiting diarrhoea, the invasive nontyphoidal form of Salmonella disease manifests as bacteraemia which often presents only with fever. If left untreated, iNTS disease often results in death. iNTS disease is more common amongst people with impaired immunity, and in developing countries particularly in Africa. Data on the epidemiology of iNTS disease in Africa are sparse, making it difficult to estimate the true disease burden. This study aggregates all published cases of iNTS disease in Africa from 1 Jan 1966 up to 31 Dec 2014 and shows the relative importance of NTS as a cause of community acquired bacteraemia and deaths in Africa. The findings are important in raising awareness of iNTS disease and driving research to help develop effective control and preventive strategies to limit the disease. Important interventions needed for iNTS disease include rapid diagnostic tests and vaccines, as well as measures to improve hygiene.
Introduction
Nontyphoidal Salmonellae (NTS) are a major cause of food borne infections throughout the developed and developing world [1]. Although infection most often results in self-limited acute gastroenteritis, NTS have been identified as a major cause of invasive bacterial infections in infants and young children in sub-Saharan Africa and HIV-infected individuals of all ages [2,3]. Invasive NTS (iNTS) disease is recognized as a problem in developed countries in young infants, the elderly and immunocompromised [4]. iNTS disease is caused mainly by Salmonella enterica serovars Typhimurium and Enteritidis [5,6].
NTS gastroenteritis is generally understood to be acquired from animal reservoirs, unlike Salmonella Typhi and Salmonella Paratyphi, where the only recognized reservoir is man. Transmission of gastroenteritis-causing NTS to humans can occur by many routes, including consumption of animal food products, especially eggs, poultry, undercooked meat, produce contaminated with animal waste, contact with animals or their environment, and contaminated water [7,8].The African strains responsible for iNTS disease are characterized by genome degradation and appear to be increasingly adapted to an invasive lifestyle [9]. The relative role of animal reservoirs and human to human transmission of strains causing iNTS disease is unclear [10,11]. iNTS disease is diagnosed definitively by blood or bone marrow culture, usually with low sensitivity [12]. It is also impossible to diagnose using clinical symptoms alone due to the lack of pathognomonic features [5,6]. There is currently no commercially-available rapid diagnostic test for iNTS disease.
Bacteraemia is an important cause of severe and often fatal disease globally, especially in developing countries (where it substantially contributes to childhood deaths) [2,13]. Invasive forms of Salmonella disease include enteric fevers (typhoid and paratyphoid fevers) and NTS bacteraemia and are important causes of morbidity and mortality in Asia and Africa [3,14,15]. In a review article of bacteraemia in Africa from 2010, NTS was found to be responsible for 29.1% of all bloodstream infections [16]. Nevertheless, the data on NTS bacteraemia in Africa are limited with no current aggregate data, so it has been difficult to estimate the burden of NTS bacteraemia in Africa. Such information is vital for planning and implementing cost-effective solutions [17] to tackle iNTS disease, especially in Africa with its fragile health-care systems. Infectious diseases are a major obstacle to human development in the African region with people suffering from an extensive range of potentially preventable and treatable conditions including invasive Salmonella disease. Adequate evidence on the burden of Salmonella infections would enable health policy makers to make informed decisions on the need for vaccines against Salmonella infections in their country and region.
The aim of the present study is to address this knowledge gap by conducting a review of the available reports on NTS bacteraemia in Africa and to describe the epidemiology of the disease; in order to inform effective prevention and control strategies, including vaccination. Specifically, the study describes all the geographical locations in Africa that have reported iNTS disease cases, and determines incidence and proportion of bacteraemia caused by NTS (by whole population and patient groups), together with risk factors and CFR of NTS bacteraemia.
Methods
Search strategy
We searched PubMed (including MEDLINE) and Embase. The search string used was: (algeria OR angola OR benin OR botswana OR “burkina faso” OR burundi OR cameroon OR “cape verde” OR “central african republic” OR chad OR comoros OR “ivory coast” OR “cote d ivoire” OR congo OR djibouti OR egypt OR “equatorial guinea” OR eritrea OR ethiopia OR gabon OR gambia OR ghana OR guinea OR “guinea bissau” OR kenya OR lesotho OR liberia OR libya OR madagascar OR malawi OR mali OR mauritania OR mauritius OR morocco OR mozambique OR namibia OR niger OR nigeria OR rhodesia OR rwanda OR “sao tome” OR senegal OR seychelles OR “sierra leone” OR somalia OR “south africa” OR sudan OR swaziland OR tanzania OR togo OR tunisia OR uganda OR “western sahara” OR zambia OR zimbabwe OR Africa) AND (fever OR fevers OR bacteremia OR bacteremias OR bacteremic OR bacteraemia OR bacteraemias OR bacteraemic OR septicemia OR septicemias OR septicemic OR septicaemia OR septicaemias OR septicaemic OR salmonella OR salmonellas OR salmonellae OR “blood stream infection” OR “blood stream infections” OR “blood stream pathogen” OR “blood stream pathogens” OR febrile) AND (infant* OR child* OR adolescent* OR adult* OR patient OR patients OR human OR travel* OR communit* OR village* OR participant* OR volunteer* OR subject* OR incidence OR hospital OR man).
We used the United Nations list of 54 African Sovereign states as the basis for searching, modified by including Rhodesia (name had changed during the search period) and both the English and French names for Ivory Coast. Initial tests showed that “Congo” found all references to the Democratic Republic of the Congo as well as Republic of the Congo. Similarly, “Sudan” found both Sudan and South Sudan. Although it is not recognized by the United Nations as a sovereign state, we also included “Western Sahara” in case there were reports from this area.
PubMed and Embase have, an English translation for all paper titles regardless of language, English key words (i.e. MESH terms in PubMed) and usually an English Abstract. These were the only fields searched in the initial searching for papers of any language and assumes that the English terms used in the search would be sufficient to identify papers in any language for the first pass.
Initial searches failed to find any papers prior to 1966, search results were limited to publications from 1st Jan 1966 up to 31st December, 2014. We imported the full texts/abstracts of the search result into Quosa Information Manager software (Quosa) [18] and a full text search was done through Quosa for articles containing the term “Salmonell*”.
To ensure a comprehensive search of the literature, especially for reports within the last five years, we did an independent search on Embase database using a similar search string and strategy as above (with limit publication dates from 2009 to 2014). Additional reports that were not retrieved from the PubMed search were obtained and reviewed for inclusion.
Selection criteria
The full text of the search results of online articles/abstracts were reviewed independently by the study authors (IVU, CAM and AS) with the aim of including articles that used blood culture to isolate NTS from humans in Africa Inconsistences between the papers included by the different authors were resolved by consensus. The full text version either obtained on line or ordered, when only the abstract was available on line, of potentially relevant articles was retrieved and reviewed critically using predetermined inclusion and exclusion criteria for the study. This was done for articles, regardless of the published language. The reference sections of retrieved full text articles were reviewed critically in search of further potential articles for inclusion.
Inclusion criteria
Studies were included if they:
Reported NTS isolated by blood culture
Were conducted in and recruited subjects from Africa;
Exclusion criteria
Studies were excluded if they:
Described NTS disease isolated by stool culture only without blood culture result
Did not specify serovar of Salmonella enterica isolated (for example, Salmonella Typhi or one of the serotypes causing NTS)
Validity assessment
Study validity was established by use of the selection criteria described above, thereby excluding studies that were thought likely to have results that are either inaccurate, not representative of the reported population or could otherwise not be compared with studies included in the analysis. Studies were not excluded on the basis of potential variability of microbiological techniques and identification of culture isolates.
Review of the selected literature
Data extraction and collection process
Relevant descriptive and quantitative variables were extracted from each of the selected article. A standardised template was used for the data extraction in the form of a Microsoft Excel 2013 workbook with each column of the database corresponding to one of the fields in the template. Double data extraction and entry was performed to ensure accuracy. The variables extracted from each article included name of journal, title of article, publication date, study location (including city, country and region in Africa), study period, patient age, and primary eligibility criteria). Quantitative data collected included number of potential study participants, subjects enrolled, participants who had blood cultured, significant pathogens isolated, NTS pathogens isolated; iNTS disease incidence (if stated), CFR of iNTS disease, and proportion of study participants with risk factors such as infection with malaria or human immunodeficiency virus (HIV) (if known). Where publications separately included data derived from more than one cohort of subjects (e.g. different age groups), each unique study was included as a separate entry in the table. The fields used are listed in S1 Table.
Data analysis
Data were cleaned and a descriptive analysis performed with the aid of Microsoft Excel 2013. All reported measures of disease frequency were directly drawn from the literature.
Duplicate reports of iNTS disease cases were sorted and removed from the final analysis or, where this was not possible, clearly identified as potentially overlapping (See supplementary S2 Table for listing of relevant reports). The sorting was done by grouping all reports according to country and then comparing variables including study dates, name and location of the study site, age group of subjects reported, number of blood cultures taken and number of NTS isolated across the studies from each country for potential overlaps.
Incidence data were extracted directly from studies where this was stated and summarized according to sub-group classification, subjects, location, study date, groups at risk and possible risk factors. Incidence was derived in a few studies by dividing the total number of NTS isolated by the population at risk per unit time (year) and stated as cases per 100,000 person year of observation or per 100,000 population per year.
Proportion of NTS as the cause of community acquired blood stream infection was calculated using the formula: number of NTS bacteraemic cases divided by total number of significant/pathogenic bacteraemia in the relevant studies. Relevant studies included prospective and retrospective hospital-based blood culture series for subjects presenting with fever with no known focus of infection or groups of subjects not selected for possible associated risk factors as anaemia, HIV, malaria and malnutrition.
CFR data were extracted directly from the studies where given. CFR was calculated using the formula: death amongst NTS bacteraemia cases divided by total iNTS disease cases multiplied by 100. The CFR data were then summarized according to subjects, location, study period and possible risk factors.
Summary data for risk factors associated with iNTS disease in Africa were extracted directly from the studies where available. Statistically-significant measures of association, such as odds ratio (OR), risk ratio (RR) and prevalence proportion were extracted from the studies.
Results
Search results
The online database search performed on PubMed (completed in February, 2015), using the search string and limiting the results from 1st January 1966 to 31st December 2014, yielded 16,638 articles. These were retrieved in Quosa and a full text search for the term ‘Salmonella’ using Quosa yielded a subset of 1,979 articles. The abstract and full text (where available) of the 1,979 articles obtained were reviewed manually for relevance based on our criteria. 177 articles were finally selected from PubMed and entered into the database (Fig 1). We found more publications using our search string with all the countries in Africa included by name, than with “Africa” alone, and by not restricting publications by using ‘human’ as a search term. The articles retrieved in Quosa were published in 16 languages of which French (216 articles) was the most common following English.
Fig 1. Strategy for selection of eligible articles.
Adapted from the PRISMA group 2009 flow diagram [203].
A similar search strategy was used on the Embase database with the initial search results limited to articles in Embase only, humans and the period 2009 to 2014, yielding 8034 articles (in February, 2015). 736 articles were obtained following a full text search for the term ‘Salmonella’ in Quosa. Thereafter, the full texts of these articles were reviewed manually and 13 additional unique articles not obtained from the initial PubMed search were added to the database (including 9 abstract-only articles).
One hundred and ninety articles [2,3,10,11,13,19–202] were obtained following the literature search and used for the descriptive analysis in our review (Fig 1). Following a further review of these articles, 14 (S2 Table) reporting cases or cohorts of iNTS disease already reported in another published article were excluded from the quantitative analysis, leaving 176 articles including 12 studies with possibly overlapping data (S2 Table). These 176 studies included 223 distinct subject cohorts.
The reports varied in their methodology. Three of the 176 articles were reports from ill subjects in a longitudinal, community-based surveillance. 159/176 (90%) of all articles retrieved are reports of isolates obtained from studies conducted on ill subjects presenting to a health facility setting (hospital or clinic). In fourteen of the reports, we could not determine the original basis for selecting the subjects (E.g., retrospective analysis of microbiology laboratory samples, follow up analyses of bacteraemia in patients previously selected for another study). However, most of these 14 were probably from ill subjects presenting to a health facility.
Only 9 (5%) were designed to derive an estimate of the population-based incidence of iNTS disease in Africa; 76 (43.2%) were prospective hospital-based studies with patients recruited during the course of the study; 22 (12.5%) were retrospective studies with analysis of existing hospital or laboratory records; and the remaining 69 (39.2%) are case reports, series, conference abstracts or outbreak reports.
A total of 18,931 isolates of NTS were reported in this review. These included 9,084 isolates of Salmonella enterica serovar Typhimurium (S. Typhimurium) (48%), 2,801 isolates of S. Enteritidis (15%), 1,215 other serovars (6%) and 5,831 ‘not further typed’ (31%).The category ‘other serovars’ includes, but is not limited to, less common serovars: 197 Infantis, 155 Wien, 88 Dublin, 71 Newport, 71 Bovis-morbificans, 92 Isangi, 24 Heidelberg, 8 Havana and 9 Ordonez [11,40,49,65,68,71,91,104,108]. Salmonella Typhimurium is therefore the most common serovar reported for iNTS disease in Africa and approximately three times (48% vs 15%) more common than S. Enteritidis.
