Short abstract
Children with peanut allergy are often provided with adrenaline (epinephrine) in case of a severe reaction. The probability of a life threatening reaction is low, however, and the criteria for provision are controversial. How should the costs and benefits be balanced?
Case study 1
Dylan, a 20 month old boy, was referred to a paediatric allergy clinic for assessment of his peanut allergy. At 12 months of age he developed facial contact urticaria to peanut butter, which spontaneously resolved without respiratory or other symptoms. Since then, he has not had further reactions or eaten peanuts, although the rest of the family often eat peanuts and nuts. Dylan is regularly cared for by his grandparents and does not attend a childcare centre. His skin prick tests show a 9 mm (≥ 3 mm is considered positive) reaction to peanut.
The doctor recommended that he continue to avoid peanuts and be reviewed annually with skin prick testing. If the results remain positive without other clinical reactions, Dylan will be considered for a formal food challenge when he starts school. An emergency adrenaline (epinephrine) autoinjector (self or carer administered) was not recommended. Dylan's mother said, “I had heard about [autoinjectors] so I was waiting to hear what the specialist would say. I suppose that if you had to, you would give it, but I just can't see it. I hate seeing him have needles for any reason.”
Case study 2
Jarred is 23 months old and attended the same clinic. At 9 months of age, peanut butter touched his face and he developed local urticaria and lip and periorbital swelling without respiratory or systemic symptoms. His parents took him to the local hospital, where he was placed on cardiorespiratory monitors and given two adrenaline injections. He was then seen by a paediatric allergist, who prescribed an adrenaline autoinjector. Since then he has avoided peanuts and has not had further reactions. Peanuts have been removed from the household and the family's diet. After Jarred's enrolment, the childcare centre he attends two days a week completely banned peanuts, nuts, and any foods labelled “may contain nuts.”
At this consultation, skin prick tests showed a 9 mm reaction to peanut. Jarred's parents were advised that he should continue to avoid peanuts. Although his mother was informed that the risk of death was extremely low, she wished to continue Jarred's autoinjector prescription: “My biggest fear is that he could be having a reaction and he can't tell us... the autoinjector allows me to feel more in control... it's a safety net so I'm not totally helpless.”
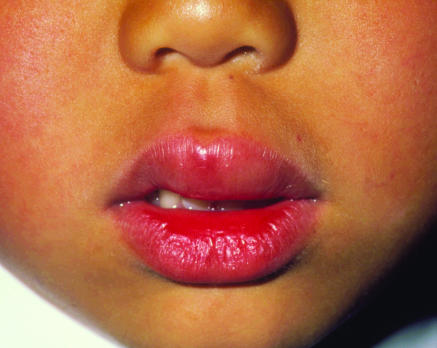
Figure 1.
Mild reaction to peanut
Credit: P MARAZZI/SPL
The issues
It will be no surprise to clinicians that patients with similar clinical features can end up being managed differently. Variation in medical practice may be deemed appropriate or inappropriatew1 and stems from many sources, some of which are unavoidable. Underlying the decisions of individual doctors and patients is the inherently uncertain nature of medical knowledge. In our example of childhood food allergies, the uncertainty is given a potent twist by a remote but dreaded outcome—the death of a child. These factors create a dilemma when weighing up the risk of a severe reaction or anaphylaxis against possible responses.
Peanut allergy
Peanuts are a commonly eaten food and often included in processed and pre-prepared foods. The prevalence of peanut allergy is rising and was estimated at 1.5% in a recent child population study.1 Peanuts have been identified as the most common precipitant for deaths from food induced anaphylaxis in the United Kingdom,2 although not in children under 13 years of age.3 Currently, there is no effective immunotherapeutic or medical treatment, so management strategies rely on avoidance of food allergens and emergency treatment of severe reactions. Analyses of fatal cases have suggested that deaths may be prevented with early administration of adrenaline,2 leading to calls for adrenaline autoinjectors to be widely available to children with food allergies and their carers.4 Although this response seems rational and empirically sound, it camouflages persisting medical, scientific, and ethical uncertainties.5 w2
Medical uncertainties
Uncertainty is inevitable in medical practice.w3 For example, the diagnosis of food allergy in both our cases relies primarily on clinical history, but equivocal histories are not uncommon. Investigations for confirming food allergy such as skin prick tests have limited predictive value, and food challenges can trigger serious allergic reactions.6 w4 Although the diagnosis of peanut allergy in Dylan and Jarred is not in question, clinical features cannot accurately predict whether they would have a life threatening reaction if they ate peanut products.7
Management uncertainties also exist. Although avoiding trigger foods and treating serious reactions are widely accepted, determining the degree of avoidance required is difficult,w5 and the prescribing of adrenaline autoinjectors is debated.w6 Published recommendations vary from the assertion that autoinjectors should be given to all patients with peanut allergy6 to statements that they should be given only to patients who have had moderate to severe reactions, including laryngeal oedema, dyspnoea, and collapse, unless the patient currently has asthma or reactions to trace amounts of allergen.8 Follow up studies have shown that even when autoinjectors are prescribed they may be not be used appropriately, or at all, for anaphylactic episodes.9 w7
To summarise, we cannot predict those at high risk of serious reactions, it is difficult to know who to treat and to what extent, and on a population basis, the evidence for providing adrenaline autoinjectors to prevent deaths from anaphylaxis is unclear.