Geographical location of sites reporting iNTS disease in Africa
iNTS disease was reported in 33 countries across the five geographical regions of Africa based on the United Nations classification (Fig 2). The majority, 53% (94/176) of the reports were from the Eastern Africa region (including 28 reports from Kenya, 24 from Malawi, 16 from Tanzania and 10 from Uganda); 26% (46/176) from the Western Africa region (including 9 reports from Cote d’Ivoire, 9 from Nigeria and 8 from Ghana); 10% (17/176) from Central Africa (including 11 reports from Democratic Republic of Congo, 3 from Gabon and 2 from Central African Republic); 7% (12/176) from Northern Africa (including 5 reports from Tunisia and 2 each from Morocco and Algeria); and 4% (7/176)from Southern Africa (South Africa) (S3 Table).
Fig 2. Map of Africa showing number of publications from countries reporting NTS blood culture isolates.
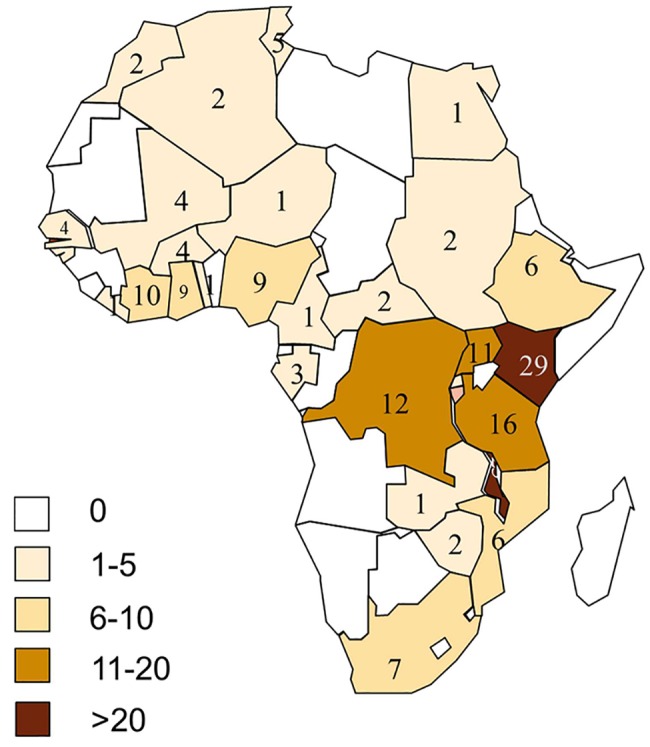
Regions are indicated using coloured boundaries based on United Nations classification
Details of the study population or study site setting could be extracted from only 113 of the 176 reports obtained. 50.4% (57/113) of the reports were from urban sites, 38.9% (44/113) from rural sites and 10.6% (12/113) from both urban and rural sites (S4 Table).
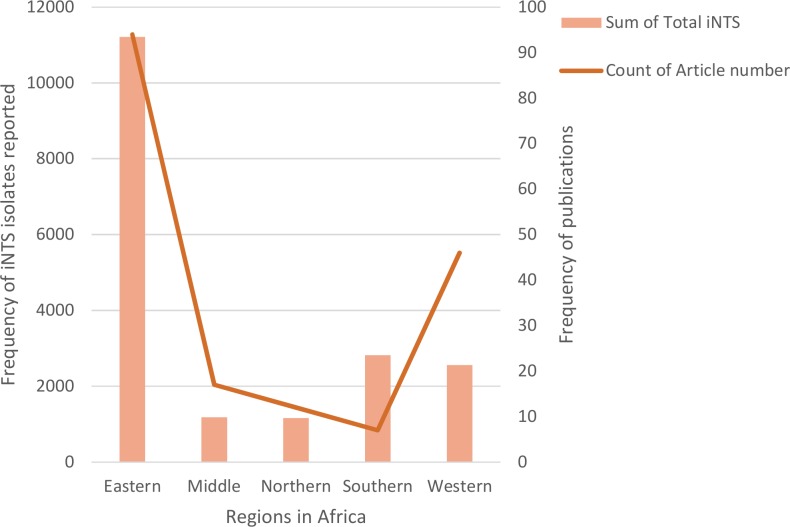
59.2% (11,211/18,931) of the total NTS isolates were reported from the Eastern Africa region (6,057 isolates from Malawi, 2,976 isolates from Kenya and 767 isolates from Uganda). 14.9% (2,819/18,931) and 13.5% (2,558/18,931) of isolates were from Southern (2,819 isolates from South Africa) and Western Africa (788 isolates from Mali, 371 from Cote d’Ivoire and 345 from Ghana) respectively. 6.2% (1,180/18,931) and 6.1% (1,163/18,931) of isolates were from Central (1,003 isolates from Democratic Republic of Congo, 138 from Gabon and 34 from Central African Republic) and Northern Africa (1,023 isolates from Algeria, 100 from Tunisia and 36 from Morocco) respectively (Fig 3).
Fig 3. Regional distribution of iNTS disease cases reported from Africa (1966 to 2014).
Graph shows total numbers of NTS isolates reported and numbers of publications in which cases were reported.
Reports based on year of publication
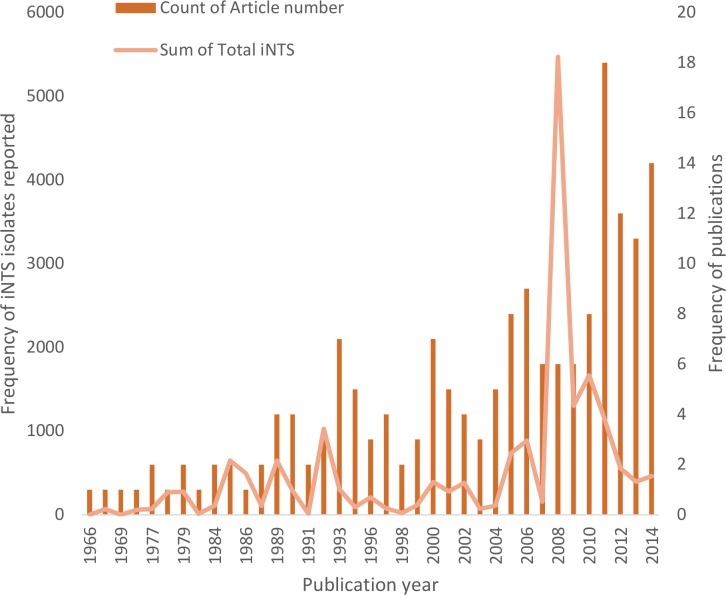
The earliest report of iNTS disease was published in 1966 [69] and the numbers of reports of iNTS disease increased with time to a peak of 18 reports in 2011 (Fig 4). 55.7% (98/176) of the reports obtained were published within the last decade (2005 to 2014), with 35.8% (63/176) were published in the last 5 years (2010 to 2014).
Fig 4. Published reports of iNTS disease in Africa by year of publication.
A similar trend can also be observed in the total number of NTS blood culture isolates with time. 67.5% (12770/18931) of the total isolates were reported within the last ten years (2005 to 2014), while 22.3% (4218/18931) were reported in the last 5 years (2010 to 2014).
Incidence of iNTS disease in Africa
Fourteen reports on the incidence of iNTS disease in Africa were obtained from eight countries–the Gambia, Ghana, Kenya, Malawi, Mozambique, South Africa, Tanzania & Uganda, spanning three regions, Eastern (ten studies), Southern (one) and Western (three) (Table 1). The incidence data were obtained using different methods of estimation and were from different population groups including specific age groups, HIV-infected patients and subjects with sickle cell disease. Overall, the estimated incidence of iNTS disease ranged from 1.4 per 100,000 population/year (in South African individuals of all ages in 2003 to 2004) to 2,520 per 100,000 population per year in children < 5 years of age from Ashanti, rural Ghana, in 2007 to 2009.
Table 1. Published reports of incidence of iNTS disease in Africa (1966 to 2014).
| Region | Ref no. | Study | Study place & time | iNTS Incidence/100,000 person years (C.I) | iNTS incidence in Sub group | Subjects | Study type | Age | Total Blood Cultures | Total bacter-aemia | Population | All iNTS | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eastern | [2] | Berkley et al, 2005 | Kilifi, rural Kenya; August 1998 to July 2002 | <1 yr old: 170; <2 yr old: 175; <5 yr old: 88 | All acute medical admissions | Hospital based, Prospective consecutive | <13 years | 16,570 | 1,094 | 189,148 (15% births in study hospital) | 166 | Estimated minimal incidence | |
| [122] | Mandomando et al, 2009 | Manhiça, rural Mozambique; May 2001 to April 2006 | S. Typhimurium: 78.6 (65.4–94.3); | <1 year: 240.4 (169.1–341.8); 1–4 years: 176.2 (141.5–219.3);≥ 5 years: 4.5 (1.7–12.1) | Febrile admissions | Prospective consecutive | <15 years | 19,896 | 1,550 | 140,000 | 263 | Minimal incidence | |
| Mandomando et al, 2009 | Manhiça, rural Mozambique; May 2001 to April 2006 | S. Enteritidis: 28.9 (21.2–38.8) | <1 year: 108.6 (64.3–183.3); 1–4 years: 59.5 (40.8–86.7); ≥ 5 years: 1.1 (0.16–8.1) | Febrile admissions | Prospective consecutive | <15 years | 19,896 | 1,550 | 140,000 | 101 | Minimal incidence | ||
| [129] | Mtove et al, 2011 | Muheza, rural Tanzania; June 2006 to May 2010 | 82 (No Confidence interval) | 2006–7: 82; 2007–8: 14; 2008–9: 17; 2009–10: 7 | Severely ill admissions | Hospital based prospective | 2 months to < 15 years | 6,836 | 684 | 123,613 (average) | 232 | Minimal incidence, pooled analysis | |
| [131] | Muyanja et al, 2011 | Entebbe, semi-urban Uganda; January 2000 to December 2008 | Pre-*ART1: 730 (290–1,830); Pre-ART2: 2,930 (1,910–4490); Interim: 1100 (600–2,020); ART1: 710 (370–1,360); ART2: 800 (100–540) | HIV-1 infected outpatients | Prospective cohort | ≥15 years | 246 | 2,540 | 66 | Periods (year) include: Pre-ART1 (2000–1); Pre-ART2 (2001–3); Interim (2003–5); ART1 (2005–7); ART2 (2007–8) | |||
| [160] | Sigauque et al, 2009 | Manhiça, rural Mozambique; May 2001 to April 2006 | 120 (103–139) | <1 year: 388 (294–512); 1-<5 years: 262 (219–314); >5 years: 6 (3–15) | Admissions with fever or malnutrition | Hospital based | <15 years | 19,896 | 1,550 | 140,000 (43% children <15 years) | 397 | Minimal incidence | |
| [164] | Tabu et al, 2012 | Asembo, rural Kenya; October 2006 to September 2009 | 580 (229–934) | 0–4 years: 2,085 (1,181–2990); 5–9 years: 389 (106–672); 10–17 years: 24 (0–62); 18–49 years: 367 (186–550); >50 years: 232 (112–351) | Febrile or Acute respiratory illness | Prospective cohort | ≤50 years | 3,578 | 155 | 25,000 | 60 | Extrapolated for patient samples not cultured or who visited another clinic (sampling) | |
| Tabu et al, 2012 | Kibera, urban Kenya; March 2007 to February 2009 | 57 (0–122) | 0–4 years: 260 (102–419); 5–9 years: 37 (1–73); 10–17 years: 0 (NA); 18–49 years: 11.2 (0–31); >50 years: 0 (NA) | Febrile or Acute respiratory illness | Prospective cohort | ≤50 years | 2,138 | 230 | 30,000 | 7 | age stratified years (1–4; 5–9; 10–17; 18–49; >50) S. Typhi more common | ||
| [174] | Williams et al, 2009 | Kilifi, rural Kenya; August 1998 to March 2008 | 1,580 (1,080–2,230) | 0–11 months: 360 (70–1050); 12–23 months: 1,080 (220–3,150);2–13 years: 1,430 (760–2,440) | All admissions with sickle cell anaemia | Case-control, retrospective | <14 years | 108 | 100,000 | 19 | Incidence in patients with Sickle cell anaemia | ||
| [123] | Mayanja et al, 2010 | Southwest Uganda; January 1996 to December 2007 | 855 (617–1223) | 13–24 yrs: 713 (328–1850); | Fever without detectable malaria | Population-based HIV clinical cohort | All ages | 703 | 159 | 20,000 | 42 | 37.7% HIV prevalence in cohort | |
| 25–34 yrs: 883 (533–15720); | |||||||||||||
| 35–44 yrs: 1395 (789–2708); | |||||||||||||
| 45+ yrs: 375 (142–1332). | |||||||||||||
| HIV negative: 37 (5–266); | |||||||||||||
| HIV, no ART:2070(1480–2980); | |||||||||||||
| HIV pos, ART:739 (237–3533) | |||||||||||||
| [76] | Feikin et al, 2013 | Asembo, Kenya; March 2007 to February 2010 | 1200 (570–1800) | Patients with severe acute respiratory illness | Hospital-based, prospective | < 5 years old | 747 | 24 | 14 | ||||
| [83] | Gordon et al, 2008 | Blantyre, Malawi; Jan 1998 to Dec 2004 | 164 | Febrile, clinical sepsis | Hospital based, prospective | All ages | 62,878 | 10,628 | 502053 | 4955 | Minimal incidence–derived from the data | ||
| Southern | [11] | Feasey et al, 2010 | South Africa; January 2003 to December 2004 | 1.4 | All | Active laboratory surveillance data | All ages | ND | ND | 46,000,000 | 1318 | Population based; Derived from data | |
| Western | [67] | Enwere et al, 2006 | Basse & Bansang, rural Gambia; August 2000 to April 2004 | Group A- 262 (190–354) | 2–5 months: 227 (74–530); 6–11 months: 407 (237–652); 12–17 months: 238 (114–438); 18–23 months: 233 (100–458); 24–29 months: 126 (26–369) | Vaccine trial participants suspected with bacteraemia | Part of vaccine trial | 2 to 29 months | 7369 | 295 | ND | 92 | Prospective cohort from the population |
| Enwere et al, 2006 | Basse & Bansang, rural Gambia; August 2000 to April 2004 | Group B- 300 (222–397) | 2–5 mo: 408 (187–775); 6–11 mo: 360 (201–594); 12–17 mo: 334 (183–561); 18–23 mo: 293 (140–539); 24–29 mo: 42 (1–236) | Vaccine trial participants | Part of vaccine trial | 2 to 29 months | 7369 | 295 | ND | 93 | Prospective cohort from the population | ||
| [184] | Marks et al, 2012 | Agogo, rural Ghana; Jan 2010 to Oct 2011 | <5 years: >600 | Febrile in & outpatients | 2 year Surveillance data | All ages | 5134 | 389 | 38882 | ND | Abstract only, survey used to adjust incidence calculation | ||
| [137] | Nielsen et al, 2012 | Ashanti, rural Ghana; September 2007 to July 2009 | 2520 (2110–2940) | Nil | Admissions | Prospective consecutive | < 5 years | 1196 | 238 | 149500 (15% aged <5 years) | 129 | Incidence adjusted based on health-seeking behaviour |
*ART anti-retroviral therapy
The incidence rates were much higher in Eastern and Western Africa compared to Southern Africa. Across the studies, the estimated incidence rates were higher amongst HIV-infected subjects [123,131], subjects with sickle cell disease[174], young children[122,160,164] and in a rural setting compared to an urban setting [164].