Risks and values
Although families and the public may wish to hear that there is no risk of childhood anaphylaxis or other feared outcomes such as adverse reactions to measles vaccine, it is not possible to prove a zero risk conclusively.w8 A single case report of fatal anaphylaxis to nut in a 2 year old is sufficient to say that a risk exists.10 Although the probability of death from anaphylaxis in young children is very low, uncertainty about degrees of risk and scientific disagreement over appropriate responses heightens public perceptions of danger.w9
Various sources, including friends and family, mass media, and lobby groups, and the way messages are conveyed can also modify how risks are interpreted.w10 Jarred's emergency treatment at the local hospital is likely to have influenced his parents' perception of the risk of severe reactions. Public perceptions of the risk of childhood anaphylaxis are also increased by the difficulty of controlling exposure to food, the unpredictability of fatal outcomes, and the catastrophic and unjust nature of child deaths. Our reactions reflect fundamental beliefs about societal obligations to protect the vulnerable, and parental responsibilities to nurture and ensure the safety of their children.11 Thus, risk is not a value neutral probability but a socially mediated sense of threat to a cherished section of society. As one prominent UK allergist has stated, “There may be no such thing as a definitely low risk peanut allergic child.”w6
Is it better to be safe than sorry?
It could be argued that we should do everything possible to keep allergic children safe. However, the value that we attach to children does not, in itself, justify such actions. Minimising risks to children incurs costs, financial and otherwise, which may be unfairly borne by many when benefits are granted to a few. Even for those few, medical interventions of unproved effectiveness can cause physical harm and give false hope. However, this argument raises the question of how to define and measure effectiveness, benefit, and costs.
In recent years, the medical profession has tended to refer to evidence based medicine and randomised controlled trials to show the effectiveness of interventions. A randomised controlled trial to determine who should be prescribed an autoinjector would be difficult; the endpoint of death is rare and there are many confounders, so an enormous study population would be required for statistical validity. It is doubtful whether there is sufficient equipoise to ethically support such a trial. Even if such a trial were conducted, the answers are likely to be expressed as probabilities—whether an intervention is more or less likely to be effective. Although valuable, this type of information is unlikely to reduce therapeutic uncertainty to a level that will satisfy many parents and doctors, and narrowly defined quantitative analyses will fail to include benefits valued by families such as Jarred's.
In such situations, it could be argued that clinical decisions should be based on parental preferences and notions of benefit. Parents are accepted proxy decision makers for their children; they are expected to act in their child's best interests and are in the best position to weigh up the consequences to their children and themselves.11 Parents are also responsible for implementing any interventions and may have a better appreciation of potential risks, which may not be anticipated by others. More generally, patient participation in decision making promotes trust between patients and doctors and may result in greater satisfaction with care.w11 The idea that differing notions of risk and benefit should be accommodated is reflected in the evolving literature on risk; the emphasis is shifting from correcting public “misperceptions” to acknowledging that lay perceptions of risk have their own validity and logic.12
However, if broader definitions of benefit are to be accepted, wider definitions of cost must also be considered. The financial cost of providing every child under 16 years old who has peanut allergy in Australia with two autoinjectors has been estimated at $A51.7m (£20.1m, €29.7m) for each life saved.7 Rigorously minimising accidental exposure and carrying an autoinjector requires families to be in a continual state of hypervigilance and may reduce their quality of life.13,14 Keeping children “safe” may also mean restricting their activities and opportunities to develop independence.w12 Although parents may agree to these burdens in return for indefinite benefits, others can become involved. Teachers and child carers may be obliged to undertake training to recognise reactions and administer adrenaline, and the choices of other parents and children will be restricted by nut and peanut bans at schools and childcare centres. Ultimately, society as a whole will bear an opportunity cost when resources are not allocated so that benefits will be maximised to all.
Summary points
Peanut allergy is an increasingly common problem.
The risk of anaphylaxis is difficult to predict
Providing adrenaline autoinjectors to every child with food allergy is costly and criteria for provision are uncertain
Risk perceptions are influenced by the value society places on children's lives
Management should be decided with parents after discussing the uncertainties
Thus it could be argued that at a certain level of response, the costs of minimising the risk of food anaphylaxis will outweigh the likely benefits. However, the point at which such a threshold lies depends on how the consequences are viewed, and the societal values inherent in such evaluations. Children occupy a special place in modern society, such that their safety and access to education and health care are viewed as priorities. Accordingly, the public has much sympathy for attempts to reduce risks to children and with parental predicaments created by such attempts. An argument can therefore be made on sociocultural grounds that we all gain from efforts to prevent a child's death, justifying the liberal provision of adrenaline autoinjectors and public health measures to restrict exposure to peanuts. A precautionary approach could then be acceptable, even if costs are borne by many and the benefits are unquantifiable.