Three studies estimated iNTS disease in the community. These studies were conducted on subjects from all age groups, and without bias for risk factors such as HIV infection, malaria and anaemia. Estimates obtained were 1.4, 164 and >600 per 100,000 population per year from South Africa, Malawi and Ghana respectively [11,83,184].
Proportion of NTS as a cause of community acquired blood stream infection in Africa
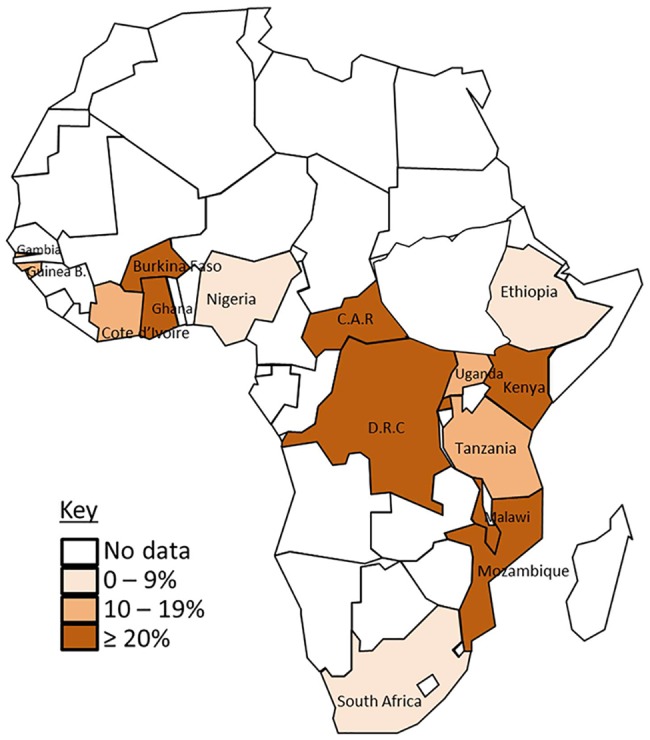
Fifty six studies, describing a total of 114,634 blood cultures (S5 Table) from four regions were eligible for analysis: hospital-based studies from Africa investigating the organisms causing bacteraemia in the community and not in any specific risk group. Eligible studies were from four of the five regions (all except the Northern region) and sixteen countries in Africa. NTS proportions varied between and within regions. Regional averages ranged from 8% in Southern Africa to 38% in Central Africa with an overall average of 25%. In Eastern Africa, with an average of 27%, the range was 9% in Ethiopia to 39% in Malawi while in Western Africa, with an average of 18%, the range was 8% in Nigeria to 34% in Burkina Faso.
Across countries, NTS was a cause of about 8% (lowest value) of community acquired bacteraemia in both Nigeria and South Africa to a peak value of 45% in one study from Central African Republic involving 131 blood cultures (Fig 5). Variations in NTS proportion within country were found in Malawi, Kenya, Tanzania and Uganda. This suggests that it might be difficult to directly extrapolate the burden of iNTS disease within a country as well as from one country to another.
Fig 5. Proportion of Community acquired blood stream infections caused by NTS in African countries (1966 to 2014).
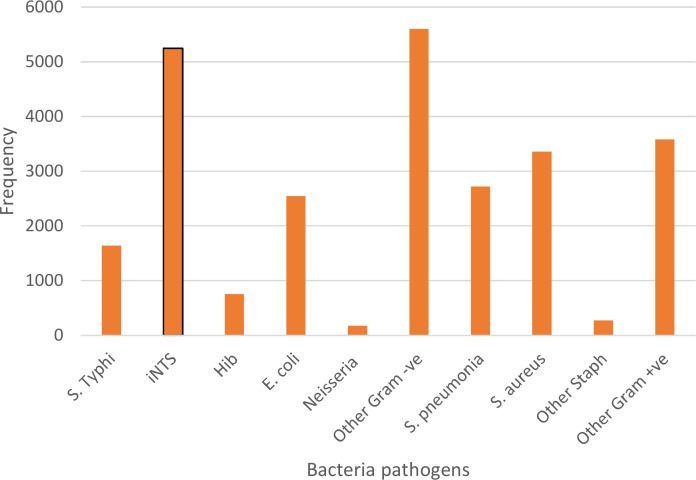
An analysis of the relative frequencies of different organisms causing community acquired bacteraemia in Africa was performed and it showed that NTS, Staphylococcus aureus and Streptococcus pneumoniae are most prevalent organisms isolated over time in Africa (Fig 6). Since the selection of articles included in this review excluded blood culture series without NTS, there is an element of bias favouring NTS compared to other organisms. However, the review of Reddy et al [16] also indicated the importance of iNTS as a cause of community acquired blood stream infection amongst adults and children in Africa [16].
Fig 6. Pathogens reported from community acquired bacteraemia cases in Africa (1966 to 2014).
Risk factors associated with iNTS disease in Africa
The risk factors known to be associated with iNTS disease in Africa include HIV infection, malnutrition, malaria, young age, anaemia and rural setting (Table 2).
Table 2. Risk factors associated with iNTS disease in Africa (1966 to 2014).
| Risk factor | Ref no | Author, Year | Measure of association reported (Confidence interval) | Comments | |
|---|---|---|---|---|---|
| 1 | HIV infection | ||||
| [31] | Archibald et al, 2000 | OR: 4.4 (0.6–93)* | P value not significant | ||
| [2] | Berkley et al, 2005 | OR: 3.21 (1.95–5.28) | OR adjusted for age, HIV infection and malnutrition | ||
| [55] | Bronzan et al, 2007 | OR: 11.6 (2.1–63.7) | Children with severe malarial anaemia | ||
| Adjusted OR: 22.1 (3.3–146) | |||||
| [156] | Seydi et al, 2005 | Prevalence: 81% vs 36% | P = 0.00001, iNTS in patients with vs without HIV | ||
| [81] | Gilks et al, 1990 | OR: 48.2 (13–176) | S. Typhimurium only | ||
| [51] | Blomberg et al, 2007 | Prevalence: 25% vs 8.4% | P = 0.022; iNTS in HIV infected vs uninfected patients | ||
| [151] | Peters et al, 2004 | OR: 5.3 (1.2–2.2) | |||
| 2 | Malnutrition | ||||
| [2] | Berkley et al, 2005 | OR: 1.68 (1.15–2.44) | OR adjusted for age, HIV infection and malnutrition | ||
| Severe (WAZ<-3) | [122] | Mandomando et al, 2009 | OR: 1.44 (1.08–1.91) | P = 0.004; iNTS vs Other bacteraemia; Multivariate analysis | |
| wasting | [137] | Nielsen et al, 2012 | OR: 1.9 (1.2–3.4) | ||
| [160] | Sigauque et al, 2009 | OR: 2.42 (1.92–3.04) | Unadjusted analysis | ||
| Severe acute | [192] | Biggs et al, 2014 | OR: 2.01 (1.15–3.52) | P = 0.014; Multivariate analysis | |
| 3 | Malaria | ||||
| P. falciparum | [122] | Mandomando et al, 2009 | OR: 1.61 (0.91–1.47) | P = 0.8; iNTS vs Other bacteraemia; Univariate analysis | |
| P. falciparum in children >6 mo old | [86] | Graham et al, 2000 | RR: 1.5 (1.2–2.2) | P<0.01; compared to other pathogens combined | |
| Recent malaria | [192] | Biggs et al, 2014 | OR: 4.13 (2.66–6.44) | P<0.001; Multivariate analysis | |
| 4 | Age | ||||
| 12–23 months vs >5 years old | [2] | Berkley et al, 2005 | Prevalence: 23% vs 7% | P value (<0.001) | |
| ≥2 vs <2 years | [55] | Bronzan et al, 2007 | OR: 4.3 (0.8–22.9) | Children with severe malarial anaemia | |
| Adjusted OR: 9.6 (1.4–64.8) | |||||
| 2–11 months | [122] | Mandomando et al, 2009 | OR: 2.07 (1.07–4.01) | P = <0.001; iNTS vs Other bacteraemia; Multivariate analysis | |
| 1–4 yrs | [122] | Mandomando et al, 2009 | OR: 3.90 (2.06–7.40) | P = <0.001; iNTS vs Other bacteraemia; Multivariate analysis | |
| 5 | Anaemia | ||||
| Moderate | [122] | Mandomando et al, 2009 | OR: 1.86 (1.34–2.58) | P = <0.001; iNTS vs Other bacteraemia; Multivariate analysis | |
| Severe | [122] | Mandomando et al, 2009 | OR: 3.48 (2.03–5.95) | P = <0.001; iNTS vs Other bacteraemia; Multivariate analysis | |
| Severe | [86] | Graham et al, 2000 | Risk Ratio: 7.2 (3.4–15.3) | P<0.0001; compared to other pathogens combined | |
| Sickle cell | [174] | Williams et al, 2009 | OR: 35.6 (16.4–76.8) | Age adjusted (<14years) | |
| Anaemia (Hb<7) | [151] | Peters et al, 2004 | OR: 2.2 (1.1–4.5) | ||
| Severe | [192] | Biggs et al, 2014 | OR: 2.19 (1.48–3.23) | P<0.001; Multivariate analysis | |
| 6 | Setting | ||||
| Rural ward residence | [192] | Biggs et al, 2014 | OR: 2.23 (1.25–3.96) | P = 0.006; Univariate analysis |
*OR Odds Ratio
Seven studies from Africa showed a positive association between HIV and iNTS disease in Africa. The estimated odds ratio (OR) from the studies for HIV-infected individuals developing iNTS disease compared with HIV-uninfected individuals ranged from 3.2 to 48.2. The strong association between HIV infection and iNTS disease in Africa was described in 1990 when Gilks et al reported an OR of 48.2 (confidence interval 13–176) in Nairobi, Kenya [81].
Malnutrition had a positive association with iNTS disease in five studies (Table 2), with the earliest study published in 2005. The OR for iNTS disease occurring in children with malnutrition compared with children without malnutrition ranged from 1.44 to 2.42. Plasmodium falciparum malaria had a positive association with iNTS disease in three African studies (Table 2) since the year 2000. The estimated OR for iNTS disease in individuals with Plasmodium falciparum malaria compared with those without Plasmodium falciparum malaria ranged from 1.5 to 4.1. In one study, recent history of malaria was positively associated with iNTS disease[192]. Young age was associated with iNTS disease in three studies (Table 2). The OR for iNTS disease in young individual compared to older individuals ranged from 2.07 to 4.30. In one study of children 1–4 years of age, iNTS disease was reported to be more associated with age when compared to other organisms causing bacteraemia[122].
Anaemia (especially moderate and severe anaemia), was shown by five studies to be associated with iNTS disease in Africa with reported OR ranging from 1.86 to 35.6. Rural settlement compared to urban was found by one study to be associated with iNTS disease [192]. The OR of iNTS disease occurring in a rural settlement compared to an urban settlement was 2.23
Case Fatality Rate (CFR) of iNTS disease in Africa
We were able to extract CFR data for iNTS disease in Africa from twenty four studies (Table 3), describing a total of 548 deaths among 2656 cases. The overall CFR was 20.6%. and ranged from 0% in individuals greater than or equal to 5 years old in Kenya [75] to 72.7% in another Kenyan study involving only HIV-infected patients [81]. The average CFR, derived from 8 studies [3,67,89,93,152,160,172,188] conducted among low risk populations (not HIV-infected, anaemic, malnourished, or having malaria), and with >90 iNTS cases isolated, is 19% (276 fatalities from 1427 cases).