Conclusions
Childhood peanut allergy presents the possibility of a rare but feared outcome without clear evidence to guide management choices. As a result, a range of clinical decisions could be justified. In such cases, the best response for doctors could be to engage families in a process of negotiation that acknowledges uncertainties, invites and considers all relevant viewpoints, and examines their basis non-judgmentally. Acknowledging uncertainty does not mean that doctors should constantly equivocate; patients may interpret this as meaning “there is nothing to be done” or that “it is simply a matter of chance.” When giving advice, doctors might consider the likely effect of different recommendations, possible costs and harms from various interventions, and the values of both the family and the broader community. They should be prepared to explain their reasoning, while recognising that parents and children may value options differently. Whatever is decided, providing information and support, and responding sensitively to parental anxiety remains essential.
This approach to the doctor-patient relationship implies commitment, trust, and open communication. If the preferences of patients and doctors are to be taken into account, practices are likely to vary between individual cases. Doctors may have to accept that in a pluralistic world, there will be varying trade-offs between effective, equitable, or cost efficient goals and between the interests of particular families and of greater society. In situations of uncertainty, clinicians can feel burdened by a perceived need to reconcile competing or incommensurable interests. Beyond finding pragmatic solutions within the clinical setting, the whole responsibility for resolving these interests cannot be shouldered by individual doctors but should be shared by all stakeholders.
Supplementary Material
This article is part of an occasional series, edited by Michael Parker (michael.parker@ethox.ox.ac.uk) and Julian Savulescu, analysing ethical issues that confront health professionals in daily practice
 References w1-12 are on bmj.com
References w1-12 are on bmj.com
We thank the parents of Dylan and Jarred for contributing their stories to this article.
Contributors and sources: All authors conceived and planned the article, critically reviewed drafts, and approved the final version. WH wrote the first draft, which arose from her research on the handling of risk and uncertainty in clinical and policy decision making, as applied to food anaphylaxis in children. WH wrote the stories using the parents' words. She is the guarantor.
WH was supported by grants from the Australian Allergy Foundation and the National Health and Medical Research Council of Australia ID297112.
Competing interests: AK's superannuation fund owns shares in Commonwealth Serum Laboratories, which distributes adrenaline autoinjectors (EpiPens) in Australia.
References
- 1.Grundy J, Matthews S, Bateman B, Dean T, Arshad SH. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol 2002;110: 784-9. [DOI] [PubMed] [Google Scholar]
- 2.Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy 2000;30: 1144-50. [DOI] [PubMed] [Google Scholar]
- 3.Macdougall CF, Cant AJ, Colver AF. How dangerous is food allergy in childhood? The incidence of severe and fatal allergic reactions across the UK and Ireland. Arch Dis Child 2002;86: 236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med 1992;327: 380-4. [DOI] [PubMed] [Google Scholar]
- 5.Warner JO. How dangerous is food allergy in childhood? Pediatr Allergy Immunol 2002;13: 149-50. [DOI] [PubMed] [Google Scholar]
- 6.Sampson H. Peanut allergy. N Engl J Med 2002;346: 1294-9. [DOI] [PubMed] [Google Scholar]
- 7.Kemp A. EpiPen epidemic: suggestions for rational prescribing in childhood food allergy. J Pediatr Child Health 2003;39: 372-5. [DOI] [PubMed] [Google Scholar]
- 8.Clark AT, Ewan PW. Food allergy in childhood: have the dangers been underestimated? Arch Dis Child 2003;88: 79-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold MS, Sainsbury R. First aid anaphylaxis management in children who were prescribed an epinephrine autoinjector device (EpiPen). J Allergy Clin Immunol 2000;106: 171-6. [DOI] [PubMed] [Google Scholar]
- 10.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol 2001;107: 191-3. [DOI] [PubMed] [Google Scholar]
- 11.Jonsen A, Siegler M, Winslade W. Clinical ethics: a practical approach to ethical decisions in clinical medicine. 5th ed. New York: McGraw-Hill, 2002.
- 12.Bennett P, Calman K, eds. Risk communication and public health. Oxford: Oxford University Press, 1999.
- 13.Munoz-Furlong A. Daily coping strategies for patients and their families. Pediatrics 2003;111: 1654-61. [PubMed] [Google Scholar]
- 14.Avery NJ, King RM, Knight S, Hourihane J O'B. Assessment of quality of life in children with peanut allergy. Pediatr Allergy Immunol 2003;14: 378-82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.