Table 3. CFR of iNTS disease from studies in Africa (1966 to 2014).
| Author | Ref no. | Location; Study dates | CFR % | Setting | Subjects | Study entry criteria | # iNTS cases | # Deaths | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Bachou et al 2006 | [41] | Kampala, Uganda; Sep 2003 to Dec 2004 | 23.3 | rural, hospital | Inpatients < 5 years old | Severe malnutrition | 30 | 7 | HIV-infected subjects |
| Bassat et al 2009 | [45] | Southern Mozambique, Mozambique; June 2003 to May 2007 | 25.0 | rural, hospital | Inpatients ≤ 5 years old | severe malaria | 8 | 2 | |
| Berkozwit et al 1984 | [50] | Soweto, South Africa; January to Dec 1982 | 19.6 | urban, hospital | Inpatients < 11 years old | All admissions | 56 | 11 | |
| Blomberg et al 2007 | [51] | Dar es Salam, Tanzania; August 2001 to August 2002 | 34.4 | Hospital | Inpatients ≤ 7 years old | clinical septicaemia | 32 | 11 | 21.6% malaria prevalence amongst participants |
| Bouallegue-Godet et al 2005 | [52] | Sousse, Tunisia; July 2002 | 66.7 | urban, hospital | inpatients, neonates | ND | 3 | 2 | Neonates |
| Brent et al 2006 | [3] | Kilifi, Kenya; August 1998 to July 2002 | 21.1 | rural, hospital | Inpatients < 13 years old | Salmonella blood culture positive | 166 | 35 | |
| Dube et al 1983 | [65] | Lusaka, Zambia; March to August 1980 | 20.0 | hospital | Inpatients ≤ 2 years old | Clinical infection | 10 | 2 | Mostly neonates |
| Enwere et al, 2006 | [67] | Basse & Bassang, Gambia; August 2000 to April 2004 | 4.3 | rural, hospital | vaccine trial subjects, 2 to 29 months | Fever | 92 | 4 | Death within 28 day period |
| Feikin et al 2012 | [75] | Asembo, Kenya; March 2007 to February 2010 | 0.0 | rural, hospital | in & outpatients ≥ 5 years old | respiratory illness | 41 | 0 | 37.9% HIV prevalence |
| Gilks et al 1990 | [81] | Nairobi, Kenya; Nov 1988 to May 1989 | 72.7 | urban, hospital | All ages | Inpatients | 11 | 8 | 10 were HIV-infected with bacteraemia on admission |
| Gordon et al 2002 | [82] | Blantyre, Malawi; | 47.0 | rural & urban, hospital | Inpatients ≥ 18 years old | Not specified | 100 | 47 | 99% HIV prevalence |
| Gordon et al 2001 | [84] | Blantyre, Malawi; Dec 1997 to Nov 1998 | 32.9 | urban, hospital | Inpatients ≥14 years old | Fever | 164 | 54 | 92% HIV prevalence amongst 25 NTS cases |
| Graham et al 2000 | [86] | Blantyre, Malawi; February 1996 to April 1998 | 24.1 | hospital | Inpatients ≤ 14 years old | Bacteraemia | 241 | 58 | HIV infected subjects, 31% malaria parasitaemia prevalence |
| Grant et al 1998 | [88] | Abidjan, Cote d'Ivoire; Dec 1995 to March 1996 | 50.0 | urban, hospital | Inpatients ≥ 18 years old | respiratory illness | 14 | 7 | HIV infected subjects, CFR includes 3 E. Coli cases |
| Green et al 1993 | [89] | Western Zaire, D.R.C; January 1982 to Dec. 1986 | 22.7 | rural, hospital | in & outpatients ≤ 5 years old | Clinical Salmonella | 172 | 39 | |
| Hadfield TL et al 1985 | [93] | Zorzor, Liberia; October 1980 to August 1982 | 27.8 | hospital | Inpatients ≤ 16 years old | S. Enteritidis blood culture positive | 97 | 27 | |
| Mandomando et al 2009 | [122] | Manhiça, Mozambique; May 2001 to April 2006 | 12.2 | rural, hospital | Inpatients < 10 years old | Fever | 344 | 42 | NTS associated with severe malnutrition, anaemia and age |
| Phoba et al 2014 | [152] | Equateur, Democratic Republic of Congo; Nov 2011 to May 2012 | 12.0 | hospital | 83 | 10 | 69.7% has P. falciparum infection | ||
| Pithie et al 1993 | [154] | Harare, Zimbabwe; | 40.7 | hospital | All ages | Bacteraemia | 27 | 11 | 50% HIV prevalence |
| Sigauque et al 2009 | [160] | Manhiça, Mozambique; May 2001 to April 2006 | 12.2 | rural, hospital | Inpatients < 10 years old | All admissions | 344 | 42 | |
| Sow S.O. 2011 | [188] | Bamako, Mali; July 2002 to June 2010 | 20.0 | hospital | Inpatients ≤ 10 years old | Fever | 434 | 87 | |
| Tabu et al 2012 | [164] | Asembo, Kenya; October 2006 to September 2009 | 11.7 | rural, hospital | ≤ 50 years old | Fever, respiratory illness, medical admissions | 60 | 7 | |
| Vaagland et al 2004 | [165] | Northern Tanzania, Tanzania; July to August 2000 | 60.0 | rural, hospital | Inpatients ≤ 6 years old | Clinical septicaemia | 5 | 3 | |
| Walsh et al, 2000 | [172] | Blantyre, Malawi; Sept. 1996 to August 1997 | 26.2 | rural & urban, hospital | Inpatients < 15 years old | Fever | 122 | 32 | median age for iNTS cases—15.5 months |
| Total | 2656 | 548 |
Discussion
Our review seeks to comprehensively document reports of iNTS disease in Africa: the number and location of the reported cases, and where available, the age of the subjects, risk factors, case fatality rates and incidence. As a result, we have included many more studies (199) than a recent review by Ao et al [204] that specifically only included reports of incidence. In their database, in addition to reports from other countries they list 10 reports from 6 African countries. All 10 reports from the Ao et al study are included in the larger set of 14 reports describing incidence from 8 African countries we include in Table 1. Not surprisingly we find a similarly high incidence of iNTS disease in sub-Saharan Africa. Ao et al estimated the overall incidence of iNTS disease in Africa at 227 cases [range 152–341] per 100,000 population with 1.9 [range 1.3–2.9] million cases annually [204]. We sought to provide a comprehensive review of the published reports of NTS bacteraemia in the peer reviewed literature and found a substantial number of reports with the earliest in 1966, interestingly about the same time as genetic studies suggest the evolution of the highly African-specific highly invasive NTS genotypes [205,206].
There are limits on the comprehensiveness of our study. In particular, many of the early and non-English reports in the initial screening stage were only available to us on line as abstracts, and in some cases, just the title with MESH headings and some of these may have contained details e.g. the use of blood cultures to determine Salmonella bacteraemia in the text but not in the abstract that resulted in these articles not being ordered and reviewed in full. Despite these limitations, this survey highlights important findings.
As shown in Fig 2, no reports were found in some countries in West Africa and in South West Africa. In view of the reported cases from neighbouring countries it seems unlikely that iNTS disease does not occur in these counties, but this highlights that gaps in the published literature almost certainly exist, e.g. due to lack of infrastructure in these countries to undertake the blood culturing required to obtain a definitive diagnosis, or the lack of researchers interested in publishing reports. Some of these gaps in the database may be addressed by examining health service records, but this is beyond the scope of the article.
As shown in Table 1 in the papers we surveyed, the estimated incidence of iNTS disease ranged from 1.4 cases per 100,000 population per year over all age groups in South Africa to a yearly cumulative incidence of 2,520 per 100,000 among <5 years old children in Ghana. However, we caution against trying to over-interpret these incidence data or the range observed–a striking observation is the lack of consistency in the age groups reported, the inclusion criteria that may or may not select for populations with specific risk factors (e.g. HIV, sickle cell anaemia) and in the way in which the population denominator is determined for these largely facility-based studies.
On the basis of the whole database of reported bacteraemia, we confirm the earlier findings that NTS accounts for a large proportion of the bacteria responsible for community acquired blood stream infections and is a substantial cause of morbidity and mortality with the serovars Typhimurium and Enteritidis responsible for 91% of NTS bacteraemia in Africa in the 69% of cases where the serotype was determined.
A major knowledge gap exists in the incidence of iNTS disease in Africa owing to the paucity of reports from population-based surveillance of Salmonella in Africa. This is due to poor surveillance systems for infectious diseases in most parts of Africa. The majority of the studies on iNTS disease in Africa are hospital-based which can only provide an estimate of the minimal incidence of disease in the community, because not all febrile cases report to hospital. Denominators necessary for the detailed estimation of iNTS disease incidence in the reported publications are generally not available. This also makes it difficult to estimate the true population at risk of disease. Population-based incidence studies are best suited to estimate the incidence of an infectious disease in Africa. This can be done by either adjusting the data obtained from hospital-based study with data obtained from simultaneous health-seeking surveys carried out in the same community (for example, as has been carried out by the Typhoid Surveillance in Africa Program (TSAP) and Severe Typhoid in Africa programme [207] or designing a study with a method that will allow for identification of all the cases of the disease within the target community.
Epidemiological data on iNTS disease were available from publications in peer-reviewed journals from 33 of the 54 countries in Africa (Fig 2). Unavailable data from the remaining 21 African countries might be due to a lack of studies in these countries, or a true low burden of iNTS disease. These 21 countries are not involved in TSAP. It would therefore be valuable to conduct a thorough search of grey literature in the affected countries including reviewing existing databases of blood cultures carried out in major hospitals in various locations across the affected countries in the last five to ten years. Such an analysis would facilitate a better understanding of the relative importance of NTS as a cause of community acquired blood stream infection in Africa. New prospective hospital-based blood culture studies in these countries would be even more useful.
The average case fatality rate of community acquired severe infections is 20.6% but with a wide range from 0 to 72%. Therefore, the development of an effective vaccine against NTS for Africa would be an important intervention to help reduce the burden of disease and deaths due to NTS. The exact mechanisms of transmission of iNTS disease are currently unclear and there are no rapid diagnostic tests available for its detection. The development of such diagnostics would greatly facilitate the study and management of iNTS disease in Africa.
Supporting Information
(DOCX)
(TIF)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank our colleagues Audino Podda from GSK Vaccines Institute for Global Health Siena, who provided insight and expertise that greatly assisted the research, and Laura B Martin for assistance with data collection and entry.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
For part of this study, IVU received a fellowship from Novartis Vaccines and Diagnostics to undertake a Master of Vaccinology and the other authors were Novartis employees. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Moore C, Banura P, Pegues D, Miller S. Nontyphoidal Salmonellosis In: Guerrant R, Walker D, Weller P, editors. Tropical Infectious Diseases. Third Elsevier Health Sciences; 2011. p. 128–36. [Google Scholar]
- 2.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005. January;352(1):39–47. 10.1056/NEJMoa040275 [DOI] [PubMed] [Google Scholar]
- 3.Brent AJ, Oundo JO, Mwangi I, Ochola L, Lowe B, Berkley JA. Salmonella bacteremia in Kenyan children. Pediatr Infect Dis J. 2006;25(3):230–6. 10.1097/01.inf.0000202066.02212.ff [DOI] [PubMed] [Google Scholar]
- 4.Nataro J, Barry E. Diarrhea caused by bacteria In: Plotkin S, editor. Vaccines. Sixth Elsevier Saunders; 2013. p. 1052–9. [Google Scholar]
- 5.MacLennan CA, Levine MM. Invasive nontyphoidal Salmonella disease in Africa: Current status. Expert Rev Anti Infect Ther. 2013;11(5):443–6. 10.1586/eri.13.27 [DOI] [PubMed] [Google Scholar]
- 6.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal salmonella disease : an emerging and neglected tropical disease in Africa. Lancet. 2012;379(9835):2489–99. 10.1016/S0140-6736(11)61752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishu B, Koehler J, Lee L, Tauxe R. Outbreaks of Salmonella enteritidis infections in the United States, 1985–1991. J Infect Dis. 1994;169(3):547–52. [DOI] [PubMed] [Google Scholar]
- 8.Angulo FJ, Tippen S, Sharp DJ, Payne BJ, Collier C, Hill JE, et al. A community waterborne outbreak of salmonellosis and the effectiveness of a boil water order. Am J Public Health. 1997. April;87(4):580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. 2009;2279–87. [DOI] [PMC free article] [PubMed]
- 10.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Muyodi J, et al. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: Zoonotic or anthroponotic transmission? J Med Microbiol. 2006;55(5):585–91. [DOI] [PubMed] [Google Scholar]
- 11.Feasey NA, Archer BN, Heyderman RS, Sooka A, Dennis B, Gordon MA, et al. Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerg Infect Dis. 2010;16(9):1448–51. 10.3201/eid1609.100125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.OAHPP. Nontyphoidal Salmonella (NTS) Infection : Information for Clinicians. Ontario; 2010. p. 1–3.
- 13.Were T, Davenport GC, Hittner JB, Ouma C, Vulule JM, Ong’echa JM, et al. Bacteremia in Kenyan children presenting with malaria. J Clin Microbiol. 2011;49(2):671–6. 10.1128/JCM.01864-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyamuya M, Nadjm B, Mtove G, Reyburn H. Contrasting Epidemiology of Salmonella Typhi and Non-Typhi Salmonella Bloodstream Infections at Two Sites in Northern Tanzania. In: Abstracts of the 15th International Symposium on Infections in the Immunocompromised Host. p. S23.
- 15.Smith AM, Mthanti MA, Haumann C, Tyalisi N, Boon GPG, Sooka A, et al. Nosocomial outbreak of Salmonella enterica serovar Typhimurium primarily affecting a pediatric ward in South Africa in 2012. J Clin Microbiol. 2014. February;52(2):627–31. 10.1128/JCM.02422-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy EA, Shaw A V, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010. June;10(6):417–32. 10.1016/S1473-3099(10)70072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clemens J. Meeting on Establishment of Consortium to Study Invasive Salmonelloses in Sub-Saharan Africa. Vol. 15, Emerging infectious diseases (serial on the internet). 2009. [DOI] [PMC free article] [PubMed]
- 18.Quosa. Elsevier. 2014.
- 19.Abo Y, Minga A, Menan H, Danel C, Ouassa T, Dohoun L, et al. Incidence of serious morbidity in HIV-infected adults on antiretroviral therapy in a West African care centre, 2003–2008. BMC Infect Dis. 2013;13:607 10.1186/1471-2334-13-607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrha A, Abdissa A, T; BGG. Bacteraemia among severely malnourished children in jimma university hospital, ethiopia. Ethiop J Heal Sci. 2011;21(3):175–82. [PMC free article] [PubMed] [Google Scholar]
- 21.Acquah SEK, Quaye L, Sagoe K, Ziem JB, Bromberger PI, Amponsem AA. Susceptibility of bacterial etiological agents to commonly-used antimicrobial agents in children with sepsis at the Tamale Teaching Hospital. 2013;1–7. [DOI] [PMC free article] [PubMed]
- 22.Adeyemi AI, Sulaiman AA, Solomon BB, Chinedu OA, Victor IA. Bacterial Bloodstream Infections in HIV-infected Adults Attending a Lagos Teaching Hospital. 2010;28(4):318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aiken AM, Mturi N, Njuguna P, Mohammed S, Berkley JA, Mwangi I, et al. Risk and causes of paediatric hospital-acquired bacteraemia in Kilifi District Hospital, Kenya : a prospective cohort study. Lancet. 2009;378(9808):2021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ailal F, Bousfiha AA, Jouhadi Z, Adnane F, Abid A. Forty-one pediatric cases of non-typhoidal salmonellosis. Med Mal Infect. 2004;34(5):206–9. [DOI] [PubMed] [Google Scholar]
- 25.Akinyemi KO, Bamiro BS, Coker AO. Salmonellosis in Lagos, Nigeria : Incidence of Plasmodium falciparum -associated Co-infection, Patterns of Antimicrobial Resistance, and Emergence of Reduced Susceptibility to Fluoroquinolones. 2007;25(3):351–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Alausa K, Montefiore D, Sogbetun A, E; AJB. Septicaemia in the tropics. A prospective epidemiological study of 146 patients with a high case fatality rate. Scand J Infect Dis. 1977;9(3):181–5. [DOI] [PubMed] [Google Scholar]
- 27.Ali J, Kebede Y. Frequency of isolation and antimicrobial susceptibility pattern of bacterial isolates from blood culture, Gondar University teaching hospital, Northwest Ethiopia. Ethiop Med J. 2008;46(2):155–61. [PubMed] [Google Scholar]
- 28.Andualem G, Abebe T, S;Mihret KN-S. A comparative study of Widal test with blood culture in the diagnosis of typhoid fever in febrile patients. BMC Res Notes. 2014;7:653 10.1186/1756-0500-7-653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anglaret X, Dakoury-Dogbo N, Bonard D, Toure S, Combe P, Ouassa T, et al. Causes and empirical treatment of fever in HIV-infected adult outpatients, Abidjan, Cote d’Ivoire. AIDS. 2002;16(6):909–18. [DOI] [PubMed] [Google Scholar]
- 30.Archibald LK, Kazembe PN, Nwanyanwu O, Mwansambo C, Reller LB, Jarvis WR. Epidemiology of bloodstream infections in a bacille Calmette-Guerin-vaccinated pediatric population in Malawi. J Infect Dis. 2003;188(2):202–8. 10.1086/376507 [DOI] [PubMed] [Google Scholar]
- 31.Archibald LK, Mcdonald LC, Rachel M, Mcknight C, Byrne T, Dobbie H, et al. Comparison of BACTEC MYCO / F LYTIC and WAMPOLE ISOLATOR 10 (Lysis-Centrifugation) Systems for Detection of Bacteremia, Mycobacteremia, and Fungemia in a Developing Country. J Clin Microbiol. 2000;38(8):2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Archibald LK, Mcdonald LC, Nwanyanwu O, Kazembe P, Dobbie H, Tokars J, et al. A Hospital-Based Prevalence Survey of Bloodstream Infections in Febrile Patients in Malawi : Implications for Diagnosis and Therapy. 1997;1414–20. [DOI] [PubMed]
- 33.Archibald LK, Nwanyanwu O, Kazembe PN, Mwansambo C, Bell M, Dobbie H, et al. Detection of bloodstream pathogens in a bacille Calmette-Guerin (BCG)-vaccinated pediatric population in Malawi: A pilot study. Clin Microbiol Infect. 2003;9(3):234–8. [DOI] [PubMed] [Google Scholar]
- 34.Arthur G, Bhatt SM, Muhindi D, Grace A, Kariuki SM, Gilks CF. The changing impact of HIV / AIDS on Kenyatta National Hospital, Nairobi from 1988 / 89 through 1992 to 1997. 2000;(March):1625–31. [DOI] [PubMed]
- 35.Arthur G, Nduba VN, Kariuki SM, Kimari J, Bhatt SM, Gilks CF. Trends in bloodstream infections among human immunodeficiency virus-infected adults admitted to a hospital in Nairobi, Kenya, during the last decade. Clin Infect Dis. 2001;33(2):248–56. 10.1086/321820 [DOI] [PubMed] [Google Scholar]
- 36.Aseffa A, Gedlu E, Asmelash T. Antibiotic resistance of prevalent Salmonella and Shigella. East Afr Med J. 1997;74(11):708–13. [PubMed] [Google Scholar]
- 37.Asrat D, Amanuel Y. Prevalence and antibiotic susceptibility pattern of bacterial. Ethiop Med J. 2001;39(2):97–104. [PubMed] [Google Scholar]
- 38.Aubry P, Niyongabo T, Nizigiye J, Muhirwa G, Kamanfu G, Ndahiragije A. Bacteremiae caused by non typhous Salmonella during the course of HIV infection in African adult. Med Trop. 1992;52(4):447–50. [PubMed] [Google Scholar]
- 39.Auma MA, Siedner MJ, Nyehangane D, Nalusaji A, Nakaye M, Mwanga-amumpaire J, et al. Malaria is an uncommon cause of adult sepsis in South-western Uganda. Malar J. 2013;12:146 10.1186/1475-2875-12-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babirekere-Iriso E, Musoke P, Kekitiinwa A. Bacteraemia in severely malnourished children in an HIV-endemic setting. Ann Trop Paediatr. 2006;26(4):319–28. 10.1179/146532806X152845 [DOI] [PubMed] [Google Scholar]
- 41.Bachou H, Tylleskar T, Kaddu-Mulindwa DH, Tumwine JK. Bacteraemia among severely malnourished children infected and uninfected with the human immunodeficiency virus-1 in Kampala, Uganda. BMC Infect Dis. 2006;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bahwere P, Donnen P, Levy J. Improvements in Nutritional Management as a Determinant of Reduced Mortality From Community- Acquired Lower Respiratory Tract Infection in Hospitalized Children From Rural Central Africa. 2004;23(8):15–7. [DOI] [PubMed] [Google Scholar]
- 43.Bahwere P, Levy J, Hennart P, Donnen P, Lomoyo W, Dramaix-Wilmet M, et al. Community-acquired bacteremia among hospitalized children in rural central Africa. Int J Infect Dis. 2001;5(4):180–8. [DOI] [PubMed] [Google Scholar]
- 44.Baiden F, Webster J, Tivura M, Delimini R, Berko Y, Amenga-Etego S, et al. Accuracy of rapid tests for malaria and treatment outcomes for malaria and non-malaria cases among under-five children in rural ghana. PLoS One. 2012;7(4):e34073 10.1371/journal.pone.0034073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bassat Q, Guinovart C, Sigauque B, Mandomando I, Aide P, Sacarlal J, et al. Severe malaria and concomitant bacteraemia in children admitted to a rural Mozambican hospital. Trop Med Int Heal. 2009;14(9):1011–9. [DOI] [PubMed] [Google Scholar]
- 46.Batchelor BIF, Kimari JN, Brindle RJ. Microbiology of HIV associated bacteraemia and diarrhoea in adults from Nairobi, Kenya. Epidemil Infect. 1996;117:139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bedell RA, Anderson STB, van Lettow M, Akesson A, Corbett EL, Kumwenda M, et al. High prevalence of tuberculosis and serious bloodstream infections in ambulatory individuals presenting for antiretroviral therapy in Malawi. PLoS One. 2012;7(6):e39347 10.1371/journal.pone.0039347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bell M, Archibald LK, Nwanyanwu O, Dobbie H, Tokars J, Kazembe PN, et al. Seasonal variation in the etiology of bloodstream infections in a febrile inpatient population in a developing country. Int J Infect Dis. 2001;5(2):63–9. [DOI] [PubMed] [Google Scholar]
- 49.Ben H, Bejaoui M, Hichri A, Lakhoua R, Ben RS. [Non-typhoid Salmonella in pediatric patients]. Bull Soc Pathol Exot. 1993;86(3):190–4. [PubMed] [Google Scholar]
- 50.Berkowitz FE. Bacteremia in hospitalized Black South African children. A one-year study emphasizing nosocomial bacteremia and bacteremia in severely malnourished children. Am J Dis Child. 1984;138(6):551–6. [DOI] [PubMed] [Google Scholar]
- 51.Blomberg B, Manji KP, Urassa WK, Tamim BS, Mwakagile DSM, Jureen R, et al. Antimicrobial resistance predicts death in Tanzanian children with bloodstream infections: A prospective cohort study. BMC Infect Dis. 2007;7:43 10.1186/1471-2334-7-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouallegue-Godet O, Salem YB, Fabre L, Demartin M, Grimont PAD, Mzoughi R, et al. Nosocomial outbreak caused by Salmonella enterica serotype livingstone producing CTX-M-27 extended-spectrum (beta)-lactamase in a neonatal unit in Sousse, Tunisia. J Clin Microbiol. 2005;43(3):1037–44. 10.1128/JCM.43.3.1037-1044.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brent AJ, Ahmed I, Ndiritu M, Lewa P, Ngetsa C, Lowe B, et al. Incidence of clinically significant bacteraemia in children who present to hospital in Kenya : community-based. Lancet. 2006;367:482–8. 10.1016/S0140-6736(06)68180-4 [DOI] [PubMed] [Google Scholar]
- 54.Brindle RJ, Nunn PP, Batchelor BIF, Gathua SN, Kimari JN, Newnham RS, et al. Infection and morbidity in patients with tuberculosis in Nairobi, Kenya. AIDS. 1993;7(11):1469–74. [DOI] [PubMed] [Google Scholar]
- 55.Bronzan RN, Taylor TE, Mwenechanya J, Tembo M, Kayira K, Bwanaisa L, et al. Bacteremia in Malawian children with severe malaria: Prevalence, etiology, HIV coinfection, and outcome. J Infect Dis. 2007;195(6):895–904. 10.1086/511437 [DOI] [PubMed] [Google Scholar]
- 56.Calis J, Phiri K, Faragher E, RJ; BBILH. Severe anemia in Malawian children. N Engl J Med. 2008;358(9):888 10.1056/NEJMoa072727 [DOI] [PubMed] [Google Scholar]
- 57.Cheesbrough JS, Taxman BC, Green SDR, Mewa FI, Numbi A. Clinical definition for invasive Salmonella infection in African children. Pediatr Infect Dis J. 1997;16(3):277–83. [DOI] [PubMed] [Google Scholar]
- 58.Commey J, Quarm-Goka B, Agyepong I. Persistent fever in severe malaria in children. Cent Afr J Med. 1994;40(9):257–60. [PubMed] [Google Scholar]
- 59.Cotton MF, Burger PJ, Bodenstein WJM. Bacteraemia in children in the south-western Cape. A hospital-based survey. South African Med J. 1992;81(2):87–90. [PubMed] [Google Scholar]
- 60.Crump JA, Ramadhani HO, Morrissey AB, Msuya LJ, Yang L-Y, Chow S-C, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected children and infants in northern Tanzania. Trop Med Int Health. 2011. July;16(7):830–7. 10.1111/j.1365-3156.2011.02774.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, Yang LY, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis. 2011;52(3):341–8. 10.1093/cid/ciq103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D’Acremont V, Kilowoko M, Kyungu E, Philipina S, Sangu W, Kahama-Maro J, et al. Beyond malaria—causes of fever in outpatient Tanzanian children. N Engl J Med. 2014;370(9):809–17. 10.1056/NEJMoa1214482 [DOI] [PubMed] [Google Scholar]
- 63.Domoua K, N’Dhatz M, Coulibaly G, Traore F, Ouattara B, Achy V, et al. Acute viral pneumopathy during retroviral infection. Epidemiological, clinical, radiological, bacteriological and evolutive aspects. Med Trop. 1993;53(4):505–9. [PubMed] [Google Scholar]
- 64.Dougle M, Hendriks E, Sanders E, Dorigo-Zetsma JW. Laboratory investigations in the diagnosis of septicaemia and malaria. East Afr Med J. 1997;74(6):353–6. [PubMed] [Google Scholar]
- 65.Dube SD, Bhagwat AG. Non-typhoidal Salmonella infection in Zambian infants. Trans R Soc Trop Med Hyg. 1983;77(3):336–7. [DOI] [PubMed] [Google Scholar]
- 66.Edoh V, Ghipponi PM. Assessment of three years of blood culture in Treichville Abidjan Hospital (Ivory Coast). Med Trop. 1989;49(4):429–31. [PubMed] [Google Scholar]
- 67.Enwere G, Biney E, Cheung YB, Zaman SMA, Okoko B, Oluwalana C, et al. Epidemiologic and clinical characteristics of community-acquired invasive bacterial infections in children aged 2–29 months in The Gambia. Pediatr Infect Dis J. 2006. August;25(8):700–5. 10.1097/01.inf.0000226839.30925.a5 [DOI] [PubMed] [Google Scholar]
- 68.Erwa H. Further studies on enteric fever in Sudanese patients. J Trop Med Hyg. 1969;72(11):276–9. [PubMed] [Google Scholar]
- 69.Erwa H. Enteric fever in the Sudan. J Trop Med Hyg. 1966;69(9):197–201. [PubMed] [Google Scholar]
- 70.Evans JA, Adusei A, Timmann C, May J, Mack D, Agbenyega T, et al. High mortality of infant bacteraemia clinically indistinguishable from severe malaria. Q J Med. 2004;97(9):591–7. [DOI] [PubMed] [Google Scholar]
- 71.Fashae K, Ogunsola F, Aarestrup FM, Hendriksen RS. Antimicrobial susceptibility and serovars of Salmonella from chickens and humans in Ibadan, Nigeria. J Infect Dev Ctries. 2010;4(8):484–94. [DOI] [PubMed] [Google Scholar]
- 72.Feasey NA, Banada PP, Howson W, Sloan DJ, Mdolo A, Boehme C, et al. Evaluation of Xpert MTB/RIF for detection of tuberculosis from blood samples of HIV-infected adults confirms Mycobacterium tuberculosis bacteremia as an indicator of poor prognosis. J Clin Microbiol. 2013;51(7):2311–6. 10.1128/JCM.00330-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feasey NA, Houston A, Mukaka M, Komrower D, Mwalukomo T, Tenthani L, et al. A reduction in adult blood stream infection and case fatality at a large african hospital following antiretroviral therapy roll-out. PLoS One. 2014;9(3):e92226 10.1371/journal.pone.0092226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feikin DR, Jagero G, Aura B, Bigogo GM, Oundo J, Beall BW, et al. High rate of pneumococcal bacteremia in a prospective cohort of older children and adults in an area of high HIV prevalence in rural western Kenya. BMC Infect Dis. 2010;10:186 10.1186/1471-2334-10-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feikin DR, Njenga MK, Bigogo G, Aura B, Aol G, Audi A, et al. Etiology and incidence of viral and bacterial acute respiratory illness among older children and adults in rural western kenya, 2007–2010. PLoS One. 2012;7(8):e43656 10.1371/journal.pone.0043656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feikin DR, Njenga MK, Bigogo G, Aura B, Aol G, Audi A, et al. Viral and Bacterial Causes of Severe Acute Respiratory Illness Among Children Aged Less Than 5 Years in a High Malaria. Pediatr Infect Dis J. 2013;32(1):2007–10. [DOI] [PubMed] [Google Scholar]
- 77.Gatti F, Vandepitte J, Van O, Makulu A. Bacteriologic and epidemiologic study of salmonelloses. Ann Soc Belges Med Trop Parasitol Mycol. 1968;48(2):195–224. [PubMed] [Google Scholar]
- 78.Gbadoe AD, Lawson-Evi K, Dagnra AY, Guedenon K, Geraldo A, Djadou E, et al. Pediatric salmonellosis at the Tokoin’s teaching hospital, Lome (Togo). Med Mal Infect. 2008;38(1):8–11. 10.1016/j.medmal.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 79.Gendrel D, Kombila M, Beaudoin-Leblevec G, Richard-Lenoble D. Nontyphoidal salmonellal septicemia in Gabonese children infected with Schistosoma intercalatum. Clin Infect Dis. 1994;18(1):103–5. [DOI] [PubMed] [Google Scholar]
- 80.Georges-Courbot MC, Wachsmuth IK, Bouquety JC, Siopathis MR, Cameron DN, Georges AJ. Cluster of antibiotic-resistant Salmonella enteritidis infections in the Central African Republic. J Clin Microbiol. 1990;28(4):771–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gilks CF, Brindle RJ, Otieno LS, N SP. Life-threatening bacteraemia in HIV-1 seropositive. Lancet. 1990;336(8714):545–9. [DOI] [PubMed] [Google Scholar]
- 82.Gordon MA, Banda HT, Gondwe M, Gordon SB, Boeree MJ, Walsh AL. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults : high mortality and frequent recrudescence. AIDS. 2002;16:1633–41. [DOI] [PubMed] [Google Scholar]
- 83.Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, Molyneux E, et al. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46(7):963–9. 10.1086/529146 [DOI] [PubMed] [Google Scholar]
- 84.Gordon MA, Walsh AL, Chaponda M, Soko D, Mbvwinji M, Molyneux ME, et al. Bacteraemia and Mortality Among Adult Medical Admissions in Malawi–Predominance of Non-typhi Salmonellae and Streptococcus pneumoniae. J Infect. 2001;42(1):44–9. 10.1053/jinf.2000.0779 [DOI] [PubMed] [Google Scholar]
- 85.Graham SM, Mankhambo L, Phiri A, Kaunda S, Chikaonda T, Mukaka M, et al. Impact of human immunodeficiency virus infection on the etiology and outcome of severe pneumonia in malawian children. Pediatr Infect Dis J. 2011;30(1):33–8. 10.1097/INF.0b013e3181fcabe4 [DOI] [PubMed] [Google Scholar]
- 86.Graham SM, Walsh AL, Molyneux EM, Phiri AJ, Molyneux ME. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg. 2000;94(3):310–4. [DOI] [PubMed] [Google Scholar]
- 87.Grant AD, Djomand G, Smets P, Kadio A, Coulibaly M, Kakou A, et al. Profound immunosuppression across the spectrum of opportunistic disease among hospitalized HIV-infected adults in Abidjan, Cote d’Ivoire. AIDS. 1997;11(11):1357–64. [DOI] [PubMed] [Google Scholar]
- 88.Grant AD, Sidibe K, Domoua K, Bonard D, Sylla-Koko F, Dosso M, et al. Spectrum of disease among HIV-infected adults hospitalised in a respiratory medicine unit in Abidjan, Cote d’Ivoire. Int J Tuberc Lung Dis. 1998;2(11):926–34. [PubMed] [Google Scholar]
- 89.Green S, Cheesbrough J. Salmonella bacteraemia among young children at a rural hospital in western Zaire. Ann Trop Paediatr. 1993;13(1):45–53. [DOI] [PubMed] [Google Scholar]
- 90.Gross U, Amuzu SK, de Ciman R, Kassimova I, Gross L, Rabsch W, et al. Bacteremia and antimicrobial drug resistance over time, Ghana. Emerg Infect Dis. 2011;17(10):1879–82. 10.3201/eid1710.110327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guechi Z, Hamza A. Epidemiology of salmonella infections in Algeria. Evolution of the salmonella serovars isolated from 1986 to 1990. Arch Inst Pasteur Alger. 1992;58:7–16. [PubMed] [Google Scholar]
- 92.Gwee A, Coghlan B, Everett D, Chagoma N, Phiri A, Wilson L, et al. Bacteraemia in Malawian neonates and young infants 2002–2007: a retrospective audit. BMJ Open. 2012;2:e000906 10.1136/bmjopen-2012-000906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hadfield TL, Monson MH, Wachsmuth IK. An outbreak of antibiotic-resistant Salmonella enteritidis in Liberia, West Africa. J Infect Dis. 1985;151(5):790–5. [DOI] [PubMed] [Google Scholar]
- 94.Hammami A, Arlet G, Ben Redjeb S, Grimont F, Ben Hassen A, Rekik A, et al. Nosocomial outbreak of acute gastroenteritis in a neonatal intensive care unit in Tunisia caused by multiply drug resistant Salmonella wien producing SHV-2 beta-lactamase. Eur J Clin Microbiol Infect Dis. 1991;10(8):641–6. [DOI] [PubMed] [Google Scholar]
- 95.Hill PC, Onyeama CO, Ikumapayi UNA, Secka O, Ameyaw S, Simmonds N, et al. Bacteraemia in patients admitted to an urban hospital in West Africa. BMC Infect Dis. 2007;7:2 10.1186/1471-2334-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ibrahim Y, Adedare T, Ehinmidu J. Antibiotic sensitivity profiles of salmonella organisms isolated from presumptive typhoid patients in Zaria, northern Nigeria. Afr J Med Med Sci. 2005;34(2):109–14. [PubMed] [Google Scholar]
- 97.Ikumapayi UN, Antonio M, Sonne-Hansen J, Biney E, Enwere G, Okoko B, et al. Molecular epidemiology of community-acquired invasive non-typhoidal Salmonella among children aged 2–29 months in rural Gambia and discovery of a new serovar, Salmonella enterica Dingiri. J Med Microbiol. 2007;56(11):1479–84. [DOI] [PubMed] [Google Scholar]
- 98.Jacob S, Banura P, Baeten J, Moore C, Meya D, Nakiyingi. The impact of early monitored management on survival in hospitalized adult Ugandan patients with severe sepsis: a prospective intervention study*. Crit care med. 2012;40(7):2050–8. 10.1097/CCM.0b013e31824e65d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jacob ST, Moore CC, Banura P, Pinkerton R, Meya D, Opendi P, et al. Severe sepsis in two Ugandan hospitals: A prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS One. 2009;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kariuki S, Gilks C, Corkiu J, Kimari J, Benea A, Waiyaki P. Multi-drug resistant non-typhi salmonellae in Kenya. J Antimicrob Chemother. 1996;38(3):425–34. [DOI] [PubMed] [Google Scholar]
- 101.Kariuki S, Gilks C, Kimari J, Muyodi J, Waiyaki P, Hart C. Analysis of Salmonella enterica serotype Typhimurium by phage typing, antimicrobial susceptibility and pulsed-field gel electrophoresis. J Med Microbiol. 1999;48:1037–42. 10.1099/00222615-48-11-1037 [DOI] [PubMed] [Google Scholar]
- 102.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Hart CA. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol. 2006;6:101 10.1186/1471-2180-6-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kariuki S, Revathi G, Kariuki N, Muyodi J, Mwituria J, Munyalo A, et al. Increasing prevalence of multidrug-resistant non-typhoidal salmonellae, Kenya, 1994–2003. Int J Antimicrob Agents. 2005;25(1):38–43. 10.1016/j.ijantimicag.2004.08.015 [DOI] [PubMed] [Google Scholar]
- 104.Kariuki S, Revathi G, Kiiru J, Lowe B, Berkley JA, Hart CA. Decreasing prevalence of antimicrobial resistance in non-typhoidal Salmonella isolated from children with bacteraemia in a rural district hospital, Kenya. Int J Antimicrob Agents. 2006;28(3):166–71. 10.1016/j.ijantimicag.2006.05.026 [DOI] [PubMed] [Google Scholar]
- 105.Kassa-Kelembho E, Mbolidi CD, Service YB, Morvan J, Minssart P. Bacteremia in adults admitted to the Department of Medicine of Bangui Community Hospital (Central African Republic). Acta Trop. 2003;89(1):67–72. [DOI] [PubMed] [Google Scholar]
- 106.Keddy KH, Dwarika S, Crowther P, Perovic O, Wadula J, Hoosen A, et al. Genotypic and demographic characterization of invasive isolates of Salmonella Typhimurium in HIV co-infected patients in South Africa. J Infect Dev Ctries. 2009;3(8):585–92. [DOI] [PubMed] [Google Scholar]
- 107.Kohli R, Omuse G, Revathi G. Antibacterial susceptibility patterns of blood stream isolates in patients investigated at the Aga Khan University Hospital, Nairobi. East Afr Med J. 2010;87(2):74–80. [DOI] [PubMed] [Google Scholar]
- 108.Lafaix C, Castets M, Denis F, Diop Mar I. [Salmonellosis in Dakar: bacteriological, clinical, epidemiological and therapeutic aspects. Ten years records (author’s transl)]. Med Trop (Mars). 1979;39(4):369–79. [PubMed] [Google Scholar]
- 109.Lepage P, Bogaerts J, Nsengumuremyi F. Severe multiresistant Salmonella typhimurium systemic infections in Central Africa—clinical features and treatment in a paediatric department. J Antimicrob Chemother. 1984;14(SUPPL. B):153–9. [DOI] [PubMed] [Google Scholar]
- 110.Lepage P, Bogaerts J, Van Goethem C, Hitimana DG, Nsengumuremyi F. Multiresistant Salmonella typhimurium systemic infection in Rwanda. Clinical features and treatment with cefotaxime. J Antimicrob Chemother. 1990;26(SUPPL. A):53–7. [DOI] [PubMed] [Google Scholar]
- 111.Lepage P, Bogaerts J, Van Goethem C. Community-acquired bacteraemia in African children. Lancet. 1987;1(8548):1458–61. [DOI] [PubMed] [Google Scholar]
- 112.Levy H, Diallo S, Tennant SM, Livio S, Sow SO, Tapia M, et al. PCR method to identify Salmonella enterica serovars Typhi, Paratyphi A, and Paratyphi B among Salmonella isolates from the blood of patients with clinical enteric fever. J Clin Microbiol. 2008;46(5):1861–6. 10.1128/JCM.00109-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lewis DK, Whitty CJM, Walsh AL, Epino H, van den Broek NR, Letsky EA, et al. Treatable factors associated with severe anaemia in adults admitted to medical wards in Blantyre, Malawi, an area of high HIV seroprevalence. Trans R Soc Trop Med Hyg. 2005;99(8):561–7. 10.1016/j.trstmh.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 114.Ley B, Mtove G, Thriemer K, Amos B, von Seidlein L, Hendriksen I, et al. Evaluation of the Widal tube agglutination test for the diagnosis of typhoid fever among children admitted to a rural hdospital in Tanzania and a comparison with previous studies. BMC Infect Dis. 2010. January;10:180 10.1186/1471-2334-10-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ley B, Thriemer K, Ame SM, Mtove GM, von Seidlein L, Amos B, et al. Assessment and comparative analysis of a rapid diagnostic test (Tubex) for the diagnosis of typhoid fever among hospitalized children in rural Tanzania. BMC Infect Dis. 2011;11:147 10.1186/1471-2334-11-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lubwama SW. Human Salmonella serotypes in Uganda, 1967–1982. East Afr Med J. 1985;62(4):260–5. [PubMed] [Google Scholar]
- 117.Lunguya O, Lejon V, Phoba MF, Bertrand S, Vanhoof R, Glupczynski Y, et al. Antimicrobial Resistance in Invasive Non-typhoid Salmonella from the Democratic Republic of the Congo: Emergence of Decreased Fluoroquinolone Susceptibility and Extended-spectrum Beta Lactamases. PLoS Negl Trop Dis. 2013;7(3):e2103 10.1371/journal.pntd.0002103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mabey DCW, Brown A, Greenwood BM. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis. 1987;155(6):1319–21. [DOI] [PubMed] [Google Scholar]
- 119.Mackenzie G, Ceesay SJ, Hill PC, Walther M, Bojang KA, Satoguina J, et al. A decline in the incidence of invasive non-typhoidal salmonella infection in the gambia temporally associated with a decline in malaria infection. PLoS One. 2010;5(5):e10568 10.1371/journal.pone.0010568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maltha J, Guiraud I, Kaboré B, Lompo P, Ley B, Bottieau E, et al. Frequency of severe malaria and invasive bacterial infections among children admitted to a rural hospital in Burkina Faso. PLoS One. 2014. January;9(2):e89103 10.1371/journal.pone.0089103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maltha J, Guiraud I, Lompo P, Kaboré B, Gillet P, Geet C Van, et al. Accuracy of Pf HRP2 versus Pf -pLDH antigen detection by malaria rapid diagnostic tests in hospitalized children in a seasonal hyperendemic malaria transmission area in Burkina Faso. Malar J. 2014;13(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mandomando I, Macete E, Sigaúque B, Morais L, Quintó L, Sacarlal J, et al. Invasive non-typhoidal Salmonella in Mozambican children. Trop Med Int Health. 2009. December;14(12):1467–74. 10.1111/j.1365-3156.2009.02399.x [DOI] [PubMed] [Google Scholar]
- 123.Mayanja BN, Todd J, Hughes P, Van Der Paal L, Mugisha JO, Atuhumuza E, et al. Septicaemia in a population-based HIV clinical cohort in rural Uganda, 1996–2007: Incidence, aetiology, antimicrobial drug resistance and impact of antiretroviral therapy. Trop Med Int Heal. 2010;15(6):697–705. [DOI] [PubMed] [Google Scholar]
- 124.Meremo A, Mshana SE, Kidenya BR, Kabangila R, Peck R, Kataraihya JB. High prevalence of Non-typhoid salmonella bacteraemia among febrile HIV adult patients admitted at a tertiary Hospital, North-Western Tanzania. Int Arch Med. 2012;5(1):28 10.1186/1755-7682-5-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Milledge J, Calis J, Graham S, Molyneux E. Aetiology of Neonatal sepsis in Blantyre, Malawi: 1996–2001. Ann Trop Pediatr. 2005;25:99–108. [DOI] [PubMed] [Google Scholar]
- 126.Mirza N, Wamola I. Salmonella typhimurium outbreak at Kenyatta National Hospital (1985). East Afr Med J. 1989;66(7):453–7. [PubMed] [Google Scholar]
- 127.Moh R, Danel C, Messou E, A;Abo OTD. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21(18):2483–91. 10.1097/QAD.0b013e3282f09876 [DOI] [PubMed] [Google Scholar]
- 128.Moon TD, Silva WP, Buene M, Morais L, Valverde E, Vermund SH, et al. Bacteremia as a cause of fever in ambulatory, hiv-infected mozambican adults: Results and policy implications from a prospective observational study. PLoS One. 2013;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mtove G, Amos B, Nadjm B, Hendriksen ICE, Dondorp AM, Mwambuli A, et al. Decreasing incidence of severe malaria and community-acquired bacteraemia among hospitalized children in Muheza, north-eastern Tanzania, 2006–2010. Malar J. 2011;10(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mtove G, Hendriksen ICE, Amos B, Mrema H, Mandia V, Manjurano A, et al. Treatment guided by rapid diagnostic tests for malaria in Tanzanian children: Safety and alternative bacterial diagnoses. Malar J. 2011;10(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muyanja SZ, Larke N, Rutebarika D, Kaddu I, Nakubulwa S, Levin J, et al. Decreasing trends of bacteraemia among HIV-infected Ugandan adults: incidence, aetiology, clinical outcomes and effect of antiretroviral therapy in a semi-urban setting (2000–2008). Trop Med Int Heal. 2011;16(6):756–65. [DOI] [PubMed] [Google Scholar]
- 132.Nadjm B, Mtove G, Amos B, Walker NF, Diefendal H, Reyburn H, et al. Severe febrile illness in adult hospital admissions in Tanzania: A prospective study in an area of high malaria transmission. Trans R Soc Trop Med Hyg. 2012;106(11):688–95. 10.1016/j.trstmh.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 133.Nathoo KJ, Chigonde S, Nhembe M, Ali MH, Mason PR. Community-acquired bacteremia in human immunodeficiency virus-infected children in Harare, Zimbabwe. Pediatr Infect Dis J. 1996;15(12):1092–7. [DOI] [PubMed] [Google Scholar]
- 134.Nesbitt A, Mirza NB. Salmonella septicaemias in Kenyan children. J Trop Pediatr. 1989;35(1):35–9. [DOI] [PubMed] [Google Scholar]
- 135.Ngandjio A, Tchendjou P, Ndombo PK, Kamga HG, Fonkoua MC. Emergence of multi-drug resistant Salmonella enterica serotype Stanleyville infections among children in Yaounde, Cameroon. J Trop Pediatr. 2012;58(2):161–3. 10.1093/tropej/fmr054 [DOI] [PubMed] [Google Scholar]
- 136.Nhampossa T, Macete E, Sigau B, Fumado V, Alonso P, Bassat Q, et al. Severe malnutrition among children under the age of 5 years admitted to a rural district hospital in southern Mozambique. Public Health Nutr. 2013;16(9):1565–74. 10.1017/S1368980013001080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nielsen M V, Sarpong N, Krumkamp R, Dekker D, Loag W, Amemasor S, et al. Incidence and characteristics of bacteremia among children in rural Ghana. PLoS One. 2012;7(9):e44063 10.1371/journal.pone.0044063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Obaro S, Lawson L, Essen U, Ibrahim K, Brooks K, Otuneye A, et al. Community acquired bacteremia in young children from central Nigeria—a pilot study. BMC Infect Dis. 2011. January;11(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Dempsey TJD, McArdle TF, Lloyd-Evans N, Baldeh I, Laurence BE, Secka O, et al. Importance of enteric bacteria as a cause of pneumonia, meningitis and septicemia among children in a rural community in The Gambia, West Africa. Pediatr Infect Dis J. 1994;13(2):122–8. [DOI] [PubMed] [Google Scholar]
- 140.Ogunsola F, Arewa D, Akinsete I, Odugbemi T. Aetiology of bacteraemia among adult AIDS. Niger Postgrad Med J. 2009;16(3):186–92. [PubMed] [Google Scholar]
- 141.Ohmani F, Khedid K, Britel S, Qasmaoui A, Charof R, Filali A. Antimicrobial resistance in Salmonella enterica serovar Enteritidis in Morocco. J Infect Dev Ctries. 2010;4(12):804–9. [DOI] [PubMed] [Google Scholar]
- 142.Odhiambo F, Wamola I, Ndinya-Achola J. Aerobic and facultative bacterial isolates from blood cultures of children with clinically diagnosed septicaemia. East Afr Med J. 1991;68(11):869–74. [PubMed] [Google Scholar]
- 143.Okome-Kouakou M, Bekale J, Kombila M. Salmonellosis in HIV infection in a hospital setting in Gabon. Med Trop. 1999;59(1):46–50. [PubMed] [Google Scholar]
- 144.Omanga U, Muganga N, Kapepela M. Bacterial septicemia in children with homozygous sickle cell anemia. Ann Pediatr (Paris). 1989;36(5):315–8. [PubMed] [Google Scholar]
- 145.Ouedraogo A, Dakoure-Kissou A, Poda G, Koueta F. Epidemiology, microbiology, and outcomes of septicemia in children treated at the Charles de Gaulle University Pediatric Hospital in Burkina Faso. Sante. 2011;21(4):221–5. 10.1684/san.2011.0273 [DOI] [PubMed] [Google Scholar]
- 146.Oundo J, Kariuki S, Maghenda J, Lowe B. Antibiotic susceptibility and genotypes of non-typhi Salmonella isolates from children in Kilifi on the Kenya coast. Trans R Soc Trop Med Hyg. 2000;94(2):212–5. [DOI] [PubMed] [Google Scholar]
- 147.Oundo J, Muli F, Kariuki S, Waiyaki P, Iijima Y, Berkley J, et al. Non-typhi salmonella in children with severe malaria. East Afr Med J. 2002;79(12):633–9. [DOI] [PubMed] [Google Scholar]
- 148.Page A, Rekeneire N De, Sayadi S, Aberrane S, Janssens A, Rieux C, et al. Infections in Children Admitted with Complicated Severe Acute Malnutrition in Niger. PLoS One. 2013;8(7):e68699 10.1371/journal.pone.0068699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Papa F, Sari L. Septicemia during Salmonella wien and Salmonella typhimurium epidemics. Bacteriological and serological studies. Arch Inst Pasteur. 1970;48:53–60. [PubMed] [Google Scholar]
- 150.Petat EA, Martinet F, Lemmens P, Ghysels G, Verhaegen J, Vandepitte J. Human Salmonella and Shigella infections in Moroni, the capital of Great Comoro Island (1987–1988). Ann Soc Belg Med Trop (1920). 1990;70(4):297–302. [PubMed] [Google Scholar]
- 151.Peters RPH, Zijlstra EE, Schijffelen MJ, Walsh AL, Joaki G, Kumwenda JJ, et al. A prospective study of bloodstream infections as cause of fever in Malawi: Clinical predictors and implications for management. Trop Med Int Heal. 2004;9(8):928–34. [DOI] [PubMed] [Google Scholar]
- 152.Phoba MF, De Boeck H, Ifeka BB, Dawili J, Lunguya O, Vanhoof R, et al. Epidemic increase in Salmonella bloodstream infection in children, Bwamanda, the Democratic Republic of Congo. Eur J Clin Microbiol Infect Dis. 2014;33(1):79–87. 10.1007/s10096-013-1931-8 [DOI] [PubMed] [Google Scholar]
- 153.Phoba MF, Lunguya O, Mayimon D V, di Mputu PL, Bertrand S, Vanhoof R, et al. Multidrug-resistant Salmonella enterica, democratic republic of the Congo. Emerg Infect Dis. 2012;18(10):1693–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pithie A, Malin A, Robertson V. Salmonella and shigella bacteraemia in Zimbabwe. Cent Afr J Med. 1993;39(6):110–2. [PubMed] [Google Scholar]
- 155.Ricosse J, Saliou P, MG;Schollhammer AJM. Current aspects of human salmonellosis in Upper Volta. Study carried out at Centre Muraz (Bobo-Dioulasso) from 1966 to 1977: isolation of 1,013 Salmonella strains. Med Trop. 1979;39(4):381–94. [PubMed] [Google Scholar]
- 156.Seydi M, Soumare M, Sow AI, Diop BM, Sow PS. Current aspects of Salmonella bacteremia cases in the Ibrahima Diop Mar infectious diseases clinic, Fann national hospital center (Senegal). Med Mal Infect. 2005;35(1):23–7. 10.1016/j.medmal.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 157.Scragg J, Rubidge C, Appelbaum P. Incidence of infections with Salmonella enteritidis serotypes in Black and Indian children. A 16-year survey. South African Med J. 1978;54(11):432–3. [PubMed] [Google Scholar]
- 158.Seydi M, Soumare M, Sow AI, Diop SA, Sow I, Dieng AB, et al. Nontyphoidal Salmonella bacteremia cases in AIDS patients in a Dakar University Hospital (Senegal). Med Mal Infect. 2008;38(1):25–8. 10.1016/j.medmal.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 159.Shimeles D, Lulseged S. Clinical profile and pattern of infection in Ethiopian children with severe protein-energy malnutrition. East Afr Med J. 1994;71(4):264–7. [PubMed] [Google Scholar]
- 160.Sigaúque B, Roca A, Mandomando I, Morais L, Quintó L, Sacarlal J, et al. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J. 2009. February;28(2):108–13. 10.1097/INF.0b013e318187a87d [DOI] [PubMed] [Google Scholar]
- 161.Sow D, Cisse M, M; SEHAOM. Non typhoidic salmonellosis in the African pediatric population. Dakar Med. 1994;39(1):51–5. [PubMed] [Google Scholar]
- 162.Srikantiah P, Youssef FY, Girgis FY, Luby SP, Jennings G, Wasfy MO, et al. Population-based surveillance of typhoid fever in Egypt. Am J Trop Med Hyg. 2006;74(1):114–9. [PubMed] [Google Scholar]
- 163.Ssali FN, Kamya MR, Wabwire-Mangen F, Kasasa S, Joloba M, Williams D, et al. A prospective study of community-acquired bloodstream infections among febrile adults admitted to Mulago Hospital in Kampala, Uganda. J Acquir Immune Defic Syndr Hum Retrovirology. 1998;19(5):484–9. [DOI] [PubMed] [Google Scholar]
- 164.Tabu C, Breiman RF, Ochieng B, Aura B, Cosmas L, Audi A, et al. Differing burden and epidemiology of non-Typhi Salmonella bacteremia in rural and urban Kenya, 2006–2009. PLoS One. 2012. January;7(2):e31237 10.1371/journal.pone.0031237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vaagland H, Blomberg B, Kruger C, Naman N, Jureen R, Langeland N. Nosocomial outbreak of neonatal Salmonella enterica serotype Enteritidis meningitis in a rural hospital in northern Tanzania. BMC Infect Dis. 2004;4:35 10.1186/1471-2334-4-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Van Den Ende J, Rotter ML. An analysis of blood culture isolates from 7 South African teaching hospital centres. South African Med J. 1986;69(2):89–93. [PubMed] [Google Scholar]
- 167.Van Oosterhout JJG, Laufer MK, Graham SM, Thumba F, Perez MA, Chimbiya N, et al. A community-based study of the incidence of trimethoprim-sulfamethoxazole- preventable infections in Malawian adults living with HIV. J Acquir Immune Defic Syndr. 2005;39(5):626–31. [PubMed] [Google Scholar]
- 168.Vandenberg O, Nyarukweba DZ, Ndeba PM, Hendriksen RS, Barzilay EJ, Schirvel C, et al. Microbiologic and clinical features of salmonella species isolated from bacteremic children in eastern democratic republic of congo. Pediatr Infect Dis J. 2010;29(6):504–10. 10.1097/INF.0b013e3181cd615a [DOI] [PubMed] [Google Scholar]
- 169.Vugia DJ, Kiehlbauch JA, Yeboue K, N’Gbichi JM, Lacina D, Maran M, et al. Pathogens and predictors of fatal septicemia associated with human immunodeficiency virus infection in Ivory Coast, West Africa. J Infect Dis. 1993;168(3):564–70. [DOI] [PubMed] [Google Scholar]
- 170.Wadula J, Von Gottberg A, Kilner D, De Jong G, Cohen C, Khoosal M, et al. Nosocomial outbreak of extended-spectrum (beta)-lactamase-producing Salmonella Isangi in pediatric wards. Pediatr Infect Dis J. 2006;25(9):843–4. 10.1097/01.inf.0000233543.78070.a2 [DOI] [PubMed] [Google Scholar]
- 171.Waitt P, Mukaka M, Goodson P, SimuKonda F, Waitt C, Feasey N, et al. Sepsis carries a high mortality among hospitalised adults in Malawi in the era of antiretroviral therapy scale-up: A longitudinal cohort study. J Infect. 2015;70(1):11–9. 10.1016/j.jinf.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Walsh AL, Phiri AJ, Graham SM, Molyneux EM, Molyneux ME. Bacteremia in febrile Malawian children: Clinical and microbiologic features. Pediatr Infect Dis J. 2000;19(4):312–8. [DOI] [PubMed] [Google Scholar]
- 173.Wiktor SZ, Sassan-morokro M, Grant AD, Abouya L, Karon JM, Maurice C, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Côte d ‘ Ivoire : a randomised controlled trial. Lancet. 1999;353:1469–75. [DOI] [PubMed] [Google Scholar]
- 174.Williams TN, Uyoga S, Macharia A, Ndila C, Mcauley CF, Opi DH, et al. Bacteraemia in Kenyan children with sickle-cell anaemia : a retrospective cohort and case–control study. Lancet. 2009;374(9698):1364–70. 10.1016/S0140-6736(09)61374-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zouiten F, Ben S, Ammari L, Slim A, Kanoun F. AIDS in Tunisian women. Study of 92 cases. Tunisie Medicale. 2002;80(7):402–6. [PubMed] [Google Scholar]
- 176.Isendahl J, Manjuba C, Rodrigues A, Xu W, Henriques-normark B, Giske CG, et al. Prevalence of community-acquired bacteraemia in Guinea-Bissau : an observational study. BMC Infect Dis. 2014;14(1):715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Labi AK, Noah ON, Addison N, Sampene-Donkor E. Salmonella blood stream infections in a tertiary care setting in Ghana. BMC Infect Dis. 2014;14(1):697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Issack MI, Hendriksen RS, Hyytia-Trees E, Svendsen CA, Mikoleit M. Emergence and clonal dissemination of Salmonella enterica serovar enteritidis causing salmonellosis in Mauritius. J Infect Dev Ctries. 2014;8(4):454–60. 10.3855/jidc.3695 [DOI] [PubMed] [Google Scholar]
- 179.Mahende C, Ngasala B, Lusingu J, Butichi A, Lushino P, Lemnge M, et al. Aetiology of acute febrile episodes in children attending Korogwe District Hospital in north-eastern Tanzania. PLoS One. 2014;9(8):e104197 10.1371/journal.pone.0104197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Okome-Nkoumou M, Guiyedi V, Ondounda M, Efire N, Clevenbergh P, Dibo M, et al. Opportunistic diseases in HIV-infected patients in Gabon following the administration of highly active antiretroviral therapy: a retrospective study. Am J Trop Med Hyg. 2014;90(2):211–5. 10.4269/ajtmh.12-0780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ley B, Le Hello S, Lunguya O, Lejon V, Muyembe JJ, Weill FX, et al. Invasive Salmonella enterica serotype typhimurium infections, democratic Republic of the Congo, 2007–2011. Emerg Infect Dis. 2014;20(4):701–4. 10.3201/eid2004.131488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Feasey NA, Cain AK, Msefula CL, Pickard D, Alaerts M, Aslett M, et al. Drug resistance in salmonella enteric ser. Typhimurium bloodstream infection, Malawi. Emerg Infect Dis. 2014;20(11):1957–9. 10.3201/eid2011.141175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Issack MI, Garcia-Migura L, Ramsamy VD, Svendsen CA, Pornruangwong S, Pulsrikarn C, et al. Dissemination of clonal Salmonella enterica serovar Typhimurium isolates causing salmonellosis in Mauritius. Foodborne Pathog Dis. 2013;10(7):618–23. 10.1089/fpd.2012.1426 [DOI] [PubMed] [Google Scholar]
- 184.Marks F, Espinoza LC, May J. Incidence of invasive Salmonella infections in Agogo, Ghana. Int J Infect Dis. 2012;16S:124–5. [Google Scholar]
- 185.Nadjm B, Valero NR, Mtove G, Amos B, Reyburn H. Bacterial infection and malaria in hiv positive children admitted to hospital in northeast Tanzania; results of a one-year observational study. Am J Trop Med Hyg. 2014;91(5):158–9. [Google Scholar]
- 186.Kibuka A, Byakika P, Achan J, Yeka A, Mpimbaza A, Kayma M. Bacteremia in children under five years admitted with non-malaria febrile illness at Jinja Children’s hospital, Uganda. Am J Trop Med Hyg. 2013. March;89(5):171. [Google Scholar]
- 187.Obaro S, Olanipekun G, Ajose T, Shatima D, Ohiaeri C, Ihebuzor N, et al. Invasive salmonellosis in Nigerian children. Int J Infect Dis. 2014;21:53. [Google Scholar]
- 188.Sow S. Invasive non-typhoidal salmonella infections in children in Mali, West Africa. Trop Med Int Heal. 2011;16:31. [Google Scholar]
- 189.Sylla MB, Tapia M, Sow SO, Sissoko S, Kourouma N, Onwuchekwa U, et al. Infections associated with severe malnutrition among outpatient children in Bamako, Mali. Am J Trop Med Hyg. 2011;85(6):35. [Google Scholar]
- 190.Theurer A, Mulenga S, Chitandale E, Neuhann F, Schutt-Gerowitt H, Fatkenheuer G. Malaria and invasive bacterial infections as causes of fever among adult patients presenting to the medical department of a referral hospital in central Malawi. Trop Med Int Heal. 2011;16:59–60. [Google Scholar]
- 191.Were T, Davenport G, Hittner J, Konah S, Ogula G, Kondiek N, et al. Antibiotic resistance of invasive non-typhoidal Salmonella (NTS) isolates in children from western Kenya. Am J Trop Med Hyg. 2011;85(6):329. [Google Scholar]
- 192.Biggs HM, Lester R, Nadjm B, Mtove G, Todd JE, Kinabo GD. Invasive Salmonella infections in areas of high and low malaria transmission intensity in Tanzania. Clin Infect Dis. 2014;58(5):638–47. 10.1093/cid/cit798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dakoury-Dogbo N, Anglaret X, Ouassa T, Toure S, Bonard D, Gourvellec G, et al. Causes of fever in HIV-1 infected adults. Press Medicale. 2001;30(34):1674–80. [PubMed] [Google Scholar]
- 194.Enwere G, Cheung YB, Zaman SMA, Akano A, Oluwalana C, Brown O, et al. Epidemiology and clinical features of pneumonia according to radiographic findings in Gambian children. Trop Med Int Heal. 2007;12(11):1377–85. [DOI] [PubMed] [Google Scholar]
- 195.Jacob ST, Pavlinac PB, Nakiyingi L, Banura P, Baeten JM, Morgan K, et al. Mycobacterium tuberculosis Bacteremia in a Cohort of HIV-Infected Patients Hospitalized with Severe Sepsis in Uganda–High Frequency, Low Clinical Sand Derivation of a Clinical Prediction Score. PLoS One. 2013;8(8):e70305 10.1371/journal.pone.0070305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mandomando I, Sigauque B, Morais L, Espasa M, Valles X, Sacarlal J, et al. Antimicrobial drug resistance trends of bacteremia isolates in a rural hospital in southern Mozambique. Am J Trop Med Hyg. 2010;83(1):152–7. 10.4269/ajtmh.2010.09-0578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mtove G, Amos B, Von Seidlein L, Hendriksen I, Mwambuli A, Kimera J, et al. Invasive salmonellosis among children admitted to a Rural Tanzanian Hospital and a comparison with previous studies. PLoS One. 2010;5(2):e9244 10.1371/journal.pone.0009244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nadjm B, Amos B, Mtove G, Ostermann J, Chonya S, Wangai H, et al. WHO guidelines for antimicrobial treatment in children admitted to hospital in an area of intense Plasmodium falciparum transmission: Prospective study. BMJ. 2010;340(7751):848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schwarz NG, Sarpong N, Hunger F, Marks F, Acquah SEK, Agyekum A, et al. Systemic bacteraemia in children presenting with clinical pneumonia and the impact of non-typhoid salmonella (NTS). BMC Infect Dis. 2010;10(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Muyembe T, Maes L, G;Vandeven MM. Epidemiology and drug resistance of salmonella infections in Kinshasa 1974–1975. Ann Soc Belges Med Trop Parasitol Mycol. 1977;57(6):545–56. [PubMed] [Google Scholar]
- 201.Okomo U, Garba D, UN;Udo FAO. Bacterial Isolates and Antibiotic Sensitivity among Gambian Children with Severe Acute Malnutrition. Int J Pediatr. 2011;2011(Article ID 825123). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Akinyemi K, Coker A, Olukoya D, Oyefolu A, Amorighoye E, Omonigbehin E. Prevalence of multi-drug resistant Salmonella typhi among clinically diagnosed typhoid fever patients in Lagos, Nigeria. J Heal Popul Nutr. 2000;55:489–93. [DOI] [PubMed] [Google Scholar]
- 203.Moher D, Liberati A, Tetziaff J, Altman D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ao T, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global Burden of Invasive Nontyphoidal Salmonella Disease, 2010. Emerg Infect Dis. 2015;21(6):941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Okoro C, Kingsley R, Connor T, Harris S, Parry C, Al-Mashhadani M, et al. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet. 2012;44(11):1215–21. 10.1038/ng.2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Feasey NA, Hadfield J, Keddy KH, Dallman TJ, Jacobs J, Deng X, et al. Distinct Salmonella Enteritidis lineages associated with enterocolitis in high-income settings and invasive disease in low-income settings. Nat Genet. 2016. October;48(10):1211–7. 10.1038/ng.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.von Kalckreuth V, Konings F, Aaby P, Adu-Sarkodie Y, Ali M, Aseffa A, et al. The Typhoid Fever Surveillance in Africa Program (TSAP): Clinical, Diagnostic, and Epidemiological Methodologies. Clin Infect Dis. 2016. March;62 Suppl 1(Suppl 1):S9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(TIF)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